Abstract
The crustacean stomatogastric nervous system is a long-standing test bed for studies of circuit dynamics and neuromodulation. We give a brief update on the most recent work on this system, with an emphasis on the broader implications for understanding neural circuits. In particular, we focus on new findings underlining that different levels of dynamics taking place at different time scales all interact in multiple ways. Dynamics due to synaptic and intrinsic neuronal properties, neuromodulation, and long-term gene expression-dependent regulation are not independent, but influence each other. Extensive research on the stomatogastric system shows that these dynamic interactions convey robustness to circuit operation, while facilitating the flexibility of producing multiple circuit outputs.
Introduction
Studying neural circuits comes with a number of technical and conceptual challenges [1]. Any given circuit is not equally amenable to all technical approaches, which makes bridging levels of analysis difficult. In addition, numerical complexity, poorly defined cell types, and incomplete connectivity maps often make inferences from cellular to circuit function tentative at best. Furthermore, establishing functional boundaries for circuits embedded in larger brain areas can be difficult. Some of these problems are less severe in invertebrate preparations, which for this reason have been useful in unraveling evolutionarily conserved principles of circuit operation.
The stomatogastric nervous system (STNS) stands out for its utility in studying how neuronal and synaptic properties give rise to circuit activity and are shaped by neuromodulation and other regulatory processes [2]. The pattern-generating circuits of the STNS play an important role in feeding in all arthropods. However, the insect STNS has been studied mainly from a developmental and anatomical perspective [3], and although some of the activity patterns and neuromodulators involved in regulating feeding have been studied [4–6], the neural circuits that underlie these activities are as yet unidentified. Consequently, we will focus on the crustacean STNS.
In lobsters and crabs, the STNS is a conveniently anatomically separated system of a few ganglia that controls rhythmic activity of the foregut and can easily be studied in vitro. The stomatogastric ganglion (STG) contains only ~30 neurons, comprising two overlapping central pattern generating circuits that produce the slow gastric mill rhythm and the faster pyloric rhythm (Fig. 1). The neurons are large and easily identifiable, and their connectivity has long been established. Considering the ongoing efforts in connectomics in many systems, it is humbling that such connectivity diagrams (Fig. 1B) provide little explanation of circuit activity and dynamics. This is due to the nonlinear dynamics of membranes and synapses, neuromodulation, and long-term regulation, all of which can influence circuit activity over multiple time scales [7]. Here we review recent work on several aspects of these different time scales of circuit dynamics in the STG, and in particular how processes at different time scales interact.
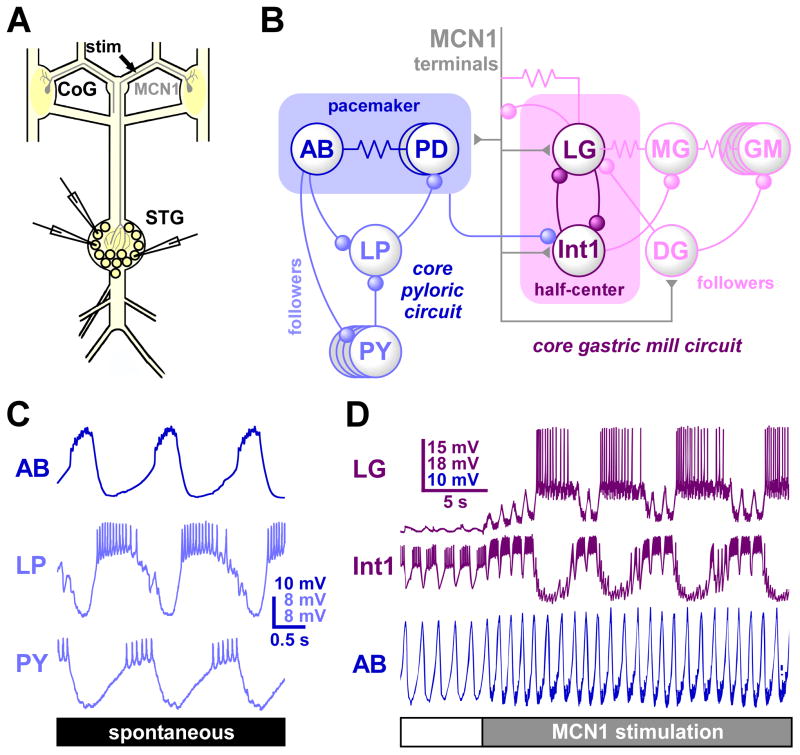
Figure 1.
