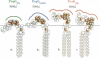Abstract
Three allelic variants of P-pilus-associated G-adhesins (lectins) with different cell-binding properties were recently described. Here we have analyzed Escherichia coli HB101 strains expressing the recombinant G-adhesin variants for their ability to agglutinate erythrocytes from various species as this relates to the glycosphingolipid (GSL) composition in the erythrocyte membranes. All three variants exhibit similar specificities for the globo-series GSLs affixed to artificial surfaces. However, only the PapGJ96 adhesin induces agglutination of erythrocytes having globotriaosylceramide (GbO3) [Gal(alpha 1-4)LacCer] as the major GSL. Furthermore, only PapGAD110 induces strong agglutination of erythrocytes having globotetraosylceramide (GbO4) [GalNAc(beta 1-3)Gal(alpha 1-4)LacCer] as the major GSL, while PrsGJ96 alone agglutinates those containing globopentaosylceramide (GbO5) [GalNAc(alpha 1-3)GalNAc(beta 1-3)Gal(alpha 1-4)LacCer]. Molecular modeling of these globo-GSLs demonstrates different saccharide orientations to the membrane surface for these isoreceptors. We suggest that the differential binding of the three G-adhesin variants results from differences in epitope presentation at the membrane among these globo-GSLs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bock K., Breimer M. E., Brignole A., Hansson G. C., Karlsson K. A., Larson G., Leffler H., Samuelsson B. E., Strömberg N., Edén C. S. Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1----4Gal-containing glycosphingolipids. J Biol Chem. 1985 Jul 15;260(14):8545–8551. [PubMed] [Google Scholar]
- Brodin N. T., Dahmén J., Nilsson B., Messeter L., Mårtensson S., Heldrup J., Sjögren H. O., Lundblad A. Monoclonal antibodies produced by immunization with neoglycoproteins containing Gal alpha 1-4Gal beta 1-4Glc beta-O and Gal alpha 1-4Gal beta 1-4GlcNAc beta-O residues: useful immunochemical and cytochemical reagents for blood group P antigens and a differentiation marker in Burkitt lymphoma and other B-cell malignancies. Int J Cancer. 1988 Aug 15;42(2):185–194. doi: 10.1002/ijc.2910420208. [DOI] [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990 Nov 5;265(31):18713–18716. [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Homans S. W., Dwek R. A., Boyd J., Mahmoudian M., Richards W. G., Rademacher T. W. Conformational transitions in N-linked oligosaccharides. Biochemistry. 1986 Oct 7;25(20):6342–6350. doi: 10.1021/bi00368a076. [DOI] [PubMed] [Google Scholar]
- Kannagi R., Levery S. B., Ishigami F., Hakomori S., Shevinsky L. H., Knowles B. B., Solter D. New globoseries glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen 3. J Biol Chem. 1983 Jul 25;258(14):8934–8942. [PubMed] [Google Scholar]
- Karlsson K. A., Strömberg N. Overlay and solid-phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Brown J. E., Strömberg N., Westling-Ryd M., Schultz J. E., Karlsson K. A. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987 Feb 5;262(4):1779–1785. [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Lindstedt R., Larson G., Falk P., Jodal U., Leffler H., Svanborg C. The receptor repertoire defines the host range for attaching Escherichia coli strains that recognize globo-A. Infect Immun. 1991 Mar;59(3):1086–1092. doi: 10.1128/iai.59.3.1086-1092.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman E., Kannagi R., Hakomori S., Parsons M., Lipinski M., Wiels J., Fellous M., Tursz T. A glycolipid antigen associated with Burkitt lymphoma defined by a monoclonal antibody. Science. 1983 Apr 29;220(4596):509–511. doi: 10.1126/science.6836295. [DOI] [PubMed] [Google Scholar]
- Nyholm P. G., Pascher I., Sundell S. The effect of hydrogen bonds on the conformation of glycosphingolipids. Methylated and unmethylated cerebroside studied by X-ray single crystal analysis and model calculations. Chem Phys Lipids. 1990 Jan;52(1):1–10. doi: 10.1016/0009-3084(90)90002-9. [DOI] [PubMed] [Google Scholar]
- Nyholm P. G., Samuelsson B. E., Breimer M., Pascher I. Conformational analysis of blood group A-active glycosphingolipids using HSEA-calculations. The possible significance of the core oligosaccharide chain for the presentation and recognition of the A-determinant. J Mol Recognit. 1989 Nov;2(3):103–113. doi: 10.1002/jmr.300020302. [DOI] [PubMed] [Google Scholar]
- Poppe L., Dabrowski J., von der Lieth C. W. Solution conformation of Forssman antigen probed by NOE and exchange interactions. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1169–1175. doi: 10.1016/0006-291x(91)91544-m. [DOI] [PubMed] [Google Scholar]
- Stern P. L., Willison K. R., Lennox E., Galfrè G., Milstein C., Secher D., Ziegler A. Monoclonal antibodies as probes for differentiation and tumor-associated antigens: a Forssman specificity on teratocarcinoma stem cells. Cell. 1978 Aug;14(4):775–783. doi: 10.1016/0092-8674(78)90333-1. [DOI] [PubMed] [Google Scholar]
- Strömberg N., Marklund B. I., Lund B., Ilver D., Hamers A., Gaastra W., Karlsson K. A., Normark S. Host-specificity of uropathogenic Escherichia coli depends on differences in binding specificity to Gal alpha 1-4Gal-containing isoreceptors. EMBO J. 1990 Jun;9(6):2001–2010. doi: 10.1002/j.1460-2075.1990.tb08328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. W., Jr, MacDonald E. M., Nowinski R. C., Hakomori S. I. Production of monoclonal antibodies specific for two distinct steric portions of the glycolipid ganglio-N-triosylceramide (asialo GM2). J Exp Med. 1979 Oct 1;150(4):1008–1019. doi: 10.1084/jem.150.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]