Abstract
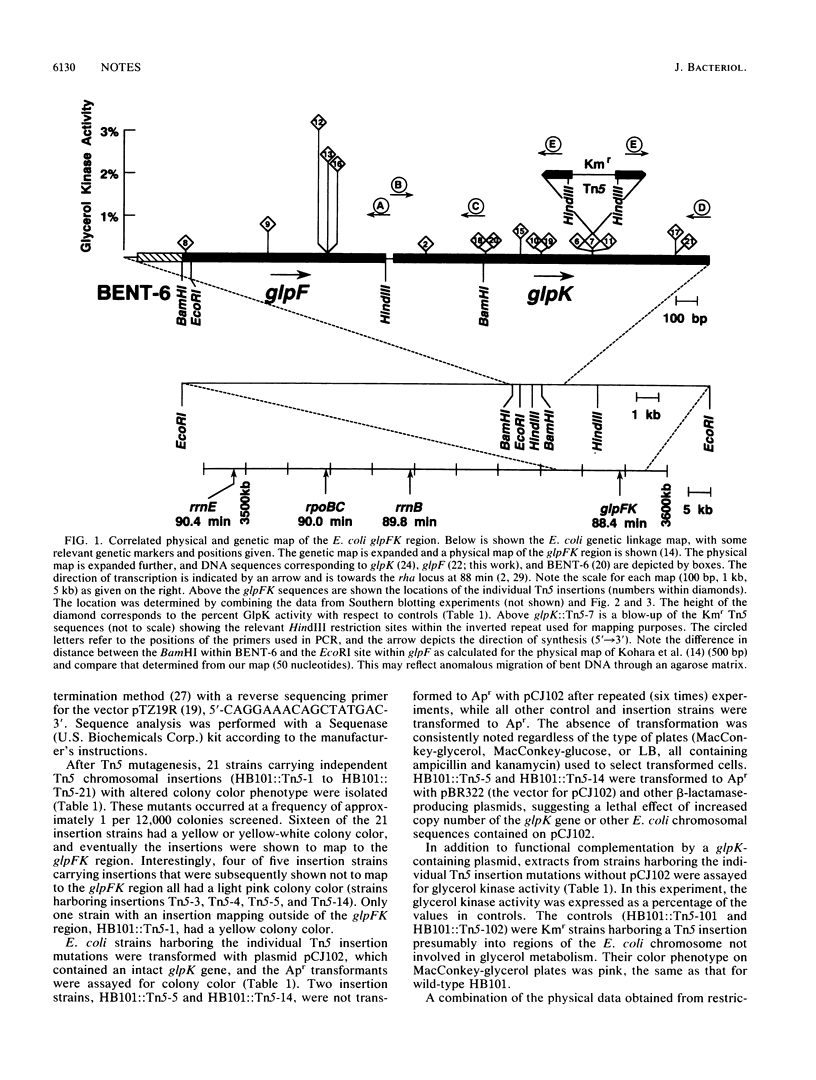
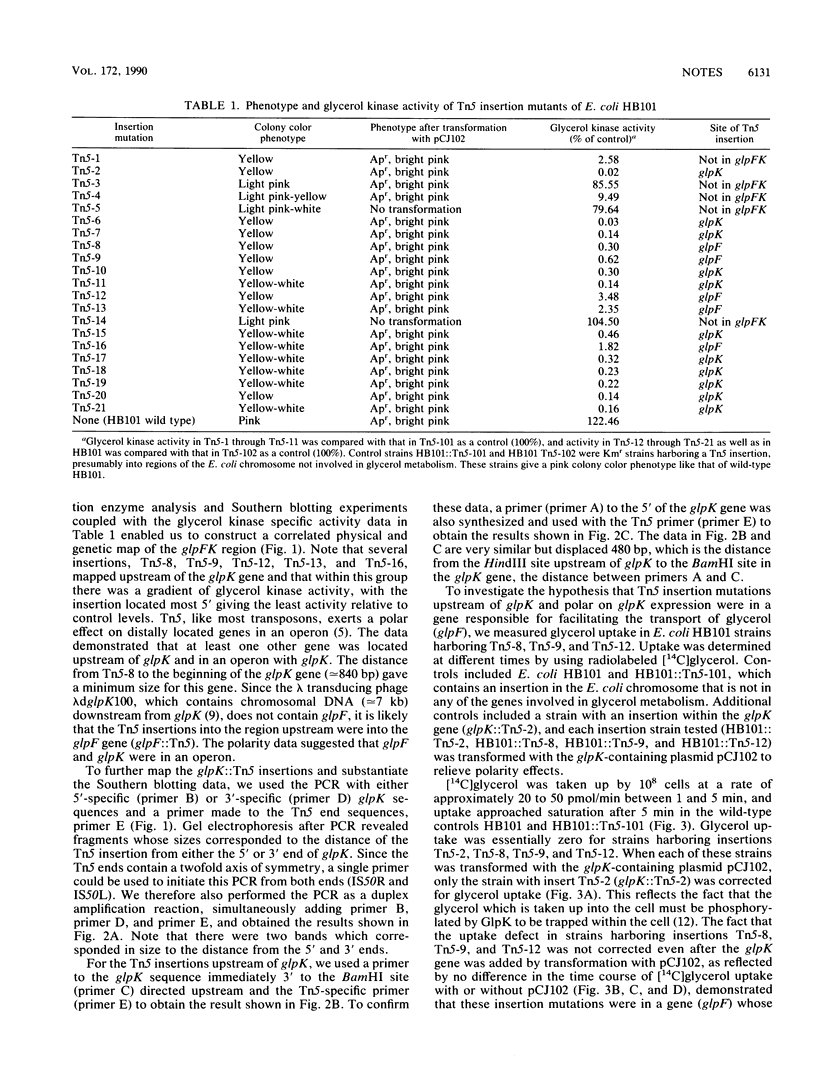
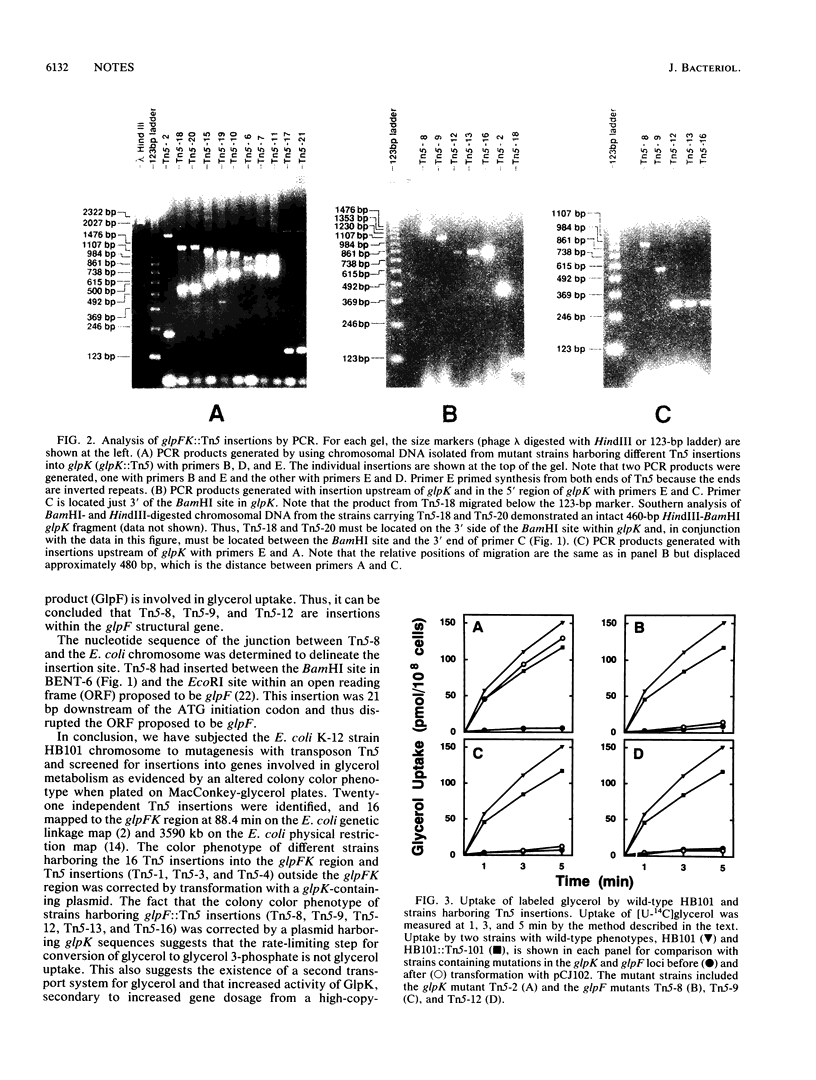
Transposon Tn5 mutagenesis of the Escherichia coli chromosome was used to isolate 21 independent insertion mutations conferring an altered colony color phenotype on MacConkey-glycerol plates. The polymerase chain reaction was used to map 16 of these Tn5 insertions within the glpFK region at 88 min. The most polar Tn5 insertion was shown by nucleotide sequencing to be in the proposed glpF open reading frame. The data suggest that the glpF and glpK genes are in an operon with a bent DNA segment (BENT-6) involved in transcriptional regulation of this operon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerswald E. A., Ludwig G., Schaller H. Structural analysis of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):107–113. doi: 10.1101/sqb.1981.045.01.019. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. E., Saier M. H., Jr A common ancestor for bovine lens fiber major intrinsic protein, soybean nodulin-26 protein, and E. coli glycerol facilitator. Cell. 1990 Jan 26;60(2):185–186. doi: 10.1016/0092-8674(90)90731-s. [DOI] [PubMed] [Google Scholar]
- Ballinger D. G., Benzer S. Targeted gene mutations in Drosophila. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9402–9406. doi: 10.1073/pnas.86.23.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bracco L., Kotlarz D., Kolb A., Diekmann S., Buc H. Synthetic curved DNA sequences can act as transcriptional activators in Escherichia coli. EMBO J. 1989 Dec 20;8(13):4289–4296. doi: 10.1002/j.1460-2075.1989.tb08615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis C. M., Molloy P. L., Both G. W., Drew H. R. Influence of the sequence-dependent flexure of DNA on transcription in E. coli. Nucleic Acids Res. 1989 Nov 25;17(22):9447–9468. doi: 10.1093/nar/17.22.9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C. A., Stearns G. W., 3rd, Prater W. E., Rheiner J. A., Johnson J. R. Characterization of a glpK transducing phage. Mol Gen Genet. 1984;193(2):376–378. doi: 10.1007/BF00330696. [DOI] [PubMed] [Google Scholar]
