Abstract
Antigenic variation of infectious organisms is a major factor in evasion of the host immune response. However, there has been no definitive demonstration of this phenomenon in the malaria parasite Plasmodium falciparum. In this study, cloned parasites were examined serologically and biochemically for the expression of erythrocyte surface antigens. A cloned line of P. falciparum gave rise to progeny that expressed antigenically distinct forms of an erythrocyte surface antigen but were otherwise identical. This demonstrates that antigenic differences on the surface of P. falciparum-infected erythrocytes can arise by antigenic variation of clonal parasite populations. The antigenic differences were shown to result from antigenic variation of the parasite-encoded protein, the P. falciparum erythrocyte membrane protein 1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnwell J. W., Howard R. J., Coon H. G., Miller L. H. Splenic requirement for antigenic variation and expression of the variant antigen on the erythrocyte membrane in cloned Plasmodium knowlesi malaria. Infect Immun. 1983 Jun;40(3):985–994. doi: 10.1128/iai.40.3.985-994.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs B. A., Culvenor J. G., Ng J. S., Kemp D. J., Brown G. V. Plasmodium falciparum: cytoadherence of a knobless clone. Exp Parasitol. 1989 Aug;69(2):189–197. doi: 10.1016/0014-4894(89)90187-2. [DOI] [PubMed] [Google Scholar]
- Biggs B. A., Goozé L., Wycherley K., Wilkinson D., Boyd A. W., Forsyth K. P., Edelman L., Brown G. V., Leech J. H. Knob-independent cytoadherence of Plasmodium falciparum to the leukocyte differentiation antigen CD36. J Exp Med. 1990 Jun 1;171(6):1883–1892. doi: 10.1084/jem.171.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs B. A., Kemp D. J., Brown G. V. Subtelomeric chromosome deletions in field isolates of Plasmodium falciparum and their relationship to loss of cytoadherence in vitro. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2428–2432. doi: 10.1073/pnas.86.7.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Cross G. A. Molecular basis for trypanosome antigenic variation. Cell. 1982 Jun;29(2):291–303. doi: 10.1016/0092-8674(82)90146-5. [DOI] [PubMed] [Google Scholar]
- Brown K. N., Brown I. N. Immunity to malaria: antigenic variation in chronic infections of Plasmodium knowlesi. Nature. 1965 Dec 25;208(5017):1286–1288. doi: 10.1038/2081286a0. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Culvenor J. G., Bianco A. E., Crewther P. E., Stahl H. D., Brown G. V., Anders R. F., Kemp D. J. Variable antigen associated with the surface of erythrocytes infected with mature stages of Plasmodium falciparum. Mol Biochem Parasitol. 1986 Sep;20(3):265–277. doi: 10.1016/0166-6851(86)90107-6. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Saint R. B., Stahl H. D., Langford C. J., Brown G. V., Anders R. F., Kemp D. J. Plasmodium falciparum: differentiation of isolates with DNA hybridization using antigen gene probes. Exp Parasitol. 1985 Aug;60(1):82–89. doi: 10.1016/s0014-4894(85)80025-4. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Saint R. B., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. Conserved sequences flank variable tandem repeats in two S-antigen genes of Plasmodium falciparum. Cell. 1985 Apr;40(4):775–783. doi: 10.1016/0092-8674(85)90337-x. [DOI] [PubMed] [Google Scholar]
- Hommel M., David P. H., Oligino L. D. Surface alterations of erythrocytes in Plasmodium falciparum malaria. Antigenic variation, antigenic diversity, and the role of the spleen. J Exp Med. 1983 Apr 1;157(4):1137–1148. doi: 10.1084/jem.157.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Barnwell J. W., Kao V. Antigenic variation of Plasmodium knowlesi malaria: identification of the variant antigen on infected erythrocytes. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4129–4133. doi: 10.1073/pnas.80.13.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Barnwell J. W., Rock E. P., Neequaye J., Ofori-Adjei D., Maloy W. L., Lyon J. A., Saul A. Two approximately 300 kilodalton Plasmodium falciparum proteins at the surface membrane of infected erythrocytes. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):207–223. doi: 10.1016/0166-6851(88)90040-0. [DOI] [PubMed] [Google Scholar]
- Hudson D. E., Wellems T. E., Miller L. H. Molecular basis for mutation in a surface protein expressed by malaria parasites. J Mol Biol. 1988 Oct 5;203(3):707–714. doi: 10.1016/0022-2836(88)90204-5. [DOI] [PubMed] [Google Scholar]
- Jensen J. B. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am J Trop Med Hyg. 1978 Nov;27(6):1274–1276. doi: 10.4269/ajtmh.1978.27.1274. [DOI] [PubMed] [Google Scholar]
- Leech J. H., Barnwell J. W., Miller L. H., Howard R. J. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med. 1984 Jun 1;159(6):1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpaiboon T., Shirley M. W., Kemp D. J., Saul A. 7H8/6, a multicopy DNA probe for distinguishing isolates of Plasmodium falciparum. Mol Biochem Parasitol. 1991 Aug;47(2):197–206. doi: 10.1016/0166-6851(91)90179-a. [DOI] [PubMed] [Google Scholar]
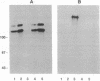
- Magowan C., Wollish W., Anderson L., Leech J. Cytoadherence by Plasmodium falciparum-infected erythrocytes is correlated with the expression of a family of variable proteins on infected erythrocytes. J Exp Med. 1988 Oct 1;168(4):1307–1320. doi: 10.1084/jem.168.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
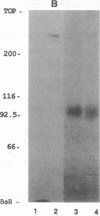
- Marsh K., Howard R. J. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science. 1986 Jan 10;231(4734):150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Klotz F. W., Tandon N. N., Jamieson G. A. Sequestrin, a CD36 recognition protein on Plasmodium falciparum malaria-infected erythrocytes identified by anti-idiotype antibodies. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3175–3179. doi: 10.1073/pnas.88.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
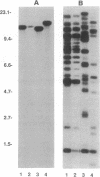
- Ockenhouse C. F., Tandon N. N., Magowan C., Jamieson G. A., Chulay J. D. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science. 1989 Mar 17;243(4897):1469–1471. doi: 10.1126/science.2467377. [DOI] [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Spitalnik S. L., Panton L. J., Howard R. J., Dixit V. M., Frazier W. A., Miller L. H., Ginsburg V. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature. 1985 Nov 7;318(6041):64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- Southwell B. R., Brown G. V., Forsyth K. P., Smith T., Philip G., Anders R. Field applications of agglutination and cytoadherence assays with Plasmodium falciparum from Papua New Guinea. Trans R Soc Trop Med Hyg. 1989 Jul-Aug;83(4):464–469. doi: 10.1016/0035-9203(89)90248-4. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Leech J., Aikawa M., Miller L. H. An in vitro assay for sequestration: binding of Plasmodium falciparum-infected erythrocytes to formalin-fixed endothelial cells and amelanotic melanoma cells. J Protozool. 1985 Feb;32(1):88–90. doi: 10.1111/j.1550-7408.1985.tb03019.x. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Miller L. H., McGregor I. A., Jensen J. B. Plasmodium falciparum strain-specific antibody blocks binding of infected erythrocytes to amelanotic melanoma cells. Nature. 1983 Jun 2;303(5916):429–431. doi: 10.1038/303429a0. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Schmidt J. A., Aikawa M., Miller L. H., Green I. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science. 1981 Jul 31;213(4507):555–557. doi: 10.1126/science.7017935. [DOI] [PubMed] [Google Scholar]