Abstract
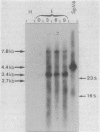
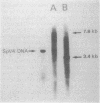
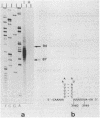
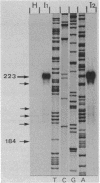
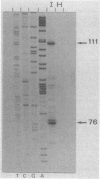
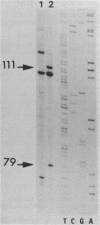
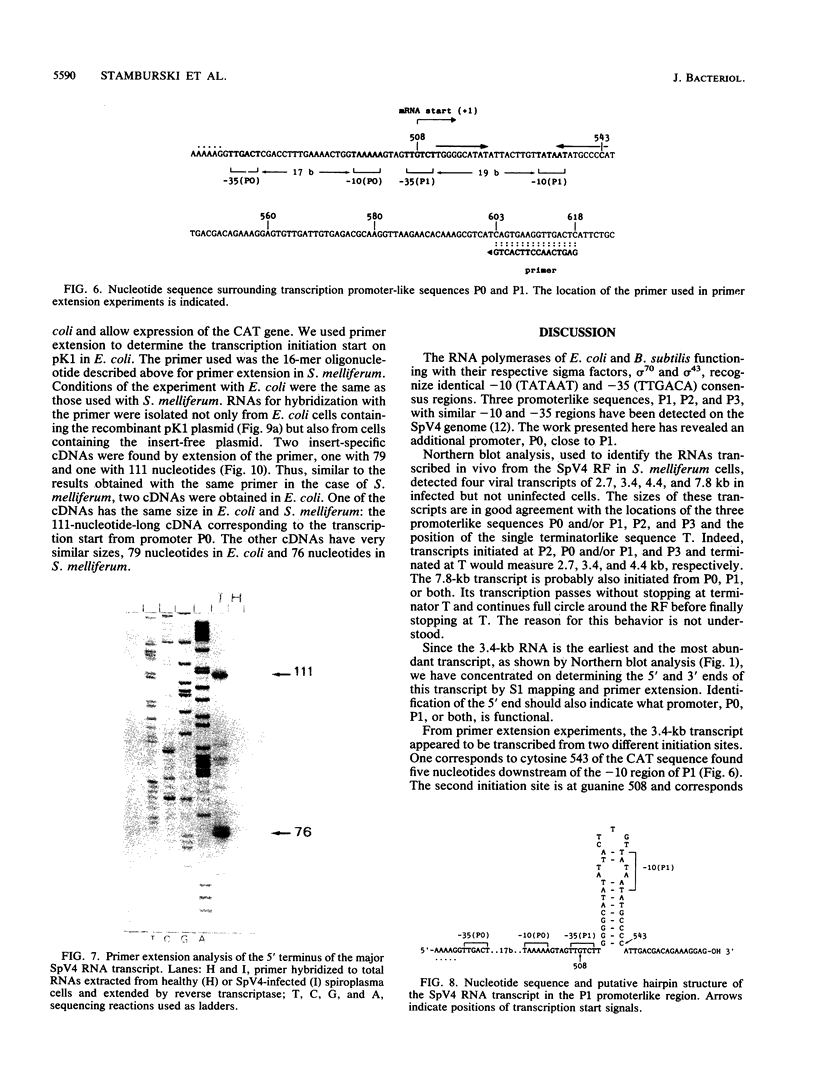
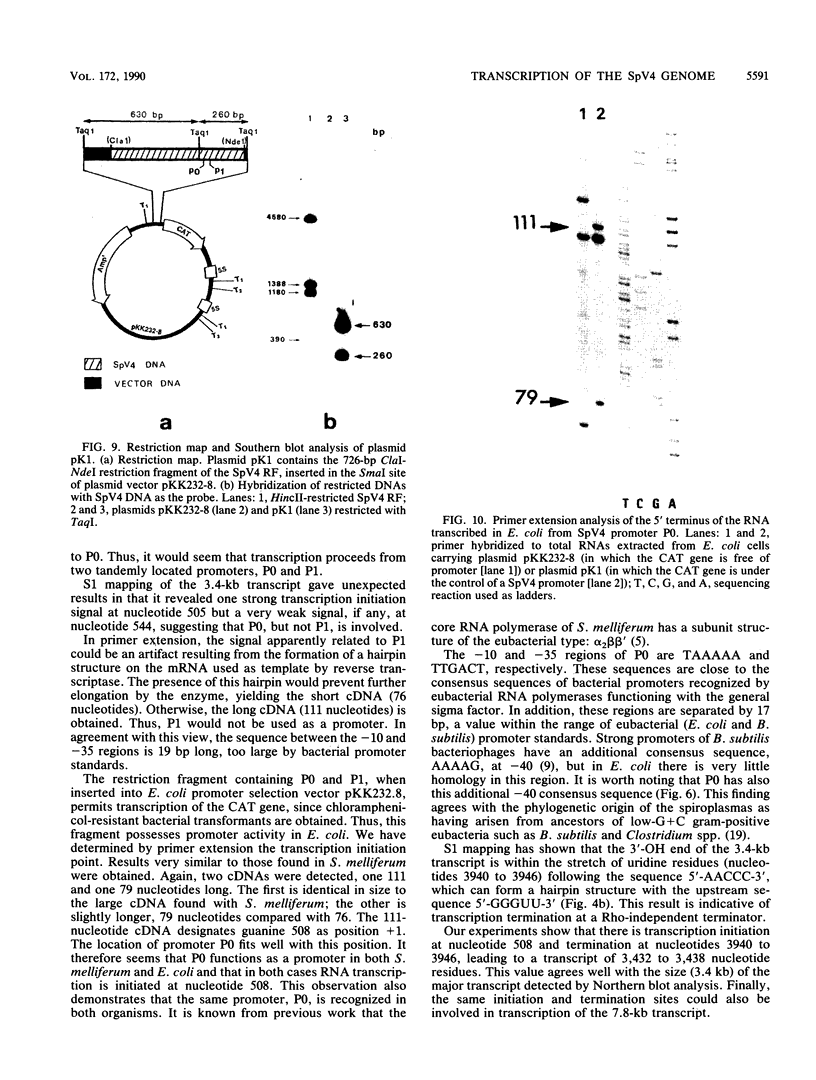
Spiroplasma virus 4 (SpV4) is an isometric virus with single-stranded, circular DNA infecting the helical mollicute Spiroplasma melliferum, a honeybee pathogen. Previous studies in our laboratory led to the determination of the base sequence of the SpV4 DNA. Nine open reading frames and three promoterlike sequences (P1, P2, and P3) were identified. An inverted repeat leading to the formation of a hairpin structure on the transcription product was also found and predicted to be a transcription terminator (T). We have now studied the in vivo transcription of the SpV4 genome by Northern (RNA) blot analysis of the total RNAs extracted from SpV4-infected spiroplasma cells. Transcripts of 7.8, 4.4, 3.4, and 2.7 kilobases (kb) were detected. The 3.4-kb RNA was the major transcript. The 5' and 3' ends of this transcript were determined by S1 mapping and primer extension. Characterization of the 3' end by S1 mapping showed that the 3.4-kb transcript terminates within the stretch of uridine residues following the hairpin structure of terminator T. Characterization of the 5' end by S1 mapping indicated that transcription proceeds from a newly recognized promoter, P0, located 36 nucleotides upstream of P1. Primer extension resulted in two cDNA signals. The short cDNA was probably a primer extension artifact due to the presence of a hairpin structure on the transcript. When reverse transcriptase stopped at this hairpin or read through, the short or the long cDNA, respectively, was obtained. The size of the long cDNA identified P0 as the transcription promoter. Promoter P0 was also shown to be functional in Escherichia coli. Indeed, when inserted upstream of the chloramphenicol acetyltransferase gene of a promoter selection vector, it promoted transcription of this gene. As in the case of S. melliferum, two cDNAs were obtained by primer extension, the longer cDNA identifying P0 as the promoter.