Abstract
Controlled cortical impact (CCI) is a commonly used and highly regarded model of brain trauma that uses a pneumatically or electromagnetically controlled piston to induce reproducible and well-controlled injury. The CCI model was originally used in ferrets and it has since been scaled for use in many other species. This chapter will describe the historical development of the CCI model, compare and contrast the pneumatic and electromagnetic models, and summarize key short- and long-term consequences of TBI that have been gleaned using this model. In accordance with the recent efforts to promote high-quality evidence through the reporting of common data elements (CDEs), relevant study details—that should be reported in CCI studies—will be noted.
Keywords: Traumatic brain injury (TBI), Experimental brain injury, Preclinical, Animal model, Controlled cortical impact (CCI), Common data elements (CDE)
1 Introduction
Animal models have been used to study traumatic brain injury (TBI) for over a century and they remain widely used today to better understand outcomes of brain trauma and test novel therapies [1–4]. Today, preclinical TBI researchers have the choice between several models including: weight drop injury (WDI), fluid percussion injury (FPI), blast-induced TBI (bTBI), and controlled cortical impact (CCI), the focus of this chapter. CCI was originally developed to study TBI in ferrets [5], and its desirable properties (e.g., reproducibility; control over injury parameters) have led researchers to scale the model and apply it to many other species. The original design uses a pneumatically driven piston to induce TBI, while a newer alternative added an element of portability by using an electromagnetically driven piston which is lighter in weight and negates the need for a cylinder filled with compressed N2 gas.
The purpose of this chapter is to introduce readers to the CCI model so that thoughtful decisions can be made in their own completion of CCI research or in their consumption of the research literature. In doing so, the following will be discussed: (1) historical development, (2) key features, (3) comparison of the pneumatic and electromagnetic devices, (4) research applications, (5) relevant common data elements, as well as (6) factors that influence outcomes and data quality. A standard protocol will be described for pneumatic CCI in rats, along with a list of all required supplies and equipment.
1.1 History of Controlled Cortical Impact and Key Features
Animals have been used to study TBI for over 100 years, with considerable refinement of methodologies within the last three decades [1–3, 6–14]. In comparison to the many experimental TBI models available today, CCI is relatively new. It was originally developed by J.W. Lighthall and colleagues in the late 1980s and early 1990s with the goal of inducing TBI in ferrets [5, 15]. The desirable properties of CCI including the control over important injury parameters and ability to induce reproducible injury led C.E. Dixon and colleagues to scale the CCI model for use in rats during the early 1990s [16]. Since that time, CCI has been further scaled for use in several other species including mice, pigs, and nonhuman primates.
The scalability and other desirable features have resulted in CCI becoming one of the most popular and widely used preclinical TBI models. One noteworthy feature is that CCI provides quantitative control over important biomechanical parameters of TBI, in particular, the velocity, depth, and force of the tip are controlled across a wide range of contact velocities; there are also different options for tip size, geometry, and positioning, as discussed later in this chapter. Taken together, the control and customization of CCI allows researchers to address a multitude of research questions as well as scale the injury as needed to study the histopathological and functional deficits of interest. Reporting the injury parameters is of critical importance when reproducing, interpreting, and comparing published study findings, as described under the Common Data Elements heading.
A detailed protocol for inducing CCI in rats is included later, but to set the stage, a brief summary of CCI will be provided first. Traditionally, CCI is an invasive model whereby the exposed cortex is subjected to trauma following a craniectomy; in invasive CCI studies sham animals are used as controls to ensure that the results seen are not due to anesthesia and craniectomy but rather the CCI itself. Following craniectomy, the CCI device is used to transfer mechanical energy onto the intact dura mater, producing a TBI. Traditionally, the tip is pneumatically driven, though a newer model affords portability by using an electromagnetic device to drive the tip [14]. Both types of CCI allow for control over the tip depth, dwell time, and velocity; additional details about the pneumatic and electromagnetic CCI models are provided in the following section. It is also worth acknowledging that while invasive CCI remains widely used, CCI has been extended to model closed head injury [17–19] as described later in this chapter. It is also notable that even with an invasive CCI model, only one surgical procedure is necessary, as opposed to standard FPI which requires two surgeries.
