Abstract
Rationale
Prescription opioid misuse and high dose opioid use may result in allostatic dysregulation of hedonic brain circuitry, leading to reduced emotion regulation capacity. In particular, opioid misuse may blunt the ability to experience and upregulate positive affect from natural rewards.
Objectives
The purpose of this study was to examine associations between opioid use/misuse and autonomic indices of emotion regulation capability in a sample of chronic pain patients receiving prescription opioid pharmacotherapy.
Methods
Chronic pain patients taking long-term opioid analgesics (N = 40) completed an emotion regulation task while heart rate variability (HRV) was recorded, as well as self-report measures of opioid misuse, craving, pain severity, and emotional distress. Based on a validated cut-point on the Current Opioid Misuse Measure, participants were grouped as opioid misusers or non-misusers. Opioid misuse status and morphine equivalent daily dose (MEDD) were examined as predictors of HRV and self-reports of emotion regulation.
Results
Opioid misusers exhibited significantly less HRV during positive and negative emotion regulation, and significantly less positive affect, than non-misusers, after controlling for confounders including pain severity and emotional distress. MEDD was inversely associated with positive emotion regulation efficacy.
Conclusion
Findings implicate the presence of reward processing deficits among chronic pain patients with opioid-misusing behaviors, and opioid dosage was associated with deficient emotion regulation, suggesting the presence of compromised top-down cognitive control over bottom-up hedonic processes. Emotion regulation among opioid misusers may represent an important treatment target.
Keywords: opioid misuse, chronic pain, emotion regulation, heart rate variability, allostatic, reward
1. Introduction
Prolonged use of prescription opioid analgesics may result in opioid misuse and addiction due to pharmacologic actions of opioids on mesocorticolimbic brain reward circuits (Volkow et al. 2016). Approximately 21% to 29% of chronic pain patients receiving long-term opioid analgesic pharmacotherapy exhibit opioid misuse behaviors including unauthorized dose escalation or attempts to self-medicate negative affect with opioids (Vowles et al. 2015). A recent investigation of treatment-seeking opioid dependent individuals found that 95% reported using opioids to alleviate negative affective states (Garland et al. 2015b). Thus, misuse of opioids to regulate emotional well-being may be prevalent in some populations.
According to the allostatic model (Koob and Le Moal 2001), the attempt to maintain hedonic homeostasis via opioid misuse may be undermined by increasing pharmacologic tolerance coupled with recurrent pain and affective distress, resulting in craving (Martel et al. 2014) and opioid dose escalation (Shurman et al. 2010). Ironically, increased opioid self-administration may shift the hedonic set point, resulting in insensitivity to natural rewards, a blunting of positive emotion, and worsening dysphoria (Koob and Le Moal 2008). When coupled with the disruptions in self-regulation that lead to and result from addictive behavior (Tang et al. 2015), these changes may drive a downward spiral of dependence on opioids (Garland et al. 2013).
Consistent with this theory implicating impaired cognitive control over reward system function, opioid misuse and opioid dose escalation among chronic pain patients should impair emotion regulation – and in particular, blunt the ability to experience and upregulate positive affect in response to naturally rewarding stimuli. Though reward processing deficits have been observed in illicit opiate dependence (Lubman et al. 2009; Huhn et al.), only one study has demonstrated this phenomenon among opioid-misusing pain patients by identifying significantly attenuated cardiac-autonomic regulation (i.e., heart rate variability - HRV) during attention to natural reward-related images in a dot probe task (Garland et al. 2015a).
Attending to emotionally-salient stimuli and regulating one’s emotional response to such stimuli is thought to elicit downstream activation of a central-autonomic network (e.g., medial prefrontal cortex → anterior cingulate cortex and anterior insula → central nucleus of the amygdala → hypothalamus → nucleus of the solitary tract → ventrolateral medullary) with downstream effects on visceral and peripheral physiology, including the beat-to-beat modulation of heart rate by the vagus nerve, known as high-frequency heart rate variability (HRV) (Thayer and Lane 2000; Appelhans and Luecken 2006; Thayer and Lane 2009; Thayer et al. 2012). HRV in the high frequency range (0.15 – 0.40 Hz) is mediated by parasympathetic influences on the sinoatrial node of the heart (Berntson et al. 1997). Among healthy individuals, heightened phasic HRV in response to emotional challenge has been shown to reflect regulatory efficacy (Butler et al. 2006; Segerstrom and Nes 2007), whereas individuals with difficulties in emotion regulation exhibit lower HRV at rest (Williams et al. 2015) and blunted phasic HRV during emotion regulation tasks (Di Simplicio et al. 2011). Similarly, substance dependent patients with deficits in regulation of attention, emotion, and appetitive urges have been shown to exhibit attenuated HRV at rest (Ingjaldsson et al. 2003; Quintana et al. 2013) and when attempting to regulate reward responses (i.e., craving) to addiction-related cues (Garland et al. 2012a). At the same time, elevated HRV is also elicited in response to a wide range of appetitive stimuli (Rajan et al. 1998; Inagaki et al. 2005; Culbertson et al. 2010; Stockhorst et al. 2011; Erblich et al. 2011; Garland et al. 2012b; Udo et al. 2013). Relatedly, upregulating positive emotions is associated with increased vagally-mediated HRV over time (Kok et al. 2013). Taken together, these findings indicate that HRV may be a useful means of assessing emotion regulation capacity and responsivity to rewarding stimuli among prescription opioid misusing chronic pain patients.