The pyloric and gastric mill central pattern generating circuits of the stomatogastric ganglion. A: Schematic of the isolated STNS. The STG contains the pyloric and gastric mill circuits. The commissural ganglia (CoG) contain the cell bodies of projection neurons like the modulatory commisural neuron 1 (MCN1), which project to the neuropil of the STG. B: The core pyloric and gastric mill circuit diagrams. Not all cells types and synapses are shown. Inhibitory chemical synapses are shown as circles, electrical coupling as resistor symbols, and excitatory inputs from MCN1 as triangles. Rhythm generation is based on intrinsic oscillatory properties of the pacemaker kernel in the pyloric circuit, and on reciprocal inhibitory connections between non-oscillatory neurons (half-center) in the gastric mill circuit. Note that both circuits are interconnected by direct synapses and through feedback to the terminals of projection neurons. C: The typical tri-phasic pyloric pattern. In each cycle, a pacemaker burst is followed by neurons burst in two different phases, in rebound from pacemaker inhibition. D: The bi-phasic gastric mill rhythm is often not spontaneously active, but can be activated by stimulating modulatory projection neurons like MCN1. Note that the interconnection between both circuits leads to substantial pyloric modulation of the much slower gastric mill neuron bursting. The pyloric pacemaker neuron AB is shown as a reference for pyloric timing. A, B, & D are modified from reference [9]; C is modified from reference [8].
Dynamics arising from intrinsic and synaptic properties
The pyloric rhythm is based on intrinsic oscillatory properties of a pacemaker kernel, and follower neurons burst in rebound from inhibition by the pacemaker [8] (Fig. 1B&C). The gastric mill rhythm arises from synaptic connectivity of non-oscillatory neurons [9] (Fig. 1B&D). The intrinsic neuronal and synaptic properties are well described in the STG, but it is not necessarily obvious how these components function within the context of circuit activity. Dynamics arising from the interactions of synaptic inputs and postsynaptic properties have recently been studied experimentally and theoretically in the context of how inhibitory feedback from follower neurons affects the pyloric pacemaker oscillation. At its usual timing with respect to the phase of oscillation, feedback inhibition has surprisingly little effect on the mean period of the rhythm, but reduces cycle-to-cycle variability and therefore stabilizes oscillations [10–12]. Similar stabilizing influences of synaptic input on irregularly firing neurons have also been theoretically demonstrated for network-based oscillations [13]. The effect of timing of synaptic input with respect to the phase of ongoing activity also allows analyzing the contributions of specific ionic conductances [14]. Another window into how neuronal and synaptic properties shape circuit activity is provided by the observation that pyloric neurons and synapses have preferred frequencies, i.e. show best responses at specific input frequencies. The distinct frequency preferences of different network components are correlated with the period of the rhythm and potentially the phasing of neurons, and are altered when neuromodulators change circuit activity [15*].
Neuromodulation
The STNS is perhaps best known for its role in uncovering principles of neuromodulation. Metabotropic actions of neuromodulators are at the root of the ability of circuits to produce different activity patterns [16–18]. The pyloric rhythm is continuously active and its stereotypical triphasic activity (Fig. 1C) can be configured by neuromodulators in vitro into different temporal patterns. The gastric mill rhythm is often not spontaneously active, but can be activated by modulatory projection neurons to generate distinct patterns (Fig. 1D). In vivo studies show that pyloric activity is indeed changed after feeding, and that distinct gastric mill rhythms exist in the intact animal [19*,20]. This flexibility stems from the fact that the STG is affected by a large number of neuromodulators, including classic neurotransmitters, biogenic amines, and many neuropeptides, which are either released from descending projection neurons, or present in the hemolymph. Even considering substantial flexibility, the sheer number of neuropeptides (>100) is puzzling. However, some isoforms of the same family may activate promiscuous receptors and not have distinct actions[21,22]. Great strides have been made identifying neuropeptides with mass spectrometry, and it is now possible to quantitatively map them to specific tissue regions in individual animals [23*, 24], or detect abundance changes in hemolymph after feeding [25].
The circuit-wide actions of biogenic amines and a few neuropeptides have been studied thoroughly, and show fairly distinct organizing principles (Fig. 2). Amines have divergent cellular and synaptic actions, i.e. modulator and cell type-specific effects on different subsets of multiple ion channel types across all neurons [16] (Fig. 2A&B). Different amines may all affect every single neuron and synapse, but the sum of effects on their multiple subcellular targets is different. Such divergent actions extend even to differential effects on synaptic dynamics [26]. Neuropeptides, on the other hand, have mostly convergent actions on a limited set of intracellular targets. In particular, they all activate the same modulator-activated inward current (IMI) [27] (Fig. 2C) which shows unusual voltage-dependence in that it is regulated by both intra- and extracellular calcium [28]. This single current represents a powerful way to activate the pyloric circuit [29], and recent experimental and theoretical work shows that just the negative slope conductance of its IV curve is sufficient to elicit oscillatory activity [30,31]. The specificity of effects of different neuropeptides stems from the fact that each activates IMI in a different subset of neurons [32] (Fig. 2D). In addition, specific effects can arise from differences in the temporal structure of release [33], and from cell type-specific differences in receptor expression levels and associated differences in the magnitude of IMI responses [34**]. On the flip side, different modulatory inputs can also result in very similar circuit activity, as the same gastric mill rhythm can arise from distinct rhythm-generating mechanisms configured by different neuropeptides [35**]. Many neuropeptides exist as co-transmitters in projection neurons, which in effect modify their actions. The spatial pattern of release may also matter, as co-transmitters can be released differentially into different target areas [36,37].
