Abstract
Atherosclerosis is initiated by the subendothelial accumulation of apolipoprotein B-containing lipoproteins (apoB LPs), which initiates a sterile inflammatory response dominated by monocyte-macrophages but including all classes of innate and adaptive immune cells. These inflammatory cells, together with proliferating smooth muscle cells and extracellular matrix, promote the formation of subendothelial lesions, or plaques. In the vast majority of cases, these lesions do not cause serious clinical symptoms, which is due in part to a resolution-repair response that limits tissue damage. However, a deadly minority of lesions progress to the point where they can trigger acute lumenal thrombosis, which may then cause unstable angina, myocardial infarction, sudden cardiac death, or stroke. Many of these clinically dangerous lesions have hallmarks of defective inflammation resolution, including defective clearance of dead cells (efferocytosis), necrosis, a defective scar response, and decreased levels of lipid mediators of the resolution response. Efferocytosis is both an effector arm of the resolution response and an inducer of resolution mediators, and thus its defect in advanced atherosclerosis amplifies plaque progression. Pre-clinical causation/treatment studies have demonstrated that "replacement therapy" with exogenously administered resolving mediators can improve lesional efferocytosis and prevent plaque progression. Work in this area has the potential to potentiate the cardiovascular benefits of apoB LP-lowering therapy.
Keywords: atherosclerosis, macrophages, inflammation resolution, efferocytosis, necrosis
This article is based on the 2016 Russell Ross Memorial Lecture in Vascular Biology presented at the American Heart Association's Scientific Sessions Annual Conference, November 12 to 16, New Orleans, LA. While atherogenesis is triggered by the sub-endothelial retention of apolipoprotein B-containing lipoproteins in focal areas of the arterial tree,1,2 the question of how this inciting event leads to the series of complex cell biological processes termed atherosclerosis is perhaps the most fundamental question in this field of research and is the topic of the lecture and this article. It is indeed an honor to be have been awarded this lectureship in memory of Dr. Ross, because it was his paradigm-shifting work in exactly this area that stimulated many of us to focus our research on this question. The evolution of Dr. Ross's concepts on this topic are highly instructive, moving from an initial theory that imagined a role for overt endothelial injury and smooth muscle cell proliferation to one that considered the role of more subtle changes in endothelial function, including inflammatory changes.3,4 Through the work of many researchers from this time forward, including leaders such as Peter Libby, Goran Hansson, and many past Ross Lectureship awardees, the concept that atherosclerosis is driven by inflammation—in essence, the most important response to lipoprotein retention—has been supported by thousands of papers using causation models in mice and observational studies in humans.5,6 Most importantly, work in this area has led to the first anti-inflammation cardiovascular causation trials in humans, which are being led by the courageous efforts of Paul Ridker and his colleagues.7,8
The Initiation of Atherosclerosis and Its Therapeutic Implications
The intense interest in and enthusiasm for the concept that inflammation drives atherosclerosis should not lead us to "forget" how atherosclerosis begins. Inflammation does not arise spontaneously but rather is a response to either invading pathogens or to non-pathogen "sterile" stimuli, including those that arise from tissue injury. While we refer to the pathogen-derived inflammatory stimuli as pathogen-associated molecular patterns (PAMPs), tissue injury molecules have been termed damage-associated molecular patterns (DAMPs).9 The inflammatory response to tissue damage probably evolved as a way to prevent secondary infection and to initiate tissue repair, which, as we shall see in the following sections, is integrated with inflammation as part of the so-called resolution response.
With this background, what is the trigger for inflammation early in atherogenesis? While a number of theories have proposed links to bacterial or viral infections, the evidence is scant. On the other hand, we know that a very early event in atherogenesis is the subendothelial retention of plasma-derived apolipoprotein B (apoB)-containing lipoproteins (LPs), notably low-density lipoprotein (LDL) and chylomicron remnants.1,2 These LPs accumulate at sites of disturbed flow in medium-sized arteries—sites that are uniquely destined to become filled with atherosclerotic plaque. LP retention occurs before the appearance of inflammatory cells,10 and blocking retention through genetic engineering or other means blocks early atherogenesis in mice.11,12 Most importantly, drugs that lower apoB LPs have shown unequivocal cardiovascular and mortality benefit in many millions of subjects and over decades of clinical study,13 and there is now convincing genetic evidence linking mutations that affect apoB LP levels to cardiovascular risk.14
From a mechanistic standpoint, molecules embedded in apoB LPs, including those that become modified after subendothelial retention, can act as DAMP-like molecules when added to immune cells in vitro. Examples include fragmented and oxidized apoB, cholesterol, oxidized sterols and phospholipids, and saturated fatty acids and ceramides.15–22 There is also evidence that an adaptive immune response is mounted against native and/or modified apoB LPs and that this response may also contribute to atherosclerosis.23 Accordingly, the response to apoB LP retention is dominated by the entry of monocyte-derived macrophages but also involves dendritic cells, neutrophils, other innate immune cells, and adaptive immune cells, notably T effector cells.5,6 Thus, the success of LDL-lowering therapy is completely predictable within the framework of the sterile inflammatory response and the response-to-retention concept, just as removing a sterile splinter is predicted to curtail the redness and pain at its insertion site and prevent further tissue damage.
