Abstract
Background
The authors compared longitudinal patient-reported outcomes and physician-rated cosmesis with conventionally fractionated whole-breast irradiation (CF-WBI) versus hypofractionated whole-breast irradiation (HF-WBI) within the context of a randomized trial.
Methods
From 2011 to 2014, a total of 287 women with American Joint Committee on Cancer stage 0 to stage II breast cancer were randomized to receive CF-WBI (at a dose of 50 grays in 25 fractions plus a tumor bed boost) or HF-WBI (at a dose of 42.56 grays in 16 fractions plus a tumor bed boost) after breast-conserving surgery. Patient-reported outcomes were assessed using the Breast Cancer Treatment Outcome Scale (BCTOS), the Functional Assessment of Cancer Therapy-Breast, and the Body Image Scale and were recorded at baseline and 0.5, 1, 2, and 3 years after radiotherapy. Physician-rated cosmesis was assessed at the same time points. Outcomes by treatment arm were compared at each time point using a 2-sided Student t test. Multivariable mixed effects growth curve models assessed the effects of treatment arm and time on longitudinal outcomes.
Results
Of the 287 patients enrolled, 149 were randomized to CF-WBI and 138 were randomized to HF-WBI. At 2 years, the Functional Assessment of Cancer Therapy-Breast Trial Outcome Index score was found to be modestly better in the HF-WBI arm (mean 79.6 vs 75.9 for CF-WBI; P5.02). In multivariable mixed effects models, treatment arm was not found to be associated with longitudinal outcomes after adjusting for time and baseline outcome measures (P≥0.14). The linear effect of time was significant for BCTOS measures of functional status (P=0.001, improved with time) and breast pain (P=.002, improved with time).
Conclusions
In this randomized trial, longitudinal outcomes did not appear to differ by treatment arm. Patient-reported functional and pain outcomes improved over time. These findings are relevant when counseling patients regarding decisions concerning radiotherapy.
Keywords: breast cancer, cosmesis, hypofractionation, patient-reported outcomes, whole-breast irradiation
Precis
In the current study, the authors assess longitudinal patient-reported outcomes and physician-rated cosmesis in a randomized trial of conventionally fractionated whole-breast irradiation plus boost versus hypofractionated whole-breast irradiation plus boost for the adjuvant treatment of patients with early-stage breast cancer. The treatment arm appears to have no significant effect on longitudinal outcomes, and patient-reported functional and pain outcomes improve with time.
Introduction
Over the past decade, large randomized trials from Canada and the United Kingdom have established hypofractionated whole-breast irradiation (HF-WBI) as a safe and effective component of breast-conserving therapy for nearly all patients with early-stage breast cancer (1, 2). In fact, despite initial concerns about the long-term effects of hypofractionation on normal tissue, 10-year provider-reported toxicity and cosmesis for patients treated with HF-WBI were equivalent to those of conventionally fractionated whole-breast irradiation (CF-WBI) in the Canadian trial and were found to be superior to CF-WBI in the UK Standardisation of Breast Radiotherapy (START) A and B trials. Furthermore, an analysis of quality of life (QOL) outcomes from the START A and B trials revealed a lower rate of patient-reported moderate to marked skin changes in patients randomized to HF-WBI (3).
The results of these 2 trials have led to broad uptake of HF-WBI in Canada and the United Kingdom (4, 5). However, to the best of our knowledge, the adoption of HF-WBI in the United States has been more limited (6), perhaps owing to skepticism regarding the applicability of these results to the practice of radiation oncology in the United States, in which a tumor bed boost is frequently used and obesity, with its attendant dosimetric challenges, is common (7–11).
To address these concerns, investigators from our group conducted a randomized trial of CF-WBI versus HF-WBI, both with a tumor bed boost, for the adjuvant treatment of patients with early-stage breast cancer, regardless of patient body mass index or central axis separation. Our group recently reported on the acute and 6-month toxicity outcomes and demonstrated that among 287 patients (nearly one-half of whom were obese), acute toxicity and physician-reported and patient-reported fatigue at 6 months were better among patients randomized to HF-WBI (12). In a multicenter cohort study published simultaneously, Jagsi et al demonstrated that patients who received HF-WBI had less acute pain, dermatitis, and fatigue compared to those treated with CF-WBI (13).
Although comparisons of acute physician- and patient-reported outcomes (PROs) after CF-WBI and HF-WBI as practiced in the United States are valuable, to the best of our knowledge little data exist regarding how these outcomes evolve with time. Such information is needed to address lingering concerns regarding the long-term toxicity of HF-WBI plus a tumor bed boost in a population with a high prevalence of obesity (7). The purpose of the current study was to compare longitudinal PROs and physician-rated cosmesis in a randomized clinical trial of CF-WBI versus HF-WBI and to assess how these outcomes change over time.
Materials and Methods
Patients
The current study was approved by the Institutional Review Board and monitored by the Institutional Data Safety and Monitoring Board. All participants provided written informed consent before enrollment. Patients were enrolled from February 2011 through February 2014 at our institution and surrounding regional centers, as well as affiliated centers in 2 other states. Eligible women were aged ≥40 years with pathologically confirmed ductal carcinoma in situ or invasive breast cancer (American Joint Committee on Cancer stage Tis-T2, N0–N1a, M0) and treated with breast-conserving surgery with final negative margins (defined as “no tumor on ink”) and for whom adjuvant radiotherapy (RT) with tangent-only WBI was recommended. Pathologic staging of the axilla was required for all patients with invasive disease. Patients were excluded if they were undergoing concomitant treatment for another malignancy, if they had a prior diagnosis of breast cancer, if they had a current diagnosis of bilateral breast cancer, if they had received prior irradiation in the same area, if they were pregnant, or if they were not fluent in English or Spanish.