To date, few studies have examined emotion regulation among individuals in pain, and those that have were largely focused on the effects of emotion regulation on acute pain experience. Among healthy individuals, downregulating negative emotions through reappraisal (Lapate et al. 2011; Hampton et al. 2015) and acceptance (Braams et al. 2012; Kohl et al. 2013), and upregulating positive emotions through positive mood induction (Finan and Garland 2015) have been associated with the ability to decrease experimentally-induced pain and pain-related cardiovascular responses. In contrast to these adaptive strategies, maladaptive forms of emotion regulation (e.g., suppressing negative emotions) have been shown to increase acute pain induced in the laboratory (Quartana et al. 2010). Among chronic pain patients, maladaptive emotion regulation strategies like emotion suppression have been shown to exacerbate pain under stressful conditions (Burns 2006) by increasing physiological reactivity to pain and stress (Burns et al. 2011). Neuroimaging research has suggested that the regulation of pain and emotion share overlapping neural substrates, including cortico-limbic-striatal circuits (Salomons et al. 2014) which partially overlap with the central autonomic network thought to govern HRV. These studies suggest a linkage between emotion regulatory capacity and pain, yet it is unclear whether chronic pain patients who use and misuse opioids evidence emotion regulation deficits. In that regard, though previous research has demonstrated associations between opioid misuse and negative affectivity in chronic pain samples (Martel et al. 2014; Wasan et al. 2015; Edwards et al. 2016), to our knowledge, no prior study has investigated emotion regulatory deficits among opioid-using and misusing chronic pain patients with a performance-based, biobehavioral measure of proactive emotion regulation.
The present study aimed to 1) establish whether prescription opioid misusers with chronic pain evidence attenuated emotion regulatory capacity (as indicated by HRV responses) on an emotion regulation task relative to chronic pain patients who take opioids as medically prescribed and 2) to determine whether opioid dose is significantly correlated with blunted positive emotion regulation capacity.
2. Method
Participants (N=40; see Table 1 for demographic/clinical characteristics) were recruited from primary care and specialty pain clinics, and met inclusion criteria if they reported having a chronic pain condition and had taken opioid analgesics daily or nearly every day for at least the past 90 days (Chou et al. 2009). Participants were instructed to take their prescribed opioid medication as usual on the day of the study. Following informed consent, participants completed self-report measures of opioid dose, pain severity and location, opioid misuse behaviors, and opioid craving. Then ECG electrodes were attached, and participants sat quietly for a 5-minute resting baseline period. After this resting baseline, participants then completed a blocked emotion regulation task while ECG was recorded. In between the four blocks of the emotion regulation task, participants made affect ratings to report their emotional response to each of the emotion regulation conditions. The study protocol was approved by the University of Utah IRB. All procedures complied with standards propounded in the Helsinki Declaration of 1975.
Table 1.
Demographic and Clinical Characteristics of the Opioid-Treated Chronic Pain Sample (N = 40).
| Measure | Non-Misusers (n = 12) |
Misusers (n = 28) |
Difference Tests |
|---|---|---|---|
| Female, N (%) | 2 (17%) | 10 (36%) | X2=1.45, p=.23 |
| Age | 42.67 ± 16.83 | 39.79 ± 13.31 | t=.58, p=.57 |
| Racial/ethnic background, N (%) | X2=.52, p=.92 | ||
| African American | 1 (8%) | 3 (11%) | |
| Caucasian | 10 (83%) | 22 (79%) | |
| Latino | 1 (8%) | 2 (7%) | |
| Asian/Pacific Islander | - | 1 (4%) | |
| Primary Pain condition, N (%) | X2=1.84, p=.61 | ||
| Low back pain | 5 (42%) | 17 (61%) | |
| Joint Pain | 4 (33%) | 6 (21%) | |
| Cervical Pain | 1 (8%) | 3 (11%) | |
| Other | 2 (17%) | 2 (7%) | |
| Pain severity (BPI) | 6.35 ± 1.81 | 5.89 ± 1.48 | t=.85, p=.40 |
| Emotional distress (DASS) | 8.25 ± 4.86 | 19.43 ± 11.91 | t=4.21, p<.001 |
| Opioid misuse (COMM) | 5.00 ± 2.34 | 16.39 ± 6.44 | t=8.19, p<.001 |
| Opioid craving in past week (VAS) | 7.00 ± 10.33 | 24.00 ± 26.23 | t=2.90, p=.006 |
| Thoughts of opioids in past week (VAS) | 5.36 ± 8.05 | 24.82 ± 28.31 | t=3.31, p=.002 |
| Current desire for opioids (VAS) | 26.82 ± 32.37 | 47.9 ± 48.53 | t=1.94, p=.06 |
| Morphine equivalent daily dose | 55.94 ± 48.53 | 157.11 ± 226.21 | t=2.25, p=.03 |
| Primary opioid type, N (%)† | X2=.82, p=.84 | ||
| Hydrocodone | 3 (25%) | 9 (32%) | |
| Oxycodone | 3 (25%) | 7 (25%) | |
| Tramadol | 5 (42%) | 8 (29%) | |
| Other | 1 (8%) | 4 (14%) |
Note: BPI = Brief Pain Inventory (Cleeland 1994); COMM = Current Opioid Misuse Measure (Butler et al. 2007); DASS =Depression Anxiety Stress Scale-21 (Antony et al. 1998); VAS = visual analogue scale (Wasan et al., 2009).
Prescription opioid misuse was determined by scores on the self-reported Current Opioid Misuse Measure (COMM; α=.83) (Butler et al. 2007). The original COMM validation study found that a score of ≥9 was suggestive of prescription opioid misuse among chronic pain patients. Based on a validated cut-point of 9 on the Current Opioid Misuse Measure (COMM) (Butler et al. 2007), participants were placed into one of two groups: a group of chronic pain patients who engaged in opioid misuse behaviors (misusers, n=28), and a group of chronic pain patients who took prescription opioids without engaging in opioid misuse (non-misusers, n=12).