Two additional points deserve attention. First, the key role of apoB LP retention in atherogenesis is not meant to diminish the importance of pre-lesional endothelial changes ("activation"), which are mediated by flow disturbances and other factors at arterial sites destined to become atherosclerotic.24,25 Indeed, endothelial activation may contribute to lipoprotein retention itself by promoting permeability and possibly transcytosis,26 and flow-mediated activation likely primes endothelial cells to respond to the subsequent inflammatory stimulus of retained LPs27. However, the fact remains that atherosclerosis will not form at sites of endothelial activation if the level of apoB LPs falls below a certain threshold level, whereas atherosclerosis will form at non-flow-disturbed sites if apoB LPs rise to very high levels.1,2
Second, how can the overall lowering of plasma LDL over the last three decades, i.e., after the introduction of statins, be reconciled with the fact that atherosclerotic vascular disease remains the leading cause of death.28 Despite the enormous life-saving success of statins, issues related to potency, real or perceived drug safety and side effects, patient compliance, and patient and provider education have limited our ability to lower LDL to the types of level, and at an early enough age, that would be needed to remove atherosclerotic disease from the leading killer list.29 The availability of PCSK9 inhibitors may help us get closer to this goal30. However, these efforts are being counterbalanced by the worldwide epidemic of obesity and insulin resistance, which are conditions that lower the atherogenic threshold to apoB LPs. 31 Thus more intense LP lowering is needed to achieve the same result in the face of this epidemic, which is predicted to continue well into the 21st century.32
The Progression of Atherosclerosis
There have been many excellent reviews on the series of atherosclerotic events that occur after LP retention and the initial entry of monocytes, and I refer to some of these with only brief outline here.33–40 The subendothelial areas that initially accumulate LPs, referred to as intima, expand as a result of (a) increasing numbers of both innate and adaptive inflammatory cells through continual entry and proliferation; (b) proliferation of myofibroblast cells that originate from vascular smooth muscle cells (VSMCs); and (c) extracellular lipid and matrix molecules. Each of these processes has been studied widely and is driven by complex processes involving hundreds of molecules and various combinations of cell-cell interactions. I would like to emphasize here two key points about the progressing lesion. First, LP retention is amplified as lesions progress,41 probably as a result of the synthesis of apoB-LP-binding proteoglycans by inflammatory cells. Therefore, the inflammatory response to these LPs is persistent and amplified. 1,2 Persistence of a sterile inflammatory stimulus creates a scenario of chronic, non-resolving inflammation, analogous to what occurs when a splinter is not removed from a finger—or, more accurately, if additional splinters were continually added to the inflamed digit. Second, despite the expansion of the intima, the lumen remains patent because of outward remodeling and compensatory enlargement of the arterial wall.42 Thus, despite the growing lesion, atherosclerosis at this stage is largely asymptomatic. Indeed, in any given individual with multiple atherosclerotic lesions, the vast majority of lesions—perhaps as many as 95%--will not cause acute thrombo-occlusive vascular disease.43
With this scenario in mind, one of the most important goals in atherosclerosis research is to understand the unique molecular and cellular events that lead to the formation of the very small minority of plaques that account for virtually all acute atherothrombotic vascular events, including unstable angina, myocardial infarction, sudden cardiac death, and stroke. Recent pathologic analyses suggest that these clinically dangerous plaques fall into two categories:44 (1) those that have numerous inflammatory cells, lipid-rich necrotic cores, and thin collagenous fibrous "caps" that overlay the core; and (2) those that are characterized by an abundance of extracellular matrix and endothelial apoptosis. Necrotic plaques, which are often called "vulnerable" plaques, can precipitate all categories of acute atherothrombotic events but are particularly associated with ST-elevation MI (STEMI). These plaques have been widely studied, and much is known about the mechanisms that lead to their formation and their thrombotic consequences, as described below. Less in known about the matrix-rich category of dangerous plaques, which is often associated with non ST-elevation MI (NSTEMI).44 Based on the results of in vitro experiments and in vivo observations, the mechanism of plaque erosion may involve pro-thrombotic activation and death of endothelial cells via activation of toll-like receptor 2 (TLR2).45
In contrast, necrotic plaques are susceptible to frank rupture, which triggers acute lumenal thrombosis via exposure of blood platelets to pro-coagulant/thrombotic factors, notably tissue factor, in the necrotic core.43 Moreover, necrotic cores are filled with DAMP-like molecules,46,47 which amplifies the inflammatory response. The mechanism of necrotic core formation involves the death of lesional cells, mostly macrophages but also smooth muscle cells,46 coupled with poor phagocytic clearance of these dead cells by a process called efferocytosis.48 In the setting of defective efferocytosis, the initially intact membranes of apoptotic cells begin to break down, leading to a type of cellular necrosis known as post-apoptotic, or "secondary," necrosis. There is also evidence that primary necrosis, or "necroptosis," of lesional macrophages triggered by receptor-interacting serine/threonine-protein kinase 3 (RIP3 kinase) contributes to plaque necrosis in advanced atherosclerosis.49–51 The consequences of necroptosis may also be exacerbated by poor phagocytic clearance of cells that die by this process.52 Plaque rupture occurs as a result of fibrous cap thinning, which has been ascribed to death of collagen-producing intimal smooth muscle cells and to the production of various types of matrix-destroying proteases by lesional inflammatory cells.53 In addition, physical properties of the lipid-rich necrotic core creates a physical strain on the overlying fibrous cap that can contribute to plaque rupture.54
