Abstract
The MR1 antigen-presenting system is conserved among mammals and enables T cells to recognize small molecules produced by bacterial pathogens, including Mycobacterium tuberculosis (M.tb). However, it is not known if MR1-mediated antigen presentation is important for protective immunity against mycobacterial disease. We hypothesized that genetic control of MR1 expression correlates with clinical outcomes of tuberculosis infection. We performed an MR1 candidate gene association study and identified an intronic SNP (rs1052632) that was significantly associated with susceptibility to tuberculosis in a discovery and validation cohort of Vietnamese adults with tuberculosis. Stratification by site of disease revealed that rs1052632 genotype GG was strongly associated with the development of meningeal tuberculosis (OR=2.99; 95%CI 1.64-5.43; p=0.00006). Among patients with meningeal disease, absence of the G allele was associated with an increased risk of death (HR=3.86; 95%CI 1.49-9.98; p=0.005). Variant annotation tools using public databases indicate that rs1052632 is strongly associated with MR1 gene expression in lymphoblastoid cells (p=0.004) and is located within a transcriptional enhancer in epithelial keratinocytes. These data support a role for MR1 in the pathogenesis of human tuberculosis by revealing that rs1052632 is associated with MR1 gene expression and susceptibility to tuberculosis in Vietnam.
Keywords: Human, Tuberculosis, Host Response, MR1, Genetic Association, Single Nucleotide Polymorphism
Introduction
Mycobacterium tuberculosis (M.tb) is a pathogen of global importance that infects more than one billion people and causes more than one million deaths annually1. Several lines of evidence in human studies and animal challenge models underscore the importance of T cells in controlling M.tb infection2-5. Most published studies have focused on secreted protein antigens that activate T cells when bound to polymorphic major histocompatibility complex (MHC) molecules6. However, human T cells have evolved to recognize both protein and non-protein antigens produced by mycobacteria, so the potential catalog of antigens mediating protective immunity extends beyond the proteome of M.tb7-9. Gamma-delta (γδ) T cells are activated by phosphorylated prenyl metabolites produced by M.tb through interaction with butorphylin molecules10, 11. CD1 molecules bind and present M.tb cell wall lipids to T cells7. Finally, mucosal-associated invariant T (MAIT) cells are activated in an MR1-dependent manner by M.tb-infected cells9, 12. The role of these non-peptide antigen-presenting systems in the pathogenesis of human tuberculosis is largely unknown.
MAIT cells are a subset of “innate-like” T cells that express a semi-invariant T cell receptor and can be activated by vitamin B metabolites derived from bacteria when bound to the MHC Class I-like related (MR1) molecule12-14. MAIT cells producing the cytokines IFN-γ and TNF-α have been shown to regulate protective immunity to pulmonary challenge with Francisella tularensis15, 16. MR1 knock out mice have a higher lung burden of M. bovis BCG compared to wild-type mice, and this effect appears to be mediated by T cells expressing the canonical semi-invariant MAIT T cell receptor17, 18. In healthy humans, MAIT cells are typically enriched in the lungs compared to peripheral blood and lymph nodes. However, in patients with pulmonary tuberculosis, MAIT cells are depleted in the peripheral circulation9, 19. One possible explanation for this finding is concomitant enrichment in the lungs, though this has not been directly demonstrated. Collectively, these data suggest that MR1-restricted MAIT cells may provide protective immunity against pulmonary bacterial infections.
MR1 is highly conserved among mammals; however, unlike MHC genes, MR1 shows low rates of genetic polymorphism that have not been associated with human disease20. We recently demonstrated that polymorphisms in CD1A, another non-classical antigen-presenting molecule, are associated with functional deficiency of lipid antigen presentation to T cells and susceptibility to tuberculosis21, 22. Thus, we hypothesized that SNPs in MR1 might also be associated with variation in function as well as clinical outcomes. We performed a candidate-gene association study in Vietnamese adults and identified an intronic SNP (rs1052632) that is reproducibly associated with susceptibility to tuberculosis. In silico analysis support a functional role for rs1052632 in MR1 gene expression. These data demonstrate a population genetics approach to complement ongoing efforts to understand the role of MR1-mediated antigen presentation in human tuberculosis.
Results
We used a case-population study design to evaluate whether SNPs in MR1 were associated with the development of active tuberculosis disease in Vietnamese adults. We genotyped eight haplotype-tagging SNPs spanning the MR1 locus in a discovery cohort of 351 cases and 392 population controls (Figure 1). All SNPs were in Hardy-Weinberg equilibrium among control subjects (Table 1). Polymorphisms rs12036052 and rs1052632 were significantly associated with the development of tuberculosis with a genotypic model (p=0.007 and p=0.024, respectively) (Table 1). The data were consistent with a recessive model of inheritance. The minor homozygous genotype (GG) of rs1052632 was associated with susceptibility to tuberculosis with an odds ratio (OR) of 1.881 (95%CI: 0.93-3.92; p=0.058). The minor homozygous genotype of rs12036052 was also associated with susceptibility to tuberculosis (p=0.004) though the absence of controls bearing this genotype precluded calculation of an odds ratio. These data reveal rs1052632 and rs12036052 as candidate susceptibility loci within MR1 and suggest a recessive model of inheritance in a Vietnamese population.
