Abstract
Despite continued research efforts, the threat of drug resistance from a variety of bacteria continues to plague clinical communities. Discovery and validation of novel biochemical targets will facilitate development of new drugs to combat these organisms. The methylerythritol phosphate (MEP) pathway to make isoprene units is a biosynthetic pathway essential to many bacteria. We and others have explored inhibitors of the MEP pathway as novel antibacterial agents. Mycobacterium tuberculosis, the causative agent of tuberculosis, and Yersinia pestis, resulting in the plague or “black death,” both rely on the MEP pathway for isoprene production. 1-Deoxy-D-xylulose 5-phosphate reductoisomerase (Dxr) catalyzes the first committed step in the MEP pathway. We examined two series of Dxr inhibitors based on the parent structure of the retrohydroxamate natural product FR900098. The compounds contain either an extended N-acyl or O-linked alkyl/aryl group, and are designed to act as bisubstrate inhibitors of the enzyme. While nearly all of the compounds inhibited both Mtb and Yp Dxr to some extent, compounds generally displayed more potent inhibition against the Yp homolog, with the best analogs displaying nM IC50 values. In bacterial growth inhibition assays, the phosphonic acids generally resulted in poor antibacterial activity, likely a reflection of inadequate permeability. Accordingly, diethyl and diPOM prodrug esters of these compounds were made. While the added lipophilicity did not enhance Yersinia activity, the compounds showed significantly improved antitubercular activities. The most potent compounds have Mtb MIC values of 3–12 µg/mL. Taken together, we have uncovered two series of analogs that potently inhibit Dxr homologs from Mtb and Yp. These inhibitors of the MEP pathway, termed MEPicides, serve as leads for future analog development.
Keywords: MEP pathway, antibiotic, phosphonate prodrug, Mycobacterium tuberculosis, Yersinia pestis
Graphical Abstract
Despite a recent surge in the search for novel antibiotics, there continues to be a significant and pressing need for additional therapies in our clinical arsenal combatting bacterial pathogens.(1–3) Disease due to drug-resistant strains of bacteria worsens public health and highlights the lack of novel chemical entities in the drug discovery pipeline.(1, 4, 5) Resistance to first- and second-line treatments has increased over the last several years, resulting in bacterial strains with very limited therapeutic options. It is therefore imperative that new drugs target biochemical pathways not currently used by existing antibiotics. These novel mechanisms-of-action must be validated to pave the way for successful drug candidates.
The goal of our work is to design small molecule inhibitors of specific biochemical pathways, which can then serve as chemical tools to validate these new drug targets. Pathways found in common across several microbial pathogens, but not in humans, are particularly attractive for drug discovery. Although pathogens may share the same rate-limiting enzyme, known structural variations within the active sites can be considered when developing specific inhibitors. Alternatively, exploring inhibition across species is an efficient and economical way to develop a broad-spectrum agent. The paucity of novel antibiotics argues strongly that both approaches should be employed.
Our work has recently focused on two organisms, Mycobacterium tuberculosis (Mtb), causing tuberculosis (TB), and Yersinia pestis (Yp), causing plague (or black death).(6–10) TB is still responsible for nearly 2 million deaths each year and threatens public health in both developed and developing countries.(11–13) Gram-negative Yersinia pestis, a bacterial cousin of Mtb, continues to infect individuals worldwide.(14) Drug resistant strains of both organisms have been well characterized and pose significant threats to public health.(15)
The methylerythritol phosphate (MEP, or nonmevalonate) pathway of isoprene synthesis is common to most bacteria, including Mtb and Yp. First identified in plants and bacteria,(16, 17) this pathway catalyzes the synthesis of five-carbon isopentenyl (IPP) and dimethylallyl (DMAPP) pyrophosphate units from pyruvate and glyceraldehyde-3-phosphate (Figure 1). IPP and DMAPP are essential building blocks of larger, more complex molecules required for several pathways including respiration, cell wall biosynthesis, and cell signaling. While humans also require the production of IPP and DMAPP, these molecules are synthesized via the mevalonate pathway, distinct from the MEP pathway. There are no human homologs of the MEP enzymes. As such, we and others have explored this pathway as a source of novel targets for antibacterial drug discovery.
Figure 1.
The Methyl Erythritol Phosphate (MEP) pathway of isoprenoid biosynthesis.
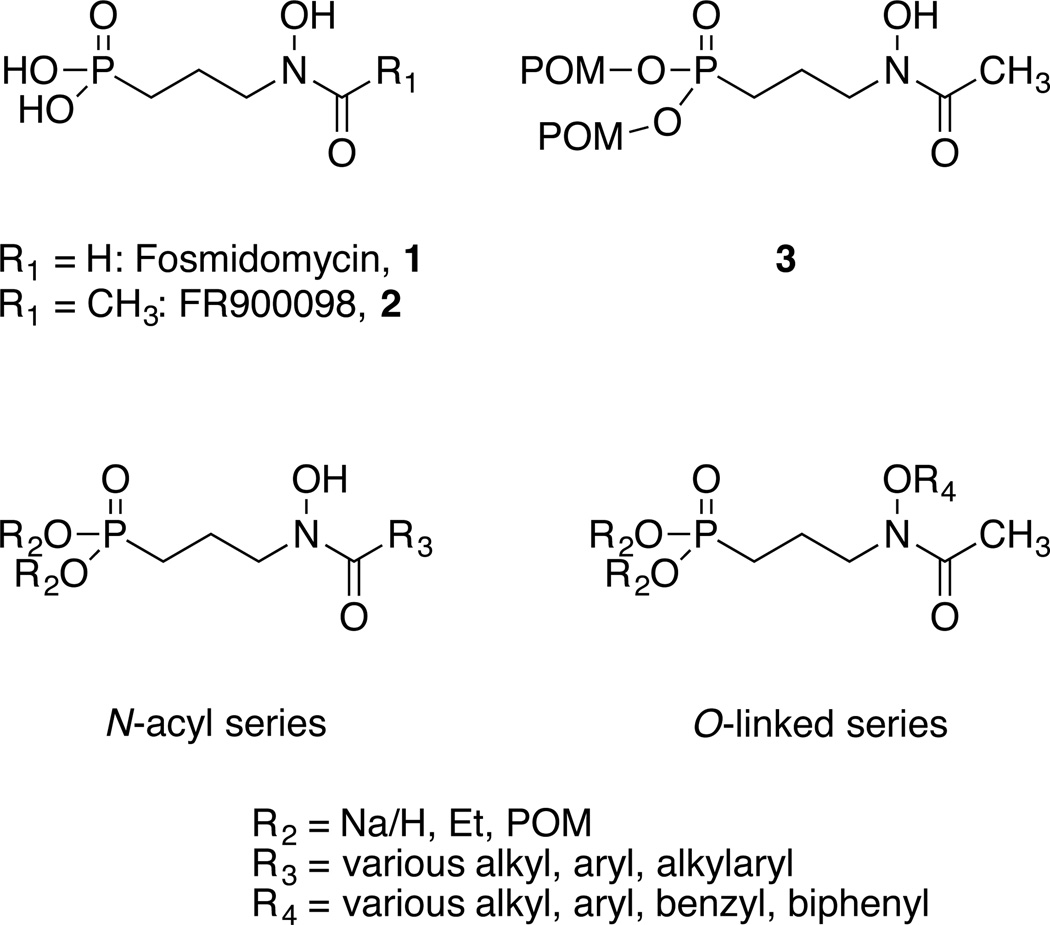
1-Deoxy-D-xylulose 5-phosphate reductoisomerase (Dxr, IspC) catalyzes the first-committed step of the MEP pathway and has been widely explored as a novel antibiotic target.(18–21) Catalyzing both the reduction and isomerization of DXP to MEP, Dxr has been shown essential in several organisms including Plasmodium falciparum (causing malaria), Mtb, and Escherichia coli.(22–26) Natural products fosmidomycin (1) and FR900098 (2) (Figure 2) potently inhibit Dxr,(27–30) however their polar nature prevents cellular penetration in organisms such as Mtb that have a cell wall rich in mycolic acids.(22) Accordingly, we have demonstrated that increasing the lipophilic character of these Dxr inhibitors using a phosphonate ester prodrug strategy significantly improves their antitubercular activity.(6) Compound 3, for example, has an MIC of 50–100 µg/mL against Mtb where FR900098 is essentially inactive. Notably, these prodrugs also demonstrate inhibitory activity towards Gram-negative bacteria harboring a mutation of the bacterial glycerol-3-phosphate transporter (Glp-T; a fosmidomycin/FR900098 influx protein), known to confer resistance to fosmidomycin/FR900098 treatment.(7) While a specific mechanism has not been reported, cleavage of the prodrug moiety is thought to occur via nonspecific esterases inside the cell.(31)
Figure 2.
Natural products fosmidomycin and FR900098, and their lipophilic esters (such as 3), are known Dxr inhibitors with antibacterial activity. N-acyl and O-linked analogs show potent Dxr inhibition and are the focus of this work.
We and others have examined the SAR of fosmidomycin and FR900098 as it relates to Dxr inhibition and antimicrobial effects. Unsaturation within the propyl chain is tolerated, while changing the chain length is not.(10) Substitution at the alpha position (relative to the phosphorus atom) enhances inhibitor potency.(32–34) Recently, we explored a bisubstrate approach where compounds were designed to occupy both the DXP and NADPH binding sites.(8) These compounds stem from two structure classes: an N-acyl series includes extensions of fosmidomycin’s formyl group, and an O-linked series explores substitution of the hydroxyl group of the retrohydroxamate (Figure 2). Early modeling efforts suggested that compounds from both series would act as Dxr ligands.(8) We refer to these compounds collectively as MEPicides (inhibitors of the MEP pathway displaying antimicrobial activity). While a small set of compounds from these series has been reported,(8, 9, 34) we describe here an expanded set of compounds from both chemical classes, SAR of these compounds against Dxr from Mtb and Yp, and antibacterial activities of these compounds against Mtb, Yp, and E. coli.
Our prior work indicated that N-acyl and O-linked compounds could act as bisubstrate inhibitors of Dxr, binding to both the DXP and NADPH binding sites.(8) An interesting conclusion from this work pertained to the importance of the retrohydroxamate in Dxr inhibition. The retrohydroxamate, found in fosmidomycin, FR900098, and most Dxr inhibitors reported to date, is expected to coordinate a divalent cation resident between the DXP and NADPH binding sites. The cation is necessary for catalysis and a prominent feature of Dxr crystal structures.(19) For retrohydroxamate inhibitors, deprotonation of the hydroxyl group proton is thought to occur, leaving the anion able to bind the metal cation.(35) This putative mode of binding is reasonable for the N-acyl analogs. The O-linked compounds, with a substituted hydroxyl group, are unable to form the corresponding anion and, thus, are not expected to interact with the cation in the same way. One prediction might be that the O-linked compounds would be significantly less active, compared with the N-acyl analogs, as a result. Here, we describe the synthesis and evaluation of larger series of N-acyl and O-linked compounds designed to test this hypothesis. Our results indicate that an anion to strongly coordinate the metal cation is not necessary for potent Dxr inhibition.
