Abstract
The hypermetabolic stress response after burn contributes to multi-organ failure, sepsis, morbidity, and mortality. The cytokine interleukin 6 (IL-6) has been hypothesized to mediate not only white adipose tissue (WAT) browning in burns, but also other hypermetabolic conditions. In addition to its inflammatory effects, IL-6 also acts as a metabolic mediator that affects metabolic tissues. Therefore, we sought to uncover the origin of circulating IL-6 post burn injury that regulates WAT browning. WAT and sera samples were collected from both adult burn patients admitted to the Ross Tilley Burn Centre at Sunnybrook Hospital and mice subjected to a burn injury. Collected tissues were analyzed for browning markers and metabolic state via histology, gene expression, and resting energy expenditure. Increased WAT browning was observed in burn patients as well as mice subjected to burn injury. Circulating IL-6 levels were significantly elevated post burn injury in mice (<0.05) and in burn patients (<0.05), the latter of which was positively correlated with elevated REE. Genetic loss of whole body IL-6 in mice prevented burn-induced WAT browning. Transplanting IL-6 KO mice with bone marrow (BM) from wild type (WT) mice, recovered the browning phenotype in these mice, as evaluated by increased UCP-1 expression (<0.05). Conversely, transplanting irradiated WT mice with BM from IL-6 KO mice impaired burn induced browning with no significant expression of UCP-1. Together, our findings implicate BM derived IL-6 as the source controlling browning of WAT post burn injury. Thus, targeting IL-6 is a promising target for hypermetabolism in burns.
Keywords: Browning, White adipose tissue, Beige adipose tissue, Burns, interleukin 6, Trauma, Hypermetabolism
INTRODUCTION
Burn injuries represent one of the most debilitating forms of trauma and rank fourth among most common types of trauma worldwide following traffic accidents, falls and interpersonal violence (1). In fact, more than 500,000 burn injuries occur annually in the United States (1). Of all cases, nearly 4000 people succumb to complications related to thermal injury in the United States, and 330,000 thermal-related deaths per year worldwide (1,2). A hallmark feature of burn injury is the development of “hypermetabolism,” which is associated with a plethora of pathological alterations in endocrine, metabolic, and inflammatory and immunological responses (3). This hypermetabolic response in burns is characterized by a profound increase in free fatty acids and glycerol release from fat, glucose production by the liver, and amino acids from muscle, ultimately resulting in significant elevations in resting energy expenditure (3) (4). In parallel to the metabolic alterations, there is hyper-activation of most inflammatory cascade systems, which may lead to failure of isolated or multiple organs over the course of the injury (5). Although this hypermetabolic response maybe viewed as an adaptive response to the injury to ensure adequate energy supply, if prolonged it becomes devastating for patient outcome. Over the last two decades, burn research has focused primarily on the hypermetabolic alterations within the liver and skeletal muscle and how these alterations affect patient prognosis and outcomes (3) (6). Advances in these areas have certainly improved post-burn outcomes, but a severe burn still continues to be associated with significant morbidity and mortality.
Perhaps the most serious gap in our understanding of the hypermetabolic response to burns is the role of the adipose tissue. We have yet to clearly identify the specific role of this critical tissue, as much of what has been documented has largely been superficial and limited to lipolysis. The response of adipose tissue to burn injury has gained significant scientific interest recently with the discovery of the browning of white adipose tissue in burn patients (7) (8). Generally, two types of distinct adipose depots have been characterized in both humans and rodents, namely white and brown adipose tissue. Brown adipose tissue (BAT) is characterized by high amounts of mitochondria and expression of the mitochondrial uncoupling protein 1 (UCP1), which uncouples electron transport from ATP production to heat production (9, 10). The rich mitochondria and UCP-1 expression has made BAT a fat burning engine and is considered one of the “good fats” (11). In contrast, white adipose tissue (12) contains very little mitochondria and is void of UCP-1 expression (9, 10). This inherent characteristic of WAT in storing energy rather than dissipating energy has contributed significantly to the obesity, diabetes, and heart disease epidemic (12) (13).
