Abstract
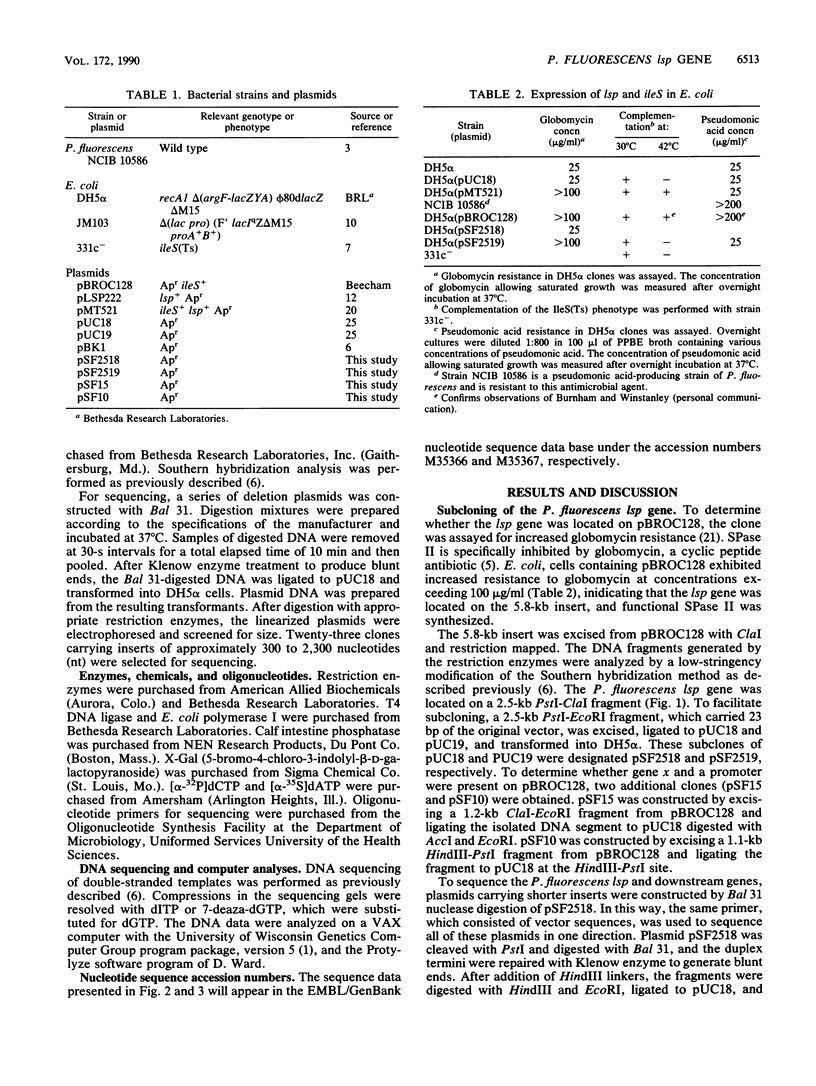
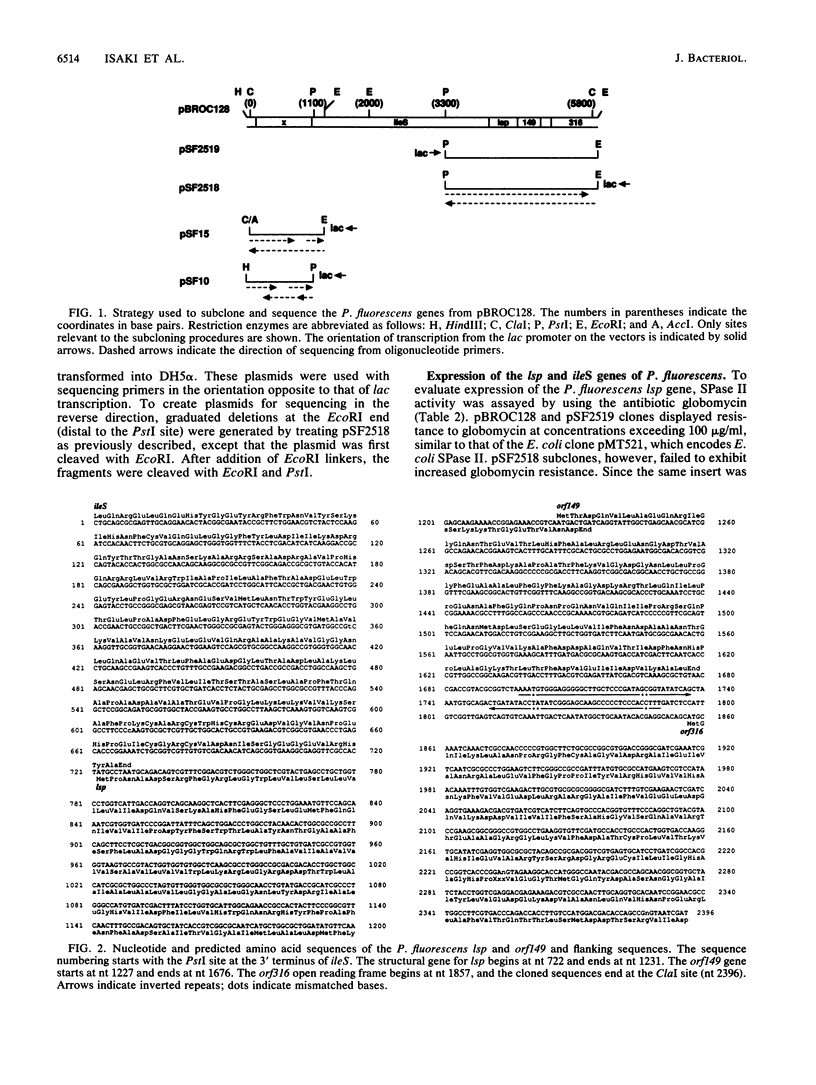
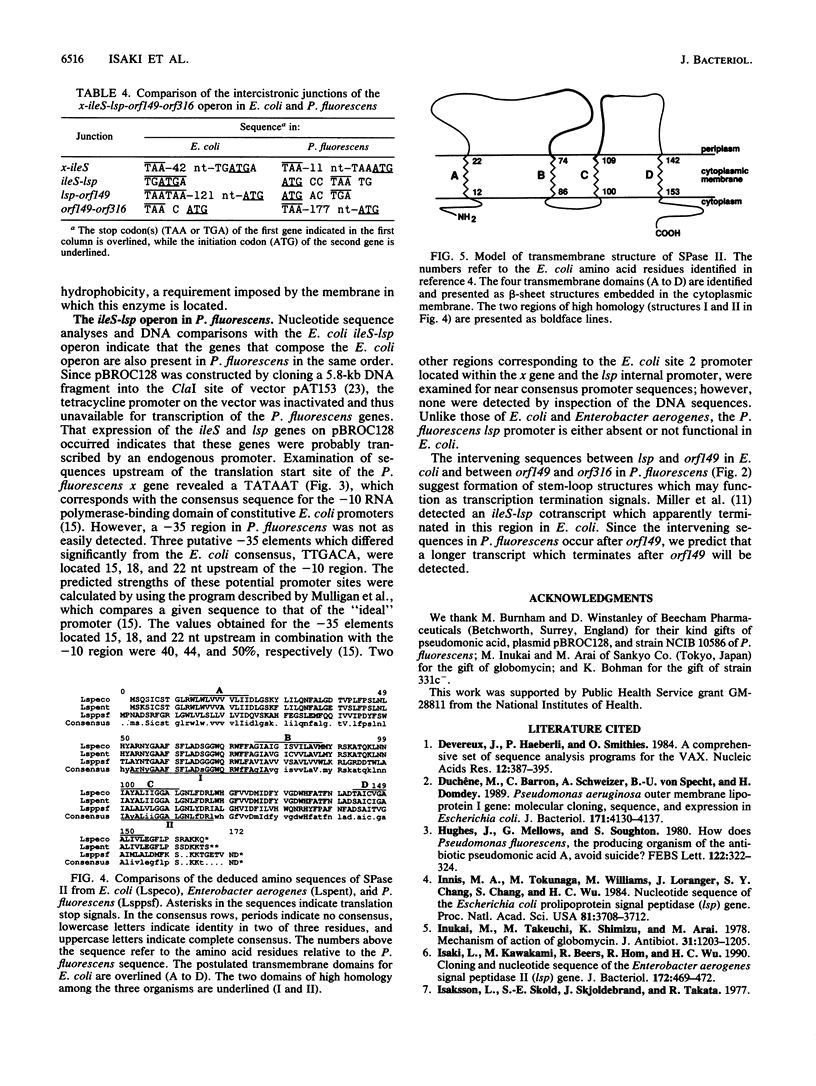
The lsp gene encoding prolipoprotein signal peptidase (signal peptidase II) is organized into an operon consisting of ileS and three open reading frames, designated genes x, orf149, and orf316 in both Escherichia coli and Enterobacter aerogenes. A plasmid, pBROC128, containing a 5.8-kb fragment of Pseudomonas fluorescens DNA was found to confer pseudomonic acid resistance on E. coli host cells and to contain the structural gene of ileS from P. fluorescens. In addition, E. coli strains carrying pBROC128 exhibited increased globomycin resistance. This indicated that the P. fluorescens lsp gene was present on the plasmid. The nucleotide sequences of the P. fluorescens lsp gene and of its flanking regions were determined. Comparison of the nucleotide sequences of the lsp genes in E. coli and P. fluorescens revealed two highly conserved domains in this enzyme. Furthermore, the five genes which constitute an operon in E. coli and Enterobacter aerogenes were found in P. fluorescens in the same order as in the first two species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchêne M., Barron C., Schweizer A., von Specht B. U., Domdey H. Pseudomonas aeruginosa outer membrane lipoprotein I gene: molecular cloning, sequence, and expression in Escherichia coli. J Bacteriol. 1989 Aug;171(8):4130–4137. doi: 10.1128/jb.171.8.4130-4137.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Mellows G., Soughton S. How does Pseudomonas fluorescens, the producing organism of the antibiotic pseudomonic acid A, avoid suicide? FEBS Lett. 1980 Dec 29;122(2):322–324. doi: 10.1016/0014-5793(80)80465-0. [DOI] [PubMed] [Google Scholar]
