Abstract
In past years, a diagnosis of bronchiolitis obliterans syndrome (BOS) after allogeneic hematopoietic cell transplant (HCT) conferred nearly universal mortality secondary to lack of consensus for diagnostic criteria, poorly understood disease pathogenesis, and very few studies of therapeutic or supportive care interventions. Recently, however, progress has been made in these areas: revised consensus diagnostic guidelines are now available, supportive care has improved, there is greater understanding of potential mechanisms of disease, and prospective trials are being conducted. This article describes these advances and provides suggestions to optimize therapy for patients with BOS after HCT.
Introduction
Despite advances in the treatment of graft-versus-host disease (GVHD) and supportive care after hematopoietic cell transplantation (HCT), lung manifestations of chronic GVHD (cGVHD) continue to confer poor prognosis.1-3 Lung cGVHD or bronchiolitis obliterans syndrome (BOS) results from immune attack of the small airways, leading to fibrotic occlusion and subsequent obliteration. BOS is often an asymptomatic, insidious disease occurring within the first 2 years of HCT with other manifestations of cGVHD. The diagnosis relies upon obstructive decline in pulmonary function in the absence of other etiologies. Diagnosis, supportive care, and treatments for this disease have improved in recent years. Here, I describe my approach to BOS diagnosis and treatment via discussion of illustrative cases.
Case reports
Case 1: diagnostic criteria
Patient 1 is a child who underwent matched sibling peripheral blood stem cell transplantation (PBSCT) for acute leukemia. Her course was complicated by cGVHD of multiple organs and declining forced expiratory volume at 1 second (FEV1) to <50% predicted, though the ratio of FEV1 to forced vital capacity (FVC) remained >0.7. Inspiratory computerized tomography (CT) scan showed reticulonodular disease. The patient underwent right upper lobe biopsy, which was complicated by pneumothorax, pneumomediastinum, pneumatoses, and subcutaneous emphysema. Biopsy showed bronchiolitis obliterans and infectious tests were negative.
Discussion of case 1
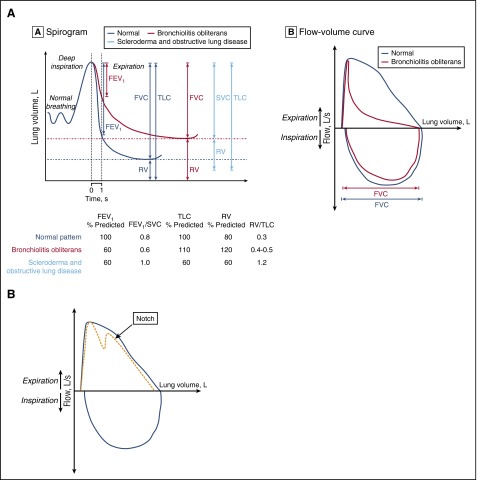
As the current case highlights, lung biopsy in these patients can be associated with high morbidity.4,5 Although historically, this was the preferred method of diagnosis, now pulmonary function test (PFT) criteria are sufficient for most patients after HCT. In the 35 years since the first accounts of lung cGVHD, noninvasive diagnostic criteria for BOS have been developed and refined by international consensus.6-9 The current definition of BOS includes: (1) FEV1 <75% predicted and an irreversible ≥10% decline in <2 years, (2) FEV1-to-vital capacity (VC) ratio <0.7 or the lower limit of the 90% confidence interval of the ratio, (3) absence of infection, and (4) either: (a) preexisting diagnosis of cGVHD, (b) air trapping by expiratory CT, or (c) air trapping on PFTs by residual volume (RV) >120% or RV/total lung capacity (TLC) exceeding the 90% confidence interval (Figure 1A).9,10 These new diagnostic criteria incorporated several important updates. First, air trapping was no longer a required criteria for patients who had obstructive disease and cGVHD, as this finding is often absent early in the disease course.10,11 Second, a standard threshold for the FEV1-to-FVC ratio (of 0.7) was supplemented by consideration of the normal age-adjusted confidence interval.12 Because the ratio of young children is 1 and declines with age, both children and the elderly may be misdiagnosed using the 0.7 ratio cutoff. Patient 1 would have met criteria for BOS by PFTs alone using current definitions using National Health and Nutrition Examination Survey equations.13 Similarly, elderly individuals may have appropriate age-related decline in FEV1 to <0.7 and not de facto meet criteria for obstruction. Finally, the slow VC may now be used rather than FVC, as some BOS patients require slow exhalation to reveal full VC due to early airway collapse with the forced expiratory maneuver. Patients with more severe disease may have evidence of air trapping by PFTs (elevated RV) or on expiratory CT scan (mosaic pattern); however, as suggested by the National Institutes of Health (NIH) 2014 guidelines, these features are not required to diagnose BOS. One final population that is particularly challenging to diagnose are those with extrapulmonary restriction, which may be due to sclerotic GVHD of the pulmonary girdle, GVHD myositis (with elevated creatine kinase or aldolase), steroid myopathy, or polyneuritis affecting respiratory muscles. The maximum inspiratory pressures (MIPs) and expiratory pressures (EPs) may be a marker of muscle weakness. The extrapulmonary restriction will also diminish TLC and VC, thus yielding a misleading picture of restriction, in contrast to intrapulmonary restriction (Figure 1A). For these patients, PFTs need to be repeated after the extrapulmonary process improves to determine whether lung cGVHD is also present.
Figure 1.
Representative flow volume. (A) Representative flow volume loop of a patient with BOS (red) compared with a patient with normal exhalation (gray) (left). Spirogram representation of BOS (red) as compared with normal lung volumes (gray) and those of a patient with sclerotic GVHD (blue) or extrapulmonary restriction (right). Within the spirogram, the FEV1 is shown first, then the FVC and RV, and then the TLC (TLC = RV + VC). Below the spirogram are representative PFT parameters for each of the 3 groups of patients: normal in gray, BOS in red, and sclerotic GVHD in blue. (B) Representative flow volume showing the notch characteristic of tracheomegaly or tracheobronchomalacia. Panel A reprinted from Williams et al10 with permission.