RESULTS and DISCUSSION
Synthesis
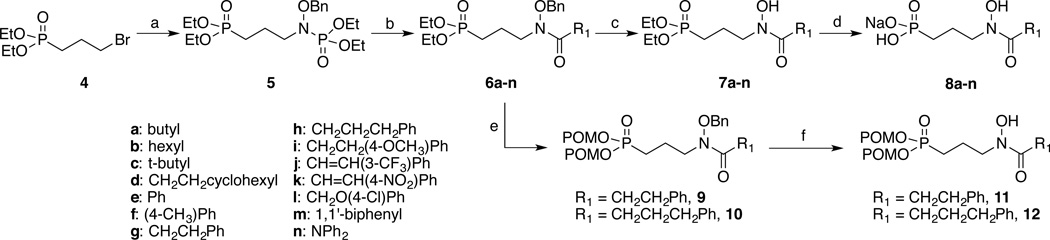
The synthetic routes used to prepare the N-acyl and O-linked compounds are shown in Schemes 1 and 2, respectively. Scheme 1 shows the synthetic route used to prepare the phosphonate salts (8a–n) and esters (7a–n, 11 and 12) of the N-acyl series. Benzyl-protected diethyl phosphoramidate(36) was deprotonated using sodium hydride and combined with diethyl phosphonate ester 4 to yield substituted phosphoramidate 5.(6, 36) Compound 5 was hydrolyzed under acidic conditions(36) and then acylated(10) with a series of acid chlorides to give N-acyl analogs 6a–n. Removal of the benzyl group using either hydrogenolysis or BCl3 (10, 37) yielded diethyl esters 7a–n. Treatment of these esters with trimethylsilylbromide(38) and BSTFA,(10, 39) followed by sodium hydroxide gave monosodium salts 8a–n. To obtain the dipivaloyloxymethyl (POM) esters (9 and 10), compounds 6g and 6h were treated with TMSBr/BSTFA and reprotected using POM chloride and triethylamine.(6) Removal of the benzyl group using either catalytic hydrogenation or BCl3 gave POM-protected N-acyl analogs 11 and 12.
Scheme 1.
aReagents and conditions: a) NaH, NH(OBn)PO(OEt)2, TBABr, NaI; b) i. 2N HCl; ii. R(CH2)nCOCl, Et3N; c) H2, Pd/C or BCl3; d) i. TMSBr, BSTFA; ii: NaOH; e) i. TMSBr; ii: NaOH; iii) POMCl, Et3N; f) H2, Pd/C or BCl3.
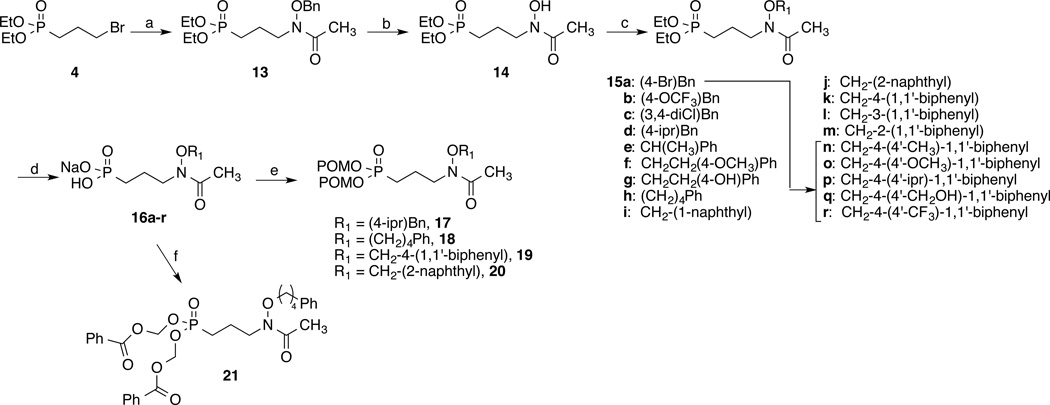
A related synthetic pathway was used to prepare the phosphonate salts (16a–r) and esters (15a–r and 17–21) of the O-linked series (Scheme 2). Compound 4 was treated with sodium hydride and acetylated O-benzylhydroxylamine(40, 41) to give compound 13. Hydrogenolysis removed the benzyl group, affording compound 14(10). Ether formation using sodium hydride and a series of alkyl/aryl halides gave the diethyl ester compounds 15a–m. Suzuki coupling using the benzyl analog (15a) and a series of boronic acids gave ethers 15n–r. Treatment with TMSBr and sodium hydroxide converted the diethyl esters to their sodium salts 16a–r. Dipivaloyloxymethyl esters (17–20) were obtained by treating the acids of 16d, 16h, 16k and 16j, respectively, with POM chloride and triethylamine. Alternate ester 21 was similarly obtained by treating 16h with chloromethyl benzoate(42) and triethylamine.
Scheme 2.
aReagents and conditions: a) NaH, NH(OBn)Ac, NaI; b) H2, Pd/C; c) NaH, NaI, Ar(CH2)nBr; d) i. TMSBr; ii: NaOH; e) POMCl, Et3N; f) PhCO2CH2Cl, Et3N (16h).
Biological Evaluation
The target compounds were examined for biological activity in two ways. First, all phosphonic acids (8a–n and 16a–r) were examined as inhibitors of Dxr from both Mtb and Yp at 100 µM (Figure 3A). This data yielded information about the intrinsic activity of each compound, as well as trends seen across compound classes and homologous enzymes (Figure 3B). Full inhibition curves were generated, and IC50 values were determined for compounds displaying >75% inhibition of the enzyme (Table 1). Second, the phosphonic acids (8a–n and 16a–r) and lipophilic esters (7a–n, 11, 12, 15a–r, and 17–21) were evaluated for growth inhibition activity against E. coli, Yp, and Mtb. The Mtb MIC values are shown in Table 2.
Figure 3.
Inhibition of Mtb and Yp Dxr by N-acyl and O-linked MEPicides. A) Percent residual activity of Yp Dxr (grey) and Mtb Dxr (black) when treated with 100 µM of inhibitor, as compared to an uninhibited sample (DMSO). Fosmidomycin (1) and FR900098 (2) are positive controls. B) Inhibition of homologous Dxr enzymes by N-acyl (diamonds) and O-linked MEPicides (squares) were correlated, especially when outlier N-acyl analogs 8e and 8f were ignored. Pearson coefficients: 0.76 (O-linked), 0.37 (all N-acyl analogs), and 0.71 (N-acyl analogs leaving 8e and 8f out).
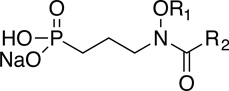
Table 1.
Inhibition of Mtb and Yp Dxr by selected N-acyl and O-linked MEPicides
 | ||||
|---|---|---|---|---|
| Cmpd No. | R1 | R2 | Mtb Dxr IC50, µM |
Yp Dxr IC50, µM |
| 1 (Fos) | H | H | 0.44 | 0.71 |
| 2 (FR) | H | CH3 | 2.91 | 0.23 |
| 8b | H | Hexyl | (43)* | (27) |
| 8e | H | Ph | (70) | 4.45 |
| 8f | H | (4-CH3)Ph | (74) | 16.03 |
| 8h | H | CH2CH2CH2Ph | 17.8 | (34) |
| 16d | (4-ipr)Bn | CH3 | 48.4 | 1.19 |
| 16e | CH(CH3)Ph | CH3 | (38.1) | (19) |
| 16g | CH2CH2(4-OH)Ph | CH3 | (59) | (22) |
| 16i | CH2-(1-naphthyl) | CH3 | (29) | 22.35 |
| 16j | CH2-(2-naphthyl) | CH3 | 1.45 | 0.33 |
| 16k | CH2-4-(1,1’-biphenyl) | CH3 | (36.8) | 8.40 |
| 16r | CH2-4-(4’-CF3)-1,1’-biphenyl) | CH3 | (70) | (19) |
Values in parentheses are percent remaining enzyme activity at 100 µM.
Table 2.
Antitubercular activities of saturated acid and ester MEPicides
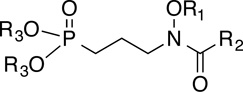 | ||||
|---|---|---|---|---|
| Cmpd No. | R1 | R2 | R3 |
M. tuberculosis MIC (µg/mL)* |
| INH | N/A | N/A | N/A | 0.01 |
| 1 (Fos) | H | H | H/Na | >500 |
| 2 (FR900098) | H | CH3 | H/Na | >500 |
| 3 | H | CH3 | CH2OCOtBu | 50–100 (62.5) |
| 8e | H | Ph | H/Na | ≥200 |
| 8h | H | (CH2)3Ph | H/Na | >200 |
| 7h | H | (CH2)3Ph | CH2CH3 | 200 |
| 12 | H | (CH2)3Ph | CH2OCOtBu | 150 |
| 8d | H | (CH2)2cyclohexyl | H/Na | >200 (25) |
| 7d | H | (CH2)2cyclohexyl | CH2CH3 | >200 (37) |
| 8l | H | CH2O(4-Cl)Ph | H/Na | >200 (25) |
| 16d | (4-ipr)Bn | CH3 | H/Na | 200 (25) |
| 17 | (4-ipr)Bn | CH3 | CH2OCOtBu | 12.5 (6.25–12.5) |
| 16k | CH2-4-(1,1’-biphenyl) | CH3 | H/Na | 25–50 |
| 15k | CH2-4-(1,1’-biphenyl) | CH3 | CH2CH3 | 200 (100) |
| 19 | CH2-4-(1,1’-biphenyl) | CH3 | CH2OCOtBu | 12.5 (3.13–6.25) |
| 16p | CH2-4-(4’-ipr)-1,1’-biphenyl | CH3 | H/Na | 100 (6.25–12.5) |
| 16h | (CH2)4Ph | CH3 | H/Na | ≥200 |
| 15h | (CH2)4Ph | CH3 | CH2CH3 | 100–200 |
| 21 | (CH2)4Ph | CH3 | CH2OCOPh | 25 |
| 18 | (CH2)4Ph | CH3 | CH2OCOtBu | 12.5 (6.25–12.5) |
| 16j | CH2-(2-naphthyl) | CH3 | H/Na | ≥200 |
| 15j | CH2-(2-naphthyl) | CH3 | CH2CH3 | 200 (100) |
| 20 | CH2-(2-naphthyl) | CH3 | CH2OCOtBu | 18.75 (4.7) |
Data using 7H9 media. When different, MIC in GAST-Fe media is given in parentheses.
Figure 3 shows the inhibition of Mtb and Yp Dxr by both series of compounds. The percent residual enzyme activity following treatment at 100 µM of each compound is shown in Figure 3A. Overall, across the two series, the inhibition activities generally paralleled each other. That is, compounds that displayed activity against one homolog were generally active against the other, and compounds that did not inhibit one homolog generally did not inhibit the other. For example, 8b, the n-hexyl N-acyl analog, inhibited Dxr from both species, while diphenyl 8n was ineffective against both enzymes. Similarly, in the O-linked series, 16d, 16j, and 16k were potent inhibitors of both Dxr homologs, but 16b and 16c were not. This pattern of correlated activities was generally followed throughout the dataset. Exceptions, however, did exist; N-acyl analogs 8e and 8f, the phenyl and toluyl analogs, respectively, were significantly more potent against Yp Dxr compared with the Mtb homolog. O-linked compound 16g showed the same trend.
Against Yp Dxr, the most active N-acyl analogs were 8b, 8e, 8f, and 8h, whereas the most active O-linked compounds were 16d, 16e, 16g, 16i, 16j, 16k, and 16r. On the other hand, N-acyl analogs displaying the greatest inhibition of Mtb Dxr were 8a, 8b, and 8h, although the level of inhibition was not as significant as that seen against Yp Dxr. O-linked compounds displaying the greatest inhibition of Mtb Dxr were 16d, 16e, 16i, 16j, and 16k.