2.1 Measures
Emotion regulation task
The study utilized an emotion regulation paradigm (Jackson et al. 2000; Ochsner et al. 2002) that was comprised of 120 trials, each presented for 6 seconds, separated by a 500 ms fixation cross. There were four conditions, each presented in one of four randomized, counterbalanced blocks: “view negative,” “reappraise negative,” “view positive,” and “savor positive.” The “reappraise negative” condition instructed participants to reinterpret the image content to decrease emotional reactions to the image. The “savor positive” condition, which corresponded to typical “increase positive” instructions on emotion regulation tasks (e.g., Kim and Hamann 2007), instructed participants to imagine experiencing the positive event occurring in the image, and to focus on enjoyable aspects of the image and their own positive emotional response to the image. The “view” conditions instructed participants to simply view the image without trying to change their emotional experience. After each block, participants rated their current emotional state from 1 (most negative) to 9 (most positive). Stimuli included positive images of natural rewards (e.g., social affiliation, athletic victories) and negative images (e.g., violence, injury) from the International Affective Picture System (Lang et al. 1997) (see Supplementary Materials for more detail).
Opioid use and misuse
Morphine equivalent daily opioid dose (MEDD) was obtained by self-report and corroborated by medical chart review. The COMM (α=.82) (Butler et al., 2007) was used to assess prescription opioid misuse. Participants responded to 17 items rated on a Likert scale (0 = never, 4 = very often) regarding how often in the past 30 days they had engaged in behaviors linked with opioid misuse or took opioid medication in excessive doses or ways other than how it was prescribed.
Opioid craving
Opioid craving over the past week was assessed with two items rated on 100mm visual analog scales (VAS): “In the past week, how much have you craved your pain medication?” and “In the past week, how often have you found yourself thinking about the next opioid dose.” Desire for opioids at the time of the emotion regulation protocol was assessed with a single VAS item: “How much do you want to take your opioids right now?” Similar VAS items have been used to assess opioid craving among chronic pain patients (Wasan et al. 2009).
Pain severity
Pain severity was measured with the four-item pain severity subscale from the Brief Pain Inventory (BPI; α=.88) a well-validated measure widely used to assess acute and chronic pain (Cleeland, 1994). Response options ranged from 0 (no pain) to 10 (pain as bad as I can imagine).
Emotional distress
Distress was measured with the 21-item Depression Anxiety Stress Scale (DASS), which provides a composite score of negative emotional symptoms (α=.93) (Antony et al. 1998).
HRV Measurement
Ag-AgCl electrodes were attached to participants’ right and left pectoral muscles. Electrocardiogram (ECG) data were sampled at 1000 Hz and recorded continuously throughout the protocol on a Biopac MP36 or MP150 (Biopac, Goleta, CA). Respiration rate was measured concurrently via the Biopac system to confirm that breathing fell within the respiratory frequency band (0.15 – 0.40 Hz).
2.2 Data Reduction and Analysis
With respect to the HRV analyses, R-R intervals were detected in the ECG data using automated routines in Acqknowledge 4.1 software (BIOPAC, Inc.). The R-wave file was then visually inspected to correct misidentified or omitted R-waves. Kubios 2.0 (Biosignal Analysis and Medical Imaging Group, University of Finland) was used to calculate beats-per-minute (BPM) and for spectral analysis of R-waves. R-R interval data were segmented into five, 3.25 minute segments, each of which spanned the entire length of one of the five study conditions: resting baseline, “view negative,” “reappraise negative,” “view positive,” and “savor positive” blocks. HRV analyses were conducted on the entire segment length for each of these five conditions: a fast Fourier transform was applied separately to R-R interval data to extract normalized high-frequency HRV from a de-trended, end-tapered interbeat interval time series. The spectrum for the selected R-R interval segment was calculated via Welch’s periodgram method, in which R-R interval data were reduced using Kubios 2.0 default settings of a window width of 128 seconds (with a window overlap of 50%). High-frequency HRV in the respiratory frequency band (0.15 – 0.40 Hz) was selected as our estimate of vagally-mediated HRV. Analysis of respiration data showed that breathing fell within the respiratory frequency band (mean respiration rate .21 Hz at baseline, .22 Hz during “view negative,” .23 Hz during “reappraise negative,” .21 Hz during “view positive,” and .22 Hz during “savor positive” blocks). Following Berntson (1997) and Malliani et al. (1994), we calculated HRV in normalized units to elucidate shifts in this frequency component that might otherwise be obscured by use of absolute units which are dependent on total HRV power. Normalization of HRV values produces statistical averages with distributions that converge more readily to normal distributions than do raw spectral band powers, and normalization tends to produce values that are more translatable across different research studies using similar tasks with different block lengths (Burr 2007). HRV was averaged for the baseline and each of the four emotion regulation task blocks.