It is very important to exclude other diagnoses, including idiopathic pneumonia syndrome, cryptogenic-organizing pneumonia (COP), pulmonary fibrosis, and late radiation effects, infection, asthma, or chronic obstructive pulmonary disease (COPD), and rare disorders such as tracheomegaly, tracheobronchomalacia, α-1-antitrypsin deficiency. Infection must be excluded to diagnose BOS and requires a thorough investigation. The FEV1 decline in asthma is reversible with albuterol in contrast to BOS. Idiopathic pneumonia syndrome typically presents earlier after transplant, and, in addition to pulmonary fibrosis, late radiation effects should be excluded by the absence of obstruction. Tracheomegaly occurs in adulthood and is diagnosed by an enlarged trachea on CT as well as a characteristic notch in the expiratory flow volume loop (Figure 1B). This can also be seen in tracheobronchomalacia, which can mimic BOS, and may be diagnosed by bronchoalveolar lavage (BAL).14 COP usually presents with restriction but a mixed picture with obstruction may be present. COP, previously termed “bronchiolitis obliterans–organizing pneumonia (BOOP)” should include a new consolidation on CT and respond rapidly to steroids in contrast to BOS. α-1-antitrypsin deficiency also presents with obstructive disease, and although there is often some obstruction present pre-HCT, this may be much more pronounced after HCT. I typically test for this if there is family history or obstruction pre-HCT, or if other cGVHD manifestations are absent. Smokers or those with COPD present a unique challenge because airflow obstruction may progress without BOS. Although FEV1 in patients with COPD declines at twice the expected age-related loss in lung function, this should not exceed the BOS-defined 10% decline within 2 years.15 One final rare complication deserves mention, that of broncho-obliterans (obliteration of large airways), which likely represents similar alloimmunity but may be visualized by bronchoscopy and potentially treated through lysis of the webs, and may be coincident with BOS.16 Notably, BOS can occur outside the setting of allogeneic HCT and rarely reported after autologous HCT.17
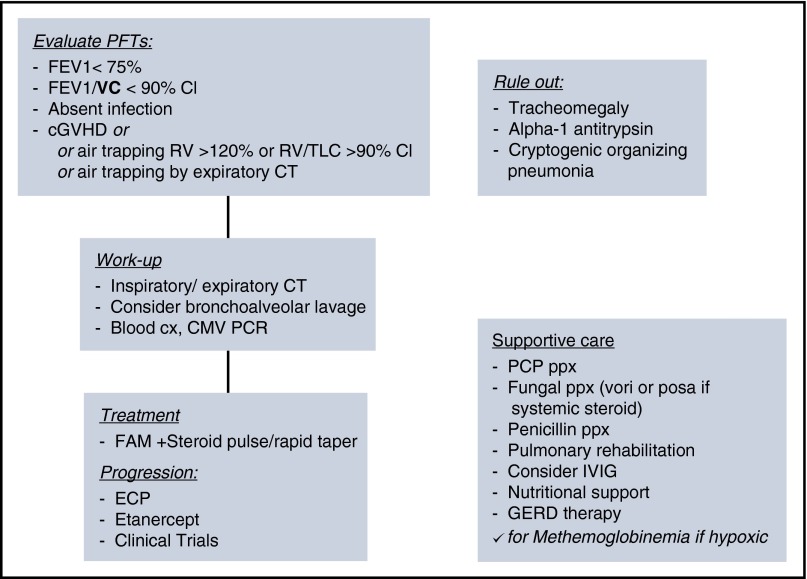
Although I view the complete PFTs, most patients may be diagnosed by FEV1% predicted below 75%, with >10% decline from pretransplant, with FEV1/slow VC (SVC) below the confidence interval. For any patients with new obstruction, I perform an intensive workup for infections, which may either cause the FEV1 decline, or often be a cofactor. Finally, I recommend BAL, which may be critical to diagnose and help guide therapy for coinfections, colonization, or resistant bacteria that may lead to subsequent infectious episodes and guide therapy, and to rule out bronchomalacia or broncho-obliterans (Figure 2). In the rare circumstance a diagnosis is not achieved through these methods, and the index of suspicion for infection remains high, I will pursue lung biopsy. Severity of disease may be classified by: mild FEV1, >60%; moderate FEV1, 40% to 59%; and severe FEV1, <39%.9
Figure 2.
Algorithm for diagnosis, work-up, and treatment of BOS. Summary algorithm for diagnosis, work-up, supportive care considerations, and treatment of BOS. CI, confidence interval; cx, culture; IVIG, intravenous immunoglobulin G; PCP, pneumocystis jirovecci pneumonia; ppx, prophylaxis.
Case 2: importance of frequent monitoring
Patient 2 is an adult woman who underwent PBSCT with busulfan and cyclophosphamide for leukemia, complicated by cytomegalovirus (CMV) reactivation and acute GHVD early after HCT, prompting resumption of cyclosporine and protracted taper. Approximately 3 years after HCT and soon after completion of taper, she developed lichenoid skin changes, hepatitis, and vaginal symptoms. She was diagnosed with cGVHD and mycophenylate mofetil administered. Despite immunosuppressive therapy, GVHD progressed to include oral and ophthalmic GVHD and a nonproductive cough. Despite pulmonary symptoms, the first PFTs were administered >2 years after initial cGVHD diagnosis, showed new obstruction, and were consistent with BOS with considerable FEV1 decline to 50% predicted with ratio of FEV1 to VC of 0.5.