Defective Resolution in Plaque Progression
We and others have sought to construct a unified conceptual framework that could help explain the series of molecular and cellular events leading to the formation of clinically dangerous plaques. The concept is based on the seminal studies of Carl Nathan, Charles Serhan, and others that have educated us about an active resolution and repair process that occurs during and immediately subsequent to the inflammatory response.55,56 In order for the inflammatory response to be effective in defense against pathogens, numerous pro-oxidant- and protease-secreting inflammatory cells must invade the site of infection, which inevitably causes collateral tissue damage. Thus, we have evolved mechanisms to repair this damage and return to tissue homeostasis through the action of numerous types of resolution mediators. These mediators are delivered at both the onset of the inflammatory response, e.g., as neutrophil-derived secretory factors in edema fluid, and after pathogen neutralization, e.g., by recruitment of reparative cells, including macrophages that have a resolution phenotype. Resolution is mediated by (a) endogenous lipids that are generated during inflammation, including lipoxins, resolvins, protectins, and maresins, called specialized pro-resolving mediators (SPMs); (b) proteins, such as IL-10, TGFβ, and annexin A1; (c) bioactive gases such as nitric oxide, hydrogen sulfide, and carbon monoxide; and (d) resolving cells, such regulatory T cells (Tregs) and resolving-type macrophages.57–59 By activating specific cell-surface receptors, resolution mediators block inflammatory cell influx and promote their egress; clear pathogens, cellular debris, inflammatory cytokines, and apoptotic cells (efferocytosis); and repair tissue damage.57,58
In chronic inflammatory diseases, resolution is defective, leading to an amplification cycle of continual tissue injury and DAMP-mediated inflammation.60 Beginning with seminal work by Lawrence Chan, Charles Serhan, and colleagues, this scenario is emerging as an important pathogenic process in the progression of atherosclerosis.61–63. We believe that defective inflammation resolution can best explain the series of events leading to the formation of clinically dangerous plaques.63 In terms of the necrotic subset of these deadly plaques, their features of defective efferocytosis, plaque necrosis, DAMP-mediated inflammation, thin fibrous cap (defective scar formation), and oxidative stress are hallmarks of defective resolution. This concept is supported by a recent study from our group in which lipid resolution mediators were measured by targeted mass spectrometry in stable and advanced regions of human carotid atherosclerotic plaques. Resolvin D1 (RvD1) and the ratio of total SPMs to pro-inflammatory leukotriene B4 were decreased in the advanced plaque regions.64 Similar results were found when we compared advanced vs. early atherosclerotic plaques in mouse aorta,64 and similar data in mice were also reported by an independent group.65 In terms of causation, restoration of resolving mediators by exogenous administration—including a study using plaque-targeted nanoparticles—has been shown to suppress the progression of mid-stage lesions to advanced plaques.64–66
Why does resolution go awry during plaque progression? As alluded to above, the overarching answer is straightforward: unlike a pathogen that gets neutralized or a splinter that gets removed, the inciting inflammatory stimulus in atherosclerosis—subendothelial apoB LPs—not only remains persistent but becomes amplified. However, the molecular-cellular mechanisms linking a persistent stimulus to a defective resolution response are poorly understood, and this is particularly the case with advanced atherosclerosis. Three classes of mechanisms can be considered: defective lipid mediator synthesis, excessive mediator inactivation, and impaired response of resolution effector cells to resolution mediators. As an example, atherosclerotic lesions have increased oxidative stress, and treatment of macrophages in vitro with a pro-oxidant oxysterol found in human lesions, 7-ketocholesterol, decreases the production of SPMs.64 Moreover, when SPMs are low, decreased SPM synthesis by macrophages can be amplified through a mechanism that involves cytosol-to-nuclear translocation of the enzyme 5-lipoygenase.67 As another example, we have shown that the SPM receptor ALX/FPR2 on lesional cells decreases as atherosclerosis progresses.66
The Interplay Between Defective Efferocytosis and Defective Resolution in Advanced Atherosclerosis
Defective efferocytosis is one of the hallmarks of both defective inflammation resolution and advanced atherosclerosis, and I would argue that it may be the linchpin in the progression to plaque vulnerability. 63,68–72 Efferocytosis is mediated through phagocyte receptors, apoptotic cell ligands, bridging proteins, and chemoattractants.73 It is normally a high-capacity and efficient process, but when it goes awry, tissue necrosis and subsequent DAMP-mediated inflammation occur.74–76 Macrophages in clinically dangerous human coronary plaques show evidence of defective efferocytosis, i.e., there are abundant uncleared dead cells, and this defect correlates with 2 key features of these plaques—necrosis and inflammation.63,68,69,72 Causation is suggested by studies using genetically altered mice. For example, when efferocytosis is compromised through gene targeting of effector molecules, there is an increase in uncleared apoptotic cells, inflammation, and plaque necrosis.77–80 Our and another laboratory demonstrated this principle using athero-prone mice lacking the macrophage efferocytosis receptor MerTK78,81.