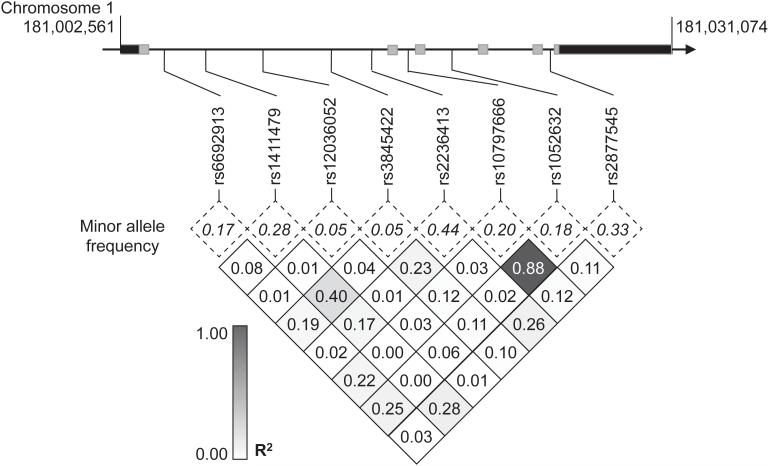
Figure 1. MR1 single nucleotide polymorphisms (SNPs) in Vietnam.
Linkage disequilibrium plot of SNPs in MR1 coding region among cord blood controls in Vietnam. MR1 spans 28.5 kB on chromosome 1 and consists of six exons (gray) and two untranslated regions (black). Minor allele frequencies (dotted box) and linkage disequilibrium as measured by R2 values (shaded box) of eight haplotype-tagging SNPs among 392 cord blood controls are indicated.
Table 1.
Association between SNPs in MR1 and tuberculosis (TB) in the discovery cohort. We genotyped eight haplotype-tagging SNPs in 351 cases and 392 population controls. SNP genotypes are shown as n(fraction) of total for common homozygous (AA), heterozygous (Aa), and minor homozygous (aa) genotypes. The p-value for deviation from Hardy-Weinberg equilibrium among cord blood controls was calculated using a χ2 goodness-of-fit test and is indicated under “HWE.” Genotypic analysis compares frequencies in a 2×3 contingency table. The z-score and unadjusted p-value shows results of genotypic trend test. For the recessive model analysis, AA+Aa is compared to aa as fractions of total. Odds ratios (OR) and unadjusted p-values of significance are highlighted.
| GenotypicAnalysis | Recessive Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Group | HWE | AA | Aa | aa | z | p-value | AA+Aa | aa | chi2 | p-value | OR | CI |
| rs6692913 G → A |
CTRL TB |
0.355 | 260 (0.69) 235 (0.68) |
110 (0.29) 94 (0.27) |
8 (0.02) 15 (0.04) |
0.68 | 0.499 | 370 (0.98) 329 (0.96) |
8 (0.02) 15 (0.04) |
2.94 | 0.086 | 2.109 | (0.83-5.81) |
|
| |||||||||||||
| rs1411479 C → T |
CTRL TB |
0.686 | 192 (0.51) 182 (0.57) |
149 (0.4) 109 (0.34) |
32 (0.09) 30 (0.09) |
−0.90 | 0.370 | 341 (0.91) 291 (0.91) |
32 (0.09) 30 (0.09) |
0.12 | 0.724 | 1.099 | (0.63-1.92) |
|
| |||||||||||||
| rs12036052 G → A |
CTRL TB |
0.462 | 362 (0.93) 299 (0.88) |
28 (0.07) 33 (0.1) |
0 (0) 7 (0.02) |
2.69 | 0.007 | 390 (1) 332 (0.98) |
0 (0) 7 (0.02) |
8.13 | 0.004 | NA | (2.13-NA) |
|
| |||||||||||||
| rs3845422 G → A |
CTRL TB |
0.758 | 96 (0.25) 103 (0.32) |
186 (0.49) 140 (0.43) |
96 (0.25) 80 (0.25) |
−1.29 | 0.199 | 282 (0.75) 243 (0.75) |
96 (0.25) 80 (0.25) |
0.04 | 0.848 | 0.967 | (0.68-1.38) |
|
| |||||||||||||
| rs2236413 C → T |
CTRL TB |
0.137 | 125 (0.33) 118 (0.36) |
170 (0.45) 124 (0.38) |
79 (0.21) 88 (0.27) |
0.56 | 0.574 | 295 (0.79) 242 (0.73) |
79 (0.21) 88 (0.27) |
2.98 | 0.084 | 1.358 | (0.94-1.95) |
|
| |||||||||||||
| rs1052632 A → G |
CTRL TB |
0.366 | 266 (0.68) 211 (0.62) |
109 (0.28) 108 (0.31) |
15 (0.04) 24 (0.07) |
2.26 | 0.024 | 375 (0.96) 319 (0.93) |
15 (0.04) 24 (0.07) |
3.60 | 0.058 | 1.881 | (0.93-3.92) |
|
| |||||||||||||
| rs10797666 C → A |
CTRL TB |
0.866 | 241 (0.65) 198 (0.61) |
117 (0.31) 111 (0.34) |
15 (0.04) 15 (0.05) |
0.94 | 0.346 | 358 (0.96) 309 (0.95) |