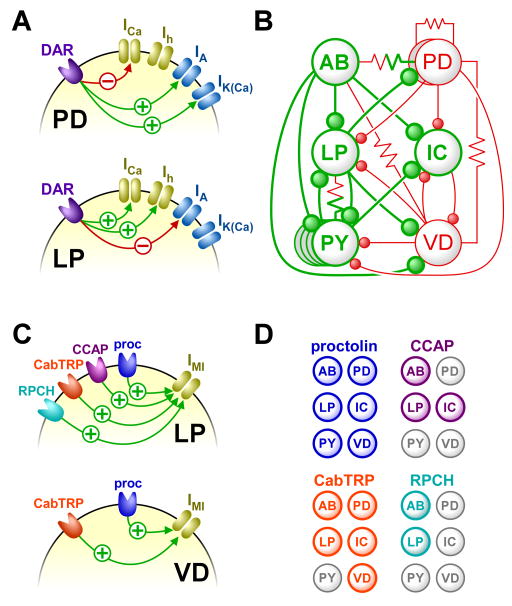
Figure 2.
Different organizing principles underlying circuit modulation by biogenic amines and neuropeptides. A: In different cell types, activation of dopamine receptors (DAR) can affect the gating properties of different subsets of ion channels, and the effects can have a different sign. Ion channels giving rise to inward currents are shown in yellow, and those giving rise to outward currents in blue. B: The sum effects of the diverse cellular loci of dopamine actions are functional enhancements (green) or recuctions (red) of excitability in all pyloric neurons and strength of all pyloric synapses. C: Neuropeptide modulation affects a limited number of intracellular targets. Different neuropeptides all converge on the same voltage-gated inward current (IMI), but different cell types respond to a different subset of neuropeptides. RPCH: red pigment concentrating hormone; CabTRP: Cancer borealis tachykinin-related peptide; CCAP: crustacean cardioactive peptide; proc: proctolin. D: Despite the convergence of neuropeptide effects on the same subcellular target, the different subsets of circuit neurons affected by each neuropetide give rise to divergent effects on circuit activity. A & B are modified from reference [16], C & D are modified from references [27] and [32].
Neuromodulation does not just affect neural circuits, but also their inputs and outputs. Input from modulatory projection neurons can be shaped by feedback from the CPG, which in turn alters CPG activity [38*]. Such bidirectional interactions themselves are modifiable, because the feedback synapses from CPG to projections neurons can also be modulated [39]. At the output level, neuromodulation does not just affect how activity is generated, but potentially also how it is propagated, as axonal spike conduction can be altered by modulators [40].
Variability and regulation of stable circuit activity
All STG neurons have qualitatively similar complements of ionic currents, but differences in their relative magnitude convey distinct intrinsic properties. In consequence, the contribution of a given ionic current can differ across neurons within one circuit, or between pyloric and gastric mill neurons [41]. The characteristic intrinsic properties of each cell type, manifest in stereotyped voltage trajectories and consistent responses to input, is surprisingly not due to tightly controlled expression levels of individual ionic currents. Current densities and mRNA expression for channel genes in a cell type can be hugely variable across individuals, even when the cell type includes only a single neuron, which raises the question how cell identities and physiological phenotypes are maintained. Stable output in the face of component variability is thought to stem from homeostatic regulatory mechanisms that allow individual animals to reach one of many permissive parameter combinations, and research using the STNS has been on the forefront of exploring this issue [42].
Ionic currents do not vary independently, but cell type-specific groups of ion channel types often covary, which is thought to have compensatory function for the variability [43,44]. Such co-variation of ionic conductances can keep features of pyloric neuron activity invariant and robust to perturbations [45], and are maintained by activity-dependent feedback [46**]. These experimental findings have inspired a series of theoretical studies that show that co-variation can support maintenance of firing phase across neurons [47], and that simple homeostatic tuning rules can easily incorporate conductance correlations [48] and may in fact depend on these correlations to give rise to stable circuit activity [49**]. Regulation of synaptic strength and dynamics may also be involved in compensating for variable circuit architectures and neuronal properties [50,51], but it is not clear to which degree they may covary with other parameters.
Neuromodulation and long-term regulation of circuit properties
Given that circuit components are both variable across individuals and malleable to many neuromodulators, it is surprising that circuit activity is robust and neuromodulators cause consistent activity changes across individuals [16,52,53]. However, at least in the long term, neuromodulation does not seem to be the cause of variability, but can reduce it. When neuromodulatory inputs are removed from the STG, rhythmic activity can slow or cease. Over the course of tens of hours, circuit properties are then reconfigured. Such reconfiguration can be sufficient to recover pyloric activity [54,55], but more recent work shows that short of recovery, long-term removal of neuromodulators and subsequent changes in circuit components increases variability of both pyloric and gastric mill activity [56,57]. Removal of neuromodulation also causes changes severe enough to prevent functional activity when inputs are restored [58].