For decades, the adipose organ was categorized into the two distinct types addressed above. Recently the lines have blurred with the discovery of beige/brite adipocytes. As there name insinuates beige/brite adipocytes share characteristics of both white (energy storage) and brown fat cells (UCP-1 expression, mitochondria rich) (11) (14). The interesting feature of the beige/brite adipose tissue is that it can be differentiated from WAT in both humans and rodents. This conversion of WAT to a more brown like phenotype has been coined ‘browning’ (14). While the browning of WAT can be beneficial in obese or diabetic patients as it facilitates fat oxidation and improves insulin sensitivity (14). In the context of hypermetabolism observed in cancer and burn patients, it has been implicated in muscle mass wasting and fueling the detrimental hypermetabolic response (8, 15).
While the occurrence of WAT browning in response to burn injury is now undisputed, many outstanding questions remain: what are the molecular regulators of WAT browning post burn injury or where are the origins of the signal? Elevation in numerous inflammatory cytokines is a hallmark observation in burn patients, yet the role of these cytokines in mediating browning is unknown (5). In fact, recently in cancer patients and mouse models of cancer, interleukin 6 (IL-6) has been implicated in mediating the browning of WAT and was associated with cachexia during tumor growth (15). Unfortunately, this link between IL-6 and browning of WAT during hypermetabolism continues to generate more questions than answers. For instance, IL-6 is a cytokine that is secreted by both metabolic tissues as well immune cells in response to injury (16, 17). Additionally, IL-6 has context-dependent pro and anti-inflammatory effects on cells of the metabolic system (16). These two unique characteristics of this cytokine have made it one of the most difficult cytokines to study. Therefore, in this study we not only answer the main regulator of WAT browning as IL-6, but our findings also reveal that the bone marrow is the origin of the IL-6 signal known to be critical for the browning of WAT during hypermetabolism.
METHODS
Human samples
Patients admitted to the Ross Tilley Burn Centre at Sunnybrook Hospital (Toronto, Canada) or non-burn patients undergoing elective surgery were consented pre-operatively for tissue collection. Approval for our study was obtained from the Research Ethics Board at Sunnybrook Hospital. We enrolled 20 severely burned adults with burns encompassing 48% ± 3.9% of their total body surface area (TBSA). Adipose tissue obtained from the last OR (≥10 days) was immediately transferred to the laboratory and either frozen (-80°C) or transferred into fixative until time of analysis. Resting energy expenditure was assessed weekly until discharge and measured with a Sensor Medics 2900 metabolic measurement cart.
Mice model
Male C57BL/6 and IL6 KO mice (Jackson, USA)were housed at ambient temperature and cared in accordance with the Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Sunnybrook Research Institute Animal Care Committee under AUP 467 (Toronto, Ontario, Canada). Mice received a 30% (TBSA), full thickness burn. Burned mice were subsequently housed individually in sterile cages and fed ad libitum until sacrifice. Tissues were harvested 5 days post burn injury and used for analysis.
Chimer Mice
C57BL/6 CD45.1 and CD45.2 congenic mice were used as either the bone marrow donor or recipient and exposed to Cesium irradiated with 9.5Gy of irradiation. 24 hours post irradiation mice were transplanted with 2×10ˆ6 donor bone marrow cells. Four weeks post transplant mice were subjected to a 30% total body surface area (TBSA) scald burn. Tissues were harvested 5 days post burn injury and used for analysis.
Histology and Immunohistochemistry
Adipose tissue was immediately fixed in formalin prior to paraffin embedding. Subsequently, tissues were sectioned and stained with Hematoxylin and Eosin (H&E) or incubated with UCP1 (Sigma) antibody followed by DAB staining. Imaging was performed on a LSM confocal microscope (Zeiss, Germany).
Quantitative PCR
Total RNA was extracted from fat tissue using TRIzol-chloroform (Life Technologies) with subsequent purification using the RNeasy Kit (Qiagen) according to the manufacturer’s instructions. RNA (2mg) was transcribed to cDNA using the high capacity cDNA reverse transcription kit (Applied Biosystems). Real-time quantitative PCR was performed using the Applied Biosystems Step One Plus Real-Time PCR System. Primer sequences used are available upon request.
