Abstract
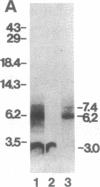
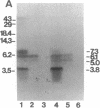
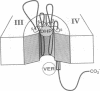
To identify regions that are involved in the formation of the dihydropyridine receptor site of skeletal muscle L-type Ca2+ channels, the alpha 1 subunit of the channel complex was specifically labeled with the 1,4-dihydropyridine-receptor-selective photoaffinity probe [3H]diazipine. Photoaffinity-labeled regions were identified by probing labeled proteolytic fragments with several anti-peptide antibodies recognizing different segments of the alpha 1 sequence. Forty to 50% of the alpha 1-associated [3H]diazipine label was contained in the tryptic fragment between Arg-988 and Ala-1023 derived from the loop between segments S5 and S6 in domain III. This region corresponds to a portion of the channel that is believed to contribute to formation of the transmembrane pore. Twenty to 30% of the labeling occurred in a V8 protease fragment between Glu-1349 and Trp-1391. This fragment contains transmembrane segment S6 of domain IV and has previously been shown to form part of the drug receptor for phenylalkylamine Ca2+ antagonists. Our data suggest that the dihydropyridine receptor is formed by close apposition of two discontinuous regions of the alpha 1 subunit sequence in domains III and IV. In light of previous work localizing this receptor site to the extracellular surface of the lipid bilayer, it is proposed that amino acid residues at the extracellular surface in the loop connecting segments IIIS5 and IIIS6 and at the extracellular end of segment IVS6 contribute to formation of the dihydropyridine receptor site.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. A., Beam K. G. Muscular dysgenesis in mice: a model system for studying excitation-contraction coupling. FASEB J. 1990 Jul;4(10):2809–2816. doi: 10.1096/fasebj.4.10.2165014. [DOI] [PubMed] [Google Scholar]
- Campbell K. P., Leung A. T., Sharp A. H. The biochemistry and molecular biology of the dihydropyridine-sensitive calcium channel. Trends Neurosci. 1988 Oct;11(10):425–430. doi: 10.1016/0166-2236(88)90193-2. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Excitation-contraction coupling in vertebrate skeletal muscle: a tale of two calcium channels. Cell. 1991 Mar 8;64(5):871–874. doi: 10.1016/0092-8674(91)90309-m. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Seagar M. J., Takahashi M. Molecular properties of dihydropyridine-sensitive calcium channels in skeletal muscle. J Biol Chem. 1988 Mar 15;263(8):3535–3538. [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-gated sodium and calcium channels. Curr Opin Neurobiol. 1991 Jun;1(1):5–13. doi: 10.1016/0959-4388(91)90004-q. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Striessnig J. Molecular properties of calcium channels. Rev Physiol Biochem Pharmacol. 1990;114:1–105. doi: 10.1007/BFb0031018. [DOI] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Guy H. R., Conti F. Pursuing the structure and function of voltage-gated channels. Trends Neurosci. 1990 Jun;13(6):201–206. doi: 10.1016/0166-2236(90)90160-c. [DOI] [PubMed] [Google Scholar]
- Herbette L. G., Vant Erve Y. M., Rhodes D. G. Interaction of 1,4 dihydropyridine calcium channel antagonists with biological membranes: lipid bilayer partitioning could occur before drug binding to receptors. J Mol Cell Cardiol. 1989 Feb;21(2):187–201. doi: 10.1016/0022-2828(89)90861-4. [DOI] [PubMed] [Google Scholar]
- Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Arena J. P. Influence of pHo on calcium channel block by amlodipine, a charged dihydropyridine compound. Implications for location of the dihydropyridine receptor. J Gen Physiol. 1989 Jun;93(6):1109–1127. doi: 10.1085/jgp.93.6.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulla S., Schneider T., Nastainczyk W., Meyer H. E., Hofmann F. Identification of the site of interaction of the dihydropyridine channel blockers nitrendipine and azidopine with the calcium-channel alpha 1 subunit. EMBO J. 1991 Jan;10(1):45–49. doi: 10.1002/j.1460-2075.1991.tb07919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sieber M., Nastainczyk W., Zubor V., Wernet W., Hofmann F. The 165-kDa peptide of the purified skeletal muscle dihydropyridine receptor contains the known regulatory sites of the calcium channel. Eur J Biochem. 1987 Aug 17;167(1):117–122. doi: 10.1111/j.1432-1033.1987.tb13311.x. [DOI] [PubMed] [Google Scholar]
- Striessnig J., Glossmann H., Catterall W. A. Identification of a phenylalkylamine binding region within the alpha 1 subunit of skeletal muscle Ca2+ channels. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9108–9112. doi: 10.1073/pnas.87.23.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki M., Kuniyasu A., Nakayama H., Kanaoka Y. Synthesis of diazipine and [3H]diazipine: novel dihydropyridines as photoaffinity probes of calcium channels. Chem Pharm Bull (Tokyo) 1991 Jul;39(7):1860–1862. doi: 10.1248/cpb.39.1860. [DOI] [PubMed] [Google Scholar]
- Taki M., Nakayama H., Kanaoka Y. Diazipine, a novel photoaffinity probe for dihydropyridine receptors of calcium channels. FEBS Lett. 1991 Jun 3;283(2):259–262. doi: 10.1016/0014-5793(91)80602-y. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]