Abstract
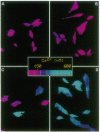
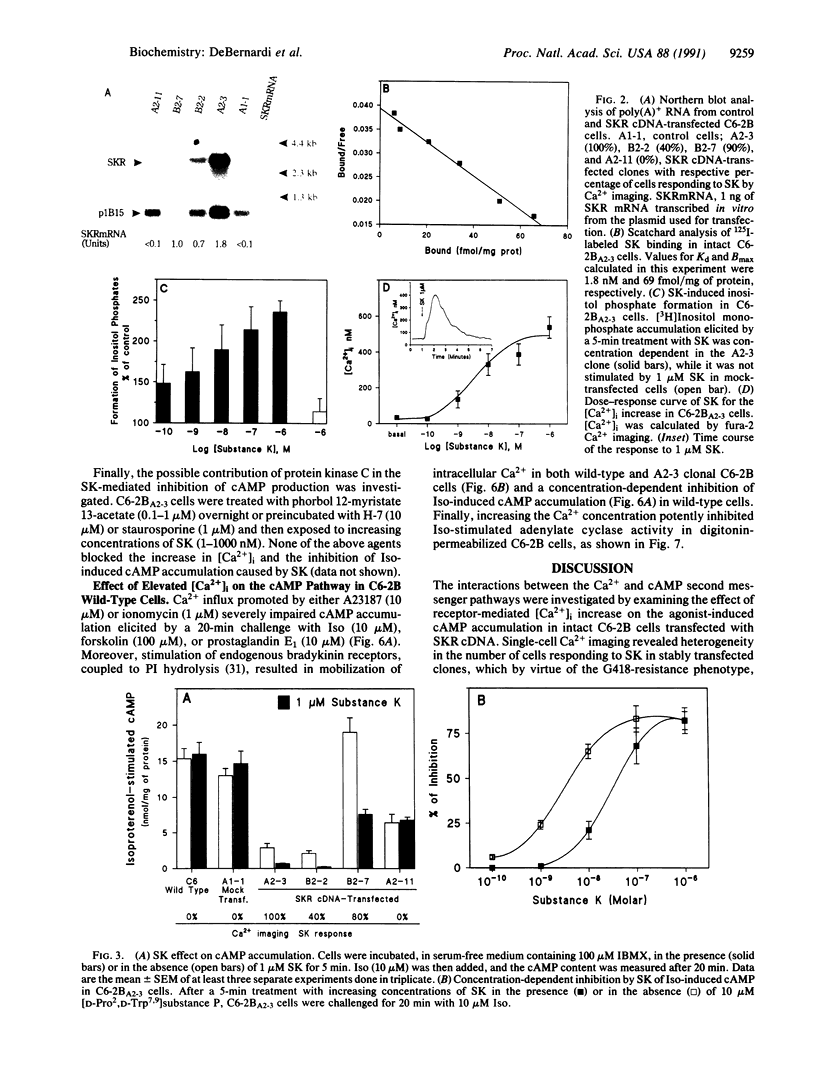
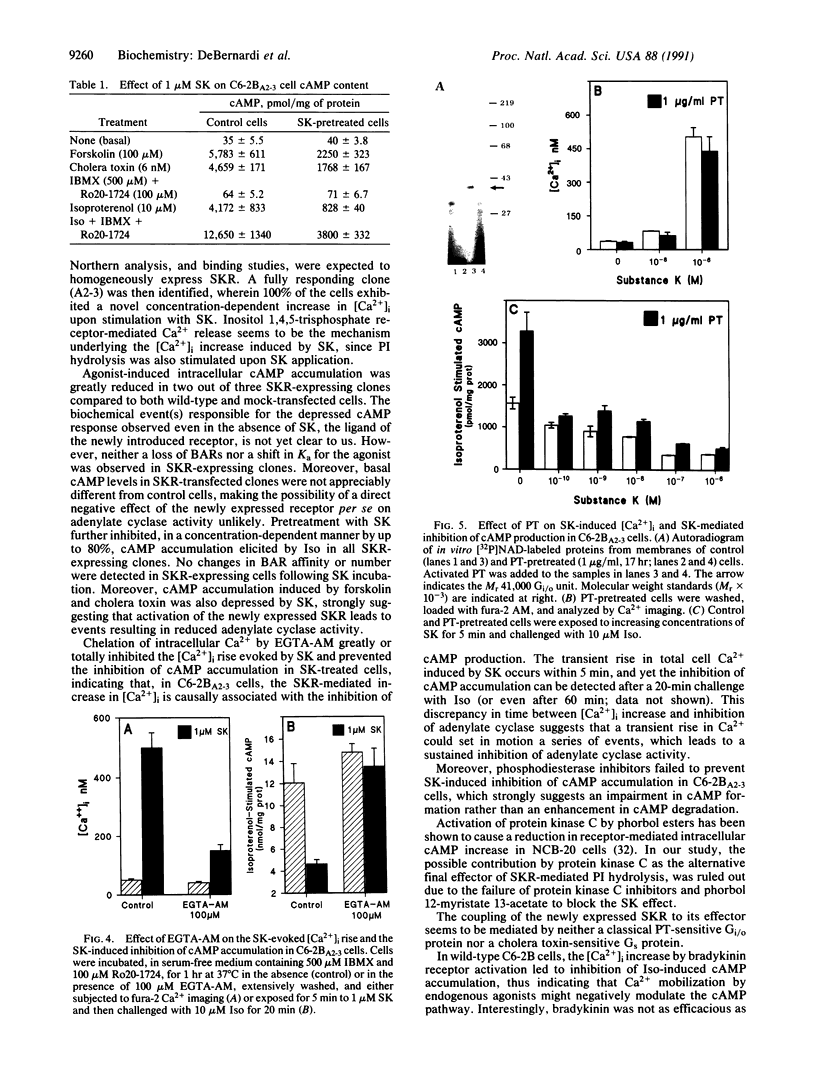
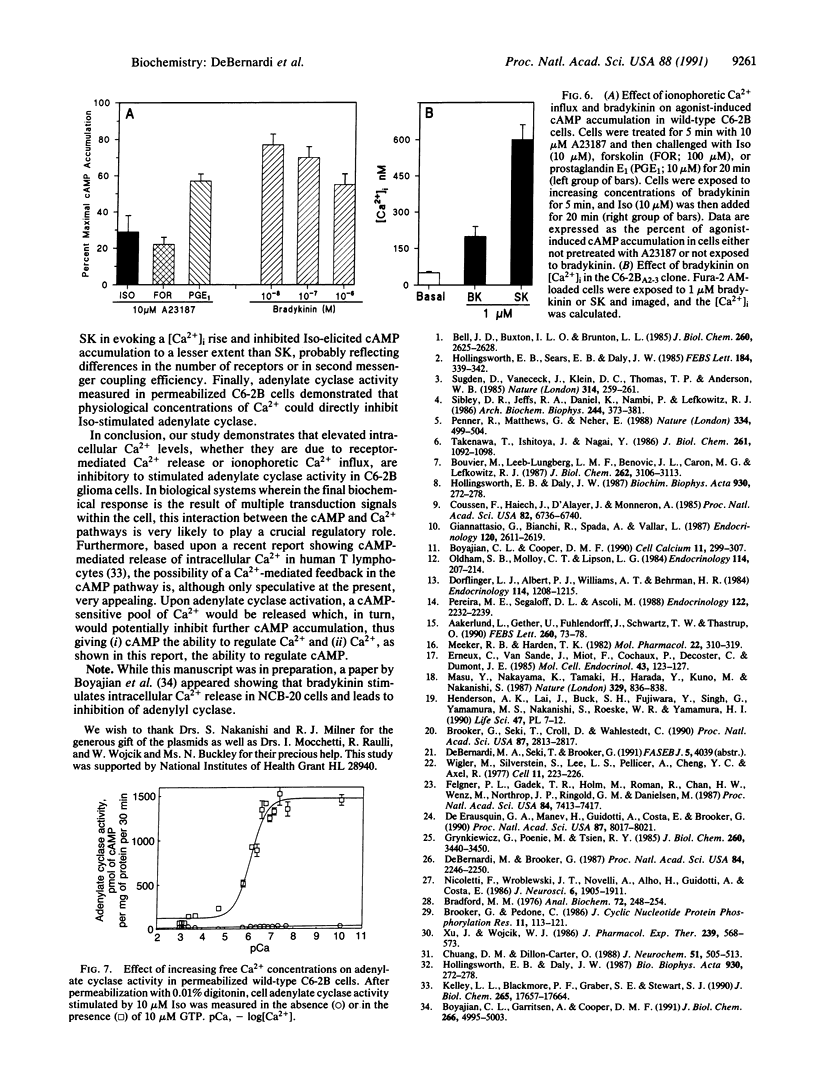
C6-2B rat glioma cells were stably transfected with substance K receptor cDNA and used to study interactions between cAMP and Ca2+ signaling pathways. Activation of the newly expressed receptors by substance K increased the intracellular free Ca2+ concentration, as monitored by single-cell fura-2 imaging, and markedly inhibited agonist-stimulated cAMP accumulation. Blockade of intracellular Ca2+ mobilization abolished the substance K receptor-mediated inhibition of isoproterenol-induced cAMP production. Phosphodiesterase inhibitors, down-regulation or inhibition of protein kinase C, and pertussis toxin failed to prevent substance K-induced inhibition of agonist-stimulated cAMP accumulation. An increased intracellular Ca2+ concentration caused by either calcium ionophores or activation of endogenous bradykinin receptors was found to markedly reduce cAMP production in wild-type cells. These results demonstrate that elevated intracellular Ca2+ concentration can negatively modulate agonist-stimulated adenylate cyclase activity in C6-2B glioma cells.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aakerlund L., Gether U., Fuhlendorff J., Schwartz T. W., Thastrup O. Y1 receptors for neuropeptide Y are coupled to mobilization of intracellular calcium and inhibition of adenylate cyclase. FEBS Lett. 1990 Jan 15;260(1):73–78. doi: 10.1016/0014-5793(90)80069-u. [DOI] [PubMed] [Google Scholar]
- Bell J. D., Buxton I. L., Brunton L. L. Enhancement of adenylate cyclase activity in S49 lymphoma cells by phorbol esters. Putative effect of C kinase on alpha s-GTP-catalytic subunit interaction. J Biol Chem. 1985 Mar 10;260(5):2625–2628. [PubMed] [Google Scholar]
- Bouvier M., Leeb-Lundberg L. M., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of adrenergic receptor function by phosphorylation. II. Effects of agonist occupancy on phosphorylation of alpha 1- and beta 2-adrenergic receptors by protein kinase C and the cyclic AMP-dependent protein kinase. J Biol Chem. 1987 Mar 5;262(7):3106–3113. [PubMed] [Google Scholar]