- Fortin M. G., Morrison N. A., Verma D. P. Nodulin-26, a peribacteroid membrane nodulin is expressed independently of the development of the peribacteroid compartment. Nucleic Acids Res. 1987 Jan 26;15(2):813–824. doi: 10.1093/nar/15.2.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin M. B., Yancey S. B., Cline J., Revel J. P., Horwitz J. The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning. Cell. 1984 Nov;39(1):49–59. doi: 10.1016/0092-8674(84)90190-9. [DOI] [PubMed] [Google Scholar]
- HAYASHI S., LIN E. C. CAPTURE OF GLYCEROL BY CELLS OF ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Mar 29;94:479–487. doi: 10.1016/0926-6585(65)90056-7. [DOI] [PubMed] [Google Scholar]
- Heller K. B., Lin E. C., Wilson T. H. Substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J Bacteriol. 1980 Oct;144(1):274–278. doi: 10.1128/jb.144.1.274-278.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Lupski J. R., Projan S. J., Ozaki L. S., Godson G. N. A temperature-dependent pBR322 copy number mutant resulting from a Tn5 position effect. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7381–7385. doi: 10.1073/pnas.83.19.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe E. R., Fennessey P. V., Guggenheim M. A., Miles B. S., Bullen W. W., Sceats D. J., Goodman S. I. Human glycerol kinase deficiency with hyperglycerolemia and glyceroluria. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1327–1333. doi: 10.1016/0006-291x(77)91437-1. [DOI] [PubMed] [Google Scholar]
- McCabe E. R., Towbin J., Chamberlain J., Baumbach L., Witkowski J., van Ommen G. J., Koenig M., Kunkel L. M., Seltzer W. K. Complementary DNA probes for the Duchenne muscular dystrophy locus demonstrate a previously undetectable deletion in a patient with dystrophic myopathy, glycerol kinase deficiency, and congenital adrenal hypoplasia. J Clin Invest. 