Abstract
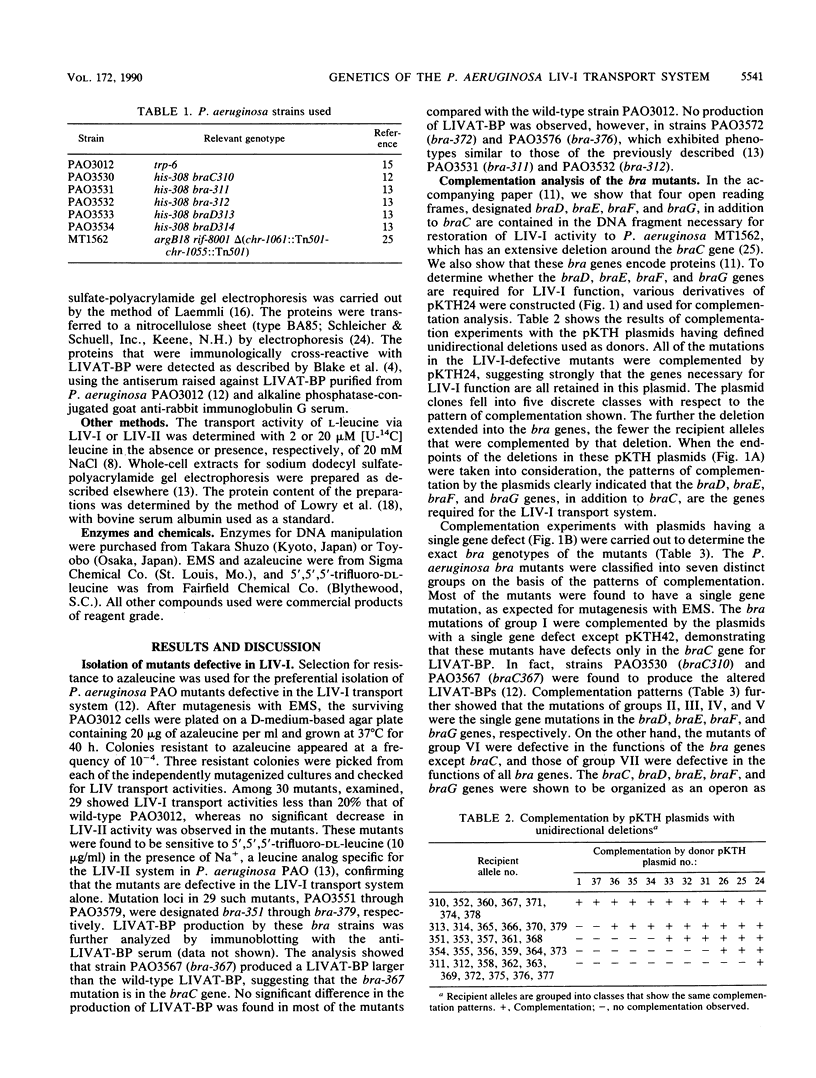
About 30 mutants of Pseudomonas aeruginosa PAO defective in the high-affinity branched-chain amino acid transport system (LIV-I) were isolated by the selection for resistance to 4-aza-DL-leucine, a toxic leucine analog for LIV-I. All of the mutants were complemented by plasmid pKTH24, harboring the braC gene, which encodes the branched-chain amino acid-binding protein, and the four open reading frames named braD, braE, braF, and braG (T. Hoshino and K. Kose, J. Bacteriol. 172:5531-5539, 1990). We identified five cistrons corresponding to these bra genes by complementation analysis with various derivatives of pKTH24, confirming that the braD, braE, braF, and braG genes are required for the LIV-I transport system. We also found mutations that seem likely to be mutations in a promoter region for the bra genes and those with polarity in the intercistronic region between braC and braD. Analysis with an omega interposon showed that the bra genes are organized as an operon and are cotranscribed in the order braC-braD-braE-braF-braG from a promoter located in the 5'-flanking region of the braC gene.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Nikaido K., Groarke J., Petithory J. Reconstitution of periplasmic transport in inside-out membrane vesicles. Energization by ATP. J Biol Chem. 1989 Mar 5;264(7):3998–4002. [PubMed] [Google Scholar]
- Bagdasarian M. M., Amann E., Lurz R., Rückert B., Bagdasarian M. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled-expression vectors. Gene. 1983 Dec;26(2-3):273–282. doi: 10.1016/0378-1119(83)90197-x. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Fellay R., Frey J., Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52(2-3):147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- Hiles I. D., Gallagher M. P., Jamieson D. J., Higgins C. F. Molecular characterization of the oligopeptide permease of Salmonella typhimurium. J Mol Biol. 1987 May 5;195(1):125–142. doi: 10.1016/0022-2836(87)90332-9. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Kageyama M. Purification and properties of a binding protein for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1980 Mar;141(3):1055–1063. doi: 10.1128/jb.141.3.1055-1063.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Kose K. Cloning and nucleotide sequence of braC, the structural gene for the leucine-, isoleucine-, and valine-binding protein of Pseudomonas aeruginosa PAO. J Bacteriol. 1989 Nov;171(11):6300–6306. doi: 10.1128/jb.171.11.6300-6306.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Kose K. Cloning, nucleotide sequences, and identification of products of the Pseudomonas aeruginosa PAO bra genes, which encode the high-affinity branched-chain amino acid transport system. J Bacteriol. 