Cytokine profile
Rodent and human sera were collected and using a Multiplex platform (Millipore, MA) IL-6 and IL-4 levels was measured.
Statistics
Unpaired student t-test was used to determine significance. Significant results were established for p<0.05 (*) and p<0.01 (**).
RESULTS
Browning of WAT in response to burn injury
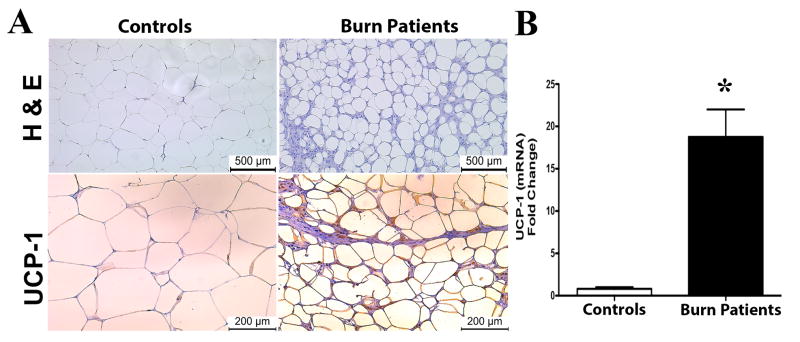
We first investigated the browning of human subcutaneous white adipose tissue (sWAT) in a cohort of patients with burn injuries. Specifically, we enrolled 20 severely burn adults (46.4 ± 5 years old, 18 males and 2 females) with an average burn encompassing 27% ± 10% TBSA (Supplemental Table 1). Seven metabolically healthy adults (44 ± 7 years, 5 males and 2 females) were compared as healthy controls (Supplemental Table 2). Others and our previous reports have shown that browning of WAT in burn patients occurs late (≥10 days), hence sWAT samples meeting this criteria were used for analysis. The browning of WAT is generally characterized by two key events; 1) the remodeling of white adipocyte morphology to one that is smaller in size and multi-ocular in appearance, and 2) the expression of the uncoupling protein 1 (UCP1). Histological examinations of sWAT from burn patients revealed white adipocytes were smaller in size and multi-ocular in appearance compared to sWAT from healthy patients (Figure 1A). Additionally, sWAT from burn patients showed increased gene expression of UCP1 as well as staining positive for UCP1 in comparison to healthy controls (Figure 1A-B).
Figure 1. Browning of white adipose tissue in burn patients post burn injury.
Micrographs of paraffin sections of sWAT obtained from burned patients and healthy controls. (A) H&E and uncoupling protein 1 (UCP1) staining in sWAT obtained from burned patients (≥10 days post injury) and healthy controls. (B) Quantitative RT-PCR gene expression of UCP1 in sWAT obtained from burned patients and healthy controls. Data represented as mean ± SEM, * = significant difference burned patients (n=10) vs. healthy controls (n=7).
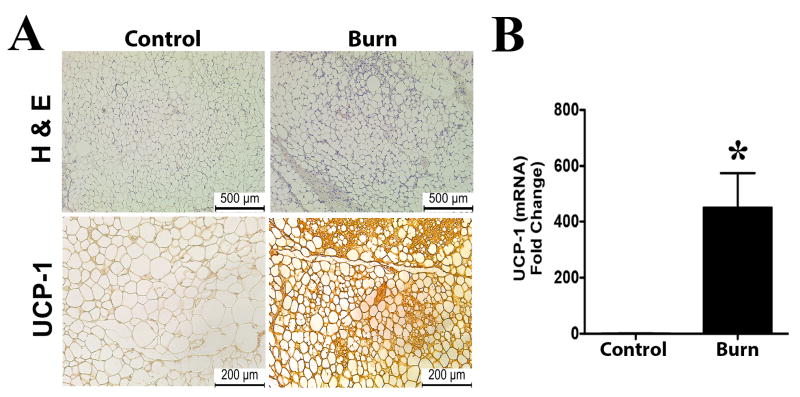
Since the molecular mechanisms regulating the browning of WAT post burn injury is poorly understood, we utilized a well-characterized animal burn model to study the mechanisms regulating browning (18). To validate and confirm that our mouse burn model was able to recapture the browning phenotype of burn patients, we assessed the sWAT from mice subjected to a burn injury. Similar to burn patients, sWAT from mice subjected to a thermal injury showed both adipocytes that were smaller, multi-ocular in appearance, as well as greater expression of UCP1 compared to control mice (Figure 2A-B). Collectively, these data provide evidence of WAT browning in response to burn injury in both humans and rodents.