- Innis M. A., Tokunaga M., Williams M. E., Loranger J. M., Chang S. Y., Chang S., Wu H. C. Nucleotide sequence of the Escherichia coli prolipoprotein signal peptidase (lsp) gene. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3708–3712. doi: 10.1073/pnas.81.12.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai M., Takeuchi M., Shimizu K., Arai M. Mechanism of action of globomycin. J Antibiot (Tokyo) 1978 Nov;31(11):1203–1205. doi: 10.7164/antibiotics.31.1203. [DOI] [PubMed] [Google Scholar]
- Isaki L., Kawakami M., Beers R., Hom R., Wu H. C. Cloning and nucleotide sequence of the Enterobacter aerogenes signal peptidase II (lsp) gene. J Bacteriol. 1990 Jan;172(1):469–472. doi: 10.1128/jb.172.1.469-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson L. A., Sköld S. E., Skjöldebrand J., Takata R. A procedure for isolation of spontaneous mutants with temperature sensitive of RNA and/or protein. Mol Gen Genet. 1977 Nov 18;156(3):233–237. doi: 10.1007/BF00267177. [DOI] [PubMed] [Google Scholar]
- Kamio Y., Lin C. K., Regue M., Wu H. C. Characterization of the ileS-lsp operon in Escherichia coli. Identification of an open reading frame upstream of the ileS gene and potential promoter(s) for the ileS-lsp operon. J Biol Chem. 1985 May 10;260(9):5616–5620. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller K. W., Bouvier J., Stragier P., Wu H. C. Identification of the genes in the Escherichia coli ileS-lsp operon. Analysis of multiple polycistronic mRNAs made in vivo. J Biol Chem. 1987 May 25;262(15):7391–7397. [PubMed] [Google Scholar]
- Miller K. W., Wu H. C. Cotranscription of the Escherichia coli isoleucyl-tRNA synthetase (ileS) and prolipoprotein signal peptidase (lsp) genes. Fine-structure mapping of the lsp internal promoter. J Biol Chem. 1987 Jan 5;262(1):389–393. [PubMed] [Google Scholar]