15 (0.04) 15 (0.05) |
0.16 | 0.693 | 1.159 | (0.52-2.59) |
|
| |||||||||||||
| rs2877545 C → T |
CTRL TB |
0.623 | 171 (0.45) 161 (0.47) |
162 (0.43) 136 (0.4) |
43 (0.11) 45 (0.13) |
0.02 | 0.981 | 333 (0.89) 297 (0.87) |
43 (0.11) 45 (0.13) |
0.49 | 0.482 | 1.173 | (0.73-1.88) |
To confirm these associations, we genotyped rs1052632 and rs12036052 in a validation cohort of 343 cases and 368 controls. We did not find an association between rs12036052 and susceptibility to tuberculosis in either a genotypic or recessive model analysis (Table 2). We confirmed the association of the minor homozygous genotype of rs1052632 in a recessive model analysis (OR=3.93, 95%CI: 1.89–8.8; p=0.00005) (Table 2). These data identify rs1052632 as an intronic SNP in MR1 that is reproducibly associated with susceptibility to tuberculosis in Vietnam.
Table 2.
Association between rs12036052 and rs1052632 in a validation cohort. We genotyped rs12036052 and rs1052632 in a validation cohort of 343 cases and 368 controls. Analysis was performed as described for the discovery cohort. Odds ratios (OR) and unadjusted p-values of significance are highlighted.
| GenotypicAnalysis | Recessive Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Group | HWE | AA | Aa | aa | z | p-value | AA+Aa | aa | chi2 | p-value | OR | CI |
| rs12036052 G → A |
CTRL TB |
0.75 | 318 (0.88) 255 (0.92) |
42 (0.12) 21 (0.08) |
1 (0) 1 (0) |
−1.53 | 0.125 | 360 (1) 276 (1) |
1 (0) 1 (0) |
0.04 | 0.85078 | 1.30 | (0.02-102.66) |
|
| |||||||||||||
| rs1052632 A → G |
CTRL TB |
0.73 | 243 (0.67) 203 (0.69) |
110 (0.3) 58 (0.2) |
11 (0.03) 32 (0.11) |
1.13 | 0.259 | 353 (0.97) 261 (0.89) |
11 (0.03) 32 (0.11) |
16.56 | 0.00005 | 3.93 | (1.89-8.8) |
Next, we evaluated whether rs1052632 was associated with clinical subtypes of tuberculosis, either pulmonary or meningeal disease. We combined cases from the discovery and validation cohorts and included an additional cohort of 450 pulmonary tuberculosis patients. We did not find an association between rs1052632 and pulmonary tuberculosis in either a genotypic or a recessive model (p=0.235 and p=0.107, respectively) (Table 3). On the other hand, rs1052632 showed a significant association with meningeal tuberculosis in both a genotypic (p=0.008) and recessive model (OR=2.99, 95%CI 1.64–5.43; p=0.00006) (Table 3). These data highlight the preferential association between an MR1 SNP and tuberculous meningitis in Vietnamese adults.
Table 3.
Association between rs1052632 and clinical subtypes of tuberculosis. We combined the discovery and validation cohorts and included an additional 450 subjects with pulmonary tuberculosis. We analyzed the association of rs1052632 with either pulmonary (PTB) or meningeal (TBM) tuberculosis using both genotypic and recessive models. Odds ratios (OR) and unadjusted p-values of significance are highlighted.