Figure 2. Browning of white adipose tissue in mice post burn injury.
Micrographs of paraffin sections of sWAT obtained from wild type mice. (A) H&E and uncoupling protein 1 (UCP1) staining in sWAT obtained from burned and control mice. (B) Quantitative RT-PCR gene expression of UCP1 in sWAT obtained from burned and control mice. Data represented as mean ± SEM, * = significant difference burned mice (n=6) vs. controls (n=6).
Increased systemic energy expenditure is associated with IL-6 cytokine post burn injury
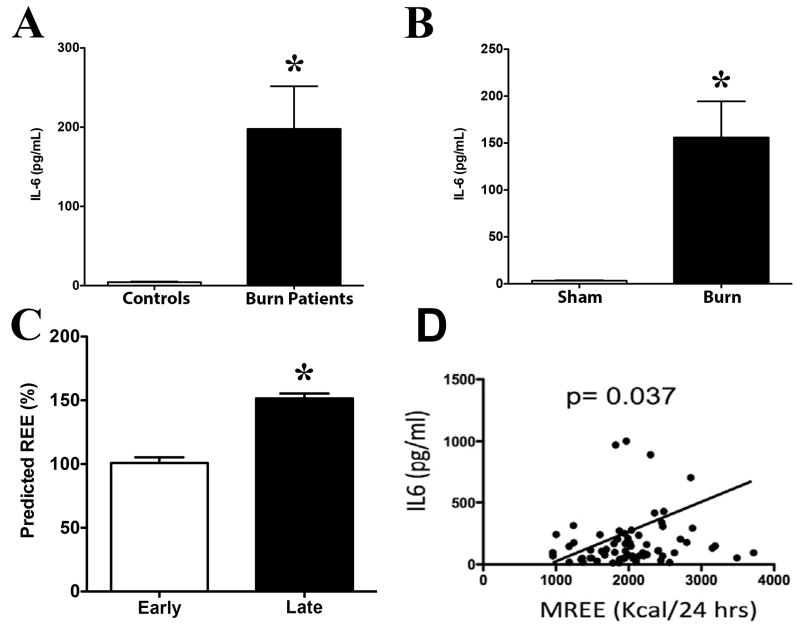
Recently, a number of cytokines, in particular the interleukin 4 (IL-4) cytokine have been implicated in mediating the browning of WAT in response to cold exposure (19). However, limited studies have examined if other cytokines are also capable of regulating the browning of WAT. One such other cytokine is the IL-6 cytokine, which has previously been shown to be chronically elevated in burn patient’s years of the initial insult (4). Plasma IL-6 levels were significantly elevated in burn patients compared to healthy controls (Figure 3A). Similarly, significantly increased plasma IL-6 levels were also observed in post-burned mice compared to controls (Figure 3B). We next looked at whole-body resting energy expenditure (20), a commonly used output measure of WAT browning, by stratifying burn patients into two cohorts based on early (<7 days) and later (≥10day) days post burn that REE was measured. Consistent with the increased browning occurring later after injury, REE was significantly elevated in the late burn group when compared to earlier time points (Figure 3C). Interestingly, REE was strongly correlated with IL-6 in burn patients (Figure 3D). Together, these data suggest that the IL-6 cytokine maybe driving WAT browning post burn injury.
Figure 3. Increased systemic energy expenditure is associated with IL-6 cytokine post burn injury.
(A) Plasma IL-6 levels were measured both in burn and healthy control patients. (B) Plasma IL-6 levels were measured both in burn and control wild type mice. (C) Average measured predicted REE as a function of days post-burn injury was measured. In patients followed prospectively after injury, average measured REE significantly increased over time. (D) Average measured REE strongly correlated with increasing IL-6 in our burn patient cohort. Data represented as mean ± SEM, * = significant difference burned mice (n=6) vs. controls (n=6), OR * = significant difference burned patients (n=10) vs. healthy controls (n=7).