1990 Oct;172(10):5531–5539. doi: 10.1128/jb.172.10.5531-5539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Nishio K. Isolation and characterization of a Pseudomonas aeruginosa PAO mutant defective in the structural gene for the LIVAT-binding protein. J Bacteriol. 1982 Aug;151(2):729–736. doi: 10.1128/jb.151.2.729-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T. Transport systems for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1979 Sep;139(3):705–712. doi: 10.1128/jb.139.3.705-712.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Tsuda M., Iino T., Nishio K., Kageyama M. Genetic mapping of bra genes affecting branched-chain amino acid transport in Pseudomonas aeruginosa. J Bacteriol. 1983 Mar;153(3):1272–1281. doi: 10.1128/jb.153.3.1272-1281.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A. K., Ahmed S., Ferro-Luzzi Ames G. Energy coupling in bacterial periplasmic transport systems. Studies in intact Escherichia coli cells. J Biol Chem. 1989 Feb 5;264(4):2126–2133. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landick R., Anderson J. J., Mayo M. M., Gunsalus R. P., Mavromara P., Daniels C. J., Oxender D. L. Regulation of high-affinity leucine transport in Escherichia coli. J Supramol Struct. 1980;14(4):527–537. doi: 10.1002/jss.400140410. [DOI] [PubMed] [Google Scholar]
- Nazos P. M., Antonucci T. K., Landick R., Oxender D. L. Cloning and characterization of livH, the structural gene encoding a component of the leucine transport system in Escherichia coli. J Bacteriol. 1986 May;166(2):565–573. doi: 10.1128/jb.166.2.565-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazos P. M., Mayo M. M., Su T. Z., Anderson J. J., Oxender D. L. Identification of livG, a membrane-associated component of the branched-chain amino acid transport in Escherichia coli. J Bacteriol. 1985 Sep;163(3):1196–1202. doi: 10.1128/jb.163.3.1196-1202.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Reznikoff W. S., Siegele D. A., Cowing D. W., Gross C. A. The regulation of transcription initiation in bacteria. Annu Rev Genet. 1985;19:355–387. doi: 10.1146/annurev.ge.19.120185.002035. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Iino T. Ordering of the flagellar genes in Pseudomonas aeruginosa by insertions of mercury transposon Tn501. J Bacteriol. 1983 Feb;153(2):1008–1017. doi: 10.1128/jb.153.2.1008-1017.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. H., Lin E. C. Evolution of membrane bioenergetics. J Supramol Struct. 1980;13(4):421–446. doi: 10.1002/jss.400130403. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


