Abstract
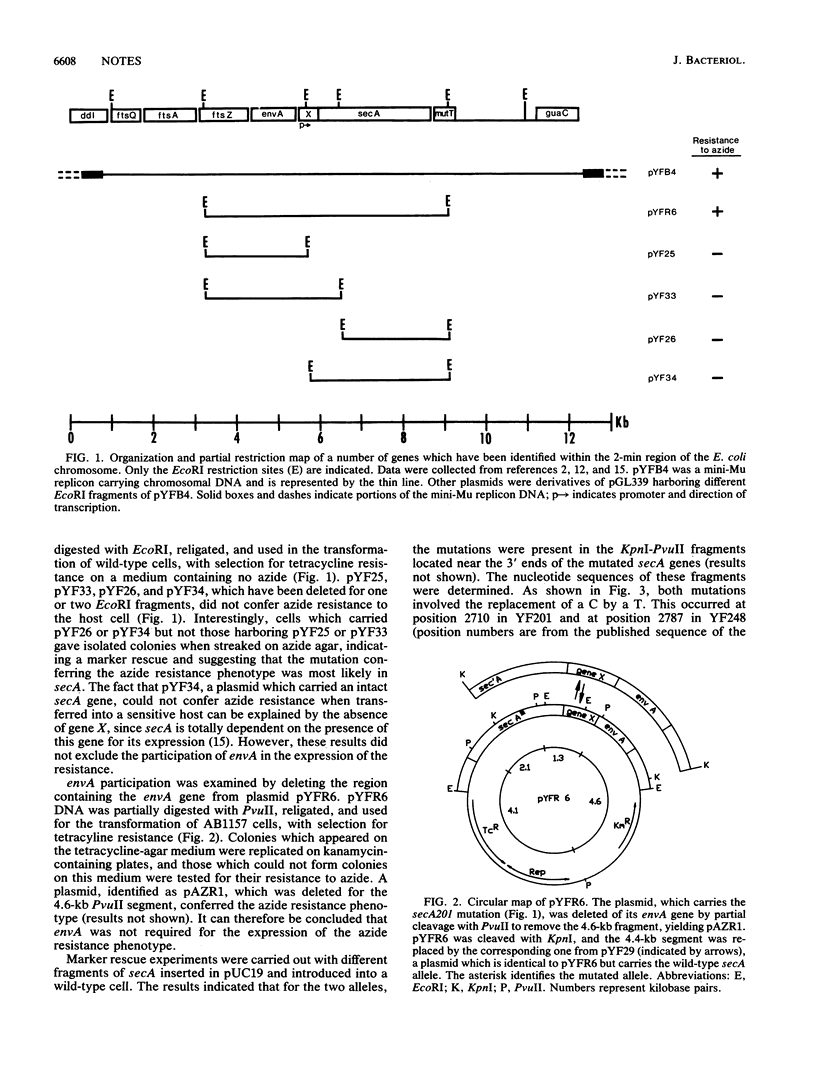
Mutant strains of Escherichia coli were screened for the ability to grow on L agar plates containing 3.4 or 4.6 mM sodium azide. Most mutants had mutations located in the leucine region, presumably at the azi locus. Two of these mutants were found to have a mutation in the secA gene, but expression of the resistance phenotype also required the presence of upstream gene X. While a plasmid carrying the X-secA mutant gene pair was able to confer azide resistance to a sensitive host, a similar plasmid harboring the wild-type secA allele rendered a resistant strain sensitive to azide, indicating codominance of the two alleles. That azide inhibits SecA is consistent with the fact that SecA has ATPase activity, an activity that is often prone to inhibition by azide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall B., Lutkenhaus J. Sequence analysis, transcriptional organization, and insertional mutagenesis of the envA gene of Escherichia coli. J Bacteriol. 1987 Dec;169(12):5408–5415. doi: 10.1128/jb.169.12.5408-5415.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieśla Z., Bagdasarian M., Szczurkiewicz W., Przygońska M., Klopotowski T. Defective cell division in thermosensitive mutants of Salmonella typhimurium. Mol Gen Genet. 1972;116(2):107–125. doi: 10.1007/BF00582221. [DOI] [PubMed] [Google Scholar]
- Cieśla Z., Mardarowicz K., Klopotowski T. Inhibition of DNA synthesis and cell division in Salmonella typhimurium by azide. Mol Gen Genet. 1974;135(4):339–348. doi: 10.1007/BF00271148. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J. The selection of genetic recombinations with bacterial growth inhibitors. J Bacteriol. 1950 Feb;59(2):211–215. doi: 10.1128/jb.59.2.211-215.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R., Cunningham K., Brundage L. A., Ito K., Oliver D., Wickner W. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 1989 Mar;8(3):961–966. doi: 10.1002/j.1460-2075.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnett P. E., Beechey R. B. Inhibitors of the ATP synthethase system. Methods Enzymol. 1979;55:472–518. doi: 10.1016/0076-6879(79)55061-7. [DOI] [PubMed] [Google Scholar]
- Noël G., Drapeau G. R. Identification of new cell division genes in Escherichia coli by using extragenic suppressors. J Bacteriol. 1986 Feb;165(2):399–404. doi: 10.1128/jb.165.2.399-404.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Identification of a new gene (secA) and gene product involved in the secretion of envelope proteins in Escherichia coli. J Bacteriol. 1982 May;150(2):686–691. doi: 10.1128/jb.150.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owais W. M., Kleinhofs A. Metabolic activation of the mutagen azide in biological systems. Mutat Res. 1988 Feb;197(2):313–323. doi: 10.1016/0027-5107(88)90101-7. [DOI] [PubMed] [Google Scholar]
- Phoenix P., Drapeau G. R. Cell division control in Escherichia coli K-12: some properties of the ftsZ84 mutation and suppression of this mutation by the product of a newly identified gene. J Bacteriol. 1988 Sep;170(9):4338–4342. doi: 10.1128/jb.170.9.4338-4342.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney S. D., Royal N. J., Neuman de Vegvar H., Herlihy W. C., Biemann K., Schimmel P. Primary structure of a large aminoacyl-tRNA synthetase. Science. 1981 Sep 25;213(4515):1497–1501. doi: 10.1126/science.7025207. [DOI] [PubMed] [Google Scholar]
- Schmidt M. G., Oliver D. B. SecA protein autogenously represses its own translation during normal protein secretion in Escherichia coli. J Bacteriol. 1989 Feb;171(2):643–649. doi: 10.1128/jb.171.2.643-649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. G., Rollo E. E., Grodberg J., Oliver D. B. Nucleotide sequence of the secA gene and secA(Ts) mutations preventing protein export in Escherichia coli. J Bacteriol. 1988 Aug;170(8):3404–3414. doi: 10.1128/jb.170.8.3404-3414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Takeda K., Miki J., Kanazawa H., Tsuchiya T., Futai M. Change of inhibitor sensitivities of Escherichia coli F1-ATPase due to a mutational substitution of Phe for Ser at residue 174 of the beta subunit. J Biochem. 1985 May;97(5):1401–1407. doi: 10.1093/oxfordjournals.jbchem.a135194. [DOI] [PubMed] [Google Scholar]
- Yura T., Wada C. Phenethyl alcohol resistance in Escherichia coli. I. Resistance of strain C600 and its relation to azide resistance. Genetics. 1968 Jun;59(2):177–190. doi: 10.1093/genetics/59.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]