It is challenging to pry apart the roles that activity-dependent or neuromodulator-dependent mechanisms play in these changes, but two lines of recent studies show that neuromodulators contribute substantially. The first set of studies shows that neuromodulatory input plays an important role in the long-term maintenance of the cell type-specific co-variation of many ionic currents, because this co-variation can be lost or changed after removal of neuromodulators [54,55,59]. These effects depend on cell identity, as they differ across cell types [55]. The second line explores the prolonged effects of tonic nanomolar levels of dopamine (DA) on network activity. In pyloric follower neurons like the lateral pyloric (LP) neuron, the balance between the transient potassium current IA and the hyperpolarization-activated current Ih plays an important role in determining the onset phase of activity. DA tone, over hours, maintains the levels of IA, thereby stabilizing phase, and counteracting reduction of IA in response to acute micromolar application of DA [60]. Interestingly, acute reduction of IA and other network influences of μM DA change the bursting activity of the LP neuron in a manner that results in an activity and DA tone-dependent decrease in Ih, thereby homeostatically recovering control levels of LP activity [61]. The second messenger pathway as well as components of the transcriptional and translational regulation underlying these effects have recently been identified [62,63, 64*].
Robustness to temperature changes
Challenges to the need for stable circuit activity can also come from changing physical parameters such as temperature. Lobsters and crabs are poikilothermic and experience substantial temperature changes. Similar network activity is achieved with variable sets of synaptic and intrinsic properties across individuals, and temperature differentially affects biological processes with a wide range of Q10 values. Therefore, temperature change could have highly variable consequences. Nevertheless, the pyloric pattern frequency consistently tracks temperature linearly over a wide range, with stable phase relationships between neurons, only revealing individual differences at high temperatures when the rhythm crashes. This is true for the full circuit in vitro [65] and in vivo [66], as well as the isolated pacemaker kernel [67]. Pacemaker models can produce similar activity with many possible combinations conductance magnitudes and Q10 values, robust over a range of temperatures [68]. Neuromodulation may play an important part in conferring robustness to the circuits. Activity levels of modulatory neurons increase with temperature, and the resulting enhancement of peptide-evoked IMI can rescue activity of the gastric mill circuit by counteracting temperature-induced increase in leak currents [69*]. Mass spectrometry-based approaches show that the neuropeptide complement changes with increasing temperature [70].
Conclusions
The STNS continues to be a valuable model for uncovering fundamental principles underlying circuit dynamics. The recent work in this system discussed here highlights the need to consider how mechanisms that span different time scales interact (Fig. 3). The dynamics of intrinsic neuron properties and synapses are constantly shaped by neuromodulation, and are continuously tuned by long-term regulatory mechanisms that maintain stable circuit function. To produce proper circuit output, these regulatory mechanisms do not rely on fixed parameter combinations, but use correlative rules which can result in many different solutions. Yet those solutions must incorporate consistent responses to neuromodulators which, themselves, can be involved in long-term regulatory mechanisms. Such findings are likely generalizable to any circuit and have been greatly aided by, or indeed only been possible because of the numerical simplicity of the STG circuits. However, both the dynamics and the many different possible parameter combinations demonstrate that even such small circuits are anything but simple, which should inform any attempts at functionally dissecting larger circuits.
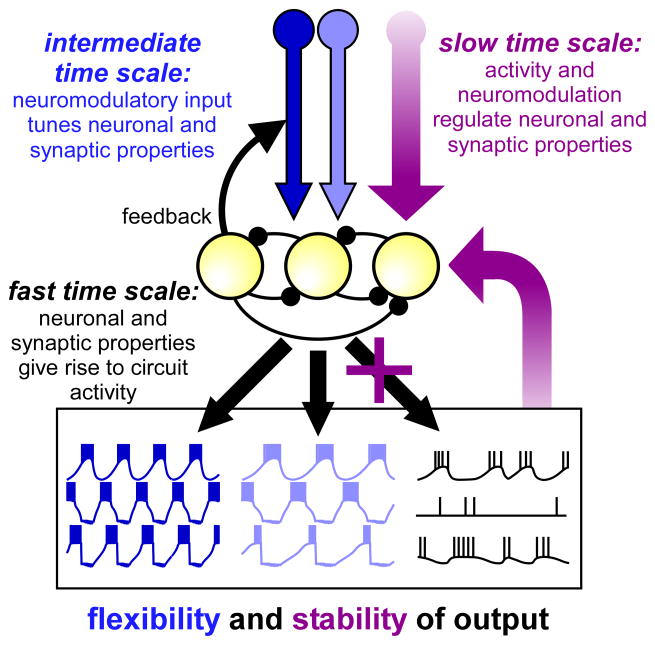
Figure 3.
Schematic of interactions of different regulatory mechanisms that affect circuit operation at different time scales. At the fast time scale, neuronal and synaptic properties give rise to circuit activity. At the intermediate time scale, neuromodulators convey flexibility, as different neuromodulators can tune neuronal and synaptic properties to generate different circuit outputs. Circuit activity itself can shape input patterns from modulatory neurons through feedback connections. At the slow time scale, long-term regulatory mechanisms dependent on neuronal activity and the presence of neuromodulators convey stability of circuit output and prevent circuit crashes.
Highlights.
Analysis of small circuits reveals interacting dynamics at different time scales.
Synaptic and intrinsic properties, neuromodulation, and long-term regulation interact.
Neuromodulation ties together short-term flexibility and long-term stability.
Circuit parameters are variable, but correlated to ensure stable function.
Acknowledgments
This work was supported in part by NIH grants NS083319 and MH060605.