Non-existence of WAT browning in IL-6 deficient mice post burn injury
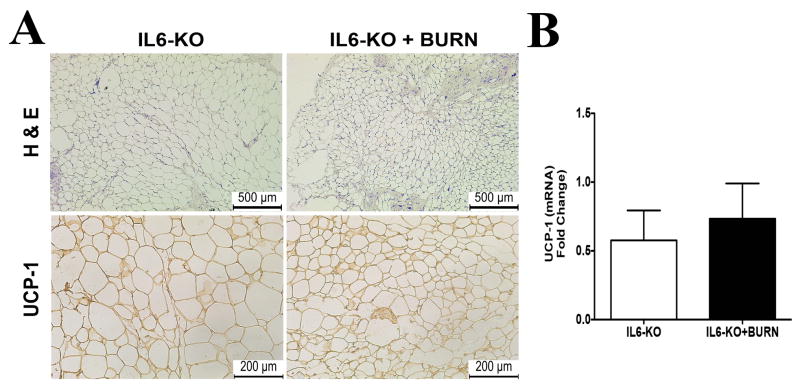
To further delineate the role of IL-6 in the regulation of WAT browning post burn injury, we employed whole body IL-6 knock out (KO) mice, which are transgenic mice that lack the IL-6 gene and thereby fail to produce this cytokine. Histological analysis of sWAT showed no change in size, morphology, and staining for UCP1 in adipocytes from IL-6 KO mice that received a burn injury as compared with those not exposed burn insult (Figure 4A). To further confirm the lack of a browning phenotype in IL-6 KO mice post burn injury, quantitative RT-PCR analysis revealed no significant expression of the UCP-1 gene in these mice as well (Figure 4B). We also measured IL-4 levels in these mice and found no significant secretion post burn injury (Supplemental Figure 1). Combined with the association of chronic serum IL-6 levels post burn injury with REE, these data establish that IL-6 signaling plays an important role in the browning of WAT observed in response to burn injury.
Figure 4. IL-6 cytokine is required for the browning of white adipose tissue in mice post burn injury.
Micrographs of paraffin sections of sWAT obtained from IL6 KO mice. (A) H&E and uncoupling protein 1 (UCP1) staining in sWAT obtained from burned and control IL6 KO mice. (B) Quantitative RT-PCR gene expression of UCP1 in sWAT obtained from burned and control IL6 KO mice. Data represented as mean ± SEM, * = significant difference burned mice (n=6) vs. controls (n=6).
Bone-marrow derived IL-6 regulates WAT browning post burn injury
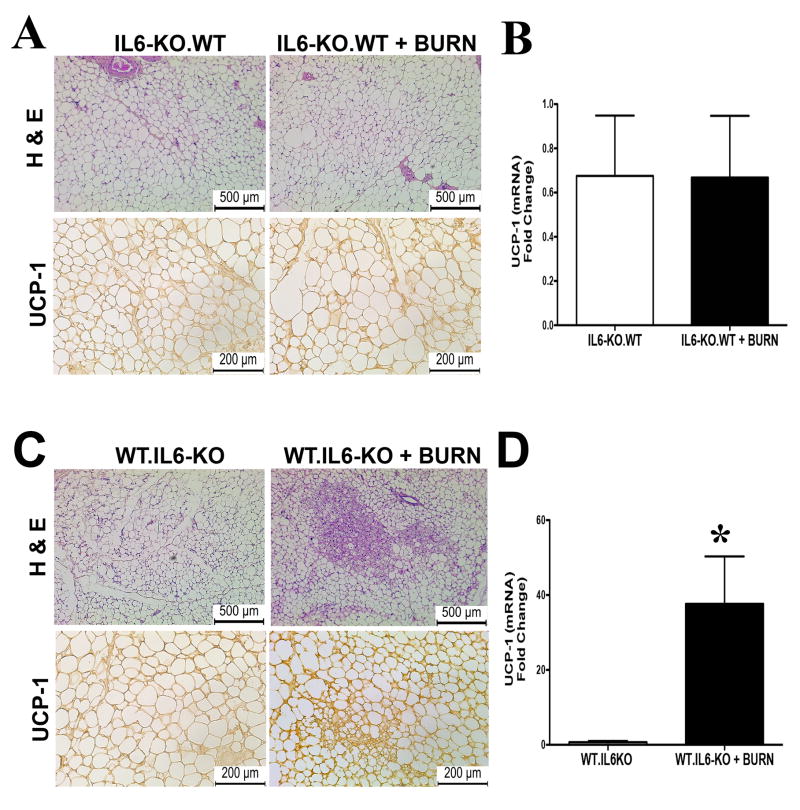
Having established a clear role of the IL-6 cytokine in regulating WAT browning in response to thermal injury, we next did a more extensive profile evaluation of the cellular source of this cytokine. Many different cell types are able to produce the IL-6 cytokine, and often depending on the source of this secretion it defines whether its metabolic functions are pro- or anti-inflammatory (16). Utilizing a chimera mouse model, we sought to uncover the location of IL-6 secretion regulating browning post burn injury (Supplemental Figure 2).We first took wild type (WT) mice and subjected them to irradiation to deplete their bone-marrow cell population, and then reconstituted these mice with bone marrow from IL6KO mice. Four weeks post reconstitution; these mice (IL6KO.WT) were now considered to only have the ability to produce IL-6 from non-hematopoietic source. Successful reconstitution of donor bone marrow (BM) in irradiated mice was evident mice transplanted with BM had 100% survival for the duration of the study (Supplemental Figure 