The mechanism of defective efferocytosis in advanced plaques represents a major gap in this field. Overwhelming apoptosis is not likely to be a major factor in view of the high-capacity nature of efferocytosis.74 For example, when apoptosis is increased in early atherosclerosis, where efferocytosis is not defective, ACs are efficiently cleared.82 While it is possible that death or a phenotypic change83 in advanced lesional macrophage death limits efferocytosis by decreasing the pool of competent efferocytes, advanced lesions have a substantial population of living phagocytes.84 Moreover, we showed that cholesteryl ester loading of macrophages does not compromise efferocytes76 and that efferocytosing macrophages acquire resistance to cell death stimuli.85 Rather, we favor the hypothesis that specific molecular-cellular processes involved in the recognition or uptake of apoptotic cells by lesional macrophages compromise efferocytosis in advanced atherosclerosis. For example, a recent study showed that some apoptotic cells in lesions continue to display a "don't-eat-me" molecule called CD47, which is usually lost upon apoptosis, thus preventing the uptake of these dead cells.86 As another example, the macrophage MerTK receptor, which as indicated above plays a very important role in advanced lesional efferocytosis, can be disabled by disintegrin and metalloproteinase domain-containing protein 17 (ADAM17)-mediated proteolytic cleavage under exactly the types of inflammatory conditions that occur in advanced atherosclerosis.87,88 Indeed, macrophages near the necrotic cores of human plaques demonstrate high ADAM17 expression and low levels of cell-surface MerTK.89 In human carotid artery endarectomy specimens, we found a strong correlation between the level of the stable product of MerTK cleavage, soluble Mer, and both advanced plaque stage and the presence of ischemic symptoms.90 Finally, Western diet-fed Ldlr−/− mice expressing a genetically engineered mutant of MerTK that cannot be cleaved showed enhanced lesional efferocytosis and decreased plaque necrosis.90
Efferocytosis is one of the most important cellular effector arms of the resolution program. Inflammation results in the accumulation of enormous amounts of dead cells, notably neutrophils, and resolution mediators have been shown to promote efferocytosis both in vitro and in vivo.66,91,92 A fascinating topic to consider is whether efferocytosis is also a mediator of the resolution response, which would amplify the response as part of a positive-feedback process. Evidence to support this concept is suggested by studies showing that phagocytes increase SPM production when engulfing apoptotic cells,93,94 and we showed recently that activation of the MerTK receptor in macrophages with an activating antibody, the MerTK ligand Gas6, or apoptotic cells promoted the synthesis of SPMs.95 The mechanism involves MerTK-mediated stimulation of nuclear-to-cytoplasmic translocation of 5-LOX, which increases the synthesis of SPMs through the 12,15-lipoxygenase pathway.95 We have shown that this pro-resolving action of MerTK is disabled by MerTK cleavage and important in several models of sterile inflammation, including atherosclerosis.90,95
Summary and Conclusions
Atherosclerosis is a heterogeneous disease despite a common initiating event, subendothelial retention of apoB LPs. In the vast majority of lesions, the sterile inflammatory response to these retained LPs does not lead to acute thrombotic complications. The most likely explanation is that an adequate resolution response is mounted, where efferocytosis prevents plaque necrosis and a reparative scarring response (the fibrous cap) prevents plaque disruption. Elements of the resolution response may also promote endothelial health in the setting of inflammation and thereby prevent plaque erosion.96 However, for reasons that remain to be elucidated, a small percentage of developing atherosclerotic lesions cannot maintain an adequate resolution response, leading to the formation of the types of clinically dangerous plaques that can trigger acute lumenal thrombosis and tissue ischemia and infarction. It is likely that a series of amplified pathophysiologic processes spin out of control to create these rare but deadly plaques. We believe that defective efferocytosis is a major contributor to this series of events, with several new studies providing plausible mechanisms of how efferocytosis becomes defective in advancing plaques. Once efferocytosis becomes defective and post-apoptotic necrosis occurs, anti-inflammatory and pro-resolving pathways downstream of efferocytosis are lost, and DAMPs arising from the necrotic cells exacerbate the inflammatory response. A fascinating question that emerges from this scenario is what determines whether any given lesion will undergo this transformation. Is it stochastic, or is there a specific determinant, such as excessive apoB LP accumulation occurring earlier in the history of the fated lesion?