Randomization
Patients were randomly assigned to treatment with either CF-WBI (50 grays [Gy] in 25 fractions to the whole breast) or HF-WBI (42.56 Gy in 16 fractions to the whole breast). Boost doses in both treatment arms were based on pathologic findings: patients with final resection margins ≥2 mm or negative re-excision received a tumor bed boost dose of 10 Gy in 5 fractions in the CF-WBI arm and 10 Gy in 4 fractions in the HF-WBI arm; patients with final resection margins <2 mm received a tumor bed boost dose of 14 Gy in 7 fractions in the CF-WBI arm and 12.5 Gy in 5 fractions in the HF-WBI arm.
Radiotherapy
Patients were required to have initiated RT within 12 weeks of either the final breast-conserving surgery or the last infusion of cytotoxic chemotherapy. In both groups, WBI was planned and delivered with megavoltage tangential fields. Prone positioning and respiratory gating with deep inspiration breath hold were allowed at the discretion of the treating radiation oncologist. Three-dimensional dose compensation with forward or inverse planning was encouraged to minimize the volume of tissue receiving >108% of the prescription dose. Modification of the tangents to cover the undissected low axilla was permitted if clinically indicated. The tumor bed boost was delivered with either photons or electrons, and could be omitted if the size of the seroma cavity precluded safe delivery.
Patient-Rated Evaluations
PROs were measured using the following validated instruments: 1) the Breast Cancer Treatment Outcome Scale (BCTOS) (14), 2) the Functional Assessment of Cancer Therapy-Breast (FACT-B; version 4) (15, 16), and 3) the Body Image Scale (BIS) (17).
The BCTOS instrument was developed to assess PROs regarding cosmetic outcomes after breast-conserving surgery and WBI (14). The instrument is divided into 3 subscales that address cosmetic status (8 items), functional status (7 items), and breast pain (3 items). Each item is rated on a scale of 1 to 4 depending on the difference between the treated and untreated breast and area, with 1 indicating no difference and 4 indicating a large difference. The subscale score is derived from the arithmetic mean of the answers for each item.
The FACT-B instrument was developed to assess multiple QOL dimensions for women with breast cancer (15). The instrument contains the FACT-General (FACT-G) scale as well as a Breast Cancer Subscale (BCS). The FACT-G is itself comprised of several subscales, including physical well-being (7 items), social/family well-being (SWB; 7 items), emotional well-being (EWB; 6 items), and functional well-being (7 items). The BCS contains 10 additional items specific to QOL in breast cancer that are not contained within the FACT-G. Each item is rated on a scale from 0 to 4; a higher score reflects a better QOL. For the overall instrument and all of its subscales, the individual items are summed to give a summary score. In addition, the physical well-being, functional well-being, and BCS subscales can be combined to give a separate outcome measure known as the trial outcome index (TOI). Version 4 of the FACT-B instrument was recently validated in the current patient cohort (16).
The BIS instrument was developed by Hopwood et al. specifically for patients with cancer (17). The instrument contains 10 items, each rated on a scale of 0 to 3, with a higher score indicating greater body image-related symptoms or distress. The overall summary score is obtained by summing the 10 items.
Participants completed the instruments at baseline and at 6 months, 1 year, 2 years, and 3 years after completion of RT. Patients were allowed to complete these evaluations either on paper or electronically on a tablet and in English or Spanish.
Physician-Rated Evaluations
Physician-rated cosmesis (“MD cosmesis”) was rated on a scale from 1 to 4 by the treating radiation oncologist using the Radiation Therapy Oncology Group scale for physician assessment (1 indicates excellent, 2 indicates good, 3 indicates fair, and 4 indicates poor) (18). Scores were obtained at baseline and at 6 months, 1 year, 2 years, and 3 years after the completion of RT.
Statistical Analyses
The sample size for the current trial was determined based on the primary endpoint of patient-reported BCTOS cosmetic status 3 years after RT. This outcome will not be reported until all patients have completed 3 years of follow-up. In the current analysis, we reported cross-sectional and longitudinal comparisons by treatment arm for pre-specified secondary endpoints including MD Cosmesis and the following PROs: BCTOS measures of cosmetic status, functional status, and breast pain; FACT-B SWB, FACT-B EWB, and FACT-B TOI; and body image as measured by BIS. For all BCTOS measures, BIS, and MD Cosmesis, a higher score indicates a worse clinical outcome. For FACT-B, a higher score indicates a better clinical outcome. Because these outcomes are considered exploratory and therefore hypothesis-generating, we did not adjust for multiple comparisons.
Baseline patient, tumor, and treatment characteristics by randomization arm were compared using the chi-square test. Means of the outcomes at each time point by randomization arm were compared using the Student t test.
Longitudinal analyses were performed using multivariable mixed effects growth curve models (19). For each outcome measure, the model included a linear term of time, randomization arm, and baseline outcome measures as covariates. Additional baseline patient and clinical variables with a P value <0.2 on univariate analysis were candidates for inclusion in the multivariable models. A likelihood ratio test was used to decide whether the variable would be retained in the final model. Results for the longitudinal analyses were expressed as estimates and standard errors with a P value to indicate statistical significance.