1.2 Controlled Cortical Impact Types
1.2.1 Pneumatic Controlled Cortical Impact
The pneumatic CCI device (Fig. 1) was the first to be developed and it remains the most commonly used today. For these reasons, the pneumatic model will be emphasized in this chapter with a brief discussion on how it differs from the electromagnetic alternative. A standard pneumatic CCI device features a small bore (19.75 mm) reciprocating a double-acting pneumatic piston with a 50 mm stroke length. The piston is used to drive a tip of a desired size and geometry into the neural tissue (or in some cases the intact skull) to induce brain trauma. The cylinder is held by a crossbar which can be stereotaxically adjusted for variable mounting positions allowing the tip to be either aligned vertically or angled with respect to the brain. The velocity of the piston is monitored by a sensor and can be controlled to promote uniform injury across test animals. Researchers using the CCI model are able to control the depth, duration (a.k.a. dwell time), and velocity of injury as well as choose what size and shape of tip to use.
Fig. 1.
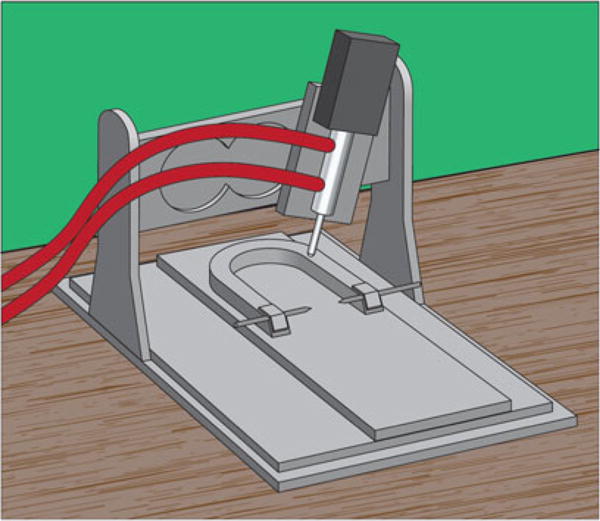
Diagram depicting a standard pneumatic CCI device (without the associated cylinder of compressed N2 gas). Depending on the research goals, researchers can choose the ideal tip (e.g., size, geometry) and injury parameters (e.g., depth, dwell time, velocity)
1.2.2 Electromagnetic Controlled Cortical Impact
The electromagnetic CCI device (Fig. 2) is very similar to the pneumatic model and also uses a stereotaxic frame for adjustability. The electromagnetic CCI devices are considered to be more portable due to their lighter weight. Another similarity is the availability of tips with varying sizes and geometry (e.g., flat, beveled, round). Generally speaking, tip scaling correlates with animal size; for example, 3 mm tips are commonly used for mice, 5–6 mm tips for rats, 10 mm for nonhuman primates, and 15 mm for pigs. Depending on the vendor, the device may come with a variety of tips. Alternatively, there may be additional tips available to purchase separately. Some researchers have also modified tips based on their unique research needs with one group using vulcanized rubber from a lacrosse ball to cover the tip [19]. A list of commercial vendors who sell both electromagnetic and pneumatic CCI devices is included in Table 1.
Fig. 2.
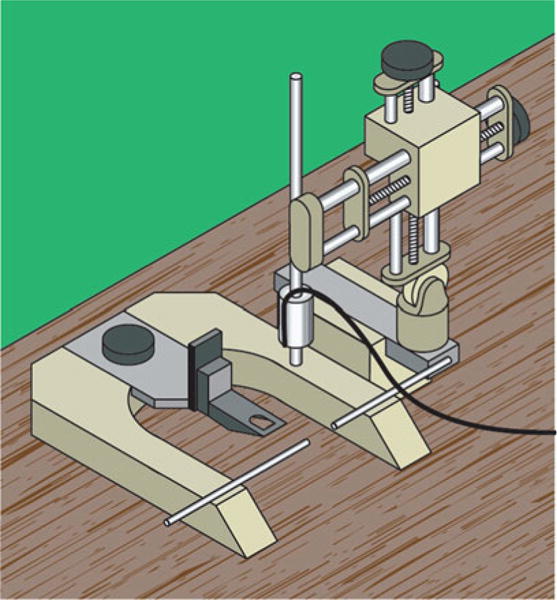
Diagram depicting a standard electromagnetic CCI device. As with pneumatic CCI, the tip and injury parameters can be adjusted. Unlike with the pneumatic model, the tip is driven by an electromagnetic actuator negating the need for N2 gas
Table 1.