Throughout this review, I have emphasized the clinical importance of advanced plaque progression as the cause of acute atherothrombotic events, and I have highlighted a number of critical questions related to mechanisms of plaque progression that remain to be addressed. However, one may legitimately question whether research in this area has therapeutic potential in the face of the logical conclusion that if apoB LPs could be brought safely below a certain threshold level at an early enough age in all individuals, atherosclerotic vascular disease would be eliminated. Even with established atherosclerosis, lesions can regress if the apoB LPs are brought to a low enough level.97 In this context, a prominent editorial 20 years ago suggested that coronary disease may no longer be a major health problem by the early 21st century 98. However, for the reasons discussed earlier in this review, i.e., cholesterol-lowering drug-related issues and the epidemic of insulin resistance, atherosclerotic vascular disease remains the leading cause of death two decades into the 21st century. While continued work on achieving lower and earlier LDL, stemming the epidemic of obesity and insulin resistance, and ameliorating other risk factors is critical, I believe that further understanding the mechanisms of advanced plaque progression should also be a priority. Research in this area may be able to suggest ways to raise the atherogenic threshold to apoB LPs such that, through combined apoB LP-lowering and arterial-wall therapy, currently achievable levels of apoB LP lowering can be disease-ending. Accordingly, we await the results of the aforementioned anti-inflammatory trials that are currently underway in humans,7,8 and several new therapeutic concepts have emerged based on the pathophysiology of plaque progression, including the use of exogenous resolving mediators and drugs that suppress cellular necrosis.51,66,99
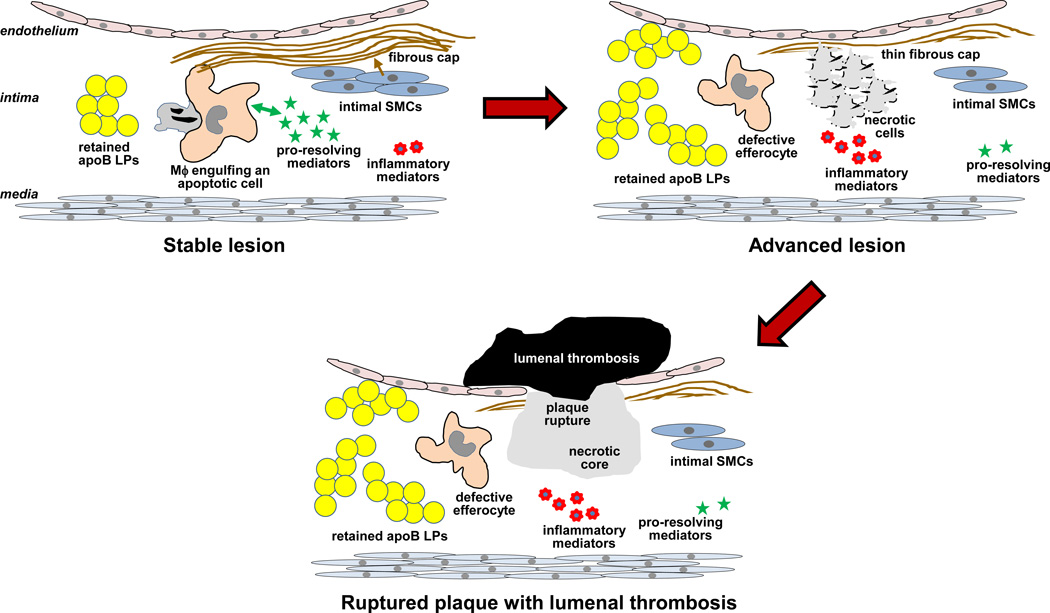
Figure.
Progression of atherosclerosis. Subendothelially retained apoB LPs incite a sterile inflammatory response, but in the majority of lesions, as depicted on the left, enough of a resolution response is mounted to prevent the formation of clinically dangerous plaques. Highlighted here is a positive-feedback cycle between efferocytosis and pro-resolving mediators, leading to the prevention of cell necrosis and a favorable pro-resolving:inflammatory mediator balance; and a scarring response in which intimal SMCs promote the formation of a protective fibrous cap. A small minority of these lesions progress, as depicted on the right. These lesions have persistent and amplified inflammatory stimuli (apoB LPs) and defective efferocytosis, which then promotes cell necrosis, an imbalance in the pro-resolving:inflammatory mediator balance, and thinning of the fibrous cap. This progression of events can lead to plaque rupture, acute lumenal thrombosis, and tissue ischemia or infarction (bottom image). Not depicted here is another type of advanced, clinically dangerous atherosclerotic lesion that is characterized by endothelial erosion rather than plaque necrosis. Whether a defective resolution response contributes to the formation of this type of plaque remains to be investigated.