Because some patients did not yet have complete follow-up data available, a subsidiary analysis was performed to evaluate the impact of missing data. For this analysis, a patient was artificially defined as a “dropout” if their outcomes were not measured at year 3. The dropout patterns were then compared using the chi-square test to assess whether the dropout was significantly different between the 2 randomization arms. A pattern-mixture model (20) was also implemented to estimate the impact of dropouts on the outcomes by coding those who did not have PRO data at year 3 as “1” and “0” if otherwise.
All analyses were intention-to-treat analyses and a 2-sided P value ≤0.05 was considered to be statistically significant. Analyses were performed using SAS statistical software (version 9.3; SAS Institute, Inc., Cary, NC).
Results
Patients
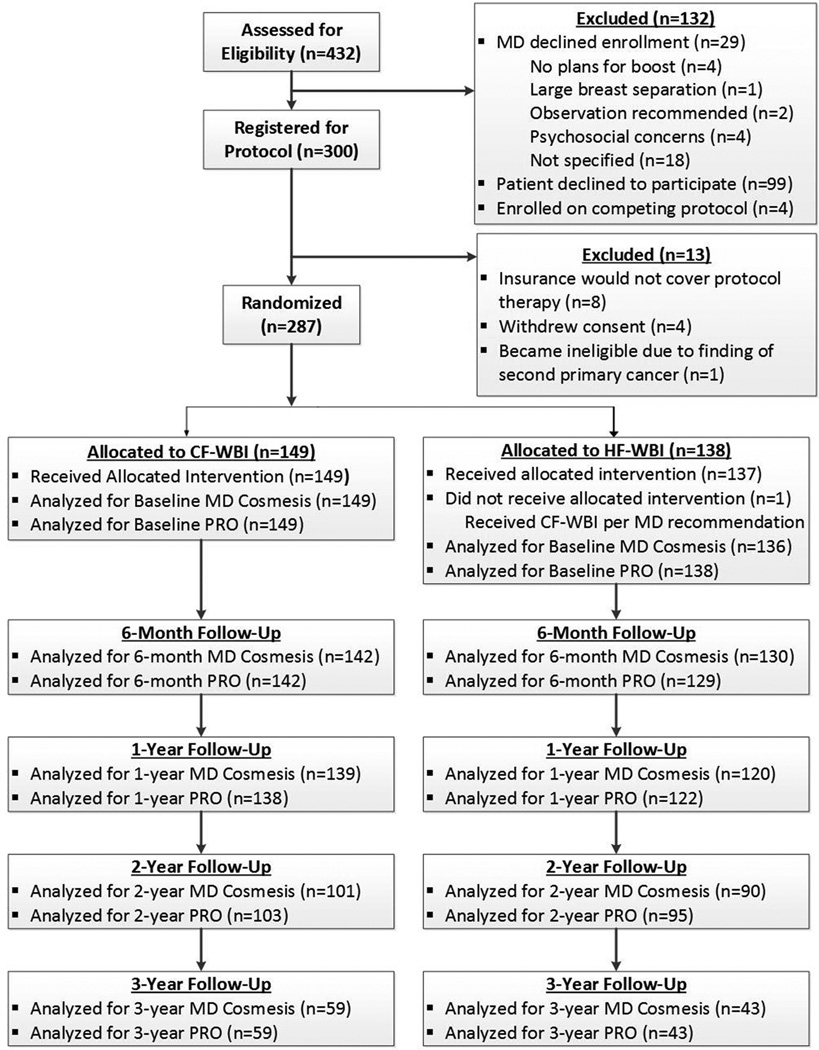
A total of 432 patients were assessed for study eligibility. Of these, 300 were registered for the protocol and 287 ultimately were randomized (Fig.1). All but one patient received the protocol-defined WBI and boost doses; this patient was randomized to HF-WBI but was treated with a conventionally fractionated schedule.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) study enrollment flowchart. CF-WBI indicates conventionally fractionated whole-breast irradiation; HF-WBI, hypofractionated whole-breast irradiation; MD, physician; PRO, patient-reported outcomes.
The median follow-up was 24.7 months (interquartile range, 13.3–36.3 months). The median age was 60 years (range, 41–81years). Approximately 75% of the patients were non-Hispanic white, and the majority of patients were either overweight (28%) or obese (44%) based on their body mass index (21). Approximately 37% of patients had a bra cup size of D or larger and 25% of patients had a central axis separation of ≥ 25.64 cm. Baseline patient, tumor, and treatment characteristics were found to be well balanced between the 2 treatment arms (Table 1).
Table 1.
Baseline demographic and clinical characteristics by randomization arm.
| No. of patients (%) | |||
|---|---|---|---|
| Characteristic | CF-WBI (n=149) |
HF-WBI (n=138) |
P* |
| Age at diagnosis, years | |||
| Median | 60 | 60 | |
| Range | 42–77 | 41–81 | |
| 40–49 | 13 (9) | 19 (14) | |
| 50–59 | 55 (37) | 46 (33) | |
| 60–69 | 59 (40) | 51 (37) | |
| ≥70 | 22 (15) | 22 (16) | 0.55 |
| Race | |||
| White non-Hispanic | 116 (78) | 99 (72) | |
| Hispanic | 16 (11) | 20 (15) | |
| Black non-Hispanic | 15 (10) | 17 (12) | |
| Asian non-Hispanic | 2 (1) | 2 (1) | 0.68 |
| Body mass index | |||
| Underweight/normal (≤24.9 kg/m2) | 38 (26) | 42 (30) | |
| Overweight (25–29.9 kg/m2) | 40 (27) | 40 (29) | |
| Obese (≥30 kg/m2) | 71 (48) | 56 (41) | 0.46 |
| Bra cup size | |||
| A | 13 (9) | 7 (5) | |
| B | 31 (21) | 34 (25) | |
| C | 50 (34) | 46 (33) | |
| D-EE | 55 (37) | 51 (37) | 0.61 |
| Breast quadrant | |||
| Central | 16 (11) | 27 (20) | |
| Lower | 31 (21) | 24 (17) | |
| Upper | 102 (68) | 87 (63) | 0.11 |
| T stage | |||
| Tis | 39 (26) | 24 (17) | |
| T1a | 16 (11) | 12 (9) | |
| T1b | 24 (16) | 23 (17) | |
| T1c | 45 (30) | 50 (36) | |
| T2 | 25 (16) | 29 (21) | 0.37 |
| Tumor behavior | |||
| DCIS | 39 (26) | 24 (17) | |
| Invasive | 110 (74) | 114 (83) | 0.07 |
| Estrogen receptor status | |||
| Positive | 130 (87) | 118 (86) | |
| Negative | 18 (12) | 16 (12) | |
| Not tested | 1 (1) | 4 (3) | 0.39 |
| HER2 status | |||
| Positive | 12 (8) | 11 (8) | |
| Negative | 102 (69) | 103 (75) | |
| Not tested | 35 (24) | 24 (17) | 0.43 |
| Chemotherapy receipt | |||
| None | 106 (71) | 96 (70) | |
| Neoadjuvant | 16 (11) | 12 (9) | |
| Adjuvant | 27 (18) | 30 (22) | 0.67 |
| Central axis separation quartile (cm) | |||
| <21.09 | 38 (26) | 34 (25) | |
| 21.09 – <23.2 | 40 (27) | 34 (25) | |
| 23.2 – <25.64 | 35 (24) | 35 (25) | |
| ≥25.64 | 36 (24) | 35 (25) | 0.96 |
Abbreviations: CF-WBI, conventionally fractionated whole-breast irradiation; HF-WBI, hypofractionated whole-breast irradiation; DCIS, ductal carcinoma in situ.
Fisher’s exact test used for race and estrogen receptor status; χ2 test used for all other comparisons.
Patient-Reported Outcomes
There were no significant differences between the 2 treatment arms for any of the baseline PROs (Table 2). Comparisons for each outcome at each time point revealed no statistically significant differences between the treatment arms for any of the PRO measures at 6 months, 1 year, and 3 years. At 2 years, the FACT-B TOI was found to be modestly higher in the HF-WBI group (mean of 79.6 v. 75.9 for CF-WBI, P=0.02).
Table 2.
Cross-sectional comparisons of outcomes by randomization arm.
| CF-WBI | HF-WBI | |||||
|---|---|---|---|---|---|---|
| Year | Outcome | No. of patients |
Mean score (95% CI) | No. of patients |
Mean score (95% CI) | P |
| 0 | BCTOS cosmetic | 144 | 1.51 (1.44–1.58) | 133 | 1.55 (1.47–1.63) | 0.48 |
| BCTOS functional | 148 | 1.21 (1.15–1.27) | 135 | 1.29 (1.21–1.37) | 0.15 | |
| BCTOS pain | 148 | 1.66 (1.56–1.76) | 134 | 1.53 (1.44–1.62) | 0.07 | |
| FACT-B SWB | 149 | 24.36 (23.65–25.07) | 138 | 25.12 (24.35–25.89) | 0.16 | |
| FACT-B EWB | 148 | 20.42 (19.98–20.86) | 138 | 20.46 (19.9–21.02) | 0.92 | |
| FACT-B TOI | 148 | 74.03 (72.23–75.83) | 138 | 74.51 (72.56–76.46) | 0.72 | |
| BIS | 140 | 3.21 (2.56–3.86) | 133 | 3.22 (2.5–3.94) | 0.98 | |
| MD cosmesis | 149 | 1.54 (1.43–1.65) | 136 | 1.51 (1.41–1.61) | 0.70 | |
| 0.5 | BCTOS cosmetic | 141 | 1.79 (1.69–1.89) | 126 | 1.76 (1.67–1.85) | 0.58 |
| BCTOS functional | 142 | 1.40 (1.30–1.50) | 128 | 1.29 (1.21–1.37) | 0.10 | |
| BCTOS pain | 142 | 1.74 (1.63–1.85) | 127 | 1.72 (1.6–1.84) | 0.78 | |
| FACT-B SWB | 140 | 24.64 (23.91–25.37) | 129 | 24.83 (24.04–25.62) | 0.73 | |
| FACT-B EWB | 140 | 21.10 (20.58–21.62) | 129 | 21.28 (20.78–21.78) | 0.63 | |
| FACT-B TOI | 139 | 76.68 (75.01–78.35) | 129 | 78.34 (76.61–80.07) | 0.18 | |
| BIS | 135 | 2.62 (1.96–3.28) | 126 | 2.94 (2.21–3.67) | 0.53 | |
| MD cosmesis | 142 | 1.76 (1.64–1.88) | 130 | 1.86 (1.73–1.99) | 0.26 | |