- Boyajian C. L., Cooper D. M. Potent and cooperative feedback inhibition of adenylate cyclase activity by calcium in pituitary-derived GH3 cells. Cell Calcium. 1990 Apr;11(4):299–307. doi: 10.1016/0143-4160(90)90007-h. [DOI] [PubMed] [Google Scholar]
- Boyajian C. L., Garritsen A., Cooper D. M. Bradykinin stimulates Ca2+ mobilization in NCB-20 cells leading to direct inhibition of adenylylcyclase. A novel mechanism for inhibition of cAMP production. J Biol Chem. 1991 Mar 15;266(8):4995–5003. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brooker G., Pedone C. Maintenance of whole cell isoproterenol and forskolin responsiveness in adenylate cyclase of permeabilized cells. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(2):113–121. [PubMed] [Google Scholar]
- Brooker G., Seki T., Croll D., Wahlestedt C. Calcium wave evoked by activation of endogenous or exogenously expressed receptors in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2813–2817. doi: 10.1073/pnas.87.7.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang D. M., Dillon-Carter O. Characterization of bradykinin-induced phosphoinositide turnover in neurohybrid NCB-20 cells. J Neurochem. 1988 Aug;51(2):505–513. doi: 10.1111/j.1471-4159.1988.tb01067.x. [DOI] [PubMed] [Google Scholar]
- Coussen F., Haiech J., d'Alayer J., Monneron A. Identification of the catalytic subunit of brain adenylate cyclase: a calmodulin binding protein of 135 kDa. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6736–6740. doi: 10.1073/pnas.82.20.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBernardi M., Brooker G. Diphtheria toxin prevents catecholamine desensitization of A431 human epidermoid carcinoma cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2246–2250. doi: 10.1073/pnas.84.8.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorflinger L. J., Albert P. J., Williams A. T., Behrman H. R. Calcium is an inhibitor of luteinizing hormone-sensitive adenylate cyclase in the luteal cell. Endocrinology. 1984 Apr;114(4):1208–1215. doi: 10.1210/endo-114-4-1208. [DOI] [PubMed] [Google Scholar]