3). Additionally, irradiation only depleted BM cells and did not ablate other immune cells like dermal macrophages (Supplementary Figure 4). After bone marrow transplantation (BMT), these mice were subjected to a burn injury and assessed for the browning phenotype. Whereas earlier WT mice subjected to a burn injury resulted in the browning of WAT, the absence of IL-6 exclusively in hematopoietic cells attenuated WAT browning in these WT mice as evidenced by lack of UCP-1 expression and WAT remodeling (Figure 5A-B).
Figure 5. Bone-marrow derived IL-6 regulates WAT browning post burn injury in mice.
Micrographs of paraffin sections of sWAT obtained from wild type mice transplanted with BM derived from IL-6 KO mice (IL6KO.WT). (A) H&E and uncoupling protein 1 (UCP1) staining in sWAT obtained from IL6KO.WT randomized into burn or control groups. (B) Quantitative RT-PCR gene expression of UCP1 in sWAT obtained from IL6KO.WT randomized into burn or control groups (no-burn injury). Data represented as mean ± SEM, * = significant difference IL6KO.WT burned mice (n=3) vs. IL6KO.WT controls (n=3). In panels C-D micrographs of paraffin sections of sWAT obtained from IL-6 KO mice transplanted with BM derived from WT mice (WT.IL6KO). (C) H&E and uncoupling protein 1 (UCP1) staining in sWAT obtained from WT.IL6KO randomized into burn or control groups. (D) Quantitative RT-PCR gene expression of UCP1 in sWAT obtained from WT.IL6KO randomized into burn or control groups (no-burn injury). Data represented as mean ± SEM, * = significant difference WT.IL6KO burned mice (n=3) vs. WT.IL6KO controls (n=3).
To further confirm the cellular source of IL-6 that regulates the browning of WAT as bone marrow derived, we next took IL-6 KO mice and subjected them to irradiation to deplete their bone-marrow cell population, and then reconstituted these mice with bone marrow from WT mice. Four weeks post reconstitution; these mice (WT.IL6KO) were now considered to only have the ability to produce IL-6 solely in hematopoietic cells. As in earlier, post BMT these mice were subjected to a burn injury and assessed for the browning phenotype. Interestingly, as we showed earlier IL6KO mice failed to brown in response a burn injury, however, transplant of BM expressing IL-6 only in hematopoietic cells was sufficient to restore the WAT browning phenotype in these mice (Figure 5C-D). In fact, WT.IL6KO mice post burn injury showed adipocytes that were smaller in size and multi-ocular in appearance, in addition to UCP1 expression compared to control mice (Figure 5C-D). Thus, these findings suggest that the source of IL-6 regulating the browning of WAT in response to thermal injury is primarily bone marrow derived.