Footnotes
Conflict of interest statement
Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Yuste R. Circuit neuroscience: the road ahead. Front Neurosci. 2008;2:6–9. doi: 10.3389/neuro.01.017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez K, Myers LG, Bowser M, Kidd T. Genetic Tools for the Analysis of Drosophila Stomatogastric Nervous System Development. PLoS One. 2015;10:e0128290. doi: 10.1371/journal.pone.0128290. 2015/06/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Audsley N, Weaver RJ. Neuropeptides associated with the regulation of feeding in insects. Gen Comp Endocrinol. 2009;162:93–104. doi: 10.1016/j.ygcen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Rand D, Knebel D, Ayali A. The effect of octopamine on the locust stomatogastric nervous system. Front Physiol. 2012;3:288. doi: 10.3389/fphys.2012.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoofs A, Spiess R. Anatomical and functional characterisation of the stomatogastric nervous system of blowfly (Calliphora vicina) larvae. J Insect Physiol. 2007;53:349–360. doi: 10.1016/j.jinsphys.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Gjorgjieva J, Drion G, Marder E. Computational implications of biophysical diversity and multiple timescales in neurons and synapses for circuit performance. Curr Opin Neurobiol. 2016;37:44–52. doi: 10.1016/j.conb.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouser C, Nadim F, Bose A. Maintaining phase of the crustacean tri-phasic pyloric rhythm. J Math Biol. 2008;57:161–181. doi: 10.1007/s00285-007-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartos M, Manor Y, Nadim F, Marder E, Nusbaum MP. Coordination of fast and slow rhythmic neuronal circuits. J Neurosci. 1999;19:6650–6660. doi: 10.1523/JNEUROSCI.19-15-06650.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooper RM, Tikidji-Hamburyan RA, Canavier CC, Prinz AA. Feedback control of variability in the cycle period of a central pattern generator. J Neurophysiol. 2015;114:2741–2752. doi: 10.1152/jn.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadim F, Zhao S, Bose A. A PRC Description of How Inhibitory Feedback Promotes Oscillation Stability. In: Schultheiss WN, Prinz AA, Butera JR, editors. Phase Response Curves in Neuroscience: Theory, Experiment, and Analysis. Springer; New York: 2012. pp. 399–417. [Google Scholar]
- 12.Nadim F, Zhao S, Zhou L, Bose A. Inhibitory feedback promotes stability in an oscillatory network. J Neural Eng. 2011;8:065001. doi: 10.1088/1741-2560/8/6/065001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reyes MB, Carelli PV, Sartorelli JC, Pinto RD. A modeling approach on why simple central pattern generators are built of irregular neurons. PLoS One. 2015;10:e0120314. doi: 10.1371/journal.pone.0120314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soofi W, Prinz AA. Differential effects of conductances on the phase resetting curve of a bursting neuronal oscillator. J Comput Neurosci. 2015;38:539–558. doi: 10.1007/s10827-015-0553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Tseng HA, Martinez D, Nadim F. The frequency preference of neurons and synapses in a recurrent oscillatory network. J Neurosci. 2014;34:12933–12945. doi: 10.1523/JNEUROSCI.2462-14.2014. Synapses and neurons often have preferred frequencies (as in resonance), an input frequency at which they produce a maximal response. This study examines pacemaker and follower neurons, connected with reciprocally inhibitory synapses, and shows that the two neurons and the synapses all have distinct preferred frequencies which can be modified by peptidergic modulation. Distinct preferred frequencies of network components may interact to shape, rather than directly determine, the network frequency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris-Warrick RM. Neuromodulation and flexibility in Central Pattern Generator networks. Curr Opin Neurobiol. 2011;21:685–692. doi: 10.1016/j.conb.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadim F, Bucher D. Neuromodulation of neurons and synapses. Curr Opin Neurobiol. 2014;29C:48–56. doi: 10.1016/j.conb.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *19.Diehl F, White RS, Stein W, Nusbaum MP. Motor circuit-specific burst patterns drive different muscle and behavior patterns. J Neurosci. 2013;33:12013–12029. doi: 10.1523/JNEUROSCI.1060-13.2013. The degree to which different firing patterns of neurons during different versions of circuit output described in vitro translate to actual different behavioral output in vivo is hard to predict, owing to the presence of sensory feedback, hormones, and other factors in intact animals. This paper demonstrates that the different versions of the gastric mill rhythm activate neuromuscular junctions differentially despite slow integrative properties, and that these differences in motor patterns are in fact translated in different movement patterns of the gastric mill in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yarger AM, Stein W. Sources and range of long-term variability of rhythmic motor patterns in vivo. J Exp Biol. 2015;218:3950–3961. doi: 10.1242/jeb.126581. [DOI] [PubMed] [Google Scholar]
- 21.Cruz-Bermudez ND, Fu Q, Kutz-Naber KK, Christie AE, Li L, Marder E. Mass spectrometric characterization and physiological actions of GAHKNYLRFamide, a novel FMRFamide-like peptide from crabs of the genus Cancer. J Neurochem. 2006;97:784–799. doi: 10.1111/j.1471-4159.2006.03747.x. [DOI] [PubMed] [Google Scholar]