| GenotypicAnalysis | Recessive Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Group | HWE | AA | Aa | aa | z | p-value | AA+Aa | aa | chi2 | p-value | OR | CI |
| rs1052632 | CTRL | 0.68 | 509 (0.68) | 219 (0.29) | 26 (0.03) | 728 (0.97) | 26 (0.03) | ||||||
| PTB | 528 (0.66) | 234 (0.29) | 41 (0.05) | 1.19 | 0.235 | 762 (0.95) | 41 (0.05) | 2.59 | 0.1072 | 1.51 | (0.89-2.59) | ||
| TBM | 176 (0.63) | 77 (0.28) | 27 (0.1) | 2.66 | 0.008 | 253 (0.9) | 27 (0.1) | 16.11 | 0.0001 | 2.99 | (1.64-5.43) | ||
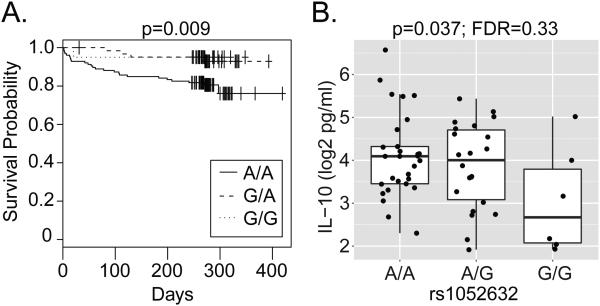
The strong association between rs1052632 and tuberculous meningitis led us to investigate whether this SNP might also be associated with mortality from this severe subtype of tuberculosis. We found that rs1052632 was associated with death in patients with tuberculous meningitis (p=0.009); however, this risk was limited to individuals carrying the common AA genotype (Figure 2A). In other words, individuals carrying the minor G allele that was associated with increased susceptibility to tuberculosis were relatively protected from death from tuberculous meningitis. This association was still significant even after adjusting for disease severity as measured by the British MRC grade (HR=3.23; 95%CI 1.25-8.40; p=0.016 by Cox proportional hazards regression). We hypothesized that specific cellular mechanisms active in the CSF might be responsible for this observation. We found no association between rs1052632 and inflammatory cell counts, glucose, lactate, or protein levels in the CSF (data not shown). We examined associations between rs1052632 and nine cytokines in the CSF (Supplementary Figure 1). We found that the common AA genotype was associated with higher IL-10 concentrations (p=0.037)(Figure 2B), suggesting an increased anti-inflammatory response in those individuals at increased risk of death from tuberculous meningitis. However, this association was not statistically significant after correcting for multiple hypothesis testing (FDR=0.33). Thus, the association between rs1052632 and death from tuberculous meningitis in a dominant model suggest cellular mechanisms that differ from those underlying susceptibility to meningitis.
Figure 2. rs1052632 is associated with mortality from tuberculous meningitis and CSF IL-10 concentration.
(A) Survival analysis was used to determine the association between rs1052632 and death among 255 patients with meningeal tuberculosis. The Kaplan-Meier curve demonstrates a statistically significant association with the A/A genotype (p=0.009 by log rank test). (B) CSF IL-10 concentrations were determined using Luminex and general linear regression was used to determine the association with rs1052632 in patients with TBM (p=0.037; FDR=0.33).
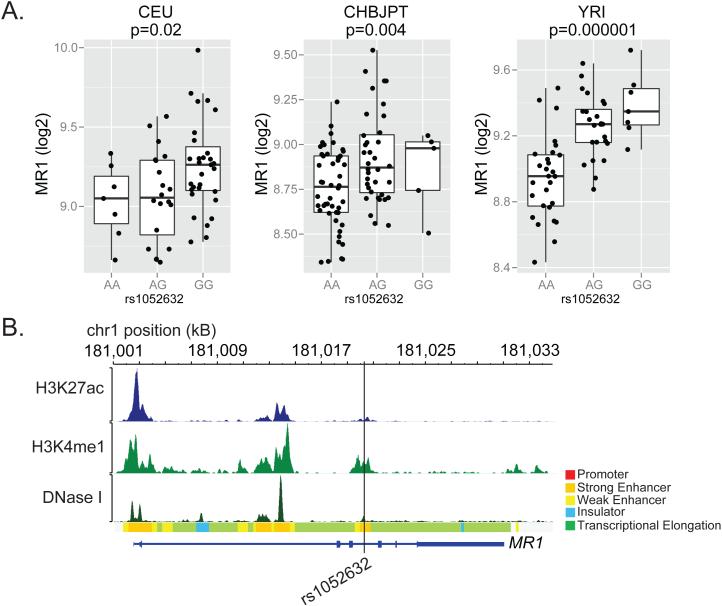
To further explore the functional basis for the association between rs1052632 and tuberculosis, we used the variant annotation tools Haploreg and RegulomeDB to query whether this intronic SNP was itself causal or in high linkage with another SNP of known functional importance23, 24. Within the CHBJPT subjects sequenced as part of the 1000 Genomes Project, we found six other SNPs in strong linkage with rs1052632 that were within 5kB, thus defining a haplotype block (Table 4). SNPs were ranked according to the likelihood they regulated MR1 gene expression based on publically available annotation data including the ENCODE and Roadmap Epigenomics Projects25-27. This analysis showed that rs1052632 itself was most likely the causal SNP. Indeed, rs1052632 is an expression quantitative trait locus (eQTL) for MR1 expression in lymphoblastoid cells in all three of the original HapMap populations (Figure 3A). Further, rs1052632 is located within a DNase hypersensitivity site as well as the activating histone marks H3K4me1 and H3K27ac suggesting its role as a transcriptional enhancer (Figure 3B). This further is supported by ChIP-Seq data showing binding with the transcription factor BRG128. Collectively, these data reveal the likely role of rs1052632 in directly regulating MR1 gene expression.