- Mizuno T. A novel peptidoglycan-associated lipoprotein found in the cell envelope of Pseudomonas aeruginosa and Escherichia coli. J Biochem. 1979 Oct;86(4):991–1000. doi: 10.1093/oxfordjournals.jbchem.a132631. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Isolation of characterization of a major outer membrane protein of Pseudomonas aeruginosa. Evidence for the occurrence of a lipoprotein. J Biochem. 1979 Jan;85(1):115–122. doi: 10.1093/oxfordjournals.jbchem.a132300. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Pirtle R. M., Inouye M. Homology of the gene coding for outer membrane lipoprotein within various Gram-negative bacteria. J Bacteriol. 1979 Jan;137(1):595–604. doi: 10.1128/jb.137.1.595-604.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regue M., Remenick J., Tokunaga M., Mackie G. A., Wu H. C. Mapping of the lipoprotein signal peptidase gene (lsp). J Bacteriol. 1984 May;158(2):632–635. doi: 10.1128/jb.158.2.632-635.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M., Loranger J. M., Wu H. C. A distinct signal peptidase for prolipoprotein in Escherichia coli. J Cell Biochem. 1984;24(2):113–120. doi: 10.1002/jcb.240240203. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Loranger J. M., Wu H. C. Isolation and characterization of an Escherichia coli clone overproducing prolipoprotein signal peptidase. J Biol Chem. 1983 Oct 25;258(20):12102–12105. [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Wu H. C. Post-translational modification and processing of Escherichia coli prolipoprotein in vitro. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2255–2259. doi: 10.1073/pnas.79.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yu F., Yamada H., Daishima K., Mizushima S. Nucleotide sequence of the lspA gene, the structural gene for lipoprotein signal peptidase of Escherichia coli. FEBS Lett. 1984 Jul 23;173(1):264–268. doi: 10.1016/0014-5793(84)81060-1. [DOI] [PubMed] [Google Scholar]