Discussion of case 2
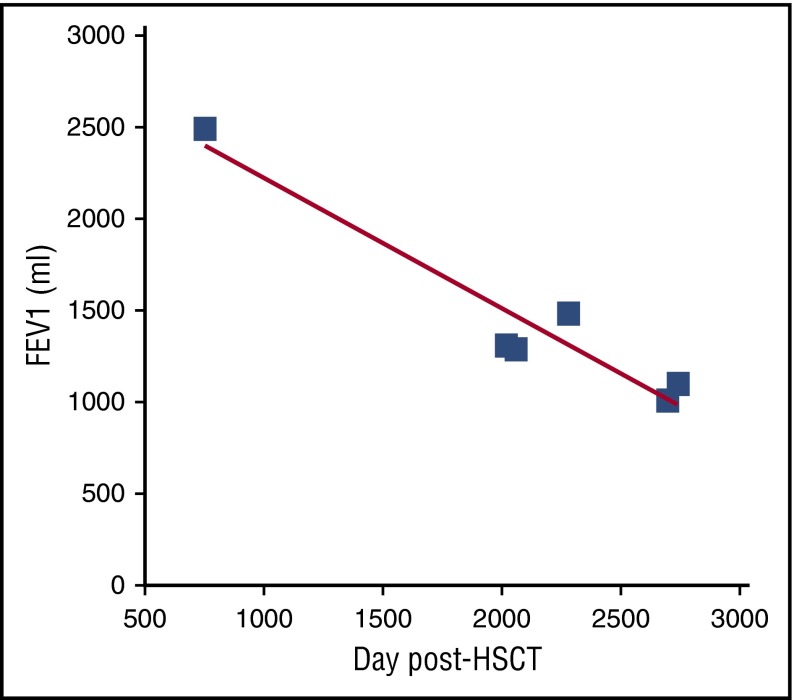
This case highlights the potential benefit of frequent screening PFTs, permitting the earliest diagnosis of disease, prior to the onset of symptoms. Symptoms of BOS may include chronic nonproductive cough, dyspnea on exertion, decrease in exercise tolerance, wheezing, or pneumomediastinum. Clinical symptoms of disease are often associated with moderate/severe FEV1 decline, suggesting that a window of time has already passed in which more mild obstruction might have been identified. However, this case is not unusual. A recent study of 82 patients diagnosed with BOS by current criteria showed that the median FEV1 was 53% at diagnosis and risk factors for poorer prognosis included diagnosis within 1 year and decreased FVC.18 Furthermore, as described in this manuscript, the modern trajectory of BOS is characterized by a rapid decline in FEV1 followed by a period of relative stabilization, likely due to recognition and interventions. Thus, theoretically, earlier detection could lead to a plateau at a higher, and less morbid, FEV1. A good way to monitor lung function is to follow the regression slope of FEV1 volume vs time (Figure 3). The slope also removes any influence of different PFT equations, which can significantly alter FEV1% predicted (up to 27%).13 In addition to quickly revealing FEV1 changes, the rate of decline can be compared interpatient to compare disease severity and intrapatient to evaluate the success of therapies. Notably, for pediatric patients, the necessity of growth is important to include, thus it is best to evaluate the percent-predicted slope for prognosis (showing sufficient recruitment of lung for growth).19 For children with pneumothorax limiting PFTs, 6-minute walk assessment can be a good surrogate for progression in the absence of other cGVHD manifestations that impair mobility. Other important means to monitor these patients include the NIH cGVHD organ grading and patient-reported outcome measures such as the Lee symptom scale.9,20
Figure 3.
Graph of FEV1 (mL) vs time (days) of patient 2. The time of cGVHD diagnosis (dx) and clinical symptoms (cough) as well as the time of BOS diagnosis are noted by arrows.
PFT monitoring is especially important for patients at highest risk of BOS, including those with cGVHD, which doubles the incidence from 5% to 6% to 13%.10,21-24 Other risk factors for BOS after HCT include: preparative regimens containing busulfan, peripheral blood stem cell source, viral infections early after HCT, history of significant acute GVHD, as seen in our patient, as well as ABO incompatibility prior lung disease, and posttransplant lung disease.25-32 Published guidelines for PFT monitoring have promoted every-3-months monitoring for a year after transplant.33 However, given that the median diagnosis of new-onset BOS was 1.5 years from HCT, in a recent prospective study, I prescribe PFTs at least every 3 months for at least the first 1 to 2 years, which was also recently endorsed by the NIH consensus and European expert groups.34,35 I also include PFTs at the time of cGVHD flare or signs of other organ progression, especially for those with risk factors for BOS present. Practitioners have reported that there are challenges to frequent PFT monitoring such as accessibility of PFT laboratories, requirements for the test, and the cross-discipline collaboration required. Hand-held spirometers may address these concerns, offering facile and economical means to frequently assess FEV1. Although not yet used in general practice, these devices may be used in the practitioner’s office or even distributed for home use. These have shown sensitivity to detect FEV1 decline, which may then prompt full PFTs to potentially yield earlier, more efficient diagnosis of BOS.36
A novel imaging modality termed parametric response mapping (PRM) may aid in the diagnosis of BOS. PRM permits spatial assessment and quantification of heterogeneity of lung parenchyma. Clinical high-resolution helical chest CTs obtained upon inspiration and expiration can be manipulated to overlay an expanded exhalation scan “over” the inspiratory scan. Small voxels segment lung parenchyma informing the spatial position of airflow pathology, permitting differentiation of normal airflow, from airflow obstruction (normal inhalation and impaired exhalation), and restrictive disease (impaired inhalation and exhalation). This technique has been applied to BOS patients to show impaired exhalation and differentiate areas of infection from obliterative disease.37,38 PRM may be useful to monitor individuals unable to perform PFTs (eg, young children), potentially diagnose patients earlier, and provide a quantitative measure of airspace disease over time.
Another important consideration is whether we could identify patients who have begun the pathological process of obliterative bronchiolitis prior to the severe decline of FEV1 below 75% predicted. An early stage has been defined in lung transplant BOS (host-vs-raft disease) and labeled BOS-0p, which identifies patients at higher risk to progress to BOS. In lung transplantation, 30% of these patients will progress to BOS.39 A similar definition has recently been evaluated in HCT patients.40 BOS-0p was defined as: decline in FEV1 of >10% or midflow rate (forced expiratory flow at 25%-75% [FEF25-75]) of >25% decline and not meeting BOS criteria.40 In HCT recipients, BOS-0p was sensitive for the development of BOS.40 These data suggest that more frequent PFTs could not only better identify patients at highest risk for BOS progression, but also permit early interventions in a population already suffering from alloimmune bronchiole damage. Prospective studies should test whether interventions at this point can prevent BOS altogether, potentially providing the best opportunity for averting disease.
Case 3: infectious disease and supportive care approaches
Patient 3 developed BOS in the first year following haploidentical HCT. She was referred for an intervention trial (NCT00656058) and underwent BAL as part of this workup. Chest radiograph was normal whereas chest CT showed large nodules concerning for fungus. BAL revealed: Parainfluenza III, Aspergillus, and CMV, precluding initiation of study drug. During the course of her treatment, the patient was also diagnosed with nocardia, extended-spectrum β-lactamase (ESBL) Klebsiella, Scopulariopsis, and Geosmithia/Paecilomyces–all obtained from BAL or fine-needle lung aspirations. Treatment included multiple antimicrobials including posaconazole, caspofungin, miltefosine (compassionate use), imipenem, moxifloxacin, valgancyclovir, tobramycin nebulizers, and tigecycline. Unfortunately, respiratory status worsened, resulting in tracheostomy and chronic ventilation, then death. At the time of autopsy, in addition to obliterative bronchiolitis, Aspergillus and CMV were evident in the lungs, despite negative BAL and blood tests.