- 22.Dickinson PS, Kurland SC, Qu X, Parker BO, Sreekrishnan A, Kwiatkowski MA, Williams AH, Ysasi AB, Christie AE. Distinct or shared actions of peptide family isoforms: II. Multiple pyrokinins exert similar effects in the lobster stomatogastric nervous system. J Exp Biol. 2015;218:2905–2917. doi: 10.1242/jeb.124818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Chen R, Ouyang C, Xiao M, Li L. In situ identification and mapping of neuropeptides from the stomatogastric nervous system of Cancer borealis. Rapid Commun Mass Spectrom. 2014;28:2437–2444. doi: 10.1002/rcm.7037. This study marks a significant improvement in the identification and localization of neuropeptides in the STNS. Direct tissue analysis and MALDI (matrix-assisted laser desorption/ionization) imaging allowed for neuropeptide profiling and mapping in individual STGs and associated nerves, with higher sensitivity than large scale HPLC-MALDI analysis of pooled tissues. This is a promising tool for the analysis of neuroeptide release under different physiological conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye H, Hui L, Kellersberger K, Li L. Mapping of neuropeptides in the crustacean stomatogastric nervous system by imaging mass spectrometry. J Am Soc Mass Spectrom. 2013;24:134–147. doi: 10.1007/s13361-012-0502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmerberg CM, Liang Z, Li L. Data-independent MS/MS quantification of neuropeptides for determination of putative feeding-related neurohormones in microdialysate. ACS Chem Neurosci. 2015;6:174–180. doi: 10.1021/cn500253u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kvarta MD, Harris-Warrick RM, Johnson BR. Neuromodulator-evoked synaptic metaplasticity within a central pattern generator network. J Neurophysiol. 2012;108:2846–2856. doi: 10.1152/jn.00586.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swensen AM, Marder E. Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J Neurosci. 2000;20:6752–6759. doi: 10.1523/JNEUROSCI.20-18-06752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray M, Golowasch J. Voltage Dependence of a Neuromodulator-Activated Ionic Current. eneuro. 2016;3 doi: 10.1523/ENEURO.0038-16.2016. ENEURO.0038-0016.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharp AA, O’Neil MB, Abbott LF, Marder E. Dynamic clamp: computer-generated conductances in real neurons. J Neurophysiol. 1993;69:992–995. doi: 10.1152/jn.1993.69.3.992. [DOI] [PubMed] [Google Scholar]
- 30.Bose A, Golowasch J, Guan Y, Nadim F. The role of linear and voltage-dependent ionic currents in the generation of slow wave oscillations. J Comput Neurosci. 2014;37:229–242. doi: 10.1007/s10827-014-0498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao S, Golowasch J, Nadim F. Pacemaker neuron and network oscillations depend on a neuromodulator-regulated linear current. Front Behav Neurosci. 2010;4:21. doi: 10.3389/fnbeh.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swensen AM, Marder E. Modulators with convergent cellular actions elicit distinct circuit outputs. J Neurosci. 2001;21:4050–4058. doi: 10.1523/JNEUROSCI.21-11-04050.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kintos N, Nusbaum MP, Nadim F. Convergent neuromodulation onto a network neuron can have divergent effects at the network level. J Comput Neurosci. 2016;40:113–135. doi: 10.1007/s10827-015-0587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **34.Garcia VJ, Daur N, Temporal S, Schulz DJ, Bucher D. Neuropeptide receptor transcript expression levels and magnitude of ionic current responses show cell type-specific differences in a small motor circuit. J Neurosci. 2015;35:6786–6800. doi: 10.1523/JNEUROSCI.0171-15.2015. This study shows cell type-specific differences in the magnitude and concentration-dependence of IMI evoked by the neuropeptide CCAP, consistent with differences in receptor mRNA expression levels. Occlusion experiments with another peptide suggest that in some cell types, a neuropeptide can maximally activate IMI, but not in others. On top of the differential distribution of receptors across cell types, these findings add a quantitative dimension to the specificity of neuropeptide effects, with important implications for co-modulation by several neuropeptides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **35.Rodriguez JC, Blitz DM, Nusbaum MP. Convergent rhythm generation from divergent cellular mechanisms. J Neurosci. 2013;33:18047–18064. doi: 10.1523/JNEUROSCI.3217-13.2013. Bath application of the peptide CabPK to the STG elicits a gastric mill rhythm effectively identical to that activated by the projection neuron MCN1. MCN1 has three known co-transmitters (not including CabPK), makes local circuit connections in the STG and receives local presynaptic inhibition by one of its target neurons (LG), all of which are essential in its rhythm generation. This study shows that CabPK additionally elicits a putative low-threshold calcium current in LG that, together with IMI, allow for the production of the rhythm without need for co-modulators or the circuit actions of MCN1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwiatkowski MA, Gabranski ER, Huber KE, Chapline MC, Christie AE, Dickinson PS. Coordination of distinct but interacting rhythmic motor programs by a modulatory projection neuron using different co-transmitters in different ganglia. J Exp Biol. 2013;216:1827–1836. doi: 10.1242/jeb.082503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blitz DM, Nusbaum MP. Distinct functions for cotransmitters mediating motor pattern selection. J Neurosci. 1999;19:6774–6783. doi: 10.1523/JNEUROSCI.19-16-06774.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *38.Spencer RM, Blitz DM. Network Feedback Regulates Motor Output across a Range of Modulatory Neuron Activity. J Neurophysiol. 216 jn 01112 02015 All pattern generating networks provide feedback to the projection neurons that initiate and sustain their activty, thus matching the timing of the projection neurons to that of the target CPG network, but the significance of this feedback is mostly unknown. This study demonstrates that feedback to the projection neuron MCN1 in the STNS modulates its influence on the target pyloric and gastric networks, independent of the MCN1 activity rate and, therefore, independent of its extent of modulatory actions. [Google Scholar]