Commercially available pneumatic- and electromagnetic-CCI devices
| CCI Type | Company | Model |
|---|---|---|
| Pneumatic | Precision Systems and Instrumentation | LLC TBI-0310 Impactor |
| Pittsburgh Precision Instruments | Pneumatic Powered Controlled Cortical Impact Device | |
| AmScien Instruments | Pneumatic (Cortical) Impact Device (Model: AMS 201) | |
|
| ||
| Electromagnetic | Leica | Impact One Stereotaxic Impactor for CCI |
| Hatteras Instruments | Pinpoint PCI3000 Precision Cortical Impactor | |
Suppliers and models are listed for commonly used pneumatic- and electromagnetic-CCI devices
1.3 Applications of Controlled Cortical Impact
1.3.1 Closed Head Injury
Though originally CCI was developed as an invasive TBI model, it has been more recently adapted to study closed head injury (CHI) including repeated concussions. CHI models have become an area of increased research emphasis as the risk to individuals who are in the military or those involved in various athletic activities is further appreciated. The aforementioned strengths of CCI make it a popular choice for researchers studying CHI [17–19]. Applications of CCI to study CHI include a study modeling sports-related concussions [19]. In this study, the researchers combined elements of CCI with elements of Marmarou’s impact acceleration model in an attempt to enhance control over clinically relevant variables. For example, a foam pad was placed under the rodent to limit rotational acceleration and instead promote linear acceleration. In this study, more deficits were observed after repeated TBI than a single TBI, as assessed using a battery of neurobehavioral tests including measures of cognition, memory, and sleep [19].
1.3.2 Long-Term Outcomes
The high survivability of CCI makes it a good choice for studying the long-term changes associated with TBI. Available evidence suggests that CCI results in chronic and progressive changes. For example, one study which assessed the test animals up to 1 year post-TBI reported ongoing deficits including progressive tissue loss and ventricular expansion [20]. In addition to the aforementioned histopathological consequences, there are several chronic behavioral deficits that have been reported. For example, after CCI (vs. sham) memory and learning deficits have been found to persist into the long term as assessed using the MWM [21–35]. Notably, persistent MWM deficits are more rarely reported in other models, including lateral FPI [36, 37] and medial FPI [38]. Long-term deficits in motor function have also been reported, as assessed using the foot fault test [22, 34, 39, 40], whereas the authors of this chapter were only able to find one FPI study reporting long-term motor deficits using this measure [41]. That is to say that CCI is a good choice when researchers are interested in exploring long-term motor and memory symptoms after brain injury. Conversely, no CCI studies were identified where motor deficits on the inclined plane task were reported, though such deficits have been reported after lateral FPI [42]. Similarly, no CCI studies were identified where chronic deficits in reversal learning were reported, although this has been reported in several lateral FPI studies [36, 37, 43]. A detailed review of long-term outcomes for the major experimental TBI models is available to interested readers [44].
1.4 Species Used
One of the assets of the CCI model is that it can be translated to induce experimental TBI in many species. This is accomplished by scaling the injury parameters so as to maintain the percent of brain volume deformed in relation to total brain volume taking into account desired extent of injury to address the research questions. In order to induce CCI in large animals (e.g., pigs), modifications may be necessary to ensure the piston is high enough. Table 2 provides a summary of the various species that CCI has been applied to including injury parameters commonly used in each species.
Table 2.
Commonly used CCI parameters for various species
| Species | Injury site | Crani. size (mm) | Tip diameter (mm) | Velocity (m/s) | Dwell time (ms) | Depth (mm) |
|---|---|---|---|---|---|---|
| Mouse | Parietal Cortex | 4–5 | 3 | 4–6 | 50–250 | 0.5–2 |
| Rat | Parietal Cortex; Midline | 6–8 | 5–6 | 4 | 50–250 | 1–3 |
| Primate | Frontal Lobe | 11–12 | 10 | 3.5 | 150 | 7 |
| Pig | Frontal Lobe | 15–18 | 15 | 2–4 | 50–400 | 12 |
For each species, injury details commonly used are provided including injury site, craniectomy size, tip diameter, velocity, dwell time, and depth
1.4.1 Rat
Graded TBI can be easily produced in rats using the CCI model. Indeed, the effects of CCI are well categorized in rats where it has been found to result in diverse histopathological and functional changes consistent with what occurs in clinical TBI cases, including but not limited to: blood–brain-barrier disruption, derangements in blood flow and pressure, axonal injury, inflammation, and edema. It is also worth noting that functional symptoms of TBI (e.g., deficits in learning, memory, and motor function) can be assessed using neurobehavioral testing, which is well characterized in rats. For example, memory is readily assessed using the Morris Water Maze (MWM), Barnes Maze, or Novel Object Recognition (NOR) task; whereas motor function can be assessed using the Beam Balance Task, Beam Walking Task, Rotarod Task, and Wire Grip Task.