Acknowledgments
Sources of Funding
The research in the author's laboratory covered in this review was supported by National Institutes of Health grants HL075662, HL127464, and HL132412.
References
- 1.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler.Thromb.Vasc.Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. The pathogenesis of atherosclerosis--an update. N.Engl.J.Med. 1986;314:488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis--an inflammatory disease. N.Engl.J.Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Libby P. Inflammation in atherosclerosis. Arterioscler.Thromb.Vasc.Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansson GK, Zhou X, Tornquist E, Paulsson G. The role of adaptive immunity in atherosclerosis. Ann.N.Y.Acad.Sci. 2000;902:53–62. doi: 10.1111/j.1749-6632.2000.tb06300.x. discussion 62-4.:53-62. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J.Thromb.Haemost. 2009;7(Suppl 1):332–339. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am.Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb.Perspect.Biol. 2012:4. doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K. Early human atherosclerosis. Accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler.Thromb.Vasc.Biol. 2007 doi: 10.1161/ATVBAHA.106.134080. [DOI] [PubMed] [Google Scholar]
- 11.Skålén K, Gustafsson M, Rydberg EK, et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 12.Brito V, Mellal K, Portelance SG, et al. Induction of anti-anti-idiotype antibodies against sulfated glycosaminoglycans reduces atherosclerosis in apolipoprotein e-deficient mice. Arterioscler.Thromb.Vasc.Biol. 2012;32:2847–2854. doi: 10.1161/ATVBAHA.112.300444. [DOI] [PubMed] [Google Scholar]
- 13.Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kathiresan S, Srivastava D. Genetics of human cardiovascular disease. Cell. 2012;148:1242–1257. doi: 10.1016/j.cell.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palinski W, Rosenfeld ME, Yla-Herttuala S, et al. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ.Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J.Clin.Invest. 2002;110:905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berliner JA, Subbanagounder G, Leitinger N, Watson AD, Vora D. Evidence for a role of phospholipid oxidation products in atherogenesis. Trends Cardiovasc.Med. 2001;11:142–147. doi: 10.1016/s1050-1738(01)00098-6. [DOI] [PubMed] [Google Scholar]
- 20.Myoishi M, Hao H, Minamino T, et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz EA, Reaven PD. Lipolysis of triglyceride-rich lipoproteins, vascular inflammation, and atherosclerosis. Biochim.Biophys.Acta. 2012;1821:858–866. doi: 10.1016/j.bbalip.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Bismuth J, Lin P, Yao Q, Chen C. Ceramide: a common pathway for atherosclerosis? Atherosclerosis. 2008;196:497–504. doi: 10.1016/j.atherosclerosis.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ketelhuth DF, Hansson GK. Cellular immunity, low-density lipoprotein and atherosclerosis: break of tolerance in the artery wall. Thromb.Haemost. 2011;106:779–786. doi: 10.1160/TH11-05-0321. [DOI] [PubMed] [Google Scholar]
- 24.Gimbrone MA, Jr, Garcia-Cardena G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc.Pathol. 2013;22:9–15. doi: 10.1016/j.carpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conway DE, Schwartz MA. Flow-dependent cellular mechanotransduction in atherosclerosis. J.Cell Sci. 2013;126:5101–5109. doi: 10.1242/jcs.138313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavlides S, Gutierrez-Pajares JL, Iturrieta J, Lisanti MP, Frank PG. Endothelial caveolin-1 plays a major role in the development of atherosclerosis. Cell Tissue Res. 2014;356:147–157. doi: 10.1007/s00441-013-1767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc.Natl.Acad.Sci.U.S.A. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAloon CJ, Boylan LM, Hamborg T, et al. The changing face of cardiovascular disease 2000–2012: An analysis of the world health organisation global health estimates data. Int.J.Cardiol. 2016;224:256–264. doi: 10.1016/j.ijcard.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Pisaniello AD, Scherer DJ, Kataoka Y, Nicholls SJ. Ongoing challenges for pharmacotherapy for dyslipidemia. Expert.Opin.Pharmacother. 2015;16:347–356. doi: 10.1517/14656566.2014.986094. [DOI] [PubMed] [Google Scholar]
- 30.Shimada YJ, Cannon CP. PCSK9 (Proprotein convertase subtilisin/kexin type 9) inhibitors: past, present, and the future. Eur.Heart J. 2015;36:2415–2424. doi: 10.1093/eurheartj/ehv174. [DOI] [PubMed] [Google Scholar]