- 39.Blitz DM, Nusbaum MP. Modulation of circuit feedback specifies motor circuit output. J Neurosci. 2012;32:9182–9193. doi: 10.1523/JNEUROSCI.1461-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballo AW, Nadim F, Bucher D. Dopamine modulation of ih improves temporal fidelity of spike propagation in an unmyelinated axon. J Neurosci. 2012;32:5106–5119. doi: 10.1523/JNEUROSCI.6320-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu L, Selverston AI, Ayers J. The role of Ih in differentiating the dynamics of the gastric mill and pyloric neurons in the stomatogastric ganglion of the lobster, Homarus americanus. J Neurophysiol. 2016 doi: 10.1152/jn.00737.2015. jn 00737 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marder E, Goeritz ML, Otopalik AG. Robust circuit rhythms in small circuits arise from variable circuit components and mechanisms. Curr Opin Neurobiol. 2014;31C:156–163. doi: 10.1016/j.conb.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golowasch J. Ionic Current Variability and Functional Stability in the Nervous System. Bioscience. 2014;64:570–580. doi: 10.1093/biosci/biu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulz DJ, Goaillard JM, Marder EE. Quantitative expression profiling of identified neurons reveals cell-specific constraints on highly variable levels of gene expression. Proc Natl Acad Sci U S A. 2007;104:13187–13191. doi: 10.1073/pnas.0705827104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao S, Golowasch J. Ionic current correlations underlie the global tuning of large numbers of neuronal activity attributes. J Neurosci. 2012;32:13380–13388. doi: 10.1523/JNEUROSCI.6500-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **46.Temporal S, Lett KM, Schulz DJ. Activity-dependent feedback regulates correlated ion channel mRNA levels in single identified motor neurons. Curr Biol. 2014;24:1899–1904. doi: 10.1016/j.cub.2014.06.067. The authors describe correlations between the mRNA expression levels of several potassium and calcium channel genes that differ across identified cell types. In a series of pertubations that pried apart influence of activity, neuromodulators, and synaptic interactions, they establish for the first time experimentally that activity-dependent feedback mechanisms are indeed involved in the maintenance of such cell type-specific corellations, which is consistent with theoretical work on homeostatic tuning rules that preserve robust cellular activity and circuit output. [DOI] [PubMed] [Google Scholar]
- 47.Soofi W, Archila S, Prinz AA. Co-variation of ionic conductances supports phase maintenance in stomatogastric neurons. J Comput Neurosci. 2012;33:77–95. doi: 10.1007/s10827-011-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Leary T, Williams AH, Caplan JS, Marder E. Correlations in ion channel expression emerge from homeostatic tuning rules. Proc Natl Acad Sci U S A. 2013;110:E2645–2654. doi: 10.1073/pnas.1309966110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **49.O’Leary T, Williams AH, Franci A, Marder E. Cell types, network homeostasis, and pathological compensation from a biologically plausible ion channel expression model. Neuron. 2014;82:809–821. doi: 10.1016/j.neuron.2014.04.002. In this theoretical paper inspired by experimental findings in the STG, the authors explore how robust activity in a small central pattern generator-like circuit can arise from homeostatic regulation of its component neurons. The tuning rules include correlated expression of different voltage-gated ion channels, and the pattern generating circuit is self-assembling from these rules, which means that cell-autonomous regulatory mechanisms can be sufficient to convey robustness at the circuit level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daur N, Bryan AS, Garcia VJ, Bucher D. Short-term synaptic plasticity compensates for variability in number of motor neurons at a neuromuscular junction. J Neurosci. 2012;32:16007–16017. doi: 10.1523/JNEUROSCI.2584-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grashow R, Brookings T, Marder E. Compensation for variable intrinsic neuronal excitability by circuit-synaptic interactions. J Neurosci. 2010;30:9145–9156. doi: 10.1523/JNEUROSCI.0980-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamood AW, Marder E. Animal-to-Animal Variability in Neuromodulation and Circuit Function. Cold Spring Harb Symp Quant Biol. 2014;79:21–28. doi: 10.1101/sqb.2014.79.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marder E, O’Leary T, Shruti S. Neuromodulation of circuits with variable parameters: single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annu Rev Neurosci. 2014;37:329–346. doi: 10.1146/annurev-neuro-071013-013958. [DOI] [PubMed] [Google Scholar]