1.4.2 Mouse
Shortly after translation of CCI from ferrets to rats, the model was further extended to mice [45–47]. Refinement of CCI in mice has paralleled the increasing application of genetically modified mice to TBI research to explore the role of genes and gene products in brain injury recovery [48]. Generally speaking, to scale CCI down for mice entails decreasing the injury depth to adjust for the relatively thinner cortex in mice compared to rats. Though slightly less well categorized, a plethora of behavioral testing strategies are available for use in mice including the MWM, NOR, and BBT [31, 33, 49, 50].
1.4.3 Pig
To adequately address some research questions, larger, more human-like brains are needed, necessitating the use of a pig or other large mammal models. The main difference from the rat and mouse models is the considerably larger impactor tip and greater depth to which the neural tissue is deformed (see Table 2). In one study, CCI was scaled to induce TBI in piglets; injury parameters were chosen after adjusting for differences in brain morphology (e.g., size and dimensions) in the study’s test animals [51]. As with rodent models of CCI, when pigs are used the histopathological changes mirror what is seen in TBI patients, including but not limited to, deranged blood flow and changes to vasculature, ongoing neurodegeneration via a number of mechanisms, and edema. To date, pig models have been used to add to the evidence surrounding TBI biomechanics [52] and as part of an effort to identify clinically relevant biomarkers of underlying brain injury [53]. However, despite these efforts there is a relative dearth of normative data specific to pigs when compared to rodents [54]. Additionally, behavioral testing is less well characterized in pigs and not to mention more challenging to perform due to their larger size and relative intelligence.
1.4.4 Nonhuman Primate
An alternative to the pig model is the nonhuman primate model of CCI, which is typically applied over the frontal cortex [55]. As with the other models, the histopathological changes reported after nonhuman primate CCI mimic what is seen clinically, including but not limited to edema, macrophage accumulation, and neurodegeneration. Nonhuman primates play a critical role in establishing the safety of novel therapies before translation to humans. Notably, due to the increased ethical considerations, care requirements, and cost, a relatively limited number of research institutions have primate research facilities. Consequently, nonhuman primate studies represent only a small fraction of TBI studies. Indeed, the use of nonhuman primate models is only justified when there are major factors that prohibit the use of a less sentient animal.
1.5 Special Considerations, Problems, and Troubleshooting
High-quality CCI research relies on thoughtful study design and careful execution. A few important confounders that have been empirically studied will be addressed as follows. When appropriate, troubleshooting strategies will be noted.
1.5.1 Tip Geometry
Commercially available tips come in round or beveled flat shapes of various sizes. When CCI was developed in ferrets, round tips were used [5, 15]; though still in use, beveled flat tips have become the norm [47, 48, 56–59] and are especially preferred for mouse CCI. Despite convention, little empirical research has tested the effects of tip geometry on outcomes. In one study, Pleasant and colleagues compared flat vs. rounded tips in a mouse (C57BL6J) model; they found more extensive cortical hemorrhage and neuronal loss (proportionally) with flat tips. The rate of neocortical loss was faster with flat tips, with a plateau in neurodegeneration 20 h earlier than rounded tips (4 h vs. 24 h) making the latter a more desirable choice when studying secondary injury cascades in the subacute period [60].
1.5.2 Anesthesia
Overall, careful choice of whether to use anesthesia and what anesthesia regimen to use is critical. In deciding, researchers should consider the study goals, along with established guidelines for the treatment of research animals. Empirical evidence shows that differences in outcomes of TBI are associated with various anesthesia agents; notably, isoflurane results in less hