Abstract
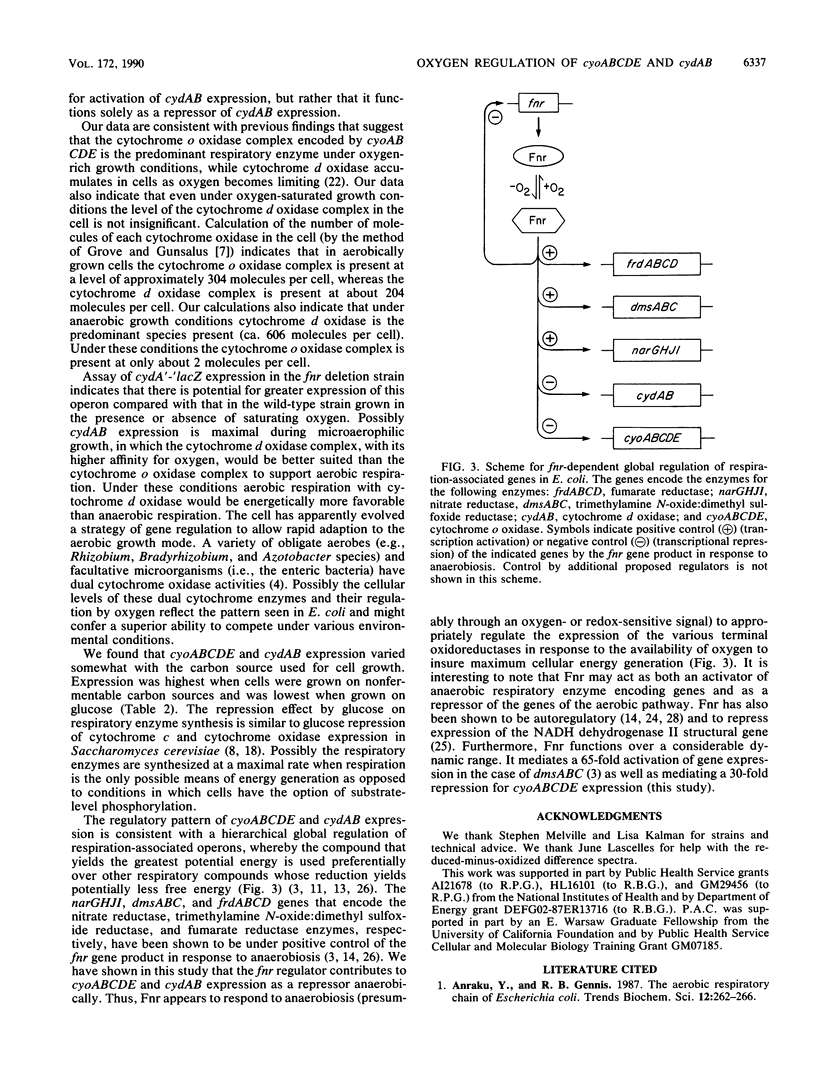
The aerobic respiratory chain of Escherichia coli contains two terminal oxidases that catalyze the oxidation of ubiquinol-8 and the reduction of oxygen to water. They are the cytochrome o oxidase complex encoded by cyoABCDE and the cytochrome d oxidase complex encoded by cydAB. To determine how these genes are regulated in response to a variety of environmental stimuli, including oxygen, we examined their expression by using lacZ protein fusions in wild-type and fnr mutant strains of E. coli. Anaerobic growth resulted in a 140-fold repression of cyoA'-'lacZ expression relative to aerobic growth and a 3-fold increase in cydA'-'lacZ expression. Anaerobic repression of both fusions was mediated in part by the fnr gene product, as evidenced by a 30-fold derepression of cyoA'-'lacZ expression and a 4-fold derepression of cydA'-'lacZ expression in an fnr deletion strain. Supplying wild-type fnr in trans restored wild-type repression for both fusions. Fnr thus functions as an anaerobic repressor of both cyoABCDE and cydAB expression. Reduced-minus-oxidized difference spectrum analyses of cell membranes confirmed the effect of the fnr gene product on the production of cytochrome d oxidase in the cell. Based on the pattern of anaerobic cydAB expression observed, we propose the existence of a second, as yet unidentified, regulatory element that must function either to activate cydAB expression as oxygen becomes limiting or to repress cydAB expression aerobically. Whereas cytochrome o oxidase encoded by cyoABCDE appears to be produced only under oxygen-rich growth conditions, in keeping with its biochemical properties, cytochrome d oxidase is expressed moderately aerobically and is elevated yet further when oxygen becomes limiting so that the organism can cope better under oxygen starvation conditions. We also examined cyoABCDE and cydAB expression in response to growth on alternative carbon compounds and to changes in the culture medium pH and osmolarity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au D. C., Lorence R. M., Gennis R. B. Isolation and characterization of an Escherichia coli mutant lacking the cytochrome o terminal oxidase. J Bacteriol. 1985 Jan;161(1):123–127. doi: 10.1128/jb.161.1.123-127.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P. A., Gunsalus R. P. Oxygen, nitrate, and molybdenum regulation of dmsABC gene expression in Escherichia coli. J Bacteriol. 1989 Jul;171(7):3817–3823. doi: 10.1128/jb.171.7.3817-3823.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey B., Jänel G., Michelsen U., Kersten H. Mutations in the Escherichia coli fnr and tgt genes: control of molybdate reductase activity and the cytochrome d complex by fnr. J Bacteriol. 1989 Mar;171(3):1524–1530. doi: 10.1128/jb.171.3.1524-1530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou C. D., Dueweke T. J., Gennis R. B. Regulation of expression of the cytochrome d terminal oxidase in Escherichia coli is transcriptional. J Bacteriol. 1988 Feb;170(2):961–966. doi: 10.1128/jb.170.2.961-966.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. N., Fang H., Lin R. J., Newton G., Mather M., Georgiou C. D., Gennis R. B. The nucleotide sequence of the cyd locus encoding the two subunits of the cytochrome d terminal oxidase complex of Escherichia coli. J Biol Chem. 1988 Sep 15;263(26):13138–13143. [PubMed] [Google Scholar]
- Green G. N., Gennis R. B. Isolation and characterization of an Escherichia coli mutant lacking cytochrome d terminal oxidase. J Bacteriol. 1983 Jun;154(3):1269–1275. doi: 10.1128/jb.154.3.1269-1275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove C. L., Gunsalus R. P. Regulation of the aroH operon of Escherichia coli by the tryptophan repressor. J Bacteriol. 1987 May;169(5):2158–2164. doi: 10.1128/jb.169.5.2158-2164.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L., Lalonde B., Gifford P., Alani E. Distinctly regulated tandem upstream activation sites mediate catabolite repression of the CYC1 gene of S. cerevisiae. Cell. 1984 Feb;36(2):503–511. doi: 10.1016/0092-8674(84)90243-5. [DOI] [PubMed] [Google Scholar]
- Iuchi S., Kuritzkes D. R., Lin E. C. Escherichia coli mutant with altered respiratory control of the frd operon. J Bacteriol. 1985 Mar;161(3):1023–1028. doi: 10.1128/jb.161.3.1023-1028.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. M., Gunsalus R. P. Regulation of Escherichia coli fumarate reductase (frdABCD) operon expression by respiratory electron acceptors and the fnr gene product. J Bacteriol. 1987 Jul;169(7):3340–3349. doi: 10.1128/jb.169.7.3340-3349.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. M., Gunsalus R. P. Transcription of the Escherichia coli fumarate reductase genes (frdABCD) and their coordinate regulation by oxygen, nitrate, and fumarate. J Bacteriol. 1985 Dec;164(3):1100–1109. doi: 10.1128/jb.164.3.1100-1109.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita K., Konishi K., Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. I. Purification and properties of cytochrome b562-o complex from cells in the early exponential phase of aerobic growth. J Biol Chem. 1984 Mar 10;259(5):3368–3374. [PubMed] [Google Scholar]
- Kita K., Konishi K., Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J Biol Chem. 1984 Mar 10;259(5):3375–3381. [PubMed] [Google Scholar]
- Kranz R. G., Barassi C. A., Gennis R. B. Immunological analysis of the heme proteins present in aerobically grown Escherichia coli. J Bacteriol. 1984 Jun;158(3):1191–1194. doi: 10.1128/jb.158.3.1191-1194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laz T. M., Pietras D. F., Sherman F. Differential regulation of the duplicated isocytochrome c genes in yeast. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4475–4479. doi: 10.1073/pnas.81.14.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Patel L., Kaback H. R. Cytochrome o type oxidase from Escherichia coli. Characterization of the enzyme and mechanism of electrochemical proton gradient generation. Biochemistry. 1984 Sep 25;23(20):4703–4714. doi: 10.1021/bi00315a028. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Gennis R. B. The purification and characterization of the cytochrome d terminal oxidase complex of the Escherichia coli aerobic respiratory chain. J Biol Chem. 1983 Aug 10;258(15):9159–9165. [PubMed] [Google Scholar]
- Peterson G. L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979 Dec;100(2):201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- Rice C. W., Hempfling W. P. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978 Apr;134(1):115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Regulation and over-expression of the fnr gene of Escherichia coli. J Gen Microbiol. 1987 Dec;133(12):3279–3288. doi: 10.1099/00221287-133-12-3279. [DOI] [PubMed] [Google Scholar]
- Spiro S., Roberts R. E., Guest J. R. FNR-dependent repression of the ndh gene of Escherichia coli and metal ion requirement for FNR-regulated gene expression. Mol Microbiol. 1989 May;3(5):601–608. doi: 10.1111/j.1365-2958.1989.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Stewart V., Berg B. L. Influence of nar (nitrate reductase) genes on nitrate inhibition of formate-hydrogen lyase and fumarate reductase gene expression in Escherichia coli K-12. J Bacteriol. 1988 Oct;170(10):4437–4444. doi: 10.1128/jb.170.10.4437-4444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V. Requirement of Fnr and NarL functions for nitrate reductase expression in Escherichia coli K-12. J Bacteriol. 1982 Sep;151(3):1320–1325. doi: 10.1128/jb.151.3.1320-1325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden G., Duchene A. On the role of cyclic AMP and the Fnr protein in Escherichia coli growing anaerobically. Arch Microbiol. 1987 Mar;147(2):195–200. doi: 10.1007/BF00415284. [DOI] [PubMed] [Google Scholar]