- 31.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 35.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N.Engl.J.Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 36.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat.Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 37.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat.Rev.Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koltsova EK, Hedrick CC, Ley K. Myeloid cells in atherosclerosis: a delicate balance of anti-inflammatory and proinflammatory mechanisms. Curr.Opin.Lipidol. 2013;24:371–380. doi: 10.1097/MOL.0b013e328363d298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat.Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 41.Schwenke DC, Carew TE. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. II. Selective retention of LDL vs. selective increases in LDL permeability in susceptible sites of arteries. Arteriosclerosis. 1989;9:908–918. doi: 10.1161/01.atv.9.6.908. [DOI] [PubMed] [Google Scholar]
- 42.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N.Engl.J.Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 43.Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv.Cardiol. 2002;15:439–446. doi: 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 44.Libby P, Pasterkamp G. Requiem for the 'vulnerable plaque'. Eur.Heart J. 2015;36:2984–2987. doi: 10.1093/eurheartj/ehv349. [DOI] [PubMed] [Google Scholar]
- 45.Quillard T, Araujo HA, Franck G, Shvartz E, Sukhova G, Libby P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur.Heart J. 2015;36:1394–1404. doi: 10.1093/eurheartj/ehv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ball RY, Stowers EC, Burton JH, Cary NR, Skepper JN, Mitchinson MJ. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 1995;114:45–54. doi: 10.1016/0021-9150(94)05463-s. [DOI] [PubMed] [Google Scholar]
- 47.Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 2009;50(Suppl):S382–S387. doi: 10.1194/jlr.R800032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler.Thromb.Vasc.Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 49.Lin J, Li H, Yang M, et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3:200–210. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Meng L, Jin W, Wang X. RIP3-mediated necrotic cell death accelerates systematic inflammation and mortality. Proc.Natl.Acad.Sci.U.S.A. 2015;112:11007–11012. doi: 10.1073/pnas.1514730112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karunakaran D, Geoffrion M, Wei L, et al. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci.Adv. 2016;2:e1600224. doi: 10.1126/sciadv.1600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krysko DV, Denecker G, Festjens N, et al. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death.Differ. 2006;13:2011–2022. doi: 10.1038/sj.cdd.4401900. [DOI] [PubMed] [Google Scholar]
- 53.Newby AC. Proteinases and plaque rupture: unblocking the road to translation. Curr.Opin.Lipidol. 2014;25:358–366. doi: 10.1097/MOL.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 54.Ohayon J, Finet G, Gharib AM, et al. Necrotic core thickness and positive arterial remodeling index: emergent biomechanical factors for evaluating the risk of plaque rupture. Am.J Physiol Heart Circ.Physiol. 2008;295:H717–H727. doi: 10.1152/ajpheart.00005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serhan CN, Brain SD, Buckley CD, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 57.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am.J.Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat.Rev.Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 59.Wallace JL, Ianaro A, Flannigan KL, Cirino G. Gaseous mediators in resolution of inflammation. Semin.Immunol. 2015;27:227–233. doi: 10.1016/j.smim.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Merched AJ, Serhan CN, Chan L. Nutrigenetic disruption of inflammation-resolution homeostasis and atherogenesis. J.Nutrigenet.Nutrigenomics. 2011;4:12–24. doi: 10.1159/000326890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nature Rev.Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fredman G, Hellmann J, Proto JD, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat.Commun. 2016;7:12859. doi: 10.1038/ncomms12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Viola J, Lemnitzer P, Jansen Y, et al. Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ.Res. 2016;119:1030–1038. doi: 10.1161/CIRCRESAHA.116.309492. [DOI] [PubMed] [Google Scholar]
- 66.Fredman G, Kamaly N, Spolitu S, et al. Targeted nanoparticles containing the pro-resolving peptide Ac2-26 protect against advanced atheosclerosis in hypercholesterolemic mice. Sci.Transl.Med. 2015;7 doi: 10.1126/scitranslmed.aaa1065. 275ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fredman G, Ozcan L, Spolitu S, et al. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proc.Natl.Acad.Sci.U.S.A. 2014;111:14530–14535. doi: 10.1073/pnas.1410851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geng Y-J, Libby P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1b-converting enzyme. Am.J.Pathol. 1995;147:251–266. [PMC free article] [PubMed] [Google Scholar]
- 69.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler.Thromb.Vasc.Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 70.Schrijvers DM, De Meyer GR, Herman AG, Martinet W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc.Res. 2007;73:470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 71.Van Vre EA, Ait-Oufella H, Tedgui A, Mallat Z. Apoptotic cell death and efferocytosis in atherosclerosis. Arterioscler.Thromb.Vasc.Biol. 2012;32:887–893. doi: 10.1161/ATVBAHA.111.224873. [DOI] [PubMed] [Google Scholar]