To test our study hypotheses, we first conducted 2 (Group: Misuser, Non-misuser) × 5 (Condition: baseline, view negative, reappraise negative, view positive, savor positive) ANOVAs on HRV and affect ratings during the emotion regulation task. Next, to isolate the effects of opioid misuse from the effects of other potential confounders on indices of emotion regulation, we controlled for MEDD, pain severity, emotional distress, and gender as covariates in a separate sensitivity analysis. To identify differential effects of opioid misuse status on various conditions of the emotion regulation task relative to the resting baseline, planned contrasts were used. Next, we examined opioid dose as a predictor of positive emotion regulatory inefficacy by correlating MEDD with reappraise-view and savor-view change scores in affect ratings (Felder et al. 2012). Then, we investigated the association between opioid craving (craving and thoughts about opioids in the past week, and current desire for opioids) and affect ratings during the emotion regulation task. The Bonferroni-Holm procedure was used to correct for multiple comparisons across these 12 correlation coefficients. Lastly, we conducted a path model with bootstrapping of the indirect effect (Preacher and Hayes 2008) to examine affect rating variables that survived correction for multiple comparisons as mediators of the association between opioid misuse and craving.
3. Results
3.1 HRV and Affective Responses During Emotion Regulation
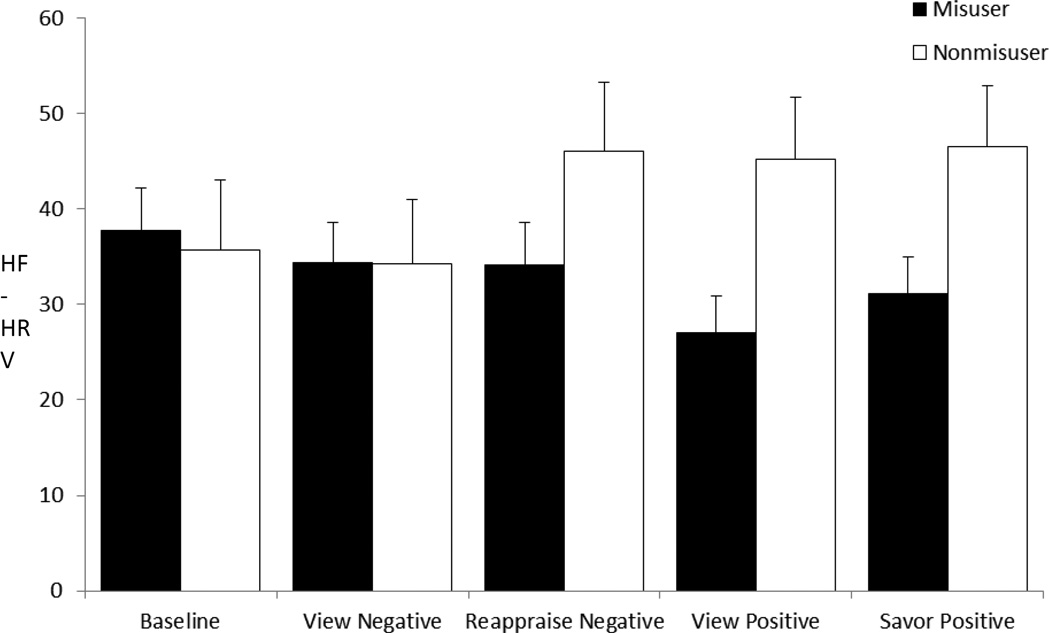
With regard to HRV responses, neither the main effect of Condition, F(4,35)=1.05, p=.37, η2partial =.03, nor the main effect of Group, were significant, F(1,35)=2.55, p=.12, η2partial=.06. The omnibus Group × Condition interaction was significant, F(4,35)=2.92, p=.035, η2partial=.07, suggesting that the pattern of HRV responses across emotion regulation conditions significantly differed between opioid misusers and non-misusers. To ensure the robustness of this between-groups difference, we then controlled for potential confounders in a sensitivity analysis, and found that neither the main effect of Condition nor the main effect of Group were significant; however, the omnibus Group X Condition interaction was significant, F(4,35)=4.32, p=.005, η2partial=.11. Planned contrasts revealed that HRV increased to a significantly greater extent for non-misusers than for misusers from resting baseline to the “view positive” block, F(1,35)=8.46, p=.006, η2partial=.20, “savor positive” block, F(1,35)=4.78, p=.036, η2partial=.12, and “reappraise negative” block, F(1,35)=4.28, p=.046, η2partial=.11 (see Figure 1). Also, misusers did not show the differences in HRV from “view negative” to “reappraise negative” exhibited by nonmisusers, F(1,35)=5.21, p=.03, η2partial=.13. With regard to affective responses, the main effect of Condition was nonsignificant, F(4,32)=2.19, p=.07, η2partial=.06. Though the Group × Condition interaction was not significant, there was a significant main effect of Group, F(1,32)=4.55, p=.04, η2partial= .13, such that misusers reported significantly less positive affect (M affect rating=4.91, SE=.15) throughout the entire protocol than non-misusers (M affect rating=5.48, SE=.23).
Figure 1.
High-frequency heart rate variability (HF-HRV, in normalized units) responses at baseline and during conditions of the emotion regulation task, by opioid misuser and nonmisuser groups, controlling for covariates including gender, morphine equivalent daily opioid dose, pain severity, and emotional distress. Means and error bar (standard error) are plotted as function of blocks across the two groups.
3.2 Association Between Opioid Dose and Emotion Regulation Efficacy
Higher MEDD was significantly associated with lower positive emotion regulation efficacy, r=−.42, p=.006, but was not significantly correlated with negative emotion regulation efficacy, r=−.14, p=.41.
3.3 Association Between Pain and Emotion Regulation Efficacy
Pain severity was not significantly associated with either positive (r=−.16, p=.34) or negative emotion regulation (r=−.09, p=.61) efficacy,
3.4 Association Between Affect Ratings and Craving
Craving in the past week was associated with lower affect ratings during the “view positive” condition (r=−.39, p=.01). Frequent thinking about the next opioid dose was associated with lower affect ratings during the “view positive” (r=−.34, p=.03) and “savor positive” (r=−.34, p=.04) conditions. Current desire for opioids at the time of the emotion regulation task was associated with lower affect ratings during the “view positive” (r=−.49, p=.001), “savor positive” (r=−.31, p=.05), and “reappraise negative” (r=−.31, p=.05) conditions. None of the other correlations were significant, and none of the significant correlations survived correction for multiple comparisons save for the association between current desire for opioids and lower affect during the “view positive” condition.