Table 4.
In-silico fine-mapping of rs1052632. Haploreg was used to identify common SNPs in high linkage disequilibrium (LD) with rs1052632. The rsID, position, distance, LD as measured by r2 and frequency in 1000 Genomes Phase I Asian subjects (ASN) is shown. These SNPs were analyzed in RegulomeDB and scored based on the strength of evidence that the SNP lies within a regulatory DNA element. The supporting data summarizing this evidence includes expression quantitative trait locus (eQTL), transcription factor binding by Chip-Seq (TF binding), predicted transcription factor binding by position weighted matrix (any motif), DNase hypersensitivity site (DNase peak), or Other.
| rsID | Position (Chr1) | distance | LD (r2) | ASN | Score | Supporting Data |
|---|---|---|---|---|---|---|
| rs10797666 | 181018799 | −1431 | 0.92 | 0.76 | 1f | eQTL + TF binding / DNase peak |
| rs1052632 | 181020230 | 0 | 1 | 0.77 | 1d | eQTL + TF binding + any motif + DNase peak |
| rs3845423 | 181021857 | 1627 | 1 | 0.77 | 6 | Other |
| rs6674136 | 181022390 | 2160 | 1 | 0.77 | 7 | |
| rs2275469 | 181022895 | 2665 | 1 | 0.77 | 7 | |
| rs2331994 | 181023708 | 3478 | 0.93 | 0.75 | 7 | |
| rs3747956 | 181024404 | 4174 | 0.93 | 0.76 | 1f | eQTL + TF binding / DNase peak |
Figure 3. rs1052632 is associated with MR1 expression and is located within a transcriptional enhancer.
(A) Genome-wide transcriptional analysis of lymphoblastoid cell lines derived from HapMap participants42 was merged with subject-specific MR1 genotypes downloaded from (http://hapmap.ncbi.nlm.nih.gov/downloads/genotypes/2007-01/fwd_strand/non-redundant/). Generalized linear regression was used to determine the association between rs105632 genotype and MR1 expression in Caucasians of European descent (CEU, n=60), Chinese in Beijing or Japanese in Tokyo (CHBJPT, n=90), or Yorubans in Nigeria (YRI, n=60). The boxplots show log2 transformed fluorescence intensity of MR1 expression as measured by microarray stratified by rs1052632 genotype. (B) Functional annotation for rs1052632 within normal human epidermal keratinocytes (NHEK) was downloaded and visualized using the RoadMap Epigenomics web browser (http://epigenomegateway.wustl.edu/browser/). DNase I represents DNase hypersensitive sites, H3K27ac represents acetylation of lysine 27 on histone H3, and H3K4me1 represents methylation of lysine 4 on histone H3. The Chromatin State is derived from a Hidden Markov Model using ChIP-Seq data for nine parameters measured in nine human cell types through the ENCODE Project 43.
Discussion
The discovery that MAIT cells respond to vitamin metabolites presented by MR1 molecules significantly expanded the catalog of bacterial T cell antigens, but the importance of this observation for human disease pathogenesis is still not clear. We found that rs1052632, an intronic SNP within MR1, was associated with susceptibility to tuberculosis in a discovery and validation cohort of Vietnamese adults. We also found that this SNP is associated with MR1 expression in lymphoblastoid cells and lies within a cis-regulatory region in epidermal keratinocytes, supporting its role in regulating MR1 expression and modulating the response of MAIT cells. These data demonstrate the utility of a population genetics approach to understanding the role of MR1-mediated antigen presentation in human tuberculosis.
Strengths of this study include a large sample size, the use of separate discovery and validation cohorts, the analysis of both pulmonary and meningeal tuberculosis, and the availability of clinical data including mortality outcomes. Possible limitations include the use of cord blood controls since some of these individuals are likely to develop tuberculosis as adults and will be misclassified as controls. However, misclassification of controls would indicate that the associations reported here underestimate the true genetic risk.
We demonstrated an association between the minor GG genotype and increased susceptibility to meningeal tuberculosis, but the risk of death in patients with meningeal tuberculosis was significant only in those carrying the common AA genotype. This suggests that different mechanisms may govern susceptibility compared to mortality. Higher MR1 expression levels associated with rs1052632 GG may lead to increased antigen-presentation and activation of MAIT cells, which have been shown to protect against pulmonary bacterial infections in mouse models15, 16. However, activation of MAIT cells may increase inflammation and paradoxically allow for the dissemination of infection to the CNS. In a zebrafish model of tuberculosis, extrapulmonary infections were mediated by macrophages that respond to inflammatory signals, migrate to the site of infection, and serve as Trojan horses to carry the bacteria to other sites29. By contrast, MR1-mediated inflammation associated with the G allele may protect against death from meningitis by promoting successful bacterial clearance, possibly through inhibition of IL-10 production as has been previously demonstrated30, 31.