| 1 | BCTOS cosmetic | 133 | 1.78 (1.69–1.87) | 121 | 1.77 (1.68–1.86) | 0.84 |
| BCTOS functional | 138 | 1.26 (1.18–1.34) | 119 | 1.24 (1.17–1.31) | 0.63 | |
| BCTOS pain | 138 | 1.64 (1.54–1.74) | 120 | 1.59 (1.48–1.7) | 0.52 | |
| FACT-B SWB | 137 | 24.07 (23.21–24.93) | 122 | 24.99 (24.15–25.83) | 0.14 | |
| FACT-B EWB | 138 | 21.30 (20.76–21.84) | 122 | 21.49 (21.02–21.96) | 0.60 | |
| FACT-B TOI | 137 | 78.02 (76.37–79.67) | 122 | 78.23 (76.58–79.88) | 0.87 | |
| BIS | 132 | 2.46 (1.83–3.09) | 119 | 3.33 (2.46–4.2) | 0.11 | |
| MD cosmesis | 139 | 1.76 (1.64–1.88) | 120 | 1.93 (1.79–2.07) | 0.08 | |
| 2 | BCTOS cosmetic | 100 | 1.79 (1.68–1.9) | 92 | 1.70 (1.59–1.81) | 0.24 |
| BCTOS functional | 101 | 1.29 (1.19–1.39) | 93 | 1.25 (1.15–1.35) | 0.62 | |
| BCTOS pain | 101 | 1.62 (1.5–1.74) | 93 | 1.54 (1.41–1.67) | 0.37 | |
| FACT-B SWB | 103 | 23.98 (22.9–25.06) | 95 | 24.71 (23.73–25.69) | 0.33 | |
| FACT-B EWB | 103 | 21.20 (20.65–21.75) | 95 | 21.45 (20.79–22.11) | 0.57 | |
| FACT-B TOI | 103 | 75.93 (73.62–78.24) | 95 | 79.58 (77.6–81.56) | 0.02 | |
| BIS | 99 | 3.34 (2.46–4.22) | 92 | 2.85 (1.97–3.73) | 0.44 | |
| MD cosmesis | 101 | 1.93 (1.79–2.07) | 90 | 1.90 (1.73–2.07) | 0.79 | |
| 3 | BCTOS cosmetic | 58 | 1.71 (1.57–1.85) | 41 | 1.70 (1.53–1.87) | 0.98 |
| BCTOS functional | 58 | 1.20 (1.09–1.31) | 44 | 1.27 (1.14–1.4) | 0.43 | |
| BCTOS pain | 58 | 1.59 (1.43–1.75) | 44 | 1.55 (1.36–1.74) | 0.75 | |
| FACT-B SWB | 59 | 24.68 (23.59–25.77) | 43 | 25.04 (23.81–26.27) | 0.67 | |
| FACT-B EWB | 59 | 21.41 (20.74–22.08) | 43 | 20.37 (19.54–21.2) | 0.06 | |
| FACT-B TOI | 59 | 77.51 (74.94–80.08) | 43 | 78.58 (75.79–81.37) | 0.58 | |
| BIS | 57 | 2.60 (1.68–3.52) | 41 | 3.39 (1.74–5.04) | 0.38 | |
| MD cosmesis | 59 | 1.80 (1.6–2) | 43 | 1.95 (1.68–2.22) | 0.35 | |
Abbreviations: CF-WBI, conventionally fractionated whole-breast irradiation; HF-WBI, hypofractionated whole-breast irradiation; CI, confidence interval; BCTOS, Breast Cancer Treatment Outcome Scale; FACT-B, Functional Assessment of Cancer Therapy for Patients with Breast Cancer; SWB, social well-being; EWB, emotional well-being; TOI, trial outcome index; BIS, Body Image Scale.
Multivariable mixed effects growth curve models demonstrated that the treatment arm had no statistically significant effect on longitudinal PROs after adjusting for time and baseline outcome measures (P≥0.16 for all) (Table 3). Baseline characteristics including breast quadrant and tumor behavior initially were entered into the multivariable models because of their trend toward significance in univariate analyses (Table 1); however, both of these characteristics were ultimately excluded from multivariable models due to lack of statistical significance.
Table 3.
Multivariable mixed-effects growth curve models for longitudinal Breast Cancer Treatment Outcome Scale (BCTOS) functional status and breast pain.*
| BCTOS functional status | BCTOS breast pain | |||||
|---|---|---|---|---|---|---|
| Parameter | Estimate | Standard error |
P | Estimate | Standard error |
P |
| Treatment arm (HF- v. CF-WBI) |
−0.06 | 0.045 | 0.18 | 0.012 | 0.061 | 0.84 |
| Time (year) | −0.048 | 0.015 | 0.001 | −0.261 | 0.085 | 0.002 |
| Baseline score | 0.392 | 0.053 | <0.0001 | 0.301 | 0.053 | <0.0001 |
Abbreviations: HF, hypofractionated; CF-WBI, conventionally fractionated whole-breast irradiation.
Multivariable mixed-effects growth curve models were also performed for the following longitudinal outcomes but are not presented here because neither treatment arm nor time were significant in the model: BCTOS cosmetic status (P=0.16 for treatment arm, P=0.35 for time), Functional Assessment of Cancer Therapy for Patients with Breast Cancer (FACT-B) Social Well-Being (P=0.72 for treatment arm, P=0.25 for time), FACT-B Emotional Well-Being (P=0.77 for treatment arm, P=0.80 for time), FACT-B Trial Outcome Index (P=0.23 for treatment arm, P=0.39 for time), Body Image Scale (P=0.30 for treatment arm, P=0.34 for time), and MD Cosmesis (P=0.14 for treatment arm, P=0.07 for time).