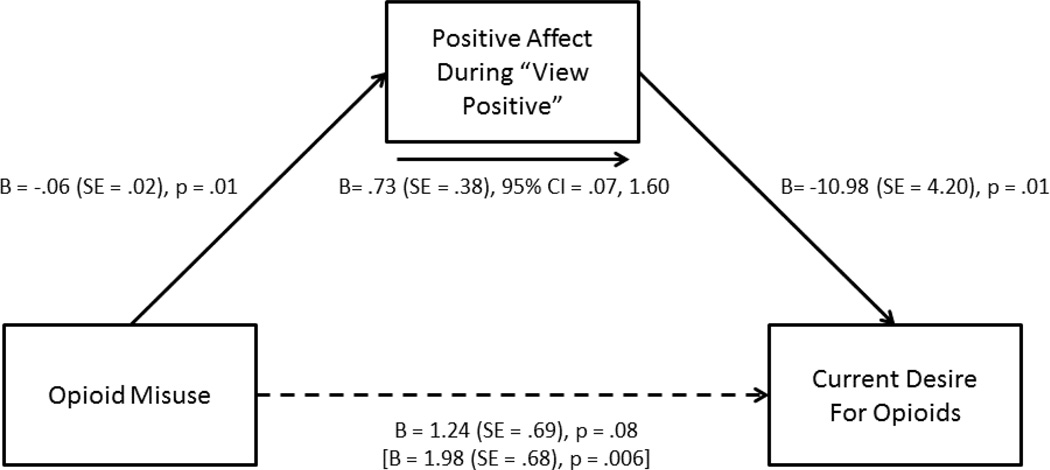
3.5 Path Model
The sole affect rating variable that survived multiple corrections (affect during “view positive”) was entered into a path model as a mediator of the association between opioid misuse and current desire for opioids (Figure 2). Because opioid dose was correlated with positive emotional responses during the emotion regulation task, MEDD was used as the single covariate in this model. The bootstrapped unstandardized indirect effect was .74, and the 95% confidence interval ranged from .08 to 1.61. Thus, the indirect effect of attenuated positive affect during processing of positive emotional stimuli on desire for opioids was statistically significant.
Figure 2.
Path model testing positive affect during visual processing of positive emotional stimuli (“view positive” condition on the emotion regulation task) as a mediator of the association between opioid misuse and current desire for opioids, controlling for morphine equivalent daily opioid dose. Statistical parameters in brackets represent the association between opioid misuse and current desire for opioids in the absence of the mediating variable. Solid arrows denote statistical significant pathways whereas dotted arrows denote non-significant pathways.
4. Discussion
In this study of chronic pain patients receiving extended opioid pharmacotherapy, patients who surpassed the validated opioid misuse cut-point on the COMM, a measure designed to assess opioid misuse, exhibited significantly blunted HRV responses to positive emotional stimuli, and less positive affect, in general, compared to opioid non-misusers. Also, patients who exceeded the opioid misuse cut-point evidenced emotion regulatory deficits, as indicated by attenuated capacity to increase autonomic regulation during reappraisal of negative stimuli. Moreover, opioid dose was significantly inversely associated with positive emotion regulation, such that patients taking comparatively higher opioid doses were less able to proactively increase positive affect via savoring positive images than patients taking low dose opioids. Finally, reduced positive affect in response to viewing positive images was associated with heightened opioid craving.
Study findings provide preliminary support for allostatic models of prescription opioid misuse (Shurman et al. 2010; Garland et al. 2013; Elman and Borsook 2016), which assert that chronic exposure to opioids in the context of chronic pain produces a downward shift in the hedonic set point, resulting in insensitivity to natural rewards and a dysphoric mood state. According to such models, opioid-induced dysregulation of hedonic homeostasis is then thought to drive opioid misuse behaviors, which eventually become habitual, undermining self-control and miring the individual in a downward spiral of craving and compulsive opioid use. In that regard, opioid misusers in the current study exhibited dampened HRV responses during negative emotion regulation relative to non-misusers, who evidenced increases in HRV when reappraising negative emotional images. Indeed, non-misusers in the present study showed a HRV response profile during emotion regulation that was similar to that of healthy controls, who exhibit increases in HRV during reappraisal relative to passive exposure to negative stimuli (Di Simplicio et al. 2012). The observed attenuation of HRV responses during reappraisal among the opioid misusers in our sample implicates the presence of poor central-autonomic integration with consequent deficits in top-down cognitive control over bottom-up affective responding (Thayer and Lane 2009), reflected by diminished flexibility in parasympathetic cardiovascular tone during cognitive regulation of negative emotions.