Recent data examining the role of MAIT cells in multiple sclerosis (MS) provide additional context for our findings. MAIT cell-specific TCR-α rearrangements (Vα7.2-Jα33) have been detected in autopsy lesions from MS patients and from the CSF of patients experiencing relapses32. CD8+CD161++ T cells are detected in post-mortem biopsies in MS patients within perivascular cuffs and chronic active lesions, supporting a role in the pathogenesis of this inflammatory central nervous system disease33. In a murine model of multiple sclerosis, mice transgenic for the MAIT cell specific TCR-α chain (iVα19) were relatively protected from disease incidence and severity, and this was confirmed using MR1 knock out strains34. The mechanism of protection in this study was partly due to the increased production of IL-10 by B cells via ICOS-mediated help from MAIT cells. Similar mechanisms may underlie the association between rs1052632 and death from tuberculous meningitis reported here.
Our data support transcriptional regulation of MR1 as potentially important for clinical outcomes of tuberculosis infection. Yet, published studies to date have focused on trafficking as the major mechanism by which MR1 expression is regulated. MR1 transcript is detected in multiple tissues, but cell surface expression is very low in the absence of infection. Recent data demonstrate that MR1 is retained in the ER until ligand binding occurs, at which time functional MR1 is rapidly transported to the cell surface35. This has also been demonstrated during M.tb infection using airway epithelial cells36. Future studies demonstrating an association between rs1052632 and MR1 expression in cells originating from human lung could provide a cellular basis for the clinical associations reported here.
MAIT cells can become activated as a result of both MR1-dependent and MR1-independent stimuli9, 37. Our data suggest that SNPs in MR1 may affect expression at the site of disease, thus directly affecting antigen presentation. Studies of airway epithelial cells as non-lymphoid targets of M.tb infection and mediators of MR1 antigen presentation to MAIT cells could provide the experimental context to confirm in-silico findings in keratinocytes reported here38. Another possibility is that SNPs in MR1 may affect the response to inflammatory stimuli, as we have recently shown for CD1A22. SNPs directly regulating MR1 expression could influence MAIT cell development and maturation in the thymus. Thus, we might expect that SNPs are associated with the frequency of MAIT cells in the peripheral blood, and future studies are designed to answer this question. If confirmed through mechanistic studies, impaired antigen presentation would be a plausible biological mechanism for the association between SNPs in MR1 and susceptibility to tuberculosis.
Materials and Methods
Human Subjects
Details of clinical characteristics and enrollment criteria for Vietnam study subjects have been previously published39. Population controls were enrolled at Hung Vuong Hospital in Ho Chi Minh City, Vietnam, where umbilical cord blood was collected from newborns. Subjects with pulmonary tuberculosis were recruited from a network of district tuberculosis control units and defined by typical clinical symptoms in addition to sputum smear positive for acid-fast bacilli and/or culture positive for M. tuberculosis. Subjects with meningeal tuberculosis (TBM) were classified as definite or probable. All cases in the discovery cohort had definite TBM with clinical meningitis and either positive Ziehl-Neelsen stain for acid-fast bacilli or positive M. tuberculosis culture from CSF. The validation cohort included subjects with both definite (N=54) and probable (N=73) meningitis defined as clinical meningitis plus one or more of the following: chest radiograph consistent with active tuberculosis, acid-fast bacilli found in any specimen other than CSF, and clinical evidence of extrapulmonary tuberculosis at an additional site. All case and control participants were unrelated and greater than 95% were of the Vietnamese Kinh ethnicity.
Ethics
Written, informed consent was obtained from patients or their relatives if the patient could not provide consent. Parents provided consent for cord-blood controls. All protocols were approved by human subject review committees at Pham Ngoc Thach Hospital for Tuberculosis, the Hospital for Tropical Diseases, Health Services of Ho Chi Minh City, and Hung Vuong Hospital in Vietnam as well as the Oxford Tropical Research Ethics Committee and the University of Washington.
Selection of Single Nucleotide Polymorphisms (SNPs) for Genotyping
We used data from the International Haplotype Mapping Project (www.hapmap.org, version 3, release 2) to select SNPs within MR1 from the Han Chinese in Beijing, China (CHB) or Japanese in Tokyo, Japan (JPT). We previously demonstrated similar genome-wide haplotype frequencies between Vietnam and CHB/JPT populations, thus validating this approach40. Eleven Haplotype-tagging SNPs with a R2 cutoff of 0.80 for linkage disequilibrium were identified using Haploview v4.2 (www.broad.mit.edu/haploview). Three of these SNPs (rs12060051, rs17374514, rs6667601) were monomorphic in our study population and not analyzed further.