- Erneux C., Van Sande J., Miot F., Cochaux P., Decoster C., Dumont J. E. A mechanism in the control of intracellular cAMP level: the activation of a calmodulin-sensitive phosphodiesterase by a rise of intracellular free calcium. Mol Cell Endocrinol. 1985 Dec;43(2-3):123–134. doi: 10.1016/0303-7207(85)90075-9. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannattasio G., Bianchi R., Spada A., Vallar L. Effect of calcium on adenylate cyclase of rat anterior pituitary gland. Endocrinology. 1987 Jun;120(6):2611–2619. doi: 10.1210/endo-120-6-2611. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hollingsworth E. B., Daly J. W. Inhibition of receptor-mediated stimulation of cyclic AMP accumulation in neuroblastoma-hybrid NCB-20 cells by a phorbol ester. Biochim Biophys Acta. 1987 Sep 14;930(2):272–278. doi: 10.1016/0167-4889(87)90040-1. [DOI] [PubMed] [Google Scholar]
- Hollingsworth E. B., Daly J. W. Inhibition of receptor-mediated stimulation of cyclic AMP accumulation in neuroblastoma-hybrid NCB-20 cells by a phorbol ester. Biochim Biophys Acta. 1987 Sep 14;930(2):272–278. doi: 10.1016/0167-4889(87)90040-1. [DOI] [PubMed] [Google Scholar]
- Hollingsworth E. B., Sears E. B., Daly J. W. An activator of protein kinase C (phorbol-12-myristate-13-acetate) augments 2-chloroadenosine-elicited accumulation of cyclic AMP in guinea pig cerebral cortical particulate preparations. FEBS Lett. 1985 May 20;184(2):339–342. doi: 10.1016/0014-5793(85)80634-7. [DOI] [PubMed] [Google Scholar]
- Kelley L. L., Blackmore P. F., Graber S. E., Stewart S. J. Agents that raise cAMP in human T lymphocytes release an intracellular pool of calcium in the absence of inositol phosphate production. J Biol Chem. 1990 Oct 15;265(29):17657–17664. [PubMed] [Google Scholar]
- Masu Y., Nakayama K., Tamaki H., Harada Y., Kuno M., Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. 1987 Oct 29-Nov 4Nature. 329(6142):836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- Meeker R. B., Harden T. K. Muscarinic cholinergic receptor-mediated activation of phosphodiesterase. Mol Pharmacol. 1982 Sep;22(2):310–319. [PubMed] [Google Scholar]
