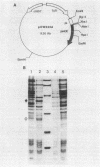
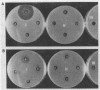
Abstract
The final step in the biosynthesis of beta-lactam antibiotics in Penicillium chrysogenum and Aspergillus nidulans involves removal of the L-alpha-aminoadipyl side chain from isopenicillin N (IPN) and exchange with a nonpolar side chain. The enzyme catalyzing this reaction, acyl-coenzyme A:isopenicillin N acyltransferase (acyltransferase), was purified from P. chrysogenum and A. nidulans. Based on NH2-terminal amino acid sequence information, the acyltransferase gene (penDE) from P. chrysogenum and A. nidulans were cloned. In both organisms, penDE was located immediately downstream from the isopenicillin N synthetase gene (pcbC) and consisted of four exons encoding an enzyme of 357 amino acids (approximately 40 kilodaltons [kDa]). The DNA coding sequences showed approximately 73% identity, while the amino acid sequences were approximately 76% identical. Noncoding DNA regions (including the region between pcbC and penDE) were not conserved. Acyltransferase activity from Escherichia coli producing the 40-kDa protein accepted either 6-aminopenicillanic acid or IPN as the substrate and made a penicillinase-sensitive antibiotic in the presence of phenylacetyl coenzyme A. Therefore, a single gene is responsible for converting IPN to penicillin G. The active form of the enzyme may result from processing of the 40-kDa monomeric precursor to a heterodimer containing subunits of 11 and 29 kDa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso M. J., Bermejo F., Reglero A., Fernández-Cañn J. M., González de Buitrago G., Luengo J. M. Enzymatic synthesis of penicillins. J Antibiot (Tokyo) 1988 Aug;41(8):1074–1084. doi: 10.7164/antibiotics.41.1074. [DOI] [PubMed] [Google Scholar]
- Alvarez E., Cantoral J. M., Barredo J. L., Díez B., Martín J. F. Purification to homogeneity and characterization of acyl coenzyme A:6-aminopenicillanic acid acyltransferase of Penicillium chrysogenum. Antimicrob Agents Chemother. 1987 Nov;31(11):1675–1682. doi: 10.1128/aac.31.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barredo J. L., van Solingen P., Díez B., Alvarez E., Cantoral J. M., Kattevilder A., Smaal E. B., Groenen M. A., Veenstra A. E., Martín J. F. Cloning and characterization of the acyl-coenzyme A: 6-aminopenicillanic-acid-acyltransferase gene of Penicillium chrysogenum. Gene. 1989 Nov 30;83(2):291–300. doi: 10.1016/0378-1119(89)90115-7. [DOI] [PubMed] [Google Scholar]
- Carr L. G., Skatrud P. L., Scheetz M. E., 2nd, Queener S. W., Ingolia T. D. Cloning and expression of the isopenicillin N synthetase gene from Penicillium chrysogenum. Gene. 1986;48(2-3):257–266. doi: 10.1016/0378-1119(86)90084-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez B., Barredo J. L., Alvarez E., Cantoral J. M., van Solingen P., Groenen M. A., Veenstra A. E., Martín J. F. Two genes involved in penicillin biosynthesis are linked in a 5.1 kb SalI fragment in the genome of Penicillium chrysogenum. Mol Gen Genet. 1989 Sep;218(3):572–576. doi: 10.1007/BF00332426. [DOI] [PubMed] [Google Scholar]
- Gatenbeck S. Acyl CoA:6-aminopenicillanic acid acyltransferase. Methods Enzymol. 1975;43:474–476. doi: 10.1016/0076-6879(75)43106-8. [DOI] [PubMed] [Google Scholar]
- Gatenbeck S., Brunsberg U. Biosynthesis of penicillins. I. Isolation of a 6-aminopenicillanic acid acyltransferase from Penicillium chrysogenum. Acta Chem Scand. 1968;22(3):1059–1061. doi: 10.3891/acta.chem.scand.22-1059. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Queener S. W. Beta-lactam biosynthetic genes. Med Res Rev. 1989 Apr-Jun;9(2):245–264. doi: 10.1002/med.2610090206. [DOI] [PubMed] [Google Scholar]
- Kovacevic S., Weigel B. J., Tobin M. B., Ingolia T. D., Miller J. R. Cloning, characterization, and expression in Escherichia coli of the Streptomyces clavuligerus gene encoding deacetoxycephalosporin C synthetase. J Bacteriol. 1989 Feb;171(2):754–760. doi: 10.1128/jb.171.2.754-760.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luengo J. M., Iriso J. L., López-Nieto M. J. Direct enzymatic synthesis of natural penicillins using phenylacetyl-CoA: 6-APA phenylacetyl transferase of Penicillium chrysogenum: minimal and maximal side chain length requirements. J Antibiot (Tokyo) 1986 Dec;39(12):1754–1759. doi: 10.7164/antibiotics.39.1754. [DOI] [PubMed] [Google Scholar]
- Luengo J. M., Iriso J. L., López-Nieto M. J. Direct evaluation of phenylacetyl-CoA: 6-aminopenicillanic acid acyltransferase of Penicillium chrysogenum by bioassay. J Antibiot (Tokyo) 1986 Nov;39(11):1565–1573. doi: 10.7164/antibiotics.39.1565. [DOI] [PubMed] [Google Scholar]
- Montenegro E., Barredo J. L., Gutiérrez S., Díez B., Alvarez E., Martín J. F. Cloning, characterization of the acyl-CoA:6-amino penicillanic acid acyltransferase gene of Aspergillus nidulans and linkage to the isopenicillin N synthase gene. Mol Gen Genet. 1990 May;221(3):322–330. doi: 10.1007/BF00259395. [DOI] [PubMed] [Google Scholar]
- Rambosek J., Leach J. Recombinant DNA in filamentous fungi: progress and prospects. Crit Rev Biotechnol. 1987;6(4):357–393. doi: 10.3109/07388558709089387. [DOI] [PubMed] [Google Scholar]
- Schoner B. E., Belagaje R. M., Schoner R. G. Translation of a synthetic two-cistron mRNA in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8506–8510. doi: 10.1073/pnas.83.22.8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Burnham M. K., Bull J. H., Hodgson J. E., Ward J. M., Browne P., Brown J., Barton B., Earl A. J., Turner G. Beta-lactam antibiotic biosynthetic genes have been conserved in clusters in prokaryotes and eukaryotes. EMBO J. 1990 Mar;9(3):741–747. doi: 10.1002/j.1460-2075.1990.tb08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel B. J., Burgett S. G., Chen V. J., Skatrud P. L., Frolik C. A., Queener S. W., Ingolia T. D. Cloning and expression in Escherichia coli of isopenicillin N synthetase genes from Streptomyces lipmanii and Aspergillus nidulans. J Bacteriol. 1988 Sep;170(9):3817–3826. doi: 10.1128/jb.170.9.3817-3826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman P. A., Abraham E. P., Baldwin J. E., Fleming M. D., Schofield C. J., Sutherland J. D., Willis A. C. Acyl coenzyme A: 6-aminopenicillanic acid acyltransferase from Penicillium chrysogenum and Aspergillus nidulans. FEBS Lett. 1990 Mar 26;262(2):342–344. doi: 10.1016/0014-5793(90)80224-7. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]