Figure 3B is a comparative plot showing the overall ability of each chemical class to inhibit the enzymes. Activities of the O-linked compounds against the two enzymes were the best correlated (Pearson coefficient = 0.76). The activities of the N-acyl compounds were best correlated when 8e and 8f were left out (Pearson coefficient = 0.71).
Compounds showing at least 75% inhibition of enzyme activity were further evaluated to determine half-maximal inhibitory concentrations (IC50 values) (Table 1). The most potent compound was O-linked analog 16j, with IC50 values of 1.45 and 0.33 µM against Mtb and Yp Dxr, respectively. Notably, against Yp Dxr, compound 16j has an IC50 value approximately equal to half the enzyme concentration used in the assay (0.89 µM). This is comparable to the IC50 values of the tight-binding inhibitors fosmidomycin (1) and FR900098 (2), hence the IC50 could be even lower than the value reported. As seen for the primary screen (Figure 3), homolog inhibition across each series was roughly in parallel, with Yp Dxr generally being inhibited to a greater extent. Compounds 8e, 16d, 16i, and 16k show selectivity between homologs and are more potent against Yp Dxr. None of the analogs showed preferential specificity towards Mtb Dxr.
To determine if the N-acyl and O-linked compounds act as bisubstrate inhibitors, we evaluated representative compounds 8e and 16j for binding modality. Using classical Lineweaver-Burk double reciprocal plots to determine inhibitor modality, both N-acyl compound 8e and O-linked compound 16j appear competitive with both NADPH and DXP (Figure 4), indicating bisubstrate binding behavior. Our prior work showed that N-acyl compound 8h binds competitively with DXP but noncompetitively with NADPH.(8) Comparing these results leads us to believe that flexibility of the N-acyl/O-linked substituent influences the exact binding modality of the inhibitor to Dxr. In other words, the longer/more flexible phenpropyl substituent of 8h may adopt an alternate binding pattern compared with the smaller CH2-2-naphthyl (16j) and phenyl (8e) substituents, which are more constrained. By plotting the apparent Km versus inhibitor concentration, the Ki values are calculated for 8e with Yp Dxr as 1.8 µM with respect to NADPH and 7.9 µM relative to DXP. The calculated Ki values for 16j with Mtb Dxr are 17.8 µM relative to NADPH and 1.0 µM with respect to DXP. Notably, these values resemble the relative differences in apparent Michaelis constants (Kmapp) between the homologous enzymes (Yp Dxr: Kmapp,NADPH=12.7 µM, Kmapp,Dxp=221.5 µM; Mtb Dxr: Kmapp,NADPH=29.7 µM, Kmapp,Dxp=47 µM),(9) suggesting that the enhanced potency of the inhibitors towards the Yp Dxr predominantly reflects an enhanced ability of the inhibitor to associate with its NADPH binding site.
Figure 4.
Lineweaver-Burk analysis. Shown against Yp Dxr, compound 8e is competitive with respect to both DXP (A) and NADPH (C), as is compound 16j (panels B and D, respectively) shown against Mtb Dxr. Each assay was performed in duplicate. Data was fit by linear regression, and R2 values are indicated.
The most potent inhibitors of Dxr were selected for evaluation in growth inhibition assays against Yp, Mtb, and E. coli. While compound 2 potently inhibits bacterial propagation in a dose-dependent manner, none of the newly synthesized MEPicide inhibitors demonstrated appreciable activity against Yp or E. coli MG1655 (data not shown). Interestingly, deletion of an outer membrane protein involved in the efflux of protein toxins and antibiotics (tolC)(43) in E. coli dramatically improved the antibacterial activities of N-acyl analog 15k (IC50 (WT) = 169.7 µg/mL; IC50 (tolC) = 35.9 µg/mL) and O-linked compound 7l (IC50 (WT) = 213.6 µg/mL; IC50 (tolC) = 54.6 µg/mL), leading to the likelihood that low MEPicide activity against E. coli (and other organisms) is due at least in part to efflux.
As a class, the MEPicides were more active against Mtb. The antitubercular activities are shown in Table 2. Two media were used to measure the minimum inhibitory concentration (MIC) values against M. tuberculosis H37Rv. Middlebrook 7H9 is a nutrient-rich media, while GASTFe is a minimal, low iron media. The low protein content in GAST-Fe helps evaluate lipophilic compounds that may suffer from high protein binding. The MIC values obtained from the N-acyl series are poor and likely reflect the low potency of the compounds against Mtb Dxr (Table 2). Better antitubercular MIC values were obtained using the O-linked series. Perhaps unsurprisingly, the most potent MIC values were obtained from the dipivaloyloxymethyl (POM) esters of compounds that displayed the highest level of enzyme inhibition. For example, 16k, 16d, and 16j yielded the best IC50 values in the series. POM esters of these compounds (17–20) yielded MIC values of 12.5–18.75 ug/mL in 7H9 media and 3.13–12.5 ug/mL in GAST-Fe media. Similarly, methyl benzoate 21, ester of 16h, yielded an MIC of 25 ug/mL in 7H9. The lipophilic character of the esters likely facilitates penetration across the mycobacterial cell wall.
CONCLUSION
We present here two series of compounds designed to further explore inhibition of Dxr. Extended N-acyl and O-linked analogs of FR900098 inhibited the enzyme. While most compounds were moderate inhibitors, some analogs inhibited Dxr to the same extent as FR900098. These results agree with N-acyl analogs previously reported.(34) Of particular interest, however, is the inhibitory activity of the O-linked compounds, as these represent a new mode of binding to the enzyme, one that does not rely on an anionic interaction with the metal cation.
Catalysis by Dxr undergoes an ordered bi bi reaction mechanism, wherein NADPH binds to the enzyme before DXP binds.(44) This mechanism is reflective of a conformational change that occurs in the protein upon NADPH binding, resulting in the formation of the DXP binding site.
Compounds 1 and 2 are competitive inhibitors with respect to DXP and uncompetitive relative to NADPH.(8, 44) That is, NADPH must bind to Dxr before either of these inhibitors can occupy the DXP binding site. Since we rationally designed the bisubstrate inhibitors to occupy both the DXP and NADPH binding sites, compounds 8e and 16j were further examined in terms of their mechanism-of-inhibition. N-acyl compound 8e and O-linked compound 16j were both found to be competitive with respect to NADPH and DXP, suggesting both binding sites were blocked upon inhibitor binding. Hence, both inhibitors appear to function via a “flip-and-lock” mechanism. That is, a portion of the inhibitor binds to the NADPH site, causing a Dxr conformational change (‘flip’), and then a second portion of the compound binds to the DXP binding site, holding the enzyme in an inhibited state (‘lock’). Flexibility of the substituent (N-acyl or O-linked) appears to be highly influential in determining the specific binding mode at the enzyme. We are seeking protein crystal structures with the bound inhibitors, the results of which will further our mechanistic understanding of how these compounds inhibit the enzyme.
Overall, compounds tend to show greater inhibition of Yp Dxr compared with the Mtb homolog. It is not clear why this occurs, but may reflect increased flexibility of the Yp Dxr active site and neighboring residues compared with those in the Mtb enzyme. In previous work, it was noted that Yp Dxr shows striking similarity in terms of sequence and proposed structure to E.coli Dxr,(9) which is known to have considerable conformational flexibility, especially in the loop region of residues 186–216.(45) This loop closes down in the active conformation to form part of the active site. As shown in Figure 5, while this loop region (as followed using loop residue Trp203) is relatively stable in Mtb between an apo and active conformation,(46) it moves quite dramatically in E. coli. Due to this movement, we speculate that the Yp Dxr can accommodate a larger variety of structures compared with the relatively static Mtb homolog.
Figure 5.
Dxr active site variation. A) The Mtb Dxr apoenzyme (PDB 2JD2, yellow) is structurally similar in the active site loop region to the catalytically active conformation of the enzyme (PDB 4AIC, green), as followed by Trp203 (circled). B) In contrast, the Ec Dxr active site conformation (PDB 2EGH, teal) has a dramatically different topology in the loop region relative to the apoenzyme (PDB 1K5H Chain A, blue), as followed by Trp212 (circled).
In contrast to the natural products fosmidomycin (1) and FR900098 (2), several of our synthesized MEPicides demonstrate significant growth inhibition of M. tuberculosis. Particularly due to the increased lipophilicity with loss of the retrohydroxamate, the antitubercular activity of the O-linked compounds is especially interesting. Our prior work indicated that using a POM ester to mask the phosphonate improved penetration across the Mtb cell wall.(6) The improved MIC values of POM esters 17–20 further corroborate this view. It is also noteworthy that the improvement in MIC parallels an increase in enzyme potency; the most striking example of this is found in the O-linked series with compounds 16k/19 and 16j/20. Further exploration into the SAR of the O-linked MEPicides will lead to a deeper understanding of Dxr inhibition and support the development of novel antimicrobial drugs.
METHODS
Synthesis
All reagents were purchased from commercial suppliers and used without further purification. THF and dichloromethane were distilled under argon immediately before use, respectively from sodium/benzophenone and calcium hydride. All air sensitive reactions were carried out under a nitrogen atmosphere. 1H and 13C NMR spectra were recorded in Acetone-d6, CDCl3, or D2O on an Agilent spectrometer at 200 or 400 MHz (1H) and 50 or 100 MHz (13C), respectively. Chemical shifts are given in parts per million (ppm, d) relative to the internal standard (TMS) or residual solvent peak. Spin multiplicities are given with the following abbreviations: s (singlet), br s (broad singlet), d (doublet), t (triplet), q (quadruplet), quin/qt (quintet), sex (sextet), and m (multiplet). Coupling constants J are given in Hertz. Mass spectra were obtained in the ESI mode on an LC-MSD Agilent 1100 (HyperSil Gold aQ). High-resolution mass spectroscopy spectra (HRMS) were recorded in negative ESI mode on a JEOL HX110/HX100 four sector tandem mass spectrometer (UMBC Mass Spectrometry Facility) or on a VG Analytical VG70SE double focusing magnetic sector mass spectrometer (JHU Mass Spectrometry Facility). Thin layer chromatography (TLC) was performed on Merck 60 F254 silica gel plates. Automated flash column chromatography was carried out using a Biotage Isolera chromatography system and Merck silica gel 60 (35–70 µm). Purity of compounds (>95%) was determined by 1H/13C NMR, LC-DAD-MS and HRMS.
General Method for preparation of compounds 8a–n
N,O-Bis(trimethylsilyl)trifluoroacetamide (3 equiv.) was added under nitrogen to 7a–n (1 equiv.) in CH2Cl2 and stirred at room temperature for 20 min. The reaction mixture was cooled to 0°C and bromotrimethylsilane (10 equiv.) was added dropwise to the reaction. The reaction was warmed to room temperature and stirred overnight under nitrogen. Ethyl bromide and excess silylating agent were removed under reduced pressure, and the residue was dissolved in aqueous NaOH (1 equiv.) and stirred for a second night. The reaction mixture was partitioned between H2O and CH2Cl2 to remove any residual impurities/organics. The aqueous fractions were combined, and the solvent was removed by lyophilization to give the desired compound in 75–100% yields.
