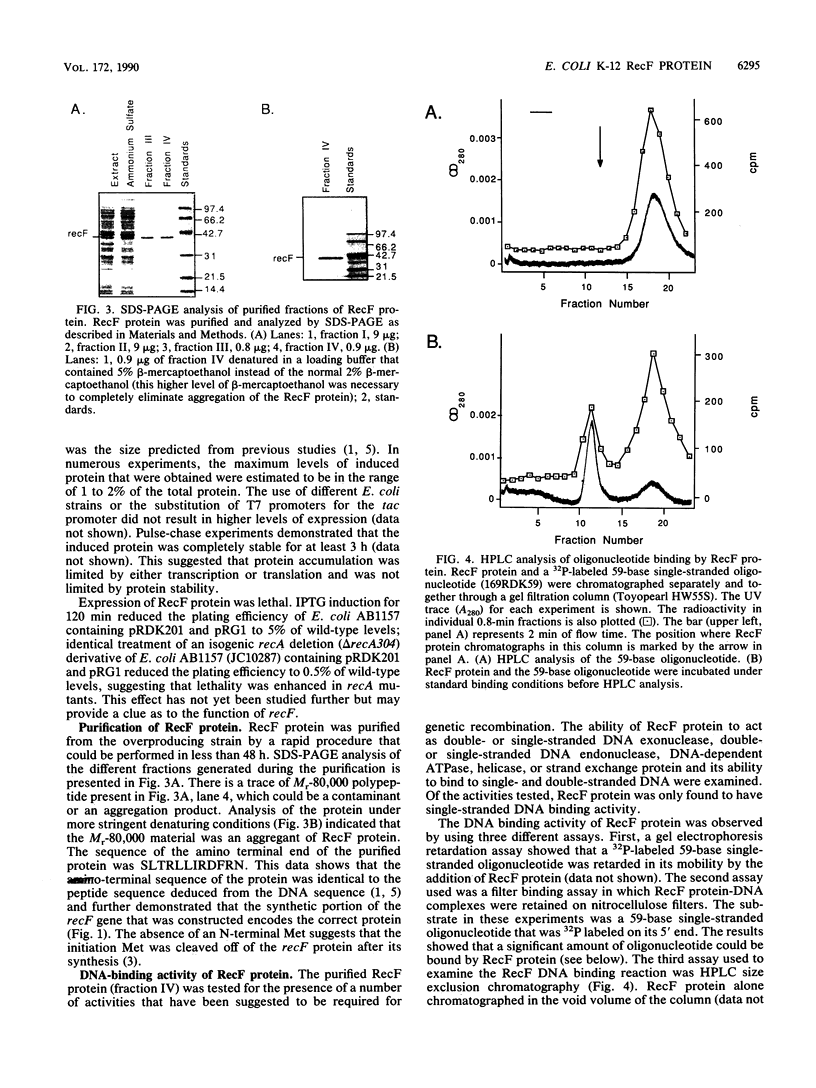
Abstract
The recF gene of Escherichia coli is known to encode an Mr-40,000 protein that is involved in DNA recombinationa nd postreplication DNA repair. To characterize the role of the recF gene product in these processes, the recF gene was cloned downstream of a tac promoter to facilitate overproduction of the recF gene product. The RecF protein was overproduced and purified to apparent homogeneity. N-terminal protein sequence analysis demonstrated that the purified protein had the sequence that was predicted from the DNA sequence of the recF gene, except that the predicted N-terminal Met was not present. The RecF protein bound to single-stranded oligonucleotides in filter binding and gel filtration assays. Maximal binding required 2 to 3 min of incubation at 37 degrees C; the binding reaction had a pH optimum of 7.0, did not require divalent cations, and was inhibited by NaCl concentrations of greater than 250 mM. The Kd of RecF protein binding to a 59-base single-stranded oligonucleotide was on the order of 1.3 X 10(-7) M, and the reaction did not show cooperativity. Experiments measuring the binding to various DNA substrates and competition binding experiments with different DNA molecules demonstrated that RecF protein binds preferentially to single-stranded, linear DNA molecules.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Mizuuchi K., Menzel R., Gellert M. DNA sequence and transcription of the region upstream of the E. coli gyrB gene. Nucleic Acids Res. 1984 Aug 24;12(16):6389–6395. doi: 10.1093/nar/12.16.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Ben-Bassat A., Bauer K., Chang S. Y., Myambo K., Boosman A., Chang S. Processing of the initiation methionine from proteins: properties of the Escherichia coli methionine aminopeptidase and its gene structure. J Bacteriol. 1987 Feb;169(2):751–757. doi: 10.1128/jb.169.2.751-757.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner M., Burke R. L., Alberts B. M. Purification of the T4 gene 32 protein free from detectable deoxyribonuclease activities. J Biol Chem. 1979 Oct 10;254(19):9565–9572. [PubMed] [Google Scholar]
- Blanar M. A., Sandler S. J., Armengod M. E., Ream L. W., Clark A. J. Molecular analysis of the recF gene of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4622–4626. doi: 10.1073/pnas.81.15.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Laban A. Plasmidic recombination in Escherichia coli K-12: the role of recF gene function. Mol Gen Genet. 1983;189(3):471–474. doi: 10.1007/BF00325911. [DOI] [PubMed] [Google Scholar]
- Doherty M. J., Morrison P. T., Kolodner R. Genetic recombination of bacterial plasmid DNA. Physical and genetic analysis of the products of plasmid recombination in Escherichia coli. J Mol Biol. 1983 Jul 5;167(3):539–560. doi: 10.1016/s0022-2836(83)80097-7. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Fishel R. A., James A. A., Kolodner R. recA-independent general genetic recombination of plasmids. Nature. 1981 Nov 12;294(5837):184–186. doi: 10.1038/294184a0. [DOI] [PubMed] [Google Scholar]
- Fishel R., Kolodner R. Gene conversion in Escherichia coli: the recF pathway for resolution of heteroduplex DNA. J Bacteriol. 1989 Jun;171(6):3046–3052. doi: 10.1128/jb.171.6.3046-3052.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer W. D., Kolodner R. D. Purification and characterization of a protein from Saccharomyces cerevisiae that binds tightly to single-stranded DNA and stimulates a cognate strand exchange protein. Biochemistry. 1989 Apr 4;28(7):2856–2862. doi: 10.1021/bi00433a017. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- James A. A., Morrison P. T., Kolodner R. Genetic recombination of bacterial plasmid DNA. Analysis of the effect of recombination-deficient mutations on plasmid recombination. J Mol Biol. 1982 Sep 25;160(3):411–430. doi: 10.1016/0022-2836(82)90305-9. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Evans D. H., Morrison P. T. Purification and characterization of an activity from Saccharomyces cerevisiae that catalyzes homologous pairing and strand exchange. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5560–5564. doi: 10.1073/pnas.84.16.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R., Fishel R. A., Howard M. Genetic recombination of bacterial plasmid DNA: effect of RecF pathway mutations on plasmid recombination in Escherichia coli. J Bacteriol. 1985 Sep;163(3):1060–1066. doi: 10.1128/jb.163.3.1060-1066.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leavitt A. D., Roberts T. M., Garcea R. L. Polyoma virus major capsid protein, VP1. Purification after high level expression in Escherichia coli. J Biol Chem. 1985 Oct 15;260(23):12803–12809. [PubMed] [Google Scholar]
- Lloyd R. G., Benson F. E., Shurvinton C. E. Effect of ruv mutations on recombination and DNA repair in Escherichia coli K12. Mol Gen Genet. 1984;194(1-2):303–309. doi: 10.1007/BF00383532. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Picksley S. M., Prescott C. Inducible expression of a gene specific to the RecF pathway for recombination in Escherichia coli K12. Mol Gen Genet. 1983;190(1):162–167. doi: 10.1007/BF00330340. [DOI] [PubMed] [Google Scholar]
- Lo K. M., Jones S. S., Hackett N. R., Khorana H. G. Specific amino acid substitutions in bacterioopsin: Replacement of a restriction fragment in the structural gene by synthetic DNA fragments containing altered codons. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2285–2289. doi: 10.1073/pnas.81.8.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman T. M., Overman L. B. Two binding modes in Escherichia coli single strand binding protein-single stranded DNA complexes. Modulation by NaCl concentration. J Biol Chem. 1985 Mar 25;260(6):3594–3603. [PubMed] [Google Scholar]
- Lovett S. T., Luisi-DeLuca C., Kolodner R. D. The genetic dependence of recombination in recD mutants of Escherichia coli. Genetics. 1988 Sep;120(1):37–45. doi: 10.1093/genetics/120.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi-DeLuca C., Lovett S. T., Kolodner R. D. Genetic and physical analysis of plasmid recombination in recB recC sbcB and recB recC sbcA Escherichia coli K-12 mutants. Genetics. 1989 Jun;122(2):269–278. doi: 10.1093/genetics/122.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiraju M. V., Templin A., Clark A. J. Properties of a mutant recA-encoded protein reveal a possible role for Escherichia coli recF-encoded protein in genetic recombination. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6592–6596. doi: 10.1073/pnas.85.18.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S. K., Datta A. R. Mechanisms of recombination by the RecBC and the RecF pathways following conjugation in Escherichia coli K12. Mol Gen Genet. 1979 Jan 16;169(1):67–78. doi: 10.1007/BF00267547. [DOI] [PubMed] [Google Scholar]
- Mahdi A. A., Lloyd R. G. Identification of the recR locus of Escherichia coli K-12 and analysis of its role in recombination and DNA repair. Mol Gen Genet. 1989 Apr;216(2-3):503–510. doi: 10.1007/BF00334397. [DOI] [PubMed] [Google Scholar]