Genomic techniques
Genomic DNA was prepared from peripheral blood (Qiagen). Because of limiting quantities, genomic DNA from cases in Vietnam was amplified using Repli-G (Qiagen). Multiplex genotyping in the discovery cohort was performed using allele-specific primer extension on the MassARRAY (Sequenom) platform. Genotyping in the validation cohorts was performed using MassARRAY and the Illumina GoldenGate platforms. In all cohorts and platforms, automatic call rates exceeded 93% and less than 3% of calls were assigned manually. All SNPs were in Hardy-Weinberg equilibrium among control subjects, effectively ruling out errors in genotyping. We have previously demonstrated a lack of population stratification in this cohort39, 40.
Statistics
Statistical analyses were performed using Stata Statistical Software: Release 11 (StataCorp LP, College Station, TX). Function ‘pwld’ was used to calculate R2 measurements of linkage disequilibrium between polymorphisms. SNPs were assessed for association with tuberculosis using “genassoc”41
Supplementary Material
Acknowledgements
We would like to acknowledge the work of the clinical staff from the Hospital of Tropical Diseases and Pham Ngoc Thach Hospital who initially diagnosed and studied the patients with TBM and PTB. We would like to thank Dr. Nguyen Thi Hieu from Hung Vuong Obstetric Hospital Vietnam, Dr Tran Tinh Hien from the Hospital for Tropical Diseases Vietnam, and all the Vietnamese Doctors and patients who participated in this study. We thank Drs. Alan Aderem (Center for Infectious Disease Research), Marta Janer and Sarah Li (Institute for Systems Biology) for advice and technical assistance.
Funding
This study was funded in part by the NIH (K24AI089794 to TRH and K08AI089938 to CS), the Burroughs Wellcome Foundation (TRH), and the Firland Foundation (CS). The clinical component of this study was funded through the Wellcome Trust Major Overseas Program in Vietnam (089276/Z/09/Z).
Footnotes
Conflict of Interest Statement
All authors declare that they have no competing financial interests with this research.
Conference Presentations
The findings described here are unpublished but were presented as an abstract at the CD1-MR1 Meeting in Lorne, Australia : November 15-19, 2015.
References
- 1.WHO Global Tuberculosis Report 2013. 2013 [Google Scholar]
- 2.Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N Engl J Med. 2013;368(8):745–55. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 3.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193(3):271–80. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999;189(12):1973–80. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin PL, Rutledge T, Green AM, Bigbee M, Fuhrman C, Klein E, et al. CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS Res Hum Retroviruses. 2012;28(12):1693–702. doi: 10.1089/aid.2012.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jasenosky LD, Scriba TJ, Hanekom WA, Goldfeld AE. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol Rev. 2015;264(1):74–87. doi: 10.1111/imr.12274. [DOI] [PubMed] [Google Scholar]
- 7.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372(6507):691–4. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin RL, et al. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci U S A. 1994;91(17):8175–9. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8(6):e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandstrom A, Peigne CM, Leger A, Crooks JE, Konczak F, Gesnel MC, et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity. 2014;40(4):490–500. doi: 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vavassori S, Kumar A, Wan GS, Ramanjaneyulu GS, Cavallari M, El Daker S, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human gammadelta T cells. Nat Immunol. 2013;14(9):908–16. doi: 10.1038/ni.2665. [DOI] [PubMed] [Google Scholar]
- 12.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–23. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 13.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164–9. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 14.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8-alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178(1):1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meierovics A, Yankelevich WJ, Cowley SC. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc Natl Acad Sci U S A. 2013;110(33):E3119–28. doi: 10.1073/pnas.1302799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georgel P, Radosavljevic M, Macquin C, Bahram S. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Molecular immunology. 2011;48(5):769–75. doi: 10.1016/j.molimm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Chua WJ, Truscott SM, Eickhoff CS, Blazevic A, Hoft DF, Hansen TH. Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect Immun. 2012;80(9):3256–67. doi: 10.1128/IAI.00279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakala IG, Kjer-Nielsen L, Eickhoff CS, Wang X, Blazevic A, Liu L, et al. Functional Heterogeneity and Antimycobacterial Effects of Mouse Mucosal-Associated Invariant T Cells Specific for Riboflavin Metabolites. J Immunol. 2015;195(2):587–601. doi: 10.4049/jimmunol.1402545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11(8):701–8. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 20.Tsukamoto K, Deakin JE, Graves JA, Hashimoto K. Exceptionally high conservation of the MHC class I-related gene, MR1, among mammals. Immunogenetics. 2013;65(2):115–24. doi: 10.1007/s00251-012-0666-5. [DOI] [PubMed] [Google Scholar]