1989 Jan;83(1):95–99. doi: 10.1172/JCI113890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Mizuno T. Random cloning of bent DNA segments from Escherichia coli chromosome and primary characterization of their structures. Nucleic Acids Res. 1987 Sep 11;15(17):6827–6841. doi: 10.1093/nar/15.17.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., McClure W. R. Analysis of the occurrence of promoter-sites in DNA. Nucleic Acids Res. 1986 Jan 10;14(1):109–126. doi: 10.1093/nar/14.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S., Mizuno T. Nucleotide sequence of the region encompassing the glpKF operon and its upstream region containing a bent DNA sequence of Escherichia coli. Nucleic Acids Res. 1989 Jun 12;17(11):4378–4378. [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Ledbetter S. A., Corbo L., Victoria M. F., Ramírez-Solis R., Webster T. D., Ledbetter D. H., Caskey C. T. Alu polymerase chain reaction: a method for rapid isolation of human-specific sequences from complex DNA sources. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6686–6690. doi: 10.1073/pnas.86.17.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew D. W., Ma D. P., Conrad C. A., Johnson J. R. Escherichia coli glycerol kinase. Cloning and sequencing of the glpK gene and the primary structure of the enzyme. J Biol Chem. 1988 Jan 5;263(1):135–139. [PubMed] [Google Scholar]
- Rao Y., Jan L. Y., Jan Y. N. Similarity of the product of the Drosophila neurogenic gene big brain to transmembrane channel proteins. Nature. 1990 May 10;345(6271):163–167. doi: 10.1038/345163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sweet G., Gandor C., Voegele R., Wittekindt N., Beuerle J., Truniger V., Lin E. C., Boos W. Glycerol facilitator of Escherichia coli: cloning of glpF and identification of the glpF product. J Bacteriol. 1990 Jan;172(1):424–430. doi: 10.1128/jb.172.1.424-430.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