Sodium hydrogen-3-(N-hydroxypentanamido)propyl phosphonate (8a)
1H NMR (200 MHz, Acetone-d6/D2O) δ (ppm): 0.95 (t, J = 7.4 Hz, 3H), 1.39 (sex, J = 13.8, 7.1 Hz, 2H), 1.61 (quin, J = 8.3, 7.8 Hz, 4H), 1.77 – 2.07 (m, 2H), 2.55 (t, J = 7.7 Hz, 2H), 3.62 – 3.72 (m, 2H). 13C NMR (50 MHz, Acetone-d6/D2O) δ (ppm): 13.52, 21.09, 22.30, 24.11, 27.02, 32.01, 49.00 (d, J = 18.8 Hz), 176.33, 214.78. LCMS (ESI) m/z 240.1 (M+H). HRMS (ESI) m/z calcd for C8H17NO5P (M−Na): 238.0838, found: 238.0833.
Sodium hydrogen-3-(N-hydroxyheptanamido)propyl phosphonate (8b)
1H NMR (200 MHz, Acetone-d6/D2O) δ (ppm): 1.25 (t, J = 6.2 Hz, 3H), 1.58 – 1.82 (m, 6H), 1.82 – 2.08 (m, 4H), 2.12 – 2.39 (m, 2H), 2.86 (t, J = 7.6 Hz, 2H), 4.04 (t, J = 6.7 Hz, 2H). 13C NMR (50 MHz, Acetone-d6/D2O) δ (ppm): 13.86, 21.26, 22.46, 24.24, 24.85, 26.91, 31.47, 32.39, 49.03 (d, J = 20.4 Hz), 175.82, 213.84. LCMS (ESI) m/z 268.0 (M+H). HRMS (ESI) m/z calcd for C10H21NO5P (M−Na): 266.1151, found: 266.1147.
Sodium hydrogen-3-(N-hydroxypivalamido)propyl phosphonate (8c)
1H NMR (200 MHz, Acetone-d6/D2O) δ (ppm): (80/20 mixture of two conformers) 1.12 – 1.34 (m, 9H), 1.42 – 1.67 (m, 2H), 1.73 – 1.95 (m, 2H), 3.10 (t, J = 6.8 Hz, 20/100 of 2H), 3.68 (t, J = 6.8 Hz, 80/100 of 2H). 13C NMR (50 MHz, Acetone-d6/D2O) δ (ppm): 23.98, 26.62 (d, J = 8.4 Hz), 39.07, 50.90 (d, J = 18.7 Hz), 180.66 (d, J = 14.8 Hz). LCMS (ESI) m/z 240.1 (M+H). HRMS (ESI) m/z calcd for C8H17NO5P (M−Na): 238.0838, found: 238.0833.
Sodium hydrogen-3-(3-cyclohexyl-N-hydroxypropanamido)propyl phosphonate (8d)
1H NMR (400 MHz, D2O) δ (ppm): (80/20 mixture of two conformers) 0.91 (q, J = 13.6, 12.9 Hz, 2H), 1.10 – 1.29 (m, 4H), 1.49 (q, J = 7.0 Hz, 2H), 1.62 – 1.77 (m, 5H), 1.81 – 1.95 (m, 2H), 2.54 (t, J = 8.0 Hz, 2H), 3.39 (t, J = 6.0 Hz, 20/100 of 2H), 3.70 (t, J = 6.8 Hz, 80/100 of 2H). 13C NMR (101 MHz, D2O) δ (ppm): 19.95, 25.78, 26.10, 29.44, 31.97, 32.49, 36.85, 48.30, 162.54. LCMS (ESI) m/z 294.1 (M+H). HRMS (ESI) m/z calcd for C12H23NO5P (M−Na): 292.1308, found: 292.1303.
Sodium hydrogen-3-(N-hydroxybenzamido)propyl phosphonate (8e)
1H NMR (200 MHz, Deuterium Oxide/Acetone-d6) δ (ppm): 1.44 – 1.87 (m, 2H), 1.87 – 2.15 (m, 2H), 3.59 – 3.99 (m, 2H), 7.55 (s, 4H). 13C NMR (50 MHz, D2O/Acetone-d6) δ (ppm): 20.74, 23.16, 25.92, 50.56, 127.51, 128.89, 131.14, 133.83, 171.84. LCMS (ESI) m/z 259.9 (M+H). HRMS (ESI) m/z calcd for C10H13NO5P (M−Na): 258.0525, found: 258.0520.
Sodium hydrogen-3-(N-hydroxy-4-methylbenzamido)propyl phosphonate (8f)
1H NMR (CDCl3, 200MHz), δ (ppm): 1.37 – 1.73 (m, 2H), 1.79 – 2.06 (m, 2H), 2.37 (s, 3H), 3.55 – 3.86 (m, 2H), 7.25 – 7.54 (m, 4Harom). 13C NMR (50 MHz, D2O) δ (ppm): 20.93 (d, J = 16.7 Hz), 23.64, 26.34, 53.60, 127.51, 129.31, 130.63, 141.89, 171.60. LCMS (ESI) m/z 274.0 (M+H). HRMS (ESI) m/z calcd for C11H15NO5P (M−Na): 272.0682, found: 272.0684.
Sodium hydrogen-3-(N-hydroxy-3-phenylpropanamido)propyl phosphonate (8g)
1H NMR (200 MHz, D2O) δ (ppm): 1.39 – 2.09 (m, 4H), 2.76 – 2.98 (m, 4H), 3.62 (t, J = 6.7 Hz, 2H), 7.19 – 7.41 (m, 5H). 13C NMR (101 MHz, D2O) δ (ppm): 20.19 (d, J = 3.8 Hz), 25.04, 30.31, 33.24, 48.52 (d, J = 19.2 Hz), 126.36, 128.36 (d, J = 7.5 Hz), 128.65, 140.88, 175.22. LCMS (ESI) m/z 288.1 (M+H). HRMS (ESI) m/z calcd for C12H18NNaO5P (M+H): 310.0814, found: 310.0813.
Sodium hydrogen-3-(N-hydroxy-4-phenylbutanamido)propyl phosphonate (8h)
1H NMR (200 MHz, D2O) δ (ppm): (58:42 mixture of two conformers) 1.42 – 2.06 (m, 6H), 2.34 (t, 42/100 of 2H, J = 7.4 Hz), 2.48 (t, 58/100 of 2H, J = 7.4 Hz), 2.64 (t, 2H, J = 7.5 Hz), 3.34 (t, 42/100 of 2H, J = 7.3 Hz), 3.62 (t, 58/100 of 2H, J = 6.7 Hz), 7.18 – 7.42 (m, 5H). 13C NMR (50 MHz, D2O) δ (ppm): 21.0, 25.1 (d, J = 134.4 Hz), 26.7, 31.9, 35.2, 49.2 (d, J = 19.0 Hz), 126.8, 129.3, 142.8, 176.8. LCMS (ESI) m/z 302.0 (M+H). HRMS (ESI) m/z calcd for C13H20NNaO5P (M+H): 324.0971, found: 324.0970.
Sodium hydrogen-3-(N-hydroxy-3-(4-methoxyphenyl)propanamido)propyl phosphonate (8i)
1H NMR (200 MHz, D2O/Acetone-d6) δ (ppm): 1.67 – 1.92 (m, 2H), 1.91 – 2.16 (m, 2H), 2.80 – 2.91 (m, 2H), 2.91 – 3.02 (m, 2H), 3.79 (t, J = 6.6 Hz, 2H), 3.88 (s, 3H), 6.98 (d, J = 8.7 Hz, 2H), 7.29 (d, J = 8.7 Hz, 2H). 13C NMR (50 MHz, D2O/Acetone-d6) δ (ppm): 20.34, 34.26, 48.68 (d, J = 17.9 Hz), 55.42 (d, J = 3.8 Hz), 114.27, 129.77, 133.79, 157.82, 174.83. LCMS (ESI) m/z 318.0 (M+H). HRMS (ESI) m/z calcd for C13H19NO5P (M−Na): 316.0944, found: 316.0946.
Sodium hydrogen3-(N-hydroxy-3-(3-(trifluoromethyl)phenyl)acrylamido)propyl phosphonate (8j)
1H NMR (400 MHz, D2O) δ (ppm): (80:20 mixture of two conformers) 1.60 – 1.65 (m, 2H), 1.87 – 1.92 (m, 2H), 1.09 – 3.19 (m, 20/100 of 2H), 3.76 – 3.81 (m, 80/100 of 2H), 7.33 – 7.38 (m, 1H), 7.57 – 7.63 (m, 2H), 7.70 – 7.75 (m, 1H), 7.83 – 7.88 (m, 1H), 7.95 – 8.00 (m, 1H). 13C NMR (101 MHz, D2O) δ (ppm): 16.28, 25.77, 45.69, 110.81, 117.62, 124.57, 126.44, 129.53, 131.56, 141.83, 144.93, 152.92, 163.34. LCMS (ESI) m/z 354.0 (M+H). HRMS (ESI) m/z calcd for C13H14F3NO5P (M−Na): 352.0556, found: 352.0558.
Sodium (E)-hydrogen3-(N-hydroxy-3-(4-nitrophenyl)acrylamido)propyl phosphonate (8k)
1H NMR (400 MHz, Acetone-d6/D2O) δ (ppm): 1.73 – 1.91 (m, 2H), 1.96 – 2.27 (m, 2H), 3.85 – 4.10 (m, 2H), 7.58 (d, J = 16.1 Hz, 1H), 7.78 (d, J = 15.1 Hz, 1H), 7.98 (d, J = 6.3 Hz, 2H), 8.42 (d, J = 7.5 Hz, 2H). 13C NMR (101 MHz, Acetone-d6/D2O) δ (ppm): 20.63, 49.45, 120.35, 124.39, 129.22, 140.85, 148.19, 167.40. HRMS (ESI) m/z calcd for C12H14N2O7P (M−Na): 329.0533, found: 329.0532.
Sodium hydrogen-3-(2-(4-chlorophenoxy)-N-hydroxyacetamido)propyl phosphonate (8l)
1H NMR (400 MHz, Acetone-d6/D2O) δ (ppm): (80:20 mixture of two conformers) 1.65 – 1.89 (m, 2H), 1.89 – 2.10 (m, 2H), 3.42 (t, J = 7.6 Hz, 20/100 of 2H), 3.77 (t, J = 6.8 Hz, 80/100 of 2H), 5.02 (s, 2H), 7.02 (d, J = 9.0 Hz, 2H), 7.36 (d, J = 9.4 Hz, 2H). 13C NMR (101 MHz, D2O/Acetone-d6) δ (ppm): 20.44, 23.31, 26.02, 49.01 (d, J = 21.1 Hz), 65.33, 116.60, 125.92, 129.66, 157.11, 169.68. LCMS (ESI) m/z 324.0 (M+H). HRMS (ESI) m/z calcd for C11H14ClNO6P (M−Na): 322.0241, found: 322.0244.
Sodium hydrogen-3-(N-hydroxybiphenyl-4-ylcarboxamido)propyl phosphonate (8m)
1H NMR (200 MHz, CD3OD) δ (ppm): 1.64 – 1.86 (m, 2H), 1.91 – 2.16 (m, 2H), 3.83 (t, J = 6.0 Hz, 2H), 7.28 – 7.51 (m, 3H), 7.60 – 7.78 (m, 6H). 13C NMR (50 MHz, CD3OD) δ (ppm): 21.89, 27.10, 127.52, 128.07, 128.93, 129.97, 134.52, 141.44, 144.56, 171.48. Peak at 50 ppm is masked by solvent. LCMS (ESI) m/z 336.0 (M+H). HRMS (ESI) m/z calcd for C16H17NO5P (M−Na): 334.0838, found: 334.0840.