At the same time, opioid misuse in the current sample was selectively associated with blunted HRV responses when passively processing positive but not negative emotional stimuli. In light of prior studies demonstrating that HRV increases in response to presentation of rewarding stimuli (Inagaki et al. 2005; e.g., Garland et al. 2012b), this finding suggests that opioid misuse may be associated with reduced reward responsiveness. Further, in the present sample, high-dose opioid use and misuse were correlated with impairments in upregulating positive affective responses, and path analysis indicated that the association between opioid misuse and desire for opioids was statistically mediated by reduced affective responses to positive stimuli. This latter finding provides support for allostatic models (Shurman et al. 2010; Garland et al. 2013; Elman and Borsook 2016) that suggest opioid misuse may lead to insensitivity to natural rewards, which in turn may impel craving for opioids as means of obtaining hedonic equilibrium. Overall, autonomic and affective results from the present study add to the emerging body of literature demonstrating hedonic dysregulation, reward processing deficits, and reward-related pathophysiology among chronic pain patients on high dose opioids and those at risk for prescription opioid misuse (Younger et al. 2011; Bunce et al. 2015; Garland et al. 2015a; Borsook et al. 2016).
However, given the cross-sectional nature of the current study, it is unknown whether the observed emotion regulatory impairments were a cause, correlate, or consequence of opioid misuse and dose escalation. In that regard, individuals with a dispositional inability to regulate distressing emotions may turn to substance misuse as a means of self-medicating emotional distress (Khantzian 1997). From this perspective, emotional distress from comorbid chronic pain and affective dysregulation might impel self-medication motives and thereby increase vulnerability to prescription opioid misuse. Indeed, epidemiological studies indicate that mood and anxiety disorders are often antecedent to the onset of opioid misuse and dependence (Green et al. 2011; Martins et al. 2012), and in a sample of prescription opioid dependent individuals in acute detoxification and long-term outpatient treatment, 94% reported frequent misuse of opioids to self-medicate negative affect (Garland et al. 2014c). Given the common occurrence of prescription opioid misuse as a means of self-medicating negative emotions, it is possible that some participants in the study who were classified as opioid misusers may have suffered from preexisting emotion dysregulation (perhaps due to untreated pain) prior to the onset of opioid use. To account for this possibility, we included trait-level emotional distress and pain severity as covariates in our analyses, and after controlling for these variables, we found that opioid misusers continued to evidence blunted emotion regulatory capacity when compared to non-misusers. Despite these statistical controls, this alternative interpretation cannot be ruled out due to the cross-sectional nature of the study. Most likely, recursive feedback loops are at play, such that individuals with dispositional emotion regulation deficits may be more likely to engage in opioid misuse and opioid dose escalation, which, when prolonged over time, might amplify their propensity towards emotion dysregulation. Longitudinal investigations are needed to yield fine-grained, temporally dynamic causal models of the relations examined in this study.
Moreover, the study was limited by the fact that we characterized patients as opioid misusers based on their score on a self-report instrument (the COMM). Because some patients may be reluctant to admit to opioid misuse on a self-report measure, reporting bias is possible. For this reason, we may not have been able to correctly classify all the misusers in the sample. Nonetheless, participants were assured of confidentiality, and the majority endorsed opioid misuse on the COMM. Future studies should triangulate self-reports of opioid misuse with clinical interviews and review of data from legally-mandated prescription monitoring programs. In addition, interpretation of study findings is limited by the fact that we did not collect data on use of medications other than opioids which might modulate HRV (e.g., sedatives, antidepressants, beta blockers, etc.). It is possible that misusers and non-misusers may have differed with regard to other medication usage. That said, the lack of significant between-groups differences in resting baseline HRV and HRV responses to viewing negative emotional stimuli suggests that the observed HRV differences in the reappraise negative, look positive, and savor positive conditions were not driven by mere pharmacologic effects of other medications that might dampen HRV. If the misuse and non-misuse groups did in fact differ on use of other medications that dampen HRV, it is likely that HRV differences would have been observed in resting HRV and HRV response during the negative look conditions. Nonetheless, future studies should carefully quantify medication usage to prevent possible confounding by other medications. Lastly, we instructed participants to take their opioids as prescribed on the day of the emotion regulation testing session to prevent opioid withdrawal which might have had deleterious effects on participants’ health and cognitive performance. The acute pharmacologic effects of opioids might have influenced performance on the emotion regulation task. To account for this possibility, we included morphine equivalent daily dose as a covariate in sensitivity analyses. Longitudinal investigations are needed to determine the effects of high dose opioids on emotion regulation, and whether such effects are undergirded by changes in brain systems implicated in salience attribution (e.g., dopaminergic) and hedonic responses (e.g., endogenous opioid) that are dysregulated in the context of chronic pain and addiction (Elman and Borsook 2016).
In sum, findings from the present exploratory study, though preliminary, add to the growing body of literature suggesting that the comorbidity of chronic pain and opioid misuse is linked with emotion regulation and reward processing deficits. Should these findings be replicated in larger and better controlled studies, they may have important implications for treatment development. Indeed, interventions that restructure reward processes by enhancing positive emotion regulation (e.g., Mindfulness-Oriented Recovery Enhancement, see Garland 2016) may effectively remediate affective deficits among opioid-misusing patients, and possibly attenuate addictive tendencies towards opioids. In that regard, enhancing natural reward responsiveness via cognitive training appears to be associated with decreased opioid craving and opioid misuse among chronic pain patients (Garland et al. 2014a; Garland et al. 2014b). More research is needed to determine the mechanistic role of emotion regulation in modulating hedonic functions underpinning the comorbidity of chronic pain and opioid misuse.
Acknowledgments
This work was supported by grant numbers R34DA037005 and R01DA042033 from the National Institutes of Health awarded to E.L.G.; and a grant from the Fahs Beck Fund for Research and Experimentation, also awarded to E.L.G.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
Contributor Information
Eric L. Garland, University of Utah
Craig J. Bryan, University of Utah
Yoshio Nakamura, University of Utah.
Brett Froeliger, Medical University of South Carolina.