DISCUSSION
Hypermetabolism is a deadly condition in burn patients and often puts these patients at risk for a number of complications such as sepsis, multi-organ failure, and hepatic and cardiac failure (5) (21) (22) (23, 24). Here, our findings show that WAT browning represents a systemic event in the hypermetabolic response to burn injury and contributes to increased energy expenditure and lipid mobilization. This WAT remodeling was confirmed at the morphological, cellular, and molecular levels, extending from burn patients to a mouse model of burn injury. However, our primary objective of this study was to determine the main regulator of this WAT browning phenomena in the context of burn injury.
To date, several cytokines have been identified to regulate WAT browning, such as IL-4 and IL-6 with the latter less characterized (15, 19). There has been much contradictory data on the effects of IL-6 metabolically speaking; with some studies linking it to insulin resistance and others reporting it improves insulin sensitivity (25) (26) (27). In the context of burn injury, we suggest that circulating IL-6 levels post burn injury remain chronically elevated and its correlation with resting energy expenditure appears to indicate that it likely regulates the browning response. Indeed, another important finding of the current study was that Il6−/− mice showed no WAT browning phenotype in response to burn injury, suggesting that IL-6 maybe the culprit that sustains the hypermetabolic response via its effects on adipose tissue.
Furthermore, the paradoxical effects of IL-6 are largely centered on the location or source of its release. For instance, IL-6 secreted from the muscle during periods of exercise has been shown to mediate the metabolic benefits of physical activity (28). Whereas IL-6 secreted from immune cells has been shown to mediate pro-inflammatory catabolic states (16) (29). To determine the exact location of the IL-6 cytokine released that regulates WAT browning, we presently employed a chimeric mouse burn model. We found that WT mice transplanted with IL6KO hematopoietic cells reversed the browning phenotype in these mice, whereas IL6KO mice transplanted with WT hematopoietic cells recovered WAT browning in these mice. These observations indicated a hematopoietic cell source of IL-6 as the main regulator of WAT browning post burn injury. To our knowledge, this is the first study to show the exact source of IL-6 that regulates browning of WAT in the context of burn injury.
In conclusion, we have demonstrated that WAT browning is part of the hypermetabolic response to burn injury, and this remodeling of WAT is due to circulating IL-6, which is increased in response to the injury. The identification of the source of IL-6 regulating browning post burn injury as hematopoietic in origin, will help in the development of therapeutic agents that modulate IL-6 production in a location-specific fashion. Thus, future studies are needed to add depth to our knowledge about the role of IL-6 in context of post burn browning by clarifying the exact signaling pathways it activates in hypermetabolic settings.
Supplementary Material
Acknowledgments
A.A. is a Vanier Scholar and a recipient of the Vanier Canada Graduate Scholarship. M.G.J. holds grants from Canadian Institutes of Health Research, Canada Fund for Innovation (CFI) Leader’s Opportunity Fund Project, and the National Institutes of Health.
Source of Funding: This study was supported by National Institutes of Health R01-GM087285-01. CFI Leader’s Opportunity Fund: Project #25407 and Canadian Institutes of Health Research (CIHR) grant #123336.
Footnotes
Conflicts of Interest: Authors have no conflicts of interest to declare.