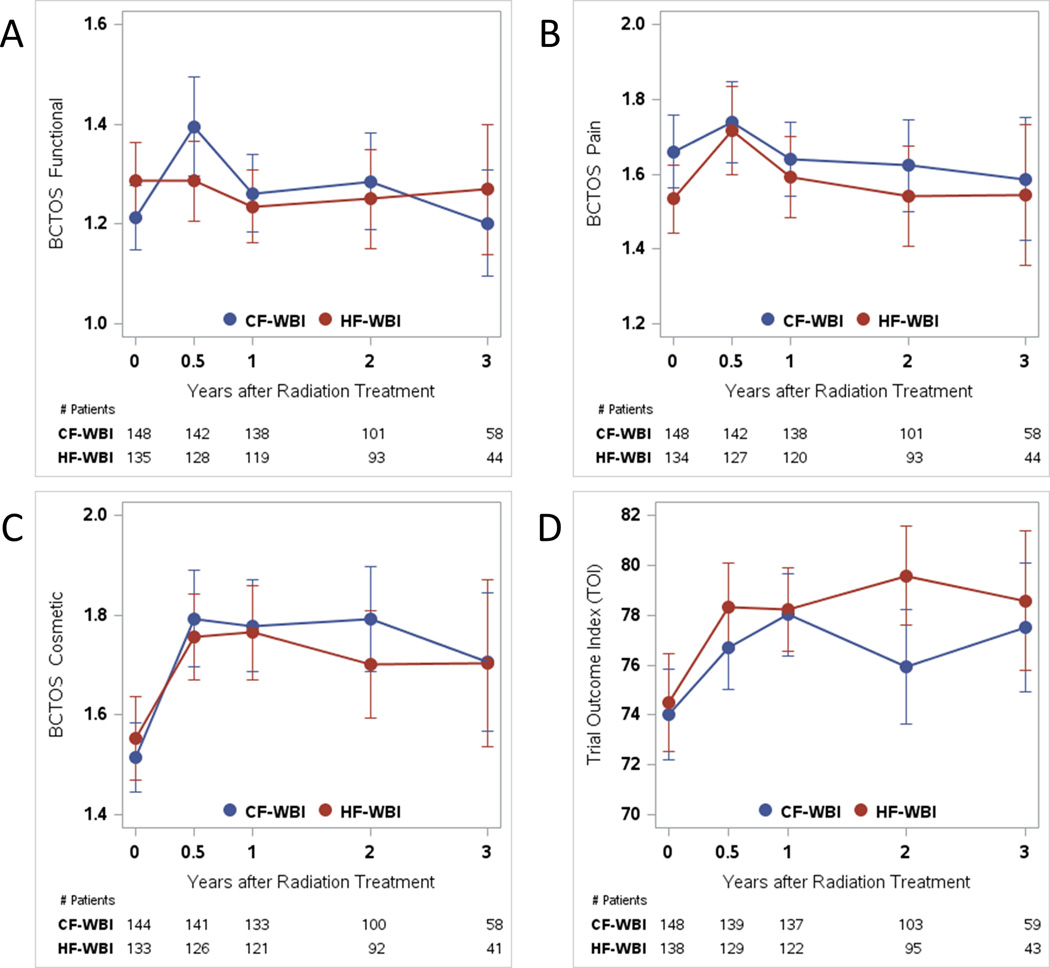
The linear effect of time was significant for BCTOS measures of functional status (P=0.001) and breast pain (P=0.002) (Table 3). Both of these scores decreased over time, signifying clinical improvement (Figs. 2A and 2B). The linear effect of time was not found to be significant for BCTOS cosmetic status (Fig. 2C), FACT-B TOI (Fig. 2D), FACT-B SWB, FACT-B EWB, or BIS outcomes (Table 3).
Figure 2.
(A) Breast Cancer Treatment Outcome Scale (BCTOS) measure of functional status, (B) BCTOS measure of breast pain, (C) BCTOS measure of cosmetic status, and (D) Functional Assessment of Cancer Therapy for Patients with Breast Cancer (FACT-B) Trial Outcome Index mean scores with 95% confidence intervals at baseline; 6 months; and 1, 2, and 3 years. CF-WBI indicates conventionally fractionated whole-breast irradiation; HF-WBI, hypofractionated whole-breast irradiation.