- 54.Khorkova O, Golowasch J. Neuromodulators, not activity, control coordinated expression of ionic currents. J Neurosci. 2007;27:8709–8718. doi: 10.1523/JNEUROSCI.1274-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Temporal S, Desai M, Khorkova O, Varghese G, Dai A, Schulz DJ, Golowasch J. Neuromodulation independently determines correlated channel expression and conductance levels in motor neurons of the stomatogastric ganglion. J Neurophysiol. 2012;107:718–727. doi: 10.1152/jn.00622.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamood AW, Haddad SA, Otopalik AG, Rosenbaum P, Marder E. Quantitative Reevaluation of the Effects of Short- and Long-Term Removal of Descending Modulatory Inputs on the Pyloric Rhythm of the Crab. eNeuro. 2015 doi: 10.1523/ENEURO.0058-14.2015. 2:ENEURO.0058-0014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamood AW, Marder E. Consequences of acute and long-term removal of neuromodulatory input on the episodic gastric rhythm of the crab Cancer borealis. J Neurophysiol. 2015;114:1677–1692. doi: 10.1152/jn.00536.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nahar J, Lett KM, Schulz DJ. Restoration of descending inputs fails to rescue activity following deafferentation of a motor network. J Neurophysiol. 2012;108:871–881. doi: 10.1152/jn.00183.2012. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Khorkova O, Rodriguez R, Golowasch J. Activity and neuromodulatory input contribute to the recovery of rhythmic output after decentralization in a central pattern generator. J Neurophysiol. 2009;101:372–386. doi: 10.1152/jn.01290.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodgers EW, Krenz WD, Jiang X, Li L, Baro DJ. Dopaminergic tone regulates transient potassium current maximal conductance through a translational mechanism requiring D1Rs, cAMP/PKA, Erk and mTOR. BMC Neurosci. 2013;14:143. doi: 10.1186/1471-2202-14-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krenz WD, Hooper RM, Parker AR, Prinz AA, Baro DJ. Activation of high and low affinity dopamine receptors generates a closed loop that maintains a conductance ratio and its activity correlate. Front Neural Circuits. 2013;7:169. doi: 10.3389/fncir.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krenz WD, Parker AR, Rodgers EW, Baro DJ. Dopaminergic tone persistently regulates voltage-gated ion current densities through the D1R-PKA axis, RNA polymerase II transcription, RNAi, mTORC1, and translation. Front Cell Neurosci. 2014;8:39. doi: 10.3389/fncel.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krenz WD, Rodgers EW, Baro DJ. Tonic 5nM DA stabilizes neuronal output by enabling bidirectional activity-dependent regulation of the hyperpolarization activated current via PKA and calcineurin. PLoS One. 2015;10:e0117965. doi: 10.1371/journal.pone.0117965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *64.Krenz WD, Parker AR, Rodgers E, Baro DJ. Monoaminergic tone supports conductance correlations and stabilizes activity features in pattern generating neurons of the lobster, Panulirus interruptus. Front Neural Circuits. 2015;9:63. doi: 10.3389/fncir.2015.00063. Nanomolar tonic dopamine application results in long-term maintenance of the correlations between IA and Ih in a follower bursting neuron, subject to rhythmic inhibition, to produce stability of bursting activity. This stability depends on an RNA-interference silencing complex pathway activated by D1 receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang LS, Taylor AL, Rinberg A, Marder E. Robustness of a rhythmic circuit to short- and long-term temperature changes. J Neurosci. 2012;32:10075–10085. doi: 10.1523/JNEUROSCI.1443-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soofi W, Goeritz ML, Kispersky TJ, Prinz AA, Marder E, Stein W. Phase maintenance in a rhythmic motor pattern during temperature changes in vivo. J Neurophysiol. 2014;111:2603–2613. doi: 10.1152/jn.00906.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rinberg A, Taylor AL, Marder E. The effects of temperature on the stability of a neuronal oscillator. PLoS Comput Biol. 2013;9:e1002857. doi: 10.1371/journal.pcbi.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caplan JS, Williams AH, Marder E. Many parameter sets in a multicompartment model oscillator are robust to temperature perturbations. J Neurosci. 2014;34:4963–4975. doi: 10.1523/JNEUROSCI.0280-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *69.Stadele C, Heigele S, Stein W. Neuromodulation to the Rescue: Compensation of Temperature-Induced Breakdown of Rhythmic Motor Patterns via Extrinsic Neuromodulatory Input. PLoS Biol. 2015;13:e1002265. doi: 10.1371/journal.pbio.1002265. This study shows the potentially very important role that neuromodulators can play in conveying robustness to temperature changes. Unlike the pyloric rhythm, the in vitro gastric mill rhythm can fall silent due to a temperature increase of a few degrees Celsius. The authors show that this is due to a temperature-dependent decrease in input resistance in gastric mill neurons. This increase in leak conductance can be effectively counterbalanced by an increase in the neuropeptide-activated current IMI. Such additional IMI can be provided by a temperature-dependent increase in the activity rate of modulatory projection neurons, which the authors show is sufficent to rescue the gastric mill rhythm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen R, Xiao M, Buchberger A, Li L. Quantitative neuropeptidomics study of the effects of temperature change in the crab Cancer borealis. J Proteome Res. 2014;13:5767–5776. doi: 10.1021/pr500742q. [DOI] [PMC free article] [PubMed] [Google Scholar]