Sodium hydrogen-3-(1-hydroxy-3,3-diphenylureido)propyl phosphonate (8n)
1H NMR (200 MHz, Acetone-d6/D2O) δ (ppm): 1.92 – 2.15 (m, 2H), 2.17 – 2.39 (m, 2H), 3.99 (t, J = 6.1 Hz, 3H), 7.57 (dd, J = 17.7, 7.9 Hz, 6H), 7.77 (t, J = 7.5 Hz, 4H). 13C NMR (50 MHz, Acetoned6/D2O) δ (ppm): 24.13, 26.83, 52.36 (d, J = 18.1 Hz), 125.72, 126.38, 129.60, 145.39, 161.44. LCMS (ESI) m/z 351.0 [M+H]. HRMS (ESI) m/z calcd for C16H18N2O5P (M−Na): 349.0947, found: 359.0951.
General procedure for the deprotection of N-acyl and O-linked ligands to give 8a–n and 16a–r
To a solution of 7a–n or 15a–r (1 eq) in CH2Cl2 (1.7 mL/mmol of phosphonate) at 0°C was added dropwise bromotrimethylsilane (8 eq). The reaction mixture was stirred overnight at room temperature. Ethyl bromide and excess silylating agent were removed by rotary evaporation at room temperature. The concentrate was solubilized in dry CH2Cl2 and evaporated again (×2). Then H2O was added to the residue and the mixture was stirred overnight at room temperature. The solution was filtered over cotton to remove the yellow oil (except for products with a low solubility in water) and concentrated in vacuo at 50°C. The crude acid was rapidly neutralized with aqueous NaOH and the mixture was stirred overnight at room temperature. The reaction mixture was concentrated in vacuo at 50°C to give the products as light yellow or white solids in 61% to quantitative yields.
Sodium Hydrogen 3-(N-(4-bromobenzyloxy)acetamido)propyl phosphonate (16a)
1H NMR (200 MHz, D2O) δ (ppm): δ 1.55–1.97 (m, 4H), 2.09 (s, 3H), 3.65–3.85 (m, 2H), 4.95 (s, 2H), 7.36–7.47 (m, 2H), 7.59–7.71 (m, 2H). 13C NMR (D2O) δ (ppm): 19.7, 20.9, 23.6, 26.3, 50.5, 75.5, 123.2, 131.4, 131.9, 132.1, 132.3, 133.1, 174.8. HRMS (ESI) m/z calculated for C12H17BrNO5P [(M−Na+H)+], 366.0106; found, 366.0110.
Sodium Hydrogen 3-(N-((4-(trifluoromethoxy)benzyl)oxy)acetamido)propyl phosphonate (16b)
1H NMR (200 MHz, D2O) δ (ppm): 1.54–1.73 (m, 2H), 1.78–2.02 (m, 2H), 2.08 (s, 3H), 3.78 (bs, 2H), 5.00 (s, 2H), 7.38 (d, J = 7.8 Hz, 2H), 7.58 (d, J = 8.6 Hz, 2H). 13C NMR (50 MHz, D2O) δ (ppm): 19.6, 21.0, 23.7, 26.4, 45.7, 46.1, 75.2, 113.0, 121.4, 123.0, 127.7, 131.7, 132.9, 149.7, 174.6. HRMS (ESI) m/z calculated for C13H17F3NNaO6P [(M+Na)+], 394.0643; found, 394.0702.
Sodium Hydrogen 3-(N-((3,4-dichlorobenzyl)oxy)acetamido)propyl phosphonate (16c)
1H NMR (200 MHz, D2O) δ (ppm): 1.43–1.72 (m, 2H), 1.72–2.01 (m, 2H), 2.09 (s, 3H), 3.74 (t, J = 6 Hz, 2H), 4.90 (s, 2H), 7.33–7.43 (m, 1H), 7.48–7.66 (m, 2H). 13C NMR (50 MHz, D2O) δ (ppm): 19.7, 21.2, 24.0, 26.6, 45.9, 46.2, 74.7, 129.5, 131.0, 131.6, 132.8, 134.5, 137.6, 174.6. LCMS (ESI+): m/z = 356.0 [M+H]+, 378.0 [M+Na]+. HRMS (ESI) m/z calculated for C12H16Cl2NNaO5P [(M+Na)+], 378.0041; found, 378.0045.
Sodium Hydrogen 3-(N-((4-isopropylbenzyl)oxy)acetamido)propyl phosphonate (16d)
1H NMR (200 MHz, D2O) δ (ppm): 1.21 (d, 6H, J = 6.9 Hz, CH3-CH ×2), 1.36–1.62 (m, 2H, CH2-P), 1.72–1.97 (m, 2H, CH2-CH2P), 2.04 (s, 3H, CH3-CO), 2.85–3.09 (m, 1H, CH-Ar), 3.57–3.83 (m, 2H, CH2-N), 4.92 (s, 2H, CH2-O-N), 7.36 (d, 2H, J = 8.2 Hz, Har), 7.43 (d, 2H, J = 8.2 Hz, Har). 13C NMR (50 MHz, CDCl3) δ (ppm): 16.4, 16.5, 20.3, 20.4, 20.6, 21.6, 24.4, 45.6, 46.1, 61.6, 61.7, 75.4, 117.9, 121.2, 123.0, 130.6, 133.2, 149.6, 172.4. LCMS (ESI+): m/z = 330.2 [M+H]+. HRMS (ESI) m/z calculated for C15H24NNaO5P [(M+Na)+], 352.1290; found, 352.1285.
Sodium Hydrogen 3-(N-(1-phenylethoxy)acetamido)propyl phosphonate (16e)
1H NMR (200 MHz, D2O) δ (ppm): 1.59 (d, 3H, J = 6.4 Hz, CH3-CH), 1.40–1.88 (m, 4H, CH2-P and CH2-CH2P), 1.96 (s, 3H, CH3-CO), 3.24–3.48 (m, 1H, CH2-N), 3.55–3.81 (m, 1H, CH2-N), 5.14 (q, 1H, J = 6.5 Hz, CH-O), 7.40–7.58 (m, 5H, Har). 13C NMR (50 MHz, D2O) δ (ppm): 19.0, 19.5, 20.4, 23.3, 26.0, 46.3, 46.6, 82.7, 128.0, 129.0, 129.5, 139.3, 175.4. LCMS (ESI+): m/z = 302.1 [M+H]+. HRMS (ESI) m/z calculated for C13H20NNaO5P [(M+Na)+], 324.0977; found, 324.1225.
Sodium Hydrogen 3-(N-(4-methoxyphenethoxy)acetamido)propyl phosphonate (16f)
1H NMR (200 MHz, D2O) δ (ppm): 1.26–1.49 (m, 2H, CH2-P), 1.64–1.86 (m, 2H, CH2-CH2P), 1.91 (s, 3H, CH3-CO), 2.94 (t, 2H, J = 6.0 Hz, CH2-Ar), 3.60 (t, 2H, J = 6.8 Hz, CH2-N), 3.82 (s, 3H, CH3-O), 4.20 (t, 2H, J = 6.0 Hz, CH2-O-N), 6.98 (d, 2H, J = 8.6 Hz, Har), 7.30 (d, 2H, J = 8.6 Hz, Har). 13C NMR (50 MHz, D2O) δ (ppm): 19.2, 21.8, 24.8, 27.4, 33.0, 45.8, 46.2, 55.7, 75.3, 114.4, 130.4, 131.1, 157.7, 174.2. LCMS (ESI+): m/z = 332.1 [M+H]+. HRMS (ESI) m/z calculated for C14H22NNaO6P [(M+Na)+], 354.1082; found, 354.1215.
Sodium Hydrogen 3-(N-(4-hydroxyphenethoxy)acetamido)propyl phosphonate (16g)
1H NMR (200 MHz, D2O) δ (ppm): 1.38–1.77 (m, 4H), 1.93 (s, 3H), 2.85 (t, J = 6.4, 6.2 Hz, 2H), 3.54 (t, J = 7, 6.2 Hz, 2H), 4.11 (t, J = 6.4, 5.8 Hz, 2H), 6.82–6.86 (m, 2H), 7.16–7.20 (m, 2H). 13C NMR (50 MHz, D2O) δ (ppm): 19.2, 20.8, 23.6, 26.3, 33.1, 45.4, 45.8, 75.3, 115.6, 130.4, 154.2, 174.2. LCMS (ESI+): m/z = 318.1 [M+H]+, 635.1 [2M+H]+. HRMS (ESI) m/z calculated for C13H20NNaO6P [(M+Na)+], 340.0926; found, 340.0978.
Sodium Hydrogen 3-(N-(4-phenylbutoxy)acetamido)propyl phosphonate (16h)
1H NMR (200 MHz, D2O) δ (ppm): 1.49–2.01 (m, 8H, CH2-P and CH2-CH2P and CH2-CH2O and CH2-CH2Ph), 2.15 (s, 3H, CH3-CO), 2.68 (t, 2H, J = 6.5 Hz, CH2-Ph), 3.70 (t, 2H, J = 7.0 Hz, CH2-N), 3.89–4.04 (m, 2H, CH2-O-N), 7.24–7.53 (m, 5H, Har). 13C NMR (50 MHz, D2O) δ (ppm): 19.6, 20.8, 23.1, 25.8, 27.3, 27.5, 35.3, 45.6, 45.9, 74.2, 125.9, 128.6, 128.7, 142.5, 173.4. LCMS (ESI+): m/z = 330.2 [M+H]+. HRMS (ESI) m/z calculated for C15H24NNaO5P [(M+Na)+], 352.1290; found, 352.1441.
Sodium Hydrogen 3-(N-(naphthalen-1-ylmethoxy)acetamido)propyl phosphonate (16i)
1H NMR (400 MHz, D2O) δ (ppm): 1.56 (bs, 2H), 1.89–2.18 (m, 5H), 3.83 (bs, 2H), 5.51 (s, 2H), 7.59–7.77 (m, 4H), 8.06–8.09 (m, 2H), 8.28–8.41 (m, 1H). 13C NMR (100 MHz, D2O) δ (ppm): 19.3, 21.0, 24.5, 25.8, 45.9, 46.0, 73.8, 123.3, 125.6, 126.4, 127.2, 128.7, 129.7, 130.2, 131.6, 133.4, 173.4. HRMS (ESI) m/z calculated for C16H20NNaO5P [(M+Na)+], 360.0977; found, 360.0978.
Sodium Hydrogen 3-(N-(naphthalen-2-ylmethoxy)acetamido)propyl phosphonate (16j)
1H NMR (200 MHz, D2O) δ (ppm): 1.23–1.51 (m, 2H, CH2-P), 1.68–1.93 (m, 2H, CH2-CH2P), 2.01 (s, 3H, CH3-CO), 3.73 (t, 2H, J = 6.6 Hz, CH2-N), 5.03 (s, 2H, CH2-O-N), 7.48–7.65 (m, 3H, Har), 7.84–8.02 (m, 4H, Har). 13C NMR (100 MHz, D2O) δ (ppm): 19.4, 20.7, 24.2, 25.5, 45.6, 45.7, 76.1, 126.7, 127.0, 127.2, 127.7, 128.0, 128.6, 129.4, 132.8, 133.2, 134.1, 174.5. LCMS (ESI+): m/z = 338.1 [M+H]+. HRMS (ESI) m/z calculated for C16H20NNaO5P [(M+Na)+], 360.0977; found, 360.0984.