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Chevalier C., Saillard C., Bové J. M. Organization and nucleotide sequences of the Spiroplasma citri genes for ribosomal protein S2, elongation factor Ts, spiralin, phosphofructokinase, pyruvate kinase, and an unidentified protein. J Bacteriol. 1990 May;172(5):2693–2703. doi: 10.1128/jb.172.5.2693-2703.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Gadeau A. P., Mouches C., Bove J. M. Probable insensitivity of mollicutes to rifampin and characterization of spiroplasmal DNA-dependent RNA polymerase. J Bacteriol. 1986 Jun;166(3):824–828. doi: 10.1128/jb.166.3.824-828.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W. M., Platt T., Rosenberg M. Termination of transcription in E. coli. Cell. 1983 Apr;32(4):1029–1032. doi: 10.1016/0092-8674(83)90287-8. [DOI] [PubMed] [Google Scholar]
- Mouchès C., Candresse T., Barroso G., Saillard C., Wroblewski H., Bové J. M. Gene for spiralin, the major membrane protein of the helical mollicute Spiroplasma citri: cloning and expression in Escherichia coli. J Bacteriol. 1985 Dec;164(3):1094–1099. doi: 10.1128/jb.164.3.1094-1099.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. L., Rabinowitz J. C. Nucleotide sequences of transcription and translation initiation regions in Bacillus phage phi 29 early genes. J Biol Chem. 1982 Jan 25;257(2):1053–1062. [PubMed] [Google Scholar]
- Pascarel-Devilder M. C., Renaudin J., Bove J. M. The spiroplasma virus 4 replicative form cloned in Escherichia coli transfects spiroplasmas. Virology. 1986 Jun;151(2):390–393. doi: 10.1016/0042-6822(86)90060-7. [DOI] [PubMed] [Google Scholar]
- Renaudin J., Pascarel M. C., Bové J. M. Spiroplasma virus 4: nucleotide sequence of the viral DNA, regulatory signals, and proposed genome organization. J Bacteriol. 1987 Nov;169(11):4950–4961. doi: 10.1128/jb.169.11.4950-4961.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff W. S., Siegele D. A., Cowing D. W., Gross C. A. The regulation of transcription initiation in bacteria. Annu Rev Genet. 1985;19:355–387. doi: 10.1146/annurev.ge.19.120185.002035. [DOI] [PubMed] [Google Scholar]
- Rogers M. J., Steinmetz A. A., Walker R. T. The nucleotide sequence of a tRNA gene cluster from Spiroplasma meliferum. Nucleic Acids Res. 1986 Apr 11;14(7):3145–3145. doi: 10.1093/nar/14.7.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]