Physician-Rated Cosmesis
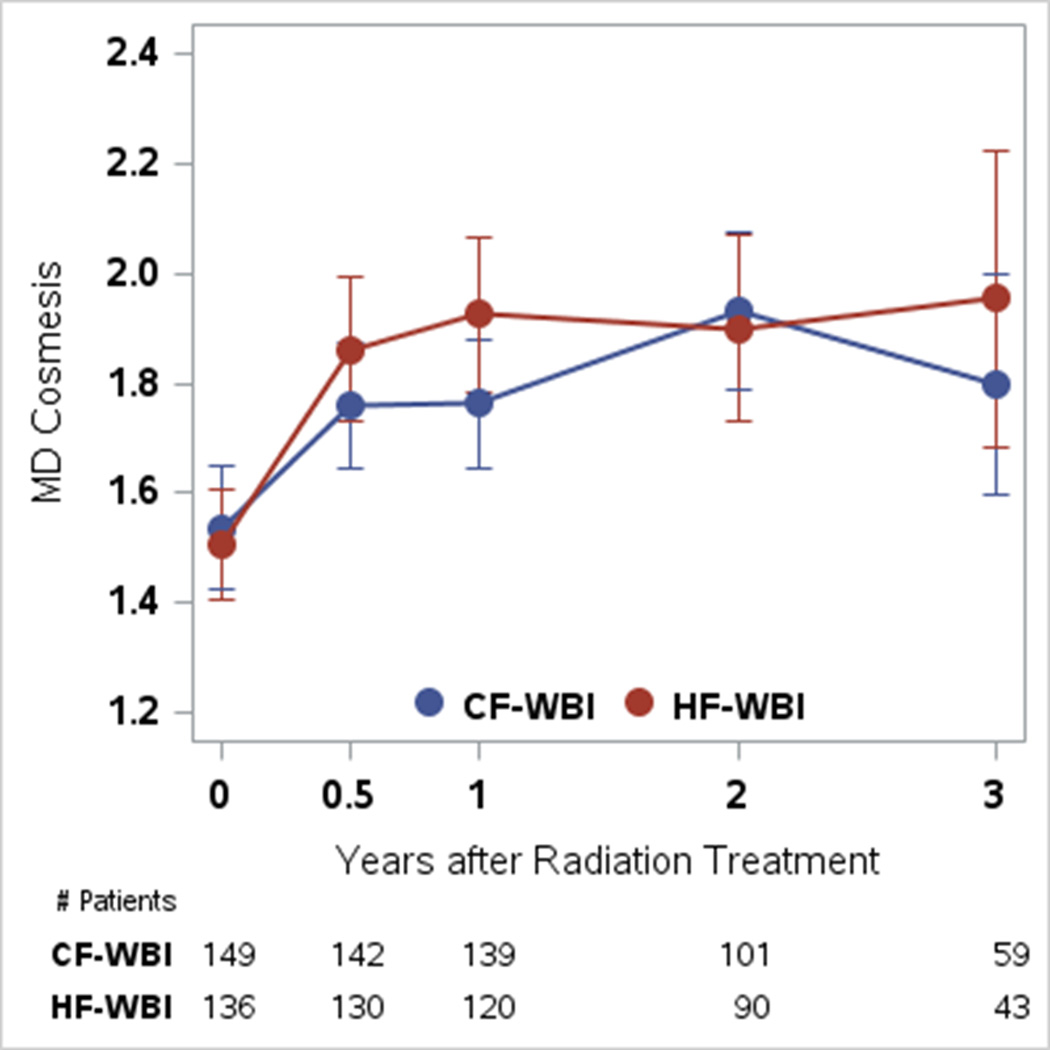
Baseline MD Cosmesis scores did not differ by randomization arm (Table 2). There were no statistically significant differences in mean scores by treatment arm at any of the time points (Table 2). A multivariable mixed effects growth curve model showed no statistically significant effect of the treatment arm on longitudinal MD Cosmesis outcomes (P=0.14), although the linear effect of time trended toward statistical significance (P=0.07, clinical outcome deteriorated with time for both treatment arms) (Fig. 3).
Figure 3.
Physician-rated cosmesis (MD Cosmesis) mean scores with 95% confidence intervals at baseline; 6 months; and 1, 2, and 3 years. CF-WBI indicates conventionally fractionated whole-breast irradiation; HF-WBI, hypofractionated whole-breast irradiation.
Effect of Missing Data
There was no statistically significant difference in the “dropout” pattern observed between the 2 treatment arms (P=0.14). The pattern mixture model that included dropout as an additional covariate in the multivariable model showed that dropout did not have a significant impact on any of the outcomes (data not shown).
Discussion
In this randomized trial of CF-WBI versus HF-WBI, there were no significant differences noted with regard to longitudinal PROs or physician-rated cosmesis by treatment arm. For both fractionation schedules, patient-reported functional status and breast pain were found to significantly improve with time.
These data represent an important contribution to the existing literature regarding hypofractionated WBI for several reasons. First, all patients in the current trial received a tumor bed boost, in contrast to the Canadian trial in which no patients received a boost (1) and the START trials in which 61% (START A trial) and 43% (START B trial) of patients received a boost (2). Second, nearly one-half of the patients in the current study were obese and approximately 25% of patients had a central axis separation of >25 cm, suggesting that the results of the current study are more generalizable to an American population than the Canadian trial, which excluded patients with a maximum breast width >25 cm (1).
The current study findings also add to the existing but to the best of our knowledge limited literature regarding the evolution of breast radiation sequalae over time. In a study of PROs from the START trials, Hopwood et al. found that for all fractionation schedules, breast symptoms improved from baseline to 5 years (3), a pattern that we were able to validate herein. However, the START trials also demonstrated an improvement in body image from baseline to 5 years, whereas the current study did not indicate any significant effect of time on body image outcomes, albeit within a shorter time frame.
A major strength of the current study is the prospective collection of physician-rated cosmesis and PROs at multiple, pre-specified time points. The resulting data have provided important insights into the natural history of radiation-related side effects in patients with breast cancer, specifically the improvement in function and pain over time. In addition, the findings of the current study should reassure clinicians that patients treated with HF-WBI experience longitudinal cosmetic and QOL outcomes that are comparable to patients treated with CF-WBI within 3 years of completing treatment.
The current study had several limitations. First, at the time of last follow-up, complete data for all patients through 3 years were not available; at last follow-up, approximately two-thirds of patients had data through 2 years of follow-up and approximately one-third of patients had data through 3 years of follow-up. We attempted to quantify the effect of incomplete data by performing a “dropout” analysis, and ultimately determined that the “dropout” pattern did not vary by randomization arm and did not significantly impact any of the outcomes examined. Given the suboptimal adoption rate of HF-WBI in the United States (6), we believe that the current study data, although incomplete, merit dissemination at this time to assuage any lingering concerns regarding the long-term toxic effects of hypofractionation, particularly when used in conjunction with a tumor bed boost in a patient population with a high prevalence of obesity.