Discussion of case 3
This case highlights several key supportive care issues in BOS. In our prospective study (NCT00656058), patients underwent extensive evaluation for infection, including BAL (n = 19). All but 1 patient (4%) presented without evidence of infection. Despite this, 25% of patients had infections identified through BAL, including: Pseudomonas infection (n = 1), mycobacteria infection (n = 2), Aspergillus infection (n = 2), CMV pneumonitis (n = 1), and nocardia (n = 1). Excluding these baseline infections, a total of 45 infections were experienced in 24 patients on study: 36% bacterial (most commonly gram negative), 47% viral (most commonly influenza), 16% fungal (mostly Aspergillus), and mycobacterial infection (2%). This high rate of coinfections requires vigilance on the part of the provider, as infections are a leading cause of death in these patients.16,22,41 Investigation for fungal disease including β-d-glucan and galactomannan in the BAL is helpful, the newer blood polymerase chain reaction (PCR) tests for Aspergillus may have a role in these patients, and strong consideration should be given to voriconazole or posaconazole for prophylaxis with frequent assay of blood levels, especially in patients on protracted systemic steroids. The high rate of mycobacterial and nocardia infections in this patient population (21% total) deserves further consideration, and suggest that BAL should be strongly considered in patients with acute decline. This relative increase in nontuberculous mycobacteria has been noted in lung transplant recipients and linked to BOS progression.42 Interestingly, other bacterial infections identified were exclusively gram negative (Pseudomonas, Moraxella, Klebsiella, Escherichia coli, Stenotrophomonas). Both these nontuberculosis mycobacterial infections and gram-negative bacteria may be related to colonized bronchiectasis, which is often associated with BOS.43,44 Treatment is likely critical not just to avoid progression of infection, but these infections may incite chemokines and accelerate BOS progression and death.45 Furthermore, similar to patients with bronchiectasis from other causes, patients with BOS can be colonized with gram-negative organisms, and experience exacerbations similar to cystic fibrosis. I have seen benefit from inhaled aminoglycosides to constrain these organisms (typically using a 1-month-on and 1-month-off approach), using spontaneous or induced sputum to follow the organism burden. In addition, early treatment with fluoroquinolones (or other sensitive antimicrobials) in the setting of sputum production and increased cough has clinically seemed beneficial for these patients. However, recommended antimicrobials for BOS patients should include prophylaxis against: Pneumocystis jirovecii pneumonia (with trimethoprim/sulfamethoxazole preferentially), Streptococcus with penicillin, and fungi as described within the paragraph above.46 I administer IVIG for patients with immunoglobulin G <500 mg/dL, though studies have not evaluated its benefit in BOS patients. Finally, influenza can be life-threatening in BOS patients, and prompt treatment with neuraminidase inhibitors is critical at the earliest sign of disease. Immunizations may be important to protect against these lung infections when patients are sufficiently immunocompetent. Further study of the best ways to diagnose, treat, and prevent these infectious complications will be important to improve outcomes in BOS.
It is important to note that chest radiographs may not reliably show infections in BOS patients, as the trapped air can effectively conceal even larger infiltrates, as in patient 3. Furthermore, for infections such as mycobacteria and fungal disease, evaluation by CT is necessary to identify the minute nodules of mild disease. CTs can also show bronchiectasis, suspicious for gram-negative bacterial colonization.
Other supportive care considerations include opportunities to minimize further infectious exposures and inflammatory sources while optimizing remaining pulmonary function. Studies have suggested that gastroesophageal reflux disease (GERD) may be associated with BOS genesis and progression, and maximizing GERD therapies may diminish progression of BOS.47,48 Furthermore, data in lung transplantation even support Nissen fundoplication procedures, though this has not been studied in BOS after HCT.49 Optimization of nutrition is important and may require supplemental nutrition while treating severe BOS. Supplemental nutrition is especially important in patients with continued weight loss. For patients who have some mild benefit by increased FEV1% by >12% with β-agonists, these agents may modestly increase endurance. For these patients, I initiate a trial with albuterol and may prescribe a long-acting β-agonist additionally. However, albuterol can also lead to bronchomalacia if overused (due to reduced smooth muscle tone), and I have seen this occur following misdiagnosis and days of continuous albuterol. Pulmonary rehabilitation improves exercise tolerance and quality of life.34,50 Similarly, if patients require antihypertensive treatment, agents other than beta-blockers or angiotensin-converting enzyme inhibitors may be better from a pulmonary perspective. Anemia should be corrected and patients with good hematopoiesis will often compensate with mildly elevated hemoglobin. Acute hypoxemia is a rare presentation of BOS, most often linked either to acute infection or methemoglobinemia (often associated with dapsone). Finally, should patients require ventilatory support, the pathophysiology of BOS is important to consider. Because these patients have significant air trapping and fixed small airway obstruction, additional positive pressure should be minimized as this will increase the risk of barotrauma and exacerbate air trapping. Furthermore, the settings should reflect that patients require added time for exhalation. Tracheostomy will increase the impedance to airflow. Finally, they should be carefully monitored for hypercapnea, which can lead to life-threatening apnea in the setting of oxygen administration.51
Case 4: therapeutic options
Patient 4 had received tacrolimus, mycophenylate mofetil, methotrexate, steroids, etanercept, oral budesonide, and extracorporeal photopheresis (ECP) prior to onset of BOS for the treatment of acute GVHD after haploidentical BMT. PFTs performed 1 year after HCT, in the setting of weaning immunosuppression showed mild BOS with FEV1 of 70% predicted and long-acting β-agonist was started. Two months later, a GVHD flare led to resumption of systemic steroids and tacrolimus and sirolimus was soon added. Six months after BOS presentation and 1.5 years after HCT, FEV1 was 45% predicted, prompting initiation of tiotropium bromide in addition to steroids, sirolimus, tacrolimus, followed by rituximab, then soon after by radiation therapy with 1 Gy total body irradiation. At 3 years post-HCT, FEV1 had declined to 36%, with FEV1 slope of −3, despite a treatment regimen that now included mycophenylate mofetil, azithromycin, advair, tacrolimus, and he was referred for a trial of montelukast at NIH.