- 72.Otsuka F, Kramer MC, Woudstra P, et al. Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study. Atherosclerosis. 2015 doi: 10.1016/j.atherosclerosis.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat.Immunol. 2015;16:907–917. doi: 10.1038/ni.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr.Biol. 2001;11:R795–R805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 75.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J.Immunol. 1999;162:3498–3503. [PubMed] [Google Scholar]
- 76.Li Y, Gerbod-Giannone MC, Seitz H, et al. Cholesterol-induced apoptotic macrophages elicit an inflammatory response in phagocytes, which is partially attenuated by the Mer receptor. J Biol.Chem. 2006;281:6707–6717. doi: 10.1074/jbc.M510579200. [DOI] [PubMed] [Google Scholar]
- 77.Tabas I. Apoptosis and efferocytosis in mouse models of atherosclerosis. Curr.Drug Targets. 2007;8:1288–1296. doi: 10.2174/138945007783220623. [DOI] [PubMed] [Google Scholar]
- 78.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of Apoe−/− mice. Arterioscler.Thromb.Vasc.Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yancey PG, Blakemore J, Ding L, et al. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and AKT activation. Arterioscler.Thromb.Vasc.Biol. 2010 doi: 10.1161/ATVBAHA.109.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tao H, Yancey PG, Babaev VR, et al. Macrophage SR-BI Mediates Efferocytosis via Src/PI3K/Rac1 Signaling and Reduces Atherosclerotic Lesion Necrosis. J.Lipid Res. 2015 doi: 10.1194/jlr.M056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ait-Oufella H, Pouresmail V, Simon T, et al. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler.Thromb.Vasc.Biol. 2008;28:1429–1431. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
- 82.Arai S, Shelton JM, Chen M, et al. A role for the apoptosis inhibitory factor AIM/Spa/Api6 in atherosclerosis development. Cell Metabolism. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 83.Vengrenyuk Y, Nishi H, Long X, et al. Cholesterol Loading Reprograms the miR-143/145-Myocardin Axis to Convert Aortic Smooth Muscle Cells to a Dysfunctional Macrophage-Like Phenotype. Arterioscler.Thromb.Vasc.Biol. 2015 doi: 10.1161/ATVBAHA.114.304029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Libby P, Geng YJ, Aikawa M, et al. Macrophages and atherosclerotic plaque stability. Curr.Opin.Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 85.Cui D, Thorp E, Li Y, et al. Pivotal advance: macrophages become resistant to cholesterol-induced death after phagocytosis of apoptotic cells. J Leukoc.Biol. 2007;82:1040–1050. doi: 10.1189/jlb.0307192. [DOI] [PubMed] [Google Scholar]
- 86.Kojima Y, Volkmer JP, McKenna K, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sather S, Kenyon KD, Lefkowitz JB, et al. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thorp E, Vaisar T, Subramanian M, Mautner L, Blobel C, Tabas I. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cdelta, and p38 mitogen-activated protein kinase (MAPK) J.Biol Chem. 2011;286:33335–33344. doi: 10.1074/jbc.M111.263020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garbin U, Baggio E, Stranieri C, et al. Expansion of necrotic core and shedding of Mertk receptor in human carotid plaques: a role for oxidized polyunsaturated fatty acids? Cardiovasc.Res. 2013;97:125–133. doi: 10.1093/cvr/cvs301. [DOI] [PubMed] [Google Scholar]
- 90.Cai B, Thorp EB, Doran AC, et al. MerTK cleavage promotes plaque necrosis and defective resolution in advanced atherosclerosis. J.Clin.Invest. 2016 doi: 10.1172/JCI90520. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Serhan CN, Fredman G, Yang R, et al. Novel proresolving aspirin-triggered DHA pathway. Chem.Biol. 2011;18:976–987. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J.Biol.Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 94.Dalli J, Serhan C. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012 doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cai B, Thorp EB, Doran AC, et al. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc.Natl.Acad.Sci.U.S.A. 2016;113:6526–6531. doi: 10.1073/pnas.1524292113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler.Thromb.Vasc.Biol. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fisher EA. Regression of Atherosclerosis: The Journey From the Liver to the Plaque and Back. Arterioscler.Thromb.Vasc.Biol. 2016;36:226–235. doi: 10.1161/ATVBAHA.115.301926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brown MS, Goldstein JL. Heart attacks: gone with the century? Science. 1996;272:629. doi: 10.1126/science.272.5262.629. [DOI] [PubMed] [Google Scholar]
- 99.Grootaert MO, Schrijvers DM, Van SH, et al. NecroX-7 reduces necrotic core formation in atherosclerotic plaques of Apoe knockout mice. Atherosclerosis. 2016;252:166–174. doi: 10.1016/j.atherosclerosis.2016.06.045. [DOI] [PubMed] [Google Scholar]