A second limitation of the current study is the lack of blinding to treatment arm. Participating patients and physicians were aware that the purpose of the study was to compare cosmetic and QOL outcomes for 2 different fractionation schedules, and we recognize the potential for such knowledge to bias the reporting of outcomes. Finally, all endpoints reported in the current study were secondary; the primary study outcome of patient-reported cosmesis at 3 years will be reported at a later date once all patients have completed 3 years of follow-up.
The results of the current study demonstrate that the fractionation schedule appeared to have no significant effect on longitudinal PROs or physician-rated cosmesis, and that patient-reported functional and pain outcomes improved with time. These results further support the use of hypofractionation in patients with early-stage breast cancer, and provide valuable information regarding the natural history of breast RT toxicity. These findings add to a growing body of evidence that, when viewed in its totality, strongly suggests that hypofractionation should be the preferred dosing regimen for patients receiving WBI.
Acknowledgments
Funding support: This work was supported by by a Career Development Award from the American Society of Clinical Oncology Conquer Cancer Foundation (to Benjamin D. Smith), funded by the Breast Cancer Research Foundation. Dr. Smith is supported by the Andrew Sabin Family Fellowship.
The authors would like to thank the Patient-Reported Outcomes, Survey & Population Research (PROSPR) Shared Resource at MD Anderson for assistance in study planning and design.
Disclosures: Dr. Shaitelman reports funding from Elekta that is unrelated to the current project. Dr. Fingeret reports funding from the NIH/NCI and the American Cancer Society that is unrelated to the current project. Dr. Smith reports funding from Varian Medical Systems that is unrelated to the current project. Drs. Shaitelman and Bloom have served as consultants to the MD Anderson Physician’s Network.
References
- 1.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 2.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 3.Hopwood P, Haviland JS, Sumo G, et al. Comparison of patient-reported breast, arm, and shoulder symptoms and body image after radiotherapy for early breast cancer: 5-year follow-up in the randomised Standardisation of Breast Radiotherapy (START) trials. Lancet Oncol. 2010;11(3):231–240. doi: 10.1016/S1470-2045(09)70382-1. [DOI] [PubMed] [Google Scholar]
- 4.Ashworth A, Kong W, Whelan T, Mackillop WJ. A population-based study of the fractionation of postlumpectomy breast radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(1):51–57. doi: 10.1016/j.ijrobp.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Harnett A. Fewer fractions of adjuvant external beam radiotherapy for early breast cancer are safe and effective and can now be the standard of care. Why the UK's NICE accepts fewer fractions as the standard of care for adjuvant radiotherapy in early breast cancer. Breast. 2010;19(3):159–162. doi: 10.1016/j.breast.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Bekelman JE, Sylwestrzak G, Barron J, et al. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008–2013. JAMA. 2014;312(23):2542–2550. doi: 10.1001/jama.2014.16616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goffman TE, Glatstein E. Hypofractionation redux? J Clin Oncol. 2004;22(4):589–591. doi: 10.1200/JCO.2004.07.174. [DOI] [PubMed] [Google Scholar]
- 8.Mowery YM, Blitzblau RC. Whole-breast radiation therapy: the long and short of it. Int J Radiat Oncol Biol Phys. 2014;90(5):990–992. doi: 10.1016/j.ijrobp.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Sartor CI, Tepper JE. Is less more? Lessons in radiation schedules in breast cancer. J Natl Cancer Inst. 2002;94(15):1114–1115. doi: 10.1093/jnci/94.15.1114. [DOI] [PubMed] [Google Scholar]
- 10.Ceilley E, Jagsi R, Goldberg S, et al. Radiotherapy for invasive breast cancer in North America and Europe: results of a survey. Int J Radiat Oncol Biol Phys. 2005;61(2):365–373. doi: 10.1016/j.ijrobp.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 11.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaitelman SF, Schlembach PJ, Arzu I, et al. Acute and Short-term Toxic Effects of Conventionally Fractionated vs Hypofractionated Whole-Breast Irradiation: A Randomized Clinical Trial. JAMA Oncol. 2015;1(7):931–941. doi: 10.1001/jamaoncol.2015.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagsi R, Griffith KA, Boike TP, et al. Differences in the Acute Toxic Effects of Breast Radiotherapy by Fractionation Schedule: Comparative Analysis of Physician-Assessed and Patient-Reported Outcomes in a Large Multicenter Cohort. JAMA Oncol. 2015;1(7):918–930. doi: 10.1001/jamaoncol.2015.2590. [DOI] [PubMed] [Google Scholar]
- 14.Stanton AL, Krishnan L, Collins CA. Form or function? Part 1. Subjective cosmetic and functional correlates of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer. 2001;91(12):2273–2281. [PubMed] [Google Scholar]
- 15.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 16.Hahn EA, Segawa E, Kaiser K, Cella D, Smith BD. Health-related quality of life among women with ductal carcinoma in situ or early invasive breast cancer: validation of the FACT-B (version 4) Expert Review of Quality of Life in Cancer Care. 2016;1(1):99–109. [Google Scholar]
- 17.Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. Eur J Cancer. 2001;37(2):189–197. doi: 10.1016/s0959-8049(00)00353-1. [DOI] [PubMed] [Google Scholar]
- 18.Harris JR, Levene MB, Svensson G, Hellman S. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys. 1979;5(2):257–261. doi: 10.1016/0360-3016(79)90729-6. [DOI] [PubMed] [Google Scholar]
- 19.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer; 2000. [Google Scholar]
- 20.Little RJA. Modeling the Drop-out Mechanism in Repeated-Measures Studies. Journal of the American Statistical Association. 1995;90(431):1112–1121. [Google Scholar]
- 21.Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Am J Clin Nutr. 1998;68(4):899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]