Discussion case 4
A rapid persistent decline in FEV1 despite interventions continues to be associated with high mortality.52 However, recent data suggest that survival is improving in BOS. In 2008, review of the literature suggested that survival was dismal, just 40% at 2 years and 20% at 5 years, and had been unchanged in the prior 20 years.10,21-23 However, recent survival estimates show that 2- to 3-year survival is 60% to 75% and 5-year survival is 40% to 50%, likely reflecting enhanced recognition and incorporation of newer therapies and better supportive care, and supported by a recent survey study of cGVHD practitioners.29,30,52,53
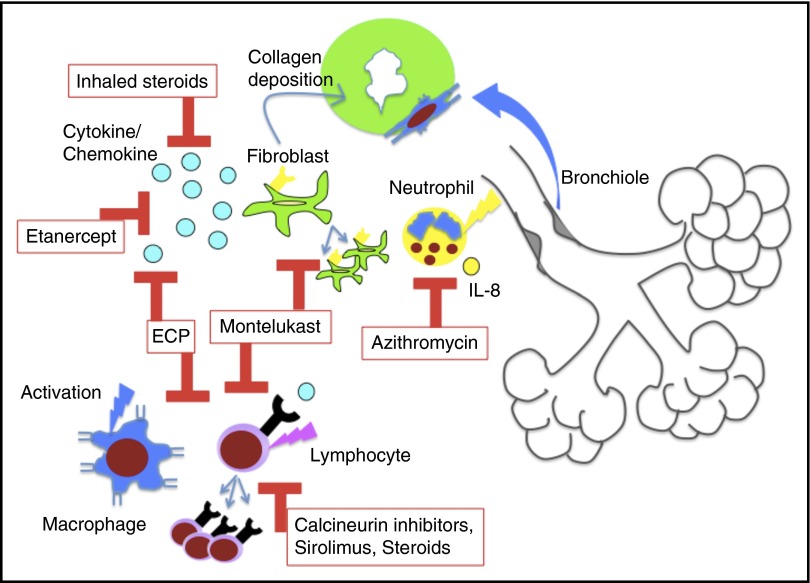
Historically, protracted systemic steroids and agents that impair lymphocyte proliferation and activation (calcineurin inhibitors, azathioprine) have formed the backbone of BOS therapy. Extended systemic steroid courses are no longer recommended for BOS therapy, as this is associated with high mortality from infectious complications. In the past 10 years, several novel options for therapy have emerged, many from studies of lung transplant BOS (host-versus-graft disease). For the treatment of new-onset BOS after HCT, a study of inhaled fluticasone, azithromycin, and montelukast (FAM) with brief steroid burst (1 mg/kg per day prednisone) and rapid taper has been reported (0.25 mg/kg per week) (Figure 2).54 This prospective, multi-institutional study of 36 patients showed stabilization or improvement in 94% of patients at 3 months and overall survival of 97% at 6 months.54 Based on these results, I initiate FAM therapy for all newly diagnosed patients in addition to continuation or resumption of classical cGVHD therapy with calcineurin inhibitor or sirolimus. The mechanistic rationale for these agents are: inhaled steroids provide local anti-inflammatory effects, azithromycin impairs interleukin-8 (IL-8) production and neutrophilia, and montelukast blocks leukotriene activity, impairing cellular homing and activation and possibly blocking fibroblast proliferation and collagen deposition (Figure 4).10,55-66 Prior studies had shown safety and some (though not all) had suggested benefit for these agents in BOS.58,63,64,67-69 Since this trial was initiated, a prospective randomized placebo-controlled study showed efficacy for inhaled budesonide/formoterol as well, supporting the use of inhaled steroids early in BOS therapy.70 In addition, there is a recent retrospective study on the use of budesonide/formoterol, montelukast and n-acetylcysteine for BOS after HCT, which showed stabilization of FEV1, though the eligibility criteria for BOS was not the consensus definition.71 I continue FAM components indefinitely as these have been nontoxic and, in many patients, permitted slow withdrawal of all other immunosuppression without decline in FEV1 and, in some patients, even increases in FEV1. To date, the longest duration of FAM treatment of BOS patients in my experience exceeds 10 years, supporting tolerability. However, the optimal length of treatment is unknown and will hopefully be addressed in future trials.
Figure 4.
Diagram of the proposed immune mechanisms linked to BOS pathogenesis, the current treatments in BOS, and the pathways blocked by these treatments. Cytokine/chemokine indicates TNF α for etanercept and multiple activating chemokines and cytokines for ECP.
For progressive disease, ECP has benefited patients with BOS (published cGVHD schedule), with 60% of HCT patients achieving stable disease, mirroring lung transplant data.72-77 Data suggest that ECP facilitates a milieu of transplantation tolerance, decreasing effector lymphocyte populations, enriching for regulatory lymphocytes and suppressor antigen-presenting cells.78-80 One study showed benefit for BOS patients with etanercept, which inhibits the tumor necrosis factor receptor, and diminishes ensuing inflammation.81 Although there are exciting translational data emerging for the role of B cells in BOS, to date, data has not shown efficacy for treatment with rituximab, nor have I witnessed benefit with this agent.82-85 For young patients with refractory severe BOS despite all interventions and best supportive care, who have no other active GVHD on minimal or no immunosuppression, without other end organ damage, >1 year post-HCT without relapse, I will recommend lung transplantation.86
Because of the rarity and severity of BOS after HCT, it is critically important to consider clinical trials to both better understand disease pathogenesis and identify the best therapeutic options. Currently, 13 interventional studies are listed on clinicaltrials.gov specifically for BOS after HCT, though many have been completed (and discussed above). Studies are in development for the prevention and treatment of this disease. In addition, translational studies to identify biomarkers to predict who will develop BOS or to identify those at high risk for progression will be invaluable. Few biomarkers have been suggested to date specifically for BOS such as serum Krebs von den Lungen-6 or surfactant protein D, but with the increase in trials and translational efforts, hopefully more will be forthcoming.87,88
Conclusions
Although the pathogenesis of BOS remains poorly understood, progress has been made in the diagnosis and treatment of these patients. Consensus of diagnostic criteria has enabled interpretation of the risk factors, prognosis, supportive care, and comparison of treatment strategies. Prospective trials have shown efficacy and offered new therapies. Incorporation of all of these advances has improved survival. However, much remains to be done in this field to better elucidate the biology underlying BOS, identify biomarkers to predict disease, develop more effective targeted therapies, monitor for disease progression, and perhaps, in the future, allow prevention or preemption of BOS after HCT entirely.