Matthew O. Howard, University of North Carolina at Chapel Hill
References
- Antony MM, Bieling PJ, Cox BJ, et al. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol Assess. 1998;10:176–181. [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psychol. 2006;10:229–240. [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Borsook D, Linnman C, Faria V, et al. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev. 2016;68:282–297. doi: 10.1016/j.neubiorev.2016.05.033. [DOI] [PubMed] [Google Scholar]
- Braams BR, Blechert J, Boden MT, Gross JJ. The effects of acceptance and suppression on anticipation and receipt of painful stimulation. J Behav Ther Exp Psychiatry. 2012;43:1014–1018. doi: 10.1016/j.jbtep.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Bunce SC, Harris JD, Bixler EO, et al. Possible Evidence for Re-regulation of HPA Axis and Brain Reward Systems Over Time in Treatment in Prescription Opioid-Dependent Patients. J Addict Med. 2015;9:53–60. doi: 10.1097/ADM.0000000000000087. [DOI] [PubMed] [Google Scholar]
- Burns JW. The role of attentional strategies in moderating links between acute pain induction and subsequent psychological stress: Evidence for symptom-specific reactivity among patients with chronic pain versus healthy nonpatients. Emotion. 2006;6:180–192. doi: 10.1037/1528-3542.6.2.180. [DOI] [PubMed] [Google Scholar]
- Burns JW, Quartana PJ, Gilliam W, et al. Suppression of anger and subsequent pain intensity and behavior among chronic low back pain patients: the role of symptom-specific physiological reactivity. J Behav Med. 2011;35:103–114. doi: 10.1007/s10865-011-9347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr RL. Interpretation of Normalized Spectral Heart Rate Variability Indices In Sleep Research: A Critical Review. Sleep. 2007;30:913–919. doi: 10.1093/sleep/30.7.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144–156. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS. Brief Pain Inventory-Short Form (BPI-SF) Houston, TX: 1994. [Google Scholar]
- Culbertson C, Nicolas S, Zaharovits I, et al. Methamphetamine craving induced in an online virtual reality environment. Pharmacol Biochem Behav. 2010;96:454–460. doi: 10.1016/j.pbb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simplicio M, Costoloni G, Western D, et al. Decreased heart rate variability during emotion regulation in subjects at risk for psychopathology. Psychol Med. 2011:1–9. doi: 10.1017/S0033291711002479. [DOI] [PubMed] [Google Scholar]
- Di Simplicio M, Costoloni G, Western D, et al. Decreased heart rate variability during emotion regulation in subjects at risk for psychopathology. Psychol Med. 2012;42:1775–1783. doi: 10.1017/S0033291711002479. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Dolman AJ, Michna E, et al. Changes in Pain Sensitivity and Pain Modulation During Oral Opioid Treatment: The Impact of Negative Affect. Pain Med pnw010. 2016 doi: 10.1093/pm/pnw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Borsook D. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron. 2016;89:11–36. doi: 10.1016/j.neuron.2015.11.027. [DOI] [PubMed] [Google Scholar]
- Erblich J, Bovbjerg DH, Sloan RP. Exposure to smoking cues: Cardiovascular and autonomic effects. Addict Behav. 2011;36:737–742. doi: 10.1016/j.addbeh.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder JN, Smoski MJ, Kozink RV, et al. Neural mechanisms of subclinical depressive symptoms in women: a pilot functional brain imaging study. BMC Psychiatry. 2012;12:152. doi: 10.1186/1471-244X-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Garland EL. The role of positive affect in pain and its treatment. Clin J Pain. 2015;31:177–187. doi: 10.1097/AJP.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL. Mindfulness-Oriented Recovery Enhancement modulates neurocognitive mechanisms and reward system function in addiction, stress, and pain. New York, NY: Sloan Kettering Memorial Hospital; 2015. [Google Scholar]
- Garland EL. Restructuring reward processing with Mindfulness-Oriented Recovery Enhancement: novel therapeutic mechanisms to remediate hedonic dysregulation in addiction, stress, and pain. Ann N Y Acad Sci. 2016;1373:25–37. doi: 10.1111/nyas.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Carter K, Ropes K, Howard MO. Thought suppression, impaired regulation of urges, and Addiction-Stroop predict affect-modulated cue-reactivity among alcohol dependent adults. Biol Psychol. 2012a;89:87–93. doi: 10.1016/j.biopsycho.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Franken IH, Howard MO. Cue-elicited heart rate variability and attentional bias predict alcohol relapse following treatment. Psychopharmacology (Berl) 2012b;222:17–26. doi: 10.1007/s00213-011-2618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Howard MO. Allostatic dysregulation of natural reward processing in prescription opioid misuse: Autonomic and attentional evidence. Biol Psychol. 2015a;105:124–129. doi: 10.1016/j.biopsycho.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Howard MO. Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology (Berl) 2014a;231:3229–3238. doi: 10.1007/s00213-014-3504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Howard MO. Neurophysiological evidence for remediation of reward processing deficits in chronic pain and opioid misuse following treatment with Mindfulness-Oriented Recovery Enhancement: exploratory ERP findings from a pilot RCT. J Behav Med. 2014b;38:327–336. doi: 10.1007/s10865-014-9607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Zeidan F, et al. The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neurosci Biobehav Rev. 2013;37:2597–2607. doi: 10.1016/j.neubiorev.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Hanley AW, Thomas EA, et al. Low Dispositional Mindfulness Predicts Self-medication of Negative Emotion With Prescription Opioids. J Addict Med. 2015b;9:61–67. doi: 10.1097/ADM.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Hanley AW, Thomas EA, et al. Low Dispositional Mindfulness Predicts Self-medication of Negative Emotion With Prescription Opioids. 2014c doi: 10.1097/ADM.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TC, Black R, Grimes Serrano JM, et al. Typologies of Prescription Opioid Use in a Large Sample of Adults Assessed for Substance Abuse Treatment. PLoS ONE. 2011;6:e27244. doi: 10.1371/journal.pone.0027244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AJ, Hadjistavropoulos T, Gagnon MM, et al. The effects of emotion regulation strategies on the pain experience: a structured laboratory investigation. Pain. 2015;156:868–879. doi: 10.1097/j.pain.0000000000000126. [DOI] [PubMed] [Google Scholar]