- Mahdi A. A., Lloyd R. G. The recR locus of Escherichia coli K-12: molecular cloning, DNA sequencing and identification of the gene product. Nucleic Acids Res. 1989 Sep 12;17(17):6781–6794. doi: 10.1093/nar/17.17.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M., Lehman I. R. recA protein-catalyzed strand assimilation: stimulation by Escherichia coli single-stranded DNA-binding protein. Proc Natl Acad Sci U S A. 1980 Feb;77(2):857–861. doi: 10.1073/pnas.77.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland A., Green L., Echols H. Control of recA gene RNA in E. coli: regulatory and signal genes. Cell. 1980 Jul;20(3):731–737. doi: 10.1016/0092-8674(80)90319-0. [DOI] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P. DNA mismatch correction. Annu Rev Biochem. 1987;56:435–466. doi: 10.1146/annurev.bi.56.070187.002251. [DOI] [PubMed] [Google Scholar]
- Morrison P. T., Lovett S. T., Gilson L. E., Kolodner R. Molecular analysis of the Escherichia coli recO gene. J Bacteriol. 1989 Jul;171(7):3641–3649. doi: 10.1128/jb.171.7.3641-3649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Nakayama K., Nakayama R., Irino N., Nakayama Y., Hanawalt P. C. Isolation and genetic characterization of a thymineless death-resistant mutant of Escherichia coli K12: identification of a new mutation (recQ1) that blocks the RecF recombination pathway. Mol Gen Genet. 1984;195(3):474–480. doi: 10.1007/BF00341449. [DOI] [PubMed] [Google Scholar]
- Pallas D. C., Schley C., Mahoney M., Harlow E., Schaffhausen B. S., Roberts T. M. Polyomavirus small t antigen: overproduction in bacteria, purification, and utilization for monoclonal and polyclonal antibody production. J Virol. 1986 Dec;60(3):1075–1084. doi: 10.1128/jvi.60.3.1075-1084.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- Richet E., Abcarian P., Nash H. A. The interaction of recombination proteins with supercoiled DNA: defining the role of supercoiling in lambda integrative recombination. Cell. 1986 Sep 26;46(7):1011–1021. doi: 10.1016/0092-8674(86)90700-2. [DOI] [PubMed] [Google Scholar]
- Rothman R. H., Clark A. J. The dependence of postreplication repair on uvrB in a recF mutant of Escherichia coli K-12. Mol Gen Genet. 1977 Oct 24;155(3):279–286. doi: 10.1007/BF00272806. [DOI] [PubMed] [Google Scholar]
- Salles B., Paoletti C. Control of UV induction of recA protein. Proc Natl Acad Sci U S A. 1983 Jan;80(1):65–69. doi: 10.1073/pnas.80.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R. Homologous recombination in procaryotes. Microbiol Rev. 1988 Mar;52(1):1–28. doi: 10.1128/mr.52.1.1-28.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Goodman H. M., Heyneker H. L., Shine J., Boyer H. W., Cozzarelli N. R. Interaction of bacteriophage T4 RNA and DNA ligases in joining of duplex DNA at base-paired ends. J Biol Chem. 1977 Jun 10;252(11):3987–3994. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoms B., Wackernagel W. Suppression of the UV-sensitive phenotype of Escherichia coli recF mutants by recA(Srf) and recA(Tif) mutations requires recJ+. J Bacteriol. 1988 Aug;170(8):3675–3681. doi: 10.1128/jb.170.8.3675-3681.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert M. R. Altered induction of the adaptive response to alkylation damage in Escherichia coli recF mutants. J Bacteriol. 1989 Jan;171(1):99–103. doi: 10.1128/jb.171.1.99-103.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert M. R., Hartke M. A. Suppression of Escherichia coli recF mutations by recA-linked srfA mutations. J Bacteriol. 1984 Feb;157(2):498–506. doi: 10.1128/jb.157.2.498-506.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- Wang T. V., Smith K. C. Effect of recB21, uvrD3, lexA101 and recF143 mutations on ultraviolet radiation sensitivity and genetic recombination in delta uvrB strains of Escherichia coli K-12. Mol Gen Genet. 1981;183(1):37–44. doi: 10.1007/BF00270135. [DOI] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]