Acknowledgments
Greg Yanik, Jennfier Holter-Chakrabarty, Ronald Gress, Stephanie Lee, and Guang-Shing Cheng reviewed the manuscript. Ronald E. Gress supported the prospective trial efforts of K.M.W. in the field of cGVHD.
This work was supported in part by the intramural research program of the National Institutes of Health, National Cancer Institute.
Authorship
Contribution: K.M.W. wrote the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Kirsten M. Williams, Center for Cancer and Blood Disorders, Children’s National Medical Center, The George Washington University Medical Center, 111 Michigan Ave, NW, Washington DC 20010; e-mail: kmwillia@cnmc.org.
References
- 1.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer J, Williams K, Inamoto Y, et al. Pulmonary symptoms measured by the national institutes of health lung score predict overall survival, nonrelapse mortality, and patient-reported outcomes in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(3):337-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird K, Steinberg SM, Grkovic L, et al. National Institutes of Health chronic graft-versus-host disease staging in severely affected patients: organ and global scoring correlate with established indicators of disease severity and prognosis. Biol Blood Marrow Transplant. 2013;19(4):632-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gassas A, Craig-Barnes H, Dell SD, et al. Severe lung injury and lung biopsy in children post-hematopoietic stem cell transplantation: the differences between allogeneic and autologous transplantation. Pediatr Transplant. 2013;17(3):278-284. [DOI] [PubMed] [Google Scholar]
- 5.White DA, Wong PW, Downey R. The utility of open lung biopsy in patients with hematologic malignancies. Am J Respir Crit Care Med. 2000;161(3 Pt 1):723-729. [DOI] [PubMed] [Google Scholar]
- 6.Beschorner WE, Saral R, Hutchins GM, Tutschka PJ, Santos GW. Lymphocytic bronchitis associated with graft-versus-host disease in recipients of bone-marrow transplants. N Engl J Med. 1978;299(19):1030-1036. [DOI] [PubMed] [Google Scholar]
- 7.Crawford SW, Clark JG. Bronchiolitis associated with bone marrow transplantation. Clin Chest Med. 1993;14(4):741-749. [PubMed] [Google Scholar]
- 8.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945-956. [DOI] [PubMed] [Google Scholar]
- 9.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21(3):389-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302(3):306-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunn ML, Godwin JD, Kanne JP, Flowers ME, Chien JW. High-resolution CT findings of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. J Thorac Imaging. 2008;23(4):244-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen JE, Sun XG, Wasserman K. Spirometric criteria for airway obstruction: Use percentage of FEV1/FVC ratio below the fifth percentile, not < 70%. Chest. 2007;131(2):349-355. [DOI] [PubMed] [Google Scholar]
- 13.Williams KM, Hnatiuk O, Mitchell SA, et al. NHANES III equations enhance early detection and mortality prediction of bronchiolitis obliterans syndrome after hematopoietic SCT. Bone Marrow Transplant. 2014;49(4):561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe J, Sato K, Fujikura Y, et al. Tracheobronchomalacia after allogeneic BMT; pulmonary function test findings similar to those of bronchiolitis obliterans. Bone Marrow Transplant. 2014;49(11):1450-1451. [DOI] [PubMed] [Google Scholar]
- 15.Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:95-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoi T, Hirabayashi N, Ito M, et al. ; Nagoya BMT Group. Broncho-bronchiolitis obliterans as a complication of bone marrow transplantation: a clinicopathological study of eight autopsy cases. Virchows Arch. 1997;431(4):275-282. [DOI] [PubMed] [Google Scholar]
- 17.Paz HL, Crilley P, Patchefsky A, Schiffman RL, Brodsky I. Bronchiolitis obliterans after autologous bone marrow transplantation. Chest. 1992;101(3):775-778. [DOI] [PubMed] [Google Scholar]
- 18.Cheng GS, Storer B, Chien JW, et al. Lung function trajectory in bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplant. Ann Am Thorac Soc. 2016;13(11):1932-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297-310. [DOI] [PubMed] [Google Scholar]
- 20.Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015;21(6):984-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark JG, Crawford SW, Madtes DK, Sullivan KM. Obstructive lung disease after allogeneic marrow transplantation. Clinical presentation and course. Ann Intern Med. 1989;111(5):368-376. [DOI] [PubMed] [Google Scholar]
- 22.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant. 2003;9(10):657-666. [DOI] [PubMed] [Google Scholar]
- 23.Nishio N, Yagasaki H, Takahashi Y, et al. Late-onset non-infectious pulmonary complications following allogeneic hematopoietic stem cell transplantation in children. Bone Marrow Transplant. 2009;44(5):303-308. [DOI] [PubMed] [Google Scholar]
- 24.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(7):1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duncan CN, Buonanno MR, Barry EV, Myers K, Peritz D, Lehmann L. Bronchiolitis obliterans following pediatric allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(11):971-975. [DOI] [PubMed] [Google Scholar]
- 26.Nakasone H, Kanda J, Yano S, et al. ; GVHD Working Group of the Japan Society for Hematopoietic Cell Transplantation. A case-control study of bronchiolitis obliterans syndrome following allogeneic hematopoietic stem cell transplantation. Transpl Int. 2013;26(6):631-639. [DOI] [PubMed] [Google Scholar]
- 27.Santo Tomas LH, Loberiza FR Jr, Klein JP, et al. Risk factors for bronchiolitis obliterans in allogeneic hematopoietic stem-cell transplantation for leukemia. Chest. 2005;128(1):153-161. [DOI] [PubMed] [Google Scholar]
- 28.Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168(2):208-214. [DOI] [PubMed] [Google Scholar]
- 29.Ditschkowski M, Elmaagacli AH, Koldehoff M, Gromke T, Trenschel R, Beelen DW. Bronchiolitis obliterans after allogeneic hematopoietic SCT: further insight--new perspectives? Bone Marrow Transplant. 2013;48(9):1224-1229. [DOI] [PubMed] [Google Scholar]
