Abstract
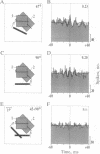
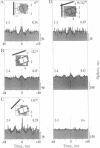
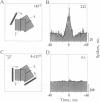
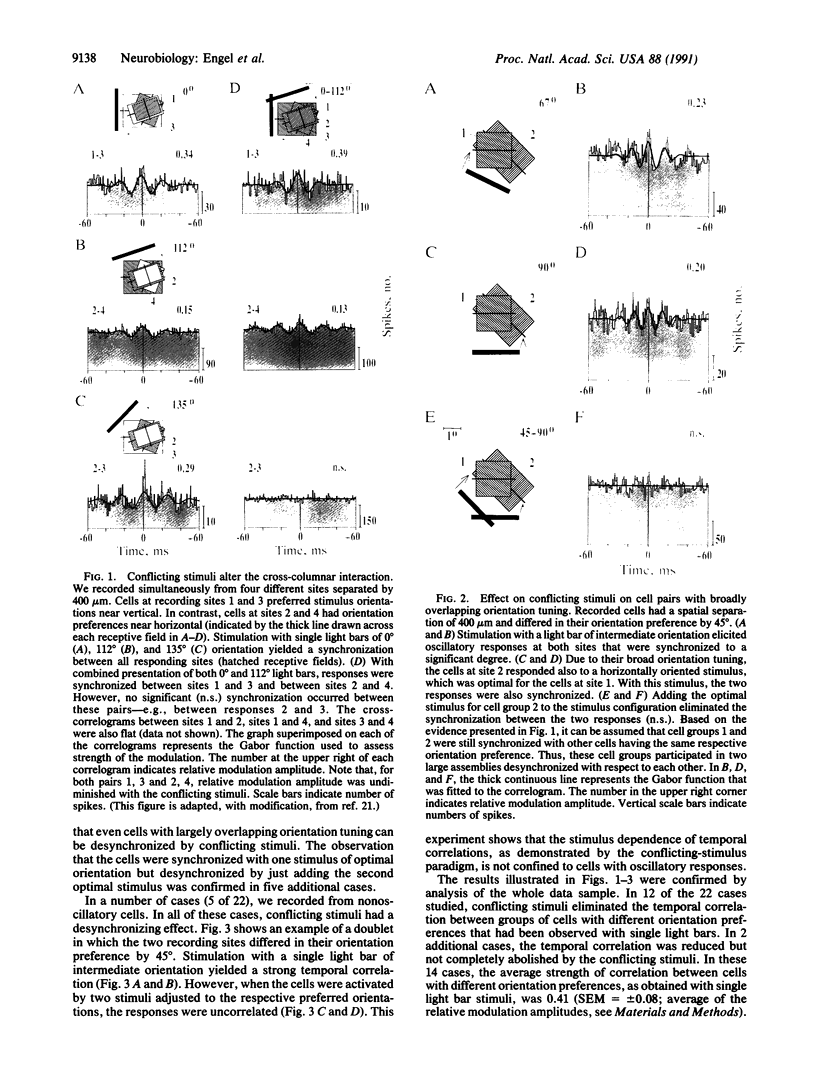
Theoretical studies have suggested that scene segmentation may be accomplished by a temporal coding mechanism using synchronization of neuronal responses. Here we report a direct experimental test of this hypothesis. Neuronal responses were recorded simultaneously from two to four sites with overlapping receptive fields in cat visual cortex. Correlation analysis revealed that all cells synchronized their responses irrespective of their orientation preference when they were activated by a single light bar. However, when stimulated with two superimposed light bars of different orientations, the same cells segregated into distinct assemblies according to their orientation preferences. Within each of these assemblies responses were synchronized, but correlation was absent between the two assemblies. These results are compatible with the hypothesis that responses to individual objects in a scene are distinguished by synchrony, whereas responses to different objects show no temporal correlation, thus allowing for the segmentation of superimposed stimuli. We conclude that stimulus-specific synchronization of spatially distributed neuronal responses may provide a physiological mechanism for scene segmentation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B. Single units and sensation: a neuron doctrine for perceptual psychology? Perception. 1972;1(4):371–394. doi: 10.1068/p010371. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhorn R., Bauer R., Jordan W., Brosch M., Kruse W., Munk M., Reitboeck H. J. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60(2):121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Engel A. K., Kreiter A. K., König P., Singer W. Synchronization of oscillatory neuronal responses between striate and extrastriate visual cortical areas of the cat. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6048–6052. doi: 10.1073/pnas.88.14.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel Andreas K., König Peter, Gray Charles M., Singer Wolf. Stimulus-Dependent Neuronal Oscillations in Cat Visual Cortex: Inter-Columnar Interaction as Determined by Cross-Correlation Analysis. Eur J Neurosci. 1990;2(7):588–606. doi: 10.1111/j.1460-9568.1990.tb00449.x. [DOI] [PubMed] [Google Scholar]
- Gray C. M., König P., Engel A. K., Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989 Mar 23;338(6213):334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Gray C. M., Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray Charles M., Engel Andreas K., König Peter, Singer Wolf. Stimulus-Dependent Neuronal Oscillations in Cat Visual Cortex: Receptive Field Properties and Feature Dependence. Eur J Neurosci. 1990;2(7):607–619. doi: 10.1111/j.1460-9568.1990.tb00450.x. [DOI] [PubMed] [Google Scholar]
- Singer W., Gray C., Engel A., König P., Artola A., Bröcher S. Formation of cortical cell assemblies. Cold Spring Harb Symp Quant Biol. 1990;55:939–952. doi: 10.1101/sqb.1990.055.01.088. [DOI] [PubMed] [Google Scholar]
- Sporns O., Tononi G., Edelman G. M. Modeling perceptual grouping and figure-ground segregation by means of active reentrant connections. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):129–133. doi: 10.1073/pnas.88.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner G. R., Albright T. D., Ramachandran V. S. Transparency and coherence in human motion perception. Nature. 1990 Mar 8;344(6262):153–155. doi: 10.1038/344153a0. [DOI] [PubMed] [Google Scholar]
- Ts'o D. Y., Gilbert C. D., Wiesel T. N. Relationships between horizontal interactions and functional architecture in cat striate cortex as revealed by cross-correlation analysis. J Neurosci. 1986 Apr;6(4):1160–1170. doi: 10.1523/JNEUROSCI.06-04-01160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Malsburg C., Schneider W. A neural cocktail-party processor. Biol Cybern. 1986;54(1):29–40. doi: 10.1007/BF00337113. [DOI] [PubMed] [Google Scholar]