- Nicoletti F., Wroblewski J. T., Novelli A., Alho H., Guidotti A., Costa E. The activation of inositol phospholipid metabolism as a signal-transducing system for excitatory amino acids in primary cultures of cerebellar granule cells. J Neurosci. 1986 Jul;6(7):1905–1911. doi: 10.1523/JNEUROSCI.06-07-01905.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S. B., Molloy C. T., Lipson L. G. Calcium inhibition of parathyroid adenylate cyclase. Endocrinology. 1984 Jan;114(1):207–214. doi: 10.1210/endo-114-1-207. [DOI] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Segaloff D. L., Ascoli M. Ca+2 is an inhibitor of adenylate cyclase in MA-10 Leydig tumor cells. Endocrinology. 1988 May;122(5):2232–2239. doi: 10.1210/endo-122-5-2232. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Jeffs R. A., Daniel K., Nambi P., Lefkowitz R. J. Phorbol diester treatment promotes enhanced adenylate cyclase activity in frog erythrocytes. Arch Biochem Biophys. 1986 Jan;244(1):373–381. doi: 10.1016/0003-9861(86)90126-8. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Ishitoya J., Nagai Y. Inhibitory effect of prostaglandin E2, forskolin, and dibutyryl cAMP on arachidonic acid release and inositol phospholipid metabolism in guinea pig neutrophils. J Biol Chem. 1986 Jan 25;261(3):1092–1098. [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Xu J., Wojcik W. J. Gamma aminobutyric acid B receptor-mediated inhibition of adenylate cyclase in cultured cerebellar granule cells: blockade by islet-activating protein. J Pharmacol Exp Ther. 1986 Nov;239(2):568–573. [PubMed] [Google Scholar]
- de Erausquin G. A., Manev H., Guidotti A., Costa E., Brooker G. Gangliosides normalize distorted single-cell intracellular free Ca2+ dynamics after toxic doses of glutamate in cerebellar granule cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8017–8021. doi: 10.1073/pnas.87.20.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]