- 30.Rhee CK, Ha JH, Yoon JH, et al. Risk factor and clinical outcome of bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation. Yonsei Med J. 2016;57(2):365-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duque-Afonso J, Ihorst G, Wäsch R, et al. Identification of risk factors for bronchiolitis obliterans syndrome after reduced toxicity conditioning before hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48(8):1098-1103. [DOI] [PubMed] [Google Scholar]
- 32.Gazourian L, Rogers AJ, Ibanga R, et al. Factors associated with bronchiolitis obliterans syndrome and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Am J Hematol. 2014;89(4):404-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12(2):138-151. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter PA, Kitko CL, Elad S, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2015;21(7):1167-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hildebrandt GC, Fazekas T, Lawitschka A, et al. Diagnosis and treatment of pulmonary chronic GVHD: report from the consensus conference on clinical practice in chronic GVHD. Bone Marrow Transplant. 2011;46(10):1283-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng GS, Campbell AP, Xie H, et al. Correlation and agreement of handheld spirometry with laboratory spirometry in allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2016;22(5):925-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galbán CJ, Boes JL, Bule M, et al. Parametric response mapping as an indicator of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(10):1592-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boes JL, Hoff BA, Bule M, et al. Parametric response mapping monitors temporal changes on lung CT scans in the subpopulations and intermediate outcome measures in COPD Study (SPIROMICS). Acad Radiol. 2015;22(2):186-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lama VN, Murray S, Mumford JA, et al. Prognostic value of bronchiolitis obliterans syndrome stage 0-p in single-lung transplant recipients. Am J Respir Crit Care Med. 2005;172(3):379-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abedin S, Yanik GA, Braun T, et al. Predictive value of bronchiolitis obliterans syndrome stage 0p in chronic graft-versus-host disease of the lung. Biol Blood Marrow Transplant. 2015;21(6):1127-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(suppl 1):S106-S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah SK, McAnally KJ, Seoane L, et al. Analysis of pulmonary non-tuberculous mycobacterial infections after lung transplantation. Transpl Infect Dis. 2016;18(4):585-591. [DOI] [PubMed] [Google Scholar]
- 43.Mirsaeidi M, Hadid W, Ericsoussi B, Rodgers D, Sadikot RT. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int J Infect Dis. 2013;17(11):e1000-e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayes D Jr, Weiland A, Kirkby S, Galantowicz M, McConnell PI, Tobias JD. Gram-negative infection and bronchiectasis in lung transplant recipients with bronchiolitis obliterans syndrome. Thorac Cardiovasc Surg. 2013;61(3):240-245. [DOI] [PubMed] [Google Scholar]
- 45.Shino MY, Weigt SS, Li N, et al. CXCR3 ligands are associated with the continuum of diffuse alveolar damage to chronic lung allograft dysfunction. Am J Respir Crit Care Med. 2013;188(9):1117-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams KM, Ahn KW, Chen M, et al. The incidence, mortality and timing of Pneumocystis jiroveci pneumonia after hematopoietic cell transplantation: a CIBMTR analysis. Bone Marrow Transplant. 2016;51(4):573-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khalid M, Aljurf M, Saleemi S, et al. Gastroesophageal reflux disease and its association with bronchiolitis obliterans syndrome in allogeneic hematopoietic stem cell transplant recipients. Exp Clin Transplant. 2013;11(3):270-273. [DOI] [PubMed] [Google Scholar]
- 48.Nicod LP. Mechanisms of airway obliteration after lung transplantation. Proc Am Thorac Soc. 2006;3(5):444-449. [DOI] [PubMed] [Google Scholar]
- 49.Lau CL, Palmer SM, Howell DN, et al. Laparoscopic antireflux surgery in the lung transplant population. Surg Endosc. 2002;16(12):1674-1678. [DOI] [PubMed] [Google Scholar]
- 50.Tran J, Norder EE, Diaz PT, et al. Pulmonary rehabilitation for bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(8):1250-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waseda K, Tanimoto Y, Ichiba S, et al. Successful extracorporeal life support for life-threatening hypercapnia with bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. Acta Med Okayama. 2011;65(6):403-406. [DOI] [PubMed] [Google Scholar]
- 52.Ahn JH, Jo KW, Song JW, et al. Prognostic role of FEV1 for survival in bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation. Clin Transplant. 2015;29(12):1133-1139. [DOI] [PubMed] [Google Scholar]
- 53.Duarte RF, Greinix H, Rabin B, et al. Uptake and use of recommendations for the diagnosis, severity scoring and management of chronic GVHD: an international survey of the EBMT-NCI Chronic GVHD Task Force. Bone Marrow Transplant. 2014;49(1):49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams KM, Cheng GS, Pusic I, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(4):710-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schultz MJ. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis. J Antimicrob Chemother. 2004;54(1):21-28. [DOI] [PubMed] [Google Scholar]
- 56.Gerhardt SG, McDyer JF, Girgis RE, Conte JV, Yang SC, Orens JB. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med. 2003;168(1):121-125. [DOI] [PubMed] [Google Scholar]
- 57.Gottlieb J, Szangolies J, Koehnlein T, Golpon H, Simon A, Welte T. Long-term azithromycin for bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2008;85(1):36-41. [DOI] [PubMed] [Google Scholar]
- 58.Khalid M, Al Saghir A, Saleemi S, et al. Azithromycin in bronchiolitis obliterans complicating bone marrow transplantation: a preliminary study. Eur Respir J. 2005;25(3):490-493. [DOI] [PubMed] [Google Scholar]
- 59.Yates B, Murphy DM, Forrest IA, et al. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2005;172(6):772-775. [DOI] [PubMed] [Google Scholar]
- 60.Medoff BD, Seung E, Wain JC, et al. BLT1-mediated T cell trafficking is critical for rejection and obliterative bronchiolitis after lung transplantation. J Exp Med. 2005;202(1):97-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilborn J, Bailie M, Coffey M, Burdick M, Strieter R, Peters-Golden M. Constitutive activation of 5-lipoxygenase in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1996;97(8):1827-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Or R, Gesundheit B, Resnick I, et al. Sparing effect by montelukast treatment for chronic graft versus host disease: a pilot study. Transplantation. 2007;83(5):577-581. [DOI] [PubMed] [Google Scholar]