Sodium Hydrogen 3-(N-([1,1'-biphenyl]-4-ylmethoxy)acetamido)propyl phosphonate (16k)
1H NMR (200 MHz, DMSO) δ (ppm): 1.29–1.60 (m, 2H), 1.60–1.89 (m, 2H), 2.03 (s, 3H), 3.66 (bs, 2H), 4.90 (s, 2H), 7.25–7.88 (m, 9H). 13C NMR (50 MHz, DMSO) δ (ppm): 20.4, 21.0, 26.4, 29.0, 45.0, 45.5, 74.9, 126.7, 127.6, 128.9, 130.0, 134.0, 139.6, 140.4, 170.9. LCMS (ESI+): m/z = 364.1 [M+H]+. HRMS (ESI) m/z calculated for C18H22NNaO5P [(M+Na)+], 386.1133; found, 386.1088.
Sodium Hydrogen 3-(N-([1,1'-biphenyl]-3-ylmethoxy)acetamido)propyl phosphonate (16l)
1H NMR (200 MHz, D2O) δ (ppm): 1.15–2.03 (m, 7H), 3.43 (bs, 2H), 4.29 (s, 2H), 6.46–7.34 (m, 9H). 13C NMR (50 MHz, D2O) δ (ppm): 20.0, 21.7, 24.3, 27.0, 46.4, 46.7, 76.1, 127.4, 127.9, 129.0, 129.3, 129.8, 134.7, 135.4, 140.3, 160.6, 141.0, 173.9. LCMS (ESI+): m/z = 364.1 [M+H]+, 727.2 [2M+H]+. HRMS (ESI) m/z calculated for C18H22NNaO5P [(M+Na)+], 386.1133; found, 386.1103.
Sodium Hydrogen 3-(N-([1,1'-biphenyl]-2-ylmethoxy)acetamido)propyl phosphonate (16m)
1H NMR (200 MHz, D2O) δ (ppm): 1.28–1.45 (m, 2H), 1.54–1.80 (m, 5H), 3.22 (t, J = 6 Hz, 2H), 4.64 (s, 2H), 7.02–7.54 (m, 9H). 13C NMR (50 MHz, D2O) δ (ppm): 19.2, 21.2, 24.1, 26.8, 45.8, 46.2, 73.6, 127.9, 128.3, 128.7, 129.3, 129.8, 130.3, 130.8, 132.2, 140.0, 143.5, 174.0. LCMS (ESI+): m/z = 364.1 [M+H]+, 727.2 [2M+H]+. HRMS (ESI) m/z calculated for C18H22NNaO5P [(M+Na)+], 386.1133; found, 386.1123.
Sodium Hydrogen 3-(N-((4'-methyl-[1,1'-biphenyl]-4-yl)methoxy)acetamido)propyl phosphonate (16n)
1H NMR (200 MHz, D2O) δ (ppm): 1.40–1.70 (m, 2H), 1.70–1.99 (m, 2H), 2.07 (s, 3H), 2.37 (s, 3H), 3.59–3.85 (m, 2H), 4.99 (s, 2H), 7.28–7.78 (m, 8H). 13C NMR (50 MHz, D2O) δ (ppm): 19.6, 20.8, 21.3, 23.9, 26.6, 46.2, 46.4, 75.6, 126.8, 129.6, 130.2, 131.8, 133.2, 136.8, 137.2, 140.6, 173.7. HRMS (ESI) m/z calculated for C19H24NNaO5P [(M+Na)+], 400.1290; found, 400.1247.
Sodium Hydrogen 3-(N-((4'-methoxy-[1,1'-biphenyl]-4-yl)methoxy)acetamido)propyl phosphonate (16o)
1H NMR (200 MHz, DMSO) δ (ppm): 1.31–1.56 (m, 2H), 1.65–1.86 (m, 2H), 2.03 (s, 3H), 3.66 (t, J = 6.6 Hz, 2H), 3.79 (s, 3H), 4.89 (s, 2H), 7.02 (d, J = 8.6 Hz, 2H), 7.36–7.71 (m, 6H). 13C NMR (50 MHz, DMSO) δ (ppm): 20.4, 20.9, 23.9, 26.6, 45.1, 45.5, 55.2, 75.0, 114.4, 126.2, 127.8, 130.1, 132.0, 133.2, 140.2, 159.1, 171.0. HRMS (ESI) m/z calculated for C19H24NNaO6P [(M+Na)+], 416.1239; found, 416.1015.
Sodium Hydrogen 3-(N-((4'-isopropyl-[1,1'-biphenyl]-4-yl)methoxy)acetamido)propyl phosphonate (16p)
1H NMR (200 MHz, D2O) δ (ppm): 0.94 (d, J = 5.8 Hz, 6H), 1.16–2.00 (m, 7H), 2.37–2.66 (m, 1H), 3.25–3.76 (m, 2H), 4.48 (s, 2H), 6.73–7.29 (m, 8H). 13C NMR (50 MHz, D2O) δ (ppm): 19.8, 21.4, 23.8, 26.4, 30.1, 33.5, 46.1, 46.6, 75.6, 126.6, 126.8, 130.1, 133.4, 137.8, 140.3, 147.1, 173.3. HRMS (ESI) m/z calculated for C21H28NNaO5P [(M+Na)+], 428.1603; found, 428.1354.
Sodium Hydrogen (3-(N-((4'-(hydroxymethyl)-[1,1'-biphenyl]-4-yl)methoxy)acetamido)propyl phosphonate (16q)
1H NMR (400 MHz, D2O) δ (ppm): 1.59–1.67 (m, 2H), 1.90–1.95 (m, 2H), 2.14 (s, 3H), 3.84 (bs, 2H), 4.74 (s, 2H), 5.06 (s, 2H), 7.55 (d, J = 8 Hz, 2H), 7.64 (d, J = 8.4 Hz, 2H), 7.79 (t, J = 8, 7.6 Hz, 4H) 13C NMR (100 MHz, D2O) δ (ppm): 19.38, 20.82, 24.36, 25.71, 45.51, 63.49, 75.64, 127.15, 127.19, 128.03, 130.55, 132.94, 139.26, 139.84, 141.10, 174.71. LCMS (ESI+): m/z = 394.2 [M+H]+, 787.2 [2M+H]+. HRMS (ESI) m/z calculated for C19H23NNaO6P [(M+H)+], 416.12390; found, 416.12281.
Sodium Hydrogen 3-(N-((4'-(trifluoromethyl)-[1,1'-biphenyl]-4-yl)methoxy)acetamido)propyl phosphonate (16r)
1H NMR (400 MHz, CD3OD) δ (ppm): 1.43–1.52 (m, 2H), 1.84–1.90 (m, 2H), 2.00 (s, 3H), 3.68 (t, J = 7.2 Hz, 2H), 4.88 (s, 2H), 7.48–7.50 (m, 2H), 7.62–7.66 (m, 4H), 7.73–7.75 (m, 2H). 13C NMR (100 MHz, D2O) δ (ppm): 19.8, 21.7, 23.3, 24.7, 48.4, 48.8, 75.8, 120.4, 123.2, 125.8, 125.9, 127.4, 128.5, 129.1 130.5, 134.4, 138.8, 139.8, 174.2. LCMS (ESI+): m/z = 432.0 [M+H]+, 863.0 2M+H]+. HRMS (ESI) m/z calculated for C19H20NNaO5P [(M+H)+], 454.10072; found 454.10024.
[({[(2,2-dimethylpropanoyl)oxy]methoxy}[3-(N-{[4-(propan-2-yl)phenyl]methoxy}acetamido)propyl]phosphoryl)oxy]methyl 2,2-dimethyl propanoate (17)
The acid of 16d (52 mg, 0.159 mmol, 1 eq) was dissolved in DMF (1.6 mL). Triethylamine (0.05 mL, 0.359 mmol, 2 eq) was added and the mixture was stirred for 5 min at room temperature, then chloromethyl pivalate (0.23 mL, 1.6 mmol, 10 eq) was added. The resulting solution was heated overnight at 60°C and concentrated. The residue was dissolved in CH2Cl2 and washed with sat. NaHCO3 and brine. The aqueous layers were extracted with CH2Cl2 three times and the combined organic layers were dried over MgSO4, filtered and evaporated under vacuum. Chromatographic separation on silica gel (toluene/acetone, 5/1) gave the expected compound as a yellow oil (45.8 mg, 52%). 1H NMR (400 MHz, CDCl3) δ (ppm): 1.21 (s, 18H), 1.25 (d, J = 6.4 Hz, 6H), 1.80–1.98 (m, 4H), 2.08 (s, 3H), 2.89–2.96 (m, 1H), 3.70 (t, J = 6.8, 6.4 Hz, 2H), 4.77 (s, 2H), 5.64 (s, 2H), 5.67 (s, 2H), 7.24–7.30 (m, 4H). 13C NMR (100 MHz, CDCl3) δ (ppm): 19.9, 20.0, 20.4, 23.3, 23.9, 24.7, 26.8, 34.0, 38.7, 45.0, 45.3, 76.3, 81.4, 81.4, 126.8, 129.4, 131.5, 150.0, 172.3, 176.8. LCMS (ESI+): m/z = 558.8 [M+H]+.
[({[(2,2-dimethylpropanoyl)oxy]methoxy}({3-[N-(4-phenylbutoxy)acetamido]propyl})phosphoryl)oxy]methyl 2,2-dimethylpropanoate (18)
A mixture of 15h (100 mg, 0.259 mmol) in CH2Cl2 (0.4 mL) was prepared at 0°C. Bromotrimethylsilane (0.27 mL, 2.072 mmol, 8 eq) was added dropwise and the reaction mixture was stirred overnight at room temperature. Ethyl bromide and excess silylating agent were removed by rotary evaporation at room temperature. The concentrate was solubilized in dry CH2Cl2 and evaporated again (×2). H2O was added to the residue and the mixture was stirred overnight at room temperature. The solution was filtered over cotton and freeze-dried on lyophilizer overnight. The crude acid (58 mg, 0.176 mmol), a yellow oil, was dissolved in DMF (1.8 mL) and triethylamine (0.05 mL, 0.352 mmol, 2 eq) was added. The mixture was stirred for 5 min at room temperature and chloromethyl pyvalate (0.25 mL, 1.760, 10 eq) was added. The resulting solution was heated overnight at 60°C. DMF was removed on the rotavapor at 60°C, and the residue was dissolved in CH2Cl2. The solution was washed with aqueous NaHCO3 and the aqueous phase was extracted three times with CH2Cl2. Then the combined organic layers were washed with brine and the aqueous phase was extracted three times with CH2Cl2. The combined organic layers were dried over MgSO4, filtered and concentrated in vacuo. Flash chromatography (toluene/acetone, 5/1) gave 18 (32 mg, 33%) as a yellow oil. 1H NMR (200 MHz, CDCl3) δ (ppm): 1.22 (s, 18H, C(CH3)3 ×2), 1.56–2.02 (m, 8H, CH2-P and CH2-CH2P and CH2-CH2O and CH2-CH2Ph), 2.65 (t, 2H, J = 7.1 Hz, CH2-Ph), 2.10 (s, 3H, CH3-CO), 3.64 (t, 2H, J = 6.4 Hz, CH2-N), 3.80 (t, 2H, J = 6.0 Hz, CH2-O-N), 5.65 (d, 4H, J = 13.0 Hz, CH2-O-P ×2), 7.13–7.36 (m, 5H, Har). 13C NMR (50 MHz, CDCl3) δ 20.1, 20.3, 22.7, 25.5, 27.0, 27.8, 28.0, 35.8, 38.9, 44.8, 45.3, 74.2, 81.4, 81.6, 126.1, 128.5, 141.8, 172.0, 177.0. LCMS (ESI+): m/z = 558.2 [M+H]+.