- Huhn A, Meyer R, Harris J, et al. Evidence of Anhedonia and Differential Reward Processing in Prefrontal Cortex Among Post-Withdrawal Patients with Prescription Opiate Dependence. Brain Res Bull. doi: 10.1016/j.brainresbull.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki H, Kuwahara M, Tsubone H. Changes in autonomic control of heart associated with classical appetitive conditioning in rats. Exp Anim. 2005;54:61–69. doi: 10.1538/expanim.54.61. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biol Psychiatry. 2003;54:1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kohl A, Rief W, Glombiewski JA. Acceptance, Cognitive Restructuring, and Distraction as Coping Strategies for Acute Pain. J Pain. 2013;14:305–315. doi: 10.1016/j.jpain.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Kok BE, Coffey KA, Cohn MA, et al. How positive emotions build physical health perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychol Sci. 2013;24:1123–1132. doi: 10.1177/0956797612470827. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert B. International affective picture system (IAPS): Technical manual and affective ratings. 1997 [Google Scholar]
- Lapate RC, Lee H, Salomons TV, et al. Amygdalar Function Reflects Common Individual Differences in Emotion and Pain Regulation Success. J Cogn Neurosci. 2011;24:148–158. doi: 10.1162/jocn_a_00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, et al. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Arch Gen Psychiatry. 2009;66:205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- Malliani A, Lombardi F, Pagani M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br Heart J. 1994;71:1–2. doi: 10.1136/hrt.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MO, Dolman AJ, Edwards RR, et al. The association between negative affect and prescription opioid misuse in patients with chronic pain: the mediating role of opioid craving. J Pain. 2014;15:90–100. doi: 10.1016/j.jpain.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins SS, Fenton MC, Keyes KM, et al. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol Med. 2012;42:1261–1272. doi: 10.1017/S0033291711002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Quartana PJ, Bounds S, Yoon KL, et al. Anger Suppression Predicts Pain, Emotional, and Cardiovascular Responses to the Cold Pressor. Ann Behav Med. 2010;39:211–221. doi: 10.1007/s12160-010-9182-8. [DOI] [PubMed] [Google Scholar]
- Quintana DS, McGregor IS, Guastella AJ, et al. A Meta-Analysis on the Impact of Alcohol Dependence on Short-Term Resting-State Heart Rate Variability: Implications for Cardiovascular Risk. Alcohol Clin Exp Res. 2013;37:E23–E29. doi: 10.1111/j.1530-0277.2012.01913.x. [DOI] [PubMed] [Google Scholar]
- Rajan I, Murthy PJ, Ramakrishnan AG, et al. Heart rate variability as an index of cue reactivity in alcoholics. Biol Psychiatry. 1998;43:544–546. doi: 10.1016/s0006-3223(97)00399-5. [DOI] [PubMed] [Google Scholar]
- Salomons TV, Nusslock R, Detloff A, et al. Neural Emotion Regulation Circuitry Underlying Anxiolytic Effects of Perceived Control over Pain. J Cogn Neurosci. 2014;27:222–233. doi: 10.1162/jocn_a_00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychol Sci. 2007;18:275–281. doi: 10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Shurman J, Koob GF, Gutstein HB. Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain Med Malden Mass. 2010;11:1092–1098. doi: 10.1111/j.1526-4637.2010.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhorst U, Huenig A, Ziegler D, Scherbaum WA. Unconditioned and conditioned effects of intravenous insulin and glucose on heart rate variability in healthy men. Physiol Behav. 2011;103:31–38. doi: 10.1016/j.physbeh.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Tang Y-Y, Posner MI, Rothbart MK, Volkow ND. Circuitry of self-control and its role in reducing addiction. Trends Cogn Sci. 2015;19:439–444. doi: 10.1016/j.tics.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, et al. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Udo T, Weinberger AH, Grilo CM, et al. Heightened vagal activity during high-calorie food presentation in obese compared with non-obese individuals—Results of a pilot study. 2013 doi: 10.1016/j.orcp.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 2016;374:363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. PAIN. 2015;156:569–576. doi: 10.1097/01.j.pain.0000460357.01998.f1. [DOI] [PubMed] [Google Scholar]
- Wasan AD, Butler SF, Budman SH, et al. Does report of craving opioid medication predict aberrant drug behavior among chronic pain patients? Clin J Pain. 2009;25:193–198. doi: 10.1097/AJP.0b013e318193a6c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan AD, Michna E, Edwards RR, et al. Psychiatric Comorbidity Is Associated Prospectively with Diminished Opioid Analgesia and Increased Opioid Misuse in Patients with Chronic Low Back Pain: Anesthesiology. 2015;123:861–872. doi: 10.1097/ALN.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DP, Cash C, Rankin C, et al. Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Emot Sci. 2015;6:261. doi: 10.3389/fpsyg.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger JW, Chu LF, D’Arcy NT, et al. Prescription opioid analgesics rapidly change the human brain. PAIN. 2011;152:1803–1810. doi: 10.1016/j.pain.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]