- 63.Bashoura L, Gupta S, Jain A, et al. Inhaled corticosteroids stabilize constrictive bronchiolitis after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(1):63-67. [DOI] [PubMed] [Google Scholar]
- 64.Bergeron A, Belle A, Chevret S, et al. Combined inhaled steroids and bronchodilatators in obstructive airway disease after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39(9):547-553. [DOI] [PubMed] [Google Scholar]
- 65.Whitford H, Orsida B, Kotsimbos T, et al. Bronchoalveolar lavage cellular profiles in lung transplantation: the effect of inhaled corticosteroids. Ann Transplant. 2000;5(3):31-37. [PubMed]
- 66.Whitford H, Walters EH, Levvey B, et al. Addition of inhaled corticosteroids to systemic immunosuppression after lung transplantation: a double-blind, placebo-controlled trial. Transplantation. 2002;73(11):1793-1799. [DOI] [PubMed] [Google Scholar]
- 67.Lam DC, Lam B, Wong MK, et al. Effects of azithromycin in bronchiolitis obliterans syndrome after hematopoietic SCT--a randomized double-blinded placebo-controlled study. Bone Marrow Transplant. 2011;46(12):1551-1556. [DOI] [PubMed] [Google Scholar]
- 68.Lemonnier F, Rivaud E, Neveu H, et al. Azithromycin in bronchiolitis obliterans syndrome after hematopoietic SCT. Bone Marrow Transplant. 2012;47(10):1374. [DOI] [PubMed] [Google Scholar]
- 69.Norman BC, Jacobsohn DA, Williams KM, et al. Fluticasone, azithromycin and montelukast therapy in reducing corticosteroid exposure in bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: a case series of eight patients. Bone Marrow Transplant. 2011;46(10):1369-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bergeron A, Chevret S, Chagnon K, et al. Budesonide/formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2015;191(11):1242-1249. [DOI] [PubMed] [Google Scholar]
- 71.Kim SW, Rhee CK, Kim YJ, Lee S, Kim HJ, Lee JW. Therapeutic effect of budesonide/formoterol, montelukast and N-acetylcysteine for bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Respir Res. 2016;17(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jaksch P, Scheed A, Keplinger M, et al. A prospective interventional study on the use of extracorporeal photopheresis in patients with bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2012;31(9):950-957. [DOI] [PubMed] [Google Scholar]
- 73.Besnier DP, Chabannes D, Mahé B, et al. Treatment of graft-versus-host disease by extracorporeal photochemotherapy: a pilot study. Transplantation. 1997;64(1):49-54. [DOI] [PubMed] [Google Scholar]
- 74.Ilhan O, Arat M, Arslan O, et al. Extracorporeal photoimmunotherapy for the treatment of steroid refractory progressive chronic graft-versus-host disease. Transfus Apheresis Sci. 2004;30(3):185-187. [DOI] [PubMed] [Google Scholar]
- 75.Smith EP, Sniecinski I, Dagis AC, et al. Extracorporeal photochemotherapy for treatment of drug-resistant graft-vs.-host disease. Biol Blood Marrow Transplant. 1998;4(1):27-37. [DOI] [PubMed] [Google Scholar]
- 76.Foss FM, DiVenuti GM, Chin K, et al. Prospective study of extracorporeal photopheresis in steroid-refractory or steroid-resistant extensive chronic graft-versus-host disease: analysis of response and survival incorporating prognostic factors. Bone Marrow Transplant. 2005;35(12):1187-1193. [DOI] [PubMed] [Google Scholar]
- 77.Lucid CE, Savani BN, Engelhardt BG, et al. Extracorporeal photopheresis in patients with refractory bronchiolitis obliterans developing after allo-SCT. Bone Marrow Transplant. 2011;46(3):426-429. [DOI] [PubMed] [Google Scholar]
- 78.Hannani D, Merlin E, Gabert F, et al. Photochemotherapy induces a faster apoptosis of alloreactive activated T cells than of nonalloreactive resting T cells in graft versus host disease. Transplantation. 2010;90(11):1232-1238. [DOI] [PubMed] [Google Scholar]
- 79.Papp G, Horvath IF, Barath S, et al. Immunomodulatory effects of extracorporeal photochemotherapy in systemic sclerosis. Clin Immunol. 2012;142(2):150-159. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez AL, Berger CL, Remington J, Girardi M, Tigelaar RE, Edelson RL. Integrin-driven monocyte to dendritic cell conversion in modified extracorporeal photochemotherapy. Clin Exp Immunol. 2014;175(3):449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yanik GA, Mineishi S, Levine JE, et al. Soluble tumor necrosis factor receptor: enbrel (etanercept) for subacute pulmonary dysfunction following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(7):1044-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whittle R, Taylor PC. Circulating B-cell activating factor level predicts clinical response of chronic graft-versus-host disease to extracorporeal photopheresis. Blood. 2011;118(24):6446-6449. [DOI] [PubMed] [Google Scholar]
- 83.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113(16):3865-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuzmina Z, Krenn K, Petkov V, et al. CD19(+)CD21(low) B cells and patients at risk for NIH-defined chronic graft-versus-host disease with bronchiolitis obliterans syndrome. Blood. 2013;121(10):1886-1895. [DOI] [PubMed] [Google Scholar]
- 85.Lorillon G, Robin M, Meignin V, et al. Rituximab in bronchiolitis obliterans after haematopoietic stem cell transplantation. Eur Respir J. 2011;38(2):470-472. [DOI] [PubMed] [Google Scholar]
- 86.Cheng GS, Edelman JD, Madtes DK, Martin PJ, Flowers ME. Outcomes of lung transplantation after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(8):1169-1175. [DOI] [PubMed] [Google Scholar]
- 87.Nakane T, Nakamae H, Kamoi H, et al. Prognostic value of serum surfactant protein D level prior to transplant for the development of bronchiolitis obliterans syndrome and idiopathic pneumonia syndrome following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;42(1):43-49. [DOI] [PubMed] [Google Scholar]
- 88.Gassas A, Schechter T, Krueger J, et al. Serum Krebs Von Den Lungen-6 as a biomarker for early detection of bronchiolitis obliterans syndrome in children undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(8):1524-1528. [DOI] [PubMed] [Google Scholar]