References
- 1.Brigham PA, McLoughlin E. Burn incidence and medical care use in the United States: estimates, trends, and data sources. J Burn Care Rehabil. 1996;17:95–107. doi: 10.1097/00004630-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Pham TN, Kramer CB, Wang J, Rivara FP, Heimbach DM, Gibran NS, Klein MB. Epidemiology and outcomes of older adults with burn injury: an analysis of the National Burn Repository. J Burn Care Res. 2009;30:30–6. doi: 10.1097/BCR.0b013e3181921efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeschke MG. Postburn Hypermetabolism: Past, Present, and Future. J Burn Care Res. 2016;37:86–96. doi: 10.1097/BCR.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, Suman OE, Mlcak RP, Herndon DN. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeschke MG, Gauglitz GG, Finnerty CC, Kraft R, Mlcak RP, Herndon DN. Survivors versus nonsurvivors postburn: differences in inflammatory and hypermetabolic trajectories. Ann Surg. 2014;259:814–23. doi: 10.1097/SLA.0b013e31828dfbf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedroso FE, Spalding PB, Cheung MC, Yang R, Gutierrez JC, Bonetto A, Zhan R, Chan HL, Namias N, Koniaris LG, et al. Inflammation, organomegaly, and muscle wasting despite hyperphagia in a mouse model of burn cachexia. J Cachexia Sarcopenia Muscle. 2011;3:199–211. doi: 10.1007/s13539-012-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patsouris D, Qi P, Abdullahi A, Stanojcic M, Chen P, Parousis A, Amini-Nik S, Jeschke MG. Burn Induces Browning of the Subcutaneous White Adipose Tissue in Mice and Humans. Cell Rep. 2015;13:1538–44. doi: 10.1016/j.celrep.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidossis LS, Porter C, Saraf MK, Borsheim E, Radhakrishnan RS, Chao T, Ali A, Chondronikola M, Mlcak R, Finnerty CC, et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015;22:219–27. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature. 2014;510:76–83. doi: 10.1038/nature13477. [DOI] [PubMed] [Google Scholar]
- 10.Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2013;62:1783–90. doi: 10.2337/db12-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125:478–86. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–31. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Contreras C, Gonzalez-Garcia I, Martinez-Sanchez N, Seoane-Collazo P, Jacas J, Morgan DA, Serra D, Gallego R, Gonzalez F, Casals N, et al. Central ceramide-induced hypothalamic lipotoxicity and ER stress regulate energy balance. Cell Rep. 2014;9:366–77. doi: 10.1016/j.celrep.2014.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 15.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20:433–47. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 17.Rohleder N, Aringer M, Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci. 2012;1261:88–96. doi: 10.1111/j.1749-6632.2012.06634.x. [DOI] [PubMed] [Google Scholar]
- 18.Abdullahi A, Amini-Nik S, Jeschke MG. Animal models in burn research. Cell Mol Life Sci. 2014;71:3241–55. doi: 10.1007/s00018-014-1612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–99. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams FN, Herndon DN, Suman OE, Lee JO, Norbury WB, Branski LK, Mlcak RP, Jeschke MG. Changes in cardiac physiology after severe burn injury. J Burn Care Res. 2011;32:269–74. doi: 10.1097/BCR.0b013e31820aafcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeschke MG. The hepatic response to thermal injury: is the liver important for postburn outcomes? Mol Med. 2009;15:337–51. doi: 10.2119/molmed.2009.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraft R, Herndon DN, Finnerty CC, Cox RA, Song J, Jeschke MG. Predictive Value of IL-8 for Sepsis and Severe Infections After Burn Injury: A Clinical Study. Shock. 2015;43:222–7. doi: 10.1097/SHK.0000000000000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauglitz GG, Toliver-Kinsky TE, Williams FN, Song J, Cui W, Herndon DN, Jeschke MG. Insulin increases resistance to burn wound infection-associated sepsis. Crit Care Med. 2010;38:202–8. doi: 10.1097/CCM.0b013e3181b43236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji C, Chen X, Gao C, Jiao L, Wang J, Xu G, Fu H, Guo X, Zhao Y. IL-6 induces lipolysis and mitochondrial dysfunction, but does not affect insulin-mediated glucose transport in 3T3-L1 adipocytes. J Bioenerg Biomembr. 2011;43:367–75. doi: 10.1007/s10863-011-9361-8. [DOI] [PubMed] [Google Scholar]
- 26.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Bronneke HS, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15:423–30. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraakman MJ, Kammoun HL, Allen TL, Deswaerte V, Henstridge DC, Estevez E, Matthews VB, Neill B, White DA, Murphy AJ, et al. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab. 2015;21:403–16. doi: 10.1016/j.cmet.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol. 2001;536:329–37. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–4. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.