[({[(2,2-dimethylpropanoyl)oxy]methoxy}(3-{N-[(4-phenylphenyl)methoxy]acetamido}propyl)phosphoryl)oxy]methyl 2,2-dimethyl propanoate (19)
The acid of 16k (41 mg, 0.113 mmol, 1 eq) in DMF (1.2 mL) was prepared. Triethylamine (0.03 mL, 0.215 mmol, 2 eq) was added and the mixture was stirred for 5 min at room temperature. Then chloromethyl pivalate (0.16 mL, 1.11 mmol, 10 eq) was added and the reaction was heated overnight at 60°C then concentrated. The residue was dissolved in CH2Cl2 and washed with sat. NaHCO3 and brine. The aqueous layers were extracted with CH2Cl2 three times and the combined organic layers were dried over MgSO4, filtered and evaporated under vacuum. Chromatographic separation on silica gel (toluene/acetone, 5/1) gave the expected compound as a yellow oil (59.1 mg, 89%). 1H NMR (200 MHz, CDCl3) δ (ppm): 1.21 (s, 18H), 1.80–2.00 (m, 4H), 2.12 (s, 3H), 3.73 (t, J = 6.2, 6.0 Hz, 2H), 4.85 (s, 2H), 5.63 (s, 2H), 5.70 (s, 2H), 7.27–7.64 (m, 9H). 13C NMR (50 MHz, CDCl3) δ (ppm): 20.1, 20.7, 22.8, 25.6, 27.0, 38.9, 45.4, 45.6, 76.4, 81.5, 81.6, 127.3, 127.7, 127.8, 129.1, 129.9, 133.4, 140.6, 142.2, 171.2, 172.5. LCMS (ESI+): m/z = 592.8 [M+H]+.
[({[(2,2-dimethylpropanoyl)oxy]methoxy}({3-[N-(naphthalen-2-ylmethoxy)acetamido]propyl})phosphoryl)oxy]methyl 2,2-dimethylpropanoate (20)
The acid of 16j (71 mg, 0.211 mmol, 1 eq), a light yellow oil, was solubilized in anhydrous DMF and triethylamine (0.06 mL, 0.451 mmol, 2 eq) was added at room temperature. After the reaction mixture was stirred at room temperature for 5 min, chloromethylpivalate (0.3 mL, 2.08 mmol, 10 eq) was added. The resulting solution was stirred at 60°C overnight, and concentrated under vacuum. The residue was solubilized in CH2Cl2, washed with aqueous saturated NaHCO3 and the aqueous phase was extracted with CH2Cl2 three times. The combined organic layers were dried with anhydrous MgSO4, filtered, and concentrated. Chromatographic separation on silica gel (CH2Cl2/EtOAc, 5/1 to 3/2) gave the expected compound as a light yellow oil (46 mg, 0.081 mmol, 32%). 1H NMR (400 MHz, CDCl3) δ (ppm): 1.20 (s, 18H), 1.81–2.01 (m, 4H), 2.12 (s, 3H), 3.72 (t, J = 6.8 Hz, 2H), 4.98 (s, 2H), 5.64 (s, 2H), 5.67 (s, 2H), 7.47–7.54 (m, 3H), 7.83–7.88 (m, 4H). 13C NMR (100 MHz, CDCl3) δ (ppm): 20.04, 20.08, 20.65, 23.41, 24.82, 26.95, 38.82, 45.66, 76.83, 77.16, 77.48, 81.46, 81.52, 126.54, 126.64, 126.80, 127.87, 128.20, 128.74, 131.86, 133.25, 133.53, 172.56, 176.97. LCMS (ESI+): m/z = 566.2 [M+H]+.
[({[(benzoyl)oxy]methoxy}({3-[N-(4-phenylbutoxy)acetamido]propyl})phosphoryl)oxy]methyl benzoate (21)
To a solution of 15h (120 mg, 0.311 mmol) in CH2Cl2 (0.5 mL) at 0°C was added dropwise bromotrimethylsilane (0.33 mL, 2.488 mmol, 8 eq). The reaction mixture was stirred overnight at room temperature. Ethyl bromide and excess silylating agent were removed by rotary evaporation at room temperature. The concentrate was dissolved in dry CH2Cl2 and evaporated again (×2). H2O was added to the residue and the mixture was stirred overnight at room temperature. The solution was freeze-dried on lyophilizer overnight. The crude acid (73 mg, 0.220 mmol), a yellow oil, was solubilized in DMF (1.2 mL) and triethylamine (0.06 mL, 0.440 mmol, 2 eq) was added. The mixture was stirred for 5 min at room temperature and chloromethyl benzoate (376 mg, 2.200 mmol, 10 eq) was added in solution in DMF (1.0 mL). The resulting solution was heated overnight at 60°C. DMF was removed on the rotavapor at 60°C and the residue was dissolved in CH2Cl2. The solution was washed with aqueous NaHCO3 and the aqueous phase was extracted three times with CH2Cl2. Then the combined organic layers were washed with brine and the aqueous phase was extracted three times with CH2Cl2. The combined organic layers were dried over MgSO4, filtered and concentrated in vacuo. Flash chromatography (toluene/acetone, 5/1) gave 21 (48 mg, 37%) as a yellow oil. 1H NMR (200 MHz, CDCl3) δ (ppm): 1.49–2.01 (m, 8H, CH2-P and CH2-CH2P and CH2-CH2O and CH2-CH2Ph), 2.04 (s, 3H, CH3-CO), 2.61 (t, 2H, J = 7.1 Hz, CH2-Ph), 3.61 (t, 2H, J = 6.2 Hz, CH2-N), 3.73 (t, 2H, J = 6.0 Hz, CH2-O-N), 5.84–6.00 (m, 4H, CH2-O-P ×2), 7.12–7.67 (m, 11H, Har), 7.99–8.13 (m, 4H, Har). 13C NMR (CDCl3): δ (ppm): 20.0, 20.3, 23.3, 24.8, 27.7, 27.9. 29.8, 35.7, 44.8, 45.0, 74.2, 81.8, 81.9, 126.1, 128.4, 128.5, 128.5, 128.7, 128.8, 130.1, 134.0, 141.8, 165.0. LCMS (ESI+): m/z = 598.2 [M+H]+.
Dxr Inhibition Assays
Dxr activity was assayed at 37°C by spectrophotometrically monitoring the enzyme-catalyzed oxidation of NADPH, as previously described.(47) To determine percent inhibition for each inhibitor, 120µL assay solutions were used, containing 100 mM Tris pH 7.8, 25 mM MgCl2, 150 µM NADPH, 0.89 µM Dxr, 100 µM inhibitor, and the appropriate KM value of DXP (Echelon Biosciences, Salt Lake City, Utah), which is 252 µM for Yp Dxr(9) and 47 µM for Mtb Dxr.(48) The assays were performed by preincubating the mixture of Tris, MgCl2, Dxr, and inhibitor for 10 min (37°C) to facilitate binding of the inhibitor, followed by the addition of NADPH, a 5 min incubation (37°C), and then the addition of DXP. The half-maximal inhibition (IC50) values were determined by plotting enzyme fractional activity as a function of inhibitor concentration (1 nM–100 µM) and using GraphPad Prism (La Jolla, CA) nonlinear curve fitting to a sigmoidal dose response curve. Lineweaver-Burk plots were generated using 120 µL assay solutions containing 100 mM Tris pH 7.8, 25 mM MgCl2, 0.89 µM Dxr, and variable concentrations of inhibitor. Each assay solution was incubated for 10 min at 37°C to facilitate inhibitor binding. NADPH was subsequently added, to a final concentration of 150 µM for the DXP-dependent plots, and at variable concentrations for the NADPH-dependent plots (ranging from 30–120 µM for compound 16j against Mtb Dxr, and 3–20 µM for compound 8e against Yp Dxr). Assays were incubated for 10 minutes (37°C) following the addition of NADPH. Enzymatic reactions were initiated with addition of 252 µM (for Yp) or 47 µM (for Mtb) DXP for the NADPH dependent plots, and at variable DXP concentrations for the DXP-dependent plots (ranging from 25–200 µM for compound 16j against Mtb Dxr and 50–300 µM for compound 8e against Yp Dxr). The resulting Lineweaver-Burk plots were fit by linear regression using Graphpad prism. Ki values were calculated by linear regression using Graphpad Prism as the negative x-intercept of a plot of apparent Km of the substrate versus inhibitor concentration.
Supplementary Material
Acknowledgments
We gratefully acknowledge technical assistance from Teresa Hawley (GWU Medical Center), Joshua Wilhide (UMBC), and Phil Mortimer (JHU) for assistance with HRMS analysis. Financial support was provided by George Washington University’s Department of Chemistry and University Facilitating Fund, NIH (AI086453 to CSD and AI103280 and AI123808 to ARO), George Mason University’s Department of Chemistry and Biochemistry, the U.S. Army Medical Research and Materiel Command (W23RYX1291N601 to RDC), and the Division of Intramural Research, NIAID, NIH.
Footnotes
Synthetic procedures and analytical information for all compounds, as well as full Dxr and antibacterial inhibition methods and data are given. This information is available free of charge via the Internet at http://pubs.acs.org/.
REFERENCES
- 1.WHO; 2014. Antimicrobial Resistance: Global Report on Surveillance. [Google Scholar]
- 2.Hampton T. Report reveals scope of US antibiotic resistance threat. Jama. 2013;310:1661–1663. doi: 10.1001/jama.2013.280695. [DOI] [PubMed] [Google Scholar]
- 3.Shlaes DM, Sahm D, Opiela C, Spellberg B. The FDA reboot of antibiotic development. Antimicrob Agents Chemother. 2013;57:4605–4607. doi: 10.1128/AAC.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 5.Silver LL. Challenges of antibacterial discovery. Clin Microbiol Rev. 2011;24:71–109. doi: 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uh E, Jackson ER, San Jose G, Maddox M, Lee RE, Lee RE, Boshoff HI, Dowd CS. Antibacterial and antitubercular activity of fosmidomycin, FR900098, and their lipophilic analogs. Bioorganic & medicinal chemistry letters. 2011;21:6973–6976. doi: 10.1016/j.bmcl.2011.09.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenney ES, Sargent M, Khan H, Uh E, Jackson ER, San Jose G, Couch RD, Dowd CS, van Hoek ML. Lipophilic prodrugs of FR900098 are antimicrobial against Francisella novicida in vivo and in vitro and show GlpT independent efficacy. PLoS One. 2012;7:e38167. doi: 10.1371/journal.pone.0038167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.San Jose G, Jackson ER, Uh E, Johny C, Haymond A, Lundberg L, Pinkham C, Kehn-Hall K, Boshoff HI, Couch RD, Dowd CS. Design of Potential Bisubstrate Inhibitors against Mycobacterium tuberculosis (Mtb) 1-Deoxy-D-Xylulose 5-Phosphate Reductoisomerase (Dxr)-Evidence of a Novel Binding Mode. Medchemcomm. 2013;4:1099–1104. doi: 10.1039/C3MD00085K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haymond A, Johny C, Dowdy T, Schweibenz B, Villarroel K, Young R, Mantooth CJ, Patel T, Bases J, San Jose G, Jackson ER, Dowd CS, Couch RD. Kinetic characterization and allosteric inhibition of the Yersinia pestis 1-deoxy-D-xylulose 5-phosphate reductoisomerase (MEP synthase) PLoS One. 2014;9:e106243. doi: 10.1371/journal.pone.0106243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson ER, San Jose G, Brothers RC, Edelstein EK, Sheldon Z, Haymond A, Johny C, Boshoff HI, Couch RD, Dowd CS. The effect of chain length and unsaturation on Mtb Dxr inhibition and antitubercular killing activity of FR900098 analogs. Bioorganic & medicinal chemistry letters. 2014;24:649–653. doi: 10.1016/j.bmcl.2013.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. ( http://www.who.int/mediacentre/factsheets/fs104/en/index.html).