- 21.Seshadri C, Thuong NT, Yen NT, Bang ND, Chau TT, Thwaites GE, et al. A polymorphism in human CD1A is associated with susceptibility to tuberculosis. Genes Immun. 2014 doi: 10.1038/gene.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seshadri C, Shenoy M, Wells RD, Hensley-McBain T, Andersen-Nissen E, McElrath MJ, et al. Human CD1a Deficiency Is Common and Genetically Regulated. J Immunol. 2013;191(4):1586–93. doi: 10.4049/jimmunol.1300575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic acids research. 2012;40:D930–4. doi: 10.1093/nar/gkr917. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22(9):1790–7. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Consortium EP The ENCODE (ENCyclopedia Of DNA Elements) Project. Science. 2004;306(5696):636–40. doi: 10.1126/science.1105136. [DOI] [PubMed] [Google Scholar]
- 26.Roadmap Epigenomics C. Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518(7539):317–30. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stranger BE, Nica AC, Forrest MS, Dimas A, Bird CP, Beazley C, et al. Population genomics of human gene expression. Nat Genet. 2007;39(10):1217–24. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu G, Schones DE, Cui K, Ybarra R, Northrup D, Tang Q, et al. Regulation of nucleosome landscape and transcription factor targeting at tissue-specific enhancers by BRG1. Genome Res. 2011;21(10):1650–8. doi: 10.1101/gr.121145.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cambier CJ, Takaki KK, Larson RP, Hernandez RE, Tobin DM, Urdahl KB, et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505(7482):218–22. doi: 10.1038/nature12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Barrera S, Aleman M, Musella R, Schierloh P, Pasquinelli V, Garcia V, et al. IL-10 down-regulates costimulatory molecules on Mycobacterium tuberculosis-pulsed macrophages and impairs the lytic activity of CD4 and CD8 CTL in tuberculosis patients. Clin Exp Immunol. 2004;138(1):128–38. doi: 10.1111/j.1365-2249.2004.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitt JM, Stavropoulos E, Redford PS, Beebe AM, Bancroft GJ, Young DB, et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol. 2012;189(8):4079–87. doi: 10.4049/jimmunol.1201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illes Z, Shimamura M, Newcombe J, Oka N, Yamamura T. Accumulation of Valpha7.2-Jalpha33 invariant T cells in human autoimmune inflammatory lesions in the nervous system. International immunology. 2004;16(2):223–30. doi: 10.1093/intimm/dxh018. [DOI] [PubMed] [Google Scholar]
- 33.Willing A, Leach OA, Ufer F, Attfield KE, Steinbach K, Kursawe N, et al. CD8(+) MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis. Eur J Immunol. 2014;44(10):3119–28. doi: 10.1002/eji.201344160. [DOI] [PubMed] [Google Scholar]
- 34.Croxford JL, Miyake S, Huang YY, Shimamura M, Yamamura T. Invariant V(alpha)19i T cells regulate autoimmune inflammation. Nat Immunol. 2006;7(9):987–94. doi: 10.1038/ni1370. [DOI] [PubMed] [Google Scholar]
- 35.McWilliam HE, Eckle SB, Theodossis A, Liu L, Chen Z, Wubben JM, et al. The intracellular pathway for the presentation of vitamin B-related antigens by the antigen-presenting molecule MR1. Nat Immunol. 2016;17(5):531–7. doi: 10.1038/ni.3416. [DOI] [PubMed] [Google Scholar]
- 36.Harriff MJ, Karamooz E, Burr A, Grant WF, Canfield ET, Sorensen ML, et al. Endosomal MR1 Trafficking Plays a Key Role in Presentation of Mycobacterium tuberculosis Ligands to MAIT Cells. PLoS Pathog. 2016;12(3):e1005524. doi: 10.1371/journal.ppat.1005524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44(1):195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harriff MJ, Cansler ME, Toren KG, Canfield ET, Kwak S, Gold MC, et al. Human lung epithelial cells contain Mycobacterium tuberculosis in a late endosomal vacuole and are efficiently recognized by CD8(+) T cells. PLoS One. 2014;9(5):e97515. doi: 10.1371/journal.pone.0097515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horne DJ, Randhawa AK, Chau TT, Bang ND, Yen NT, Farrar JJ, et al. Common Polymorphisms in the PKP3-SIGIRR-TMEM16J Gene Region Are Associated With Susceptibility to Tuberculosis. J Infect Dis. 2012 doi: 10.1093/infdis/jir785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khor CC, Chau TN, Pang J, Davila S, Long HT, Ong RT, et al. Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat Genet. 2011;43(11):1139–41. doi: 10.1038/ng.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shephard N. GENASS: Stata module to perform Genetic Case-control Association tests. Boston College Department of Economics; 2005. [Google Scholar]
- 42.Stranger BE, Montgomery SB, Dimas AS, Parts L, Stegle O, Ingle CE, et al. Patterns of cis regulatory variation in diverse human populations. PLoS Genet. 2012;8(4):e1002639. doi: 10.1371/journal.pgen.1002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473(7345):43–9. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.