- 12.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013;34:271–286. doi: 10.1146/annurev-publhealth-031912-114431. [DOI] [PubMed] [Google Scholar]
- 13.Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. Jama. 2005;293:2767–2775. doi: 10.1001/jama.293.22.2767. [DOI] [PubMed] [Google Scholar]
- 14.( http://www.cdc.gov/plague/maps/index.html) Centers for Disease Control and Prevention. Plague Maps. Centers for Disease Control and Prevention. Plague Maps
- 15.Galimand M, Carniel E, Courvalin P. Resistance of Yersinia pestis to antimicrobial agents. Antimicrobial agents and chemotherapy. 2006;50:3233–3236. doi: 10.1128/AAC.00306-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Concepcion M, Albert B. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiology. 2002;130:1079–1089. doi: 10.1104/pp.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Natural product reports. 1999;16:565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- 18.Imlay L, Odom AR. Isoprenoid metabolism in apicomplexan parasites. Curr Clin Microbiol Rep. 2014;1:37–50. doi: 10.1007/s40588-014-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson ER, Dowd CS. Inhibition of 1-deoxy-D-xylulose-5-phosphate reductoisomerase (Dxr): a review of the synthesis and biological evaluation of recent inhibitors. Curr Top Med Chem. 2012;12:706–728. doi: 10.2174/156802612799984599. [DOI] [PubMed] [Google Scholar]
- 20.Masini T, Kroezen BS, Hirsch AK. Druggability of the enzymes of the non-mevalonate-pathway. Drug Discov Today. 2013;18:1256–1262. doi: 10.1016/j.drudis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Singh N, Cheve G, Avery MA, McCurdy CR. Targeting the Methyl Erythritol Phosphate (MEP) Pathway for Novel Antimalarial, Antibacterial and Herbicidal Drug Discovery: Inhibition of 1-Deoxy-D-Xylulose-5-Phosphate Reductoisomerase (DXR) Enzyme. Current Pharmaceutical design. 2007;13:1161–1177. doi: 10.2174/138161207780618939. [DOI] [PubMed] [Google Scholar]
- 22.Brown AC, Parish T. Dxr is essential in Mycobacterium tuberculosis and fosmidomycin resistance is due to a lack of uptake. BMC Microbiol. 2008;8:78. doi: 10.1186/1471-2180-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freiberg C, Wieland B, Spaltmann F, Ehlert K, Brotz H, Labischinski H. Identification of novel essential Escherichia coli genes conserved among pathogenic bacteria. J Mol Microbiol Biotechnol. 2001;3:483–489. [PubMed] [Google Scholar]
- 24.Hunter WN. The non-mevalonate pathway of isoprenoid precursor biosynthesis. The Journal of biological chemistry. 2007;282:21573–21577. doi: 10.1074/jbc.R700005200. [DOI] [PubMed] [Google Scholar]
- 25.Nair SC, Brooks CF, Goodman CD, Sturm A, McFadden GI, Sundriyal S, Anglin JL, Song Y, Moreno SN, Striepen B. Apicoplast isoprenoid precursor synthesis and the molecular basis of fosmidomycin resistance in Toxoplasma gondii. J Exp Med. 2011;208:1547–1559. doi: 10.1084/jem.20110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odom AR, Van Voorhis WC. Functional genetic analysis of the Plasmodium falciparum deoxyxylulose 5-phosphate reductoisomerase gene. Mol Biochem Parasitol. 2010;170:108–111. doi: 10.1016/j.molbiopara.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 28.Kojo H, Shigi Y, Nishida M. FR-31564, a new phosphonic acid antibiotic: bacterial resistance and membrane permeability. The Journal of antibiotics. 1980;33:44–48. doi: 10.7164/antibiotics.33.44. [DOI] [PubMed] [Google Scholar]
- 29.Kuzuyama T, Shimizu T, Takahashi S, Seto H. Fosmidomycin, a specific inhibitor of 1-Deoxy-D-xylulose 5-phosphate reductoisomerase in the nonmevalonate pathway for terpenoid biosynthesis. Tet. Lett. 1998;39:7913–7916. [Google Scholar]
- 30.Okuhara M, Kuroda Y, Goto T, Okamoto M, Terano H, Kohsaka M, Aoki H, Imanaka H. Studies on new phosphonic acid antibiotics. I. FR-900098, isolation and characterization. J Antibiot (Tokyo) 1980;33:13–17. doi: 10.7164/antibiotics.33.13. [DOI] [PubMed] [Google Scholar]
- 31.Schultz C. Prodrugs of biologically active phosphate esters. Bioorg Med Chem. 2003;11:885–898. doi: 10.1016/s0968-0896(02)00552-7. [DOI] [PubMed] [Google Scholar]
- 32.Andaloussi M, Henriksson LM, Wieckowska A, Lindh M, Bjorkelid C, Larsson AM, Suresh S, Iyer H, Srinivasa BR, Bergfors T, Unge T, Mowbray SL, Larhed M, Jones TA, Karlen A. Design, synthesis, and X-ray crystallographic studies of alpha-aryl substituted fosmidomycin analogues as inhibitors of Mycobacterium tuberculosis 1-deoxy-D-xylulose 5-phosphate reductoisomerase. Journal of medicinal chemistry. 2011;54:4964–4976. doi: 10.1021/jm2000085. [DOI] [PubMed] [Google Scholar]
- 33.Haemers T, Wiesner J, Van Poecke S, Goeman J, Henschker D, Beck E, Jomaa H, Van Calenbergh S. Synthesis of alpha-substituted fosmidomycin analogues as highly potent Plasmodium falciparum growth inhibitors. Bioorg Med Chem Lett. 2006;16:1888–1891. doi: 10.1016/j.bmcl.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 34.Jansson AM, Wieckowska A, Bjorkelid C, Yahiaoui S, Sooriyaarachchi S, Lindh M, Bergfors T, Dharavath S, Desroses M, Suresh S, Andaloussi M, Nikhil R, Sreevalli S, Srinivasa BR, Larhed M, Jones TA, Karlen A, Mowbray SL. DXR inhibition by potent mono- and disubstituted fosmidomycin analogues. Journal of medicinal chemistry. 2013;56:6190–6199. doi: 10.1021/jm4006498. [DOI] [PubMed] [Google Scholar]
- 35.Miller MJ. Syntheses and Therapeutic Potential of Hydroxamic Acid Based Siderophores and Analogues. Chem. Rev. 1989;89:1563–1579. [Google Scholar]
- 36.Blazewska K, Gajda T. N-(diethylphosphoryl)-O-benzylhydroxylamine-a convenient substrate for the synthesis of N-substituted O-benzylhydroxylamines. Tetrahedron. 2003;59:10249–10254. [Google Scholar]
- 37.Devreux V, Wiesner J, Jomaa H, Rozenski J, Van der Eycken J, Van Calenbergh S. Divergent strategy for the synthesis of alpha-aryl-substituted fosmidomycin analogues. J Org Chem. 2007;72:3783–3789. doi: 10.1021/jo0700981. [DOI] [PubMed] [Google Scholar]
- 38.McKenna CE, Higa MT, Cheug NH, McKenna M-C. The facile dealkylation of phosphonic acid dialkyl esters by bromotrimethylsilane. Tet. Lett. 1977;18:155–158. [Google Scholar]
- 39.Verbrugghen T, Cos P, Maes L, Van Calenbergh S. Synthesis and evaluation of alpha-halogenated analogues of 3-(acetylhydroxyamino)propylphosphonic acid (FR900098) as antimalarials. Journal of medicinal chemistry. 2010;53:5342–5346. doi: 10.1021/jm100211e. [DOI] [PubMed] [Google Scholar]
- 40.Haemers T, Wiesner J, Giessmann D, Verbrugghen T, Hillaert U, Ortmann R, Jomaa H, Link A, Schlitzer M, Van Calenbergh S. Synthesis of beta- and gamma-oxa isosteres of fosmidomycin and FR900098 as antimalarial candidates. Bioorganic & medicinal chemistry. 2008;16:3361–3371. doi: 10.1016/j.bmc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Reichenberg A, Wiesner J, Weidemeyer C, Dreiseidler E, Sanderbrand S, Altincicek B, Beck E, Schlitzer M, Jomaa H. Diaryl ester prodrugs of FR900098 with improved in vivo antimalarial activity. Bioorg Med Chem Lett. 2001;11:833–835. doi: 10.1016/s0960-894x(01)00075-0. [DOI] [PubMed] [Google Scholar]
- 42.Baudy RB, Butera JA, Abou-Gharbia MA, Chen H, Harrison B, Jain U, Magolda R, Sze JY, Brandt MR, Cummons TA, Kowal D, Pangalos MN, Zupan B, Hoffmann M, May M, Mugford C, Kennedy J, Childers WE., Jr Prodrugs of perzinfotel with improved oral bioavailability. J Med Chem. 2009;52:771–778. doi: 10.1021/jm8011799. [DOI] [PubMed] [Google Scholar]
- 43.Koronakis V, Eswaran J, Hughes C. Structure and function of TolC: the bacterial exit duct for proteins and drugs. Annual review of biochemistry. 2004;73:467–489. doi: 10.1146/annurev.biochem.73.011303.074104. [DOI] [PubMed] [Google Scholar]
- 44.Koppisch AT, Fox DT, Blagg BS, Poulter CD. E. coli MEP synthase: steady-state kinetic analysis and substrate binding. Biochemistry. 2002;41:236–243. doi: 10.1021/bi0118207. [DOI] [PubMed] [Google Scholar]
- 45.Reuter K, Sanderbrand S, Jomaa H, Wiesner J, Steinbrecher I, Beck E, Hintz M, Klebe G, Stubbs MT. Crystal structure of 1-deoxy-D-xylulose-5-phosphate reductoisomerase, a crucial enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. The Journal of biological chemistry. 2002;277:5378–5384. doi: 10.1074/jbc.M109500200. [DOI] [PubMed] [Google Scholar]
- 46.Bjorkelid C, Bergfors T, Unge T, Mowbray SL, Jones TA. Structural studies on Mycobacterium tuberculosis DXR in complex with the antibiotic FR-900098. Acta Crystallogr D Biol Crystallogr. 2012;68:134–143. doi: 10.1107/S0907444911052231. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi S, Kuzuyama T, Watanabe H, Seto H. A 1-deoxy-D-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-D-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc Natl Acad Sci U S A. 1998;95:9879–9884. doi: 10.1073/pnas.95.17.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhiman RK, Schaeffer ML, Bailey AM, Testa CA, Scherman H, Crick DC. 1-Deoxy-D-xylulose 5-phosphate reductoisomerase (IspC) from Mycobacterium tuberculosis: towards understanding mycobacterial resistance to fosmidomycin. J Bacteriol. 2005;187:8395–8402. doi: 10.1128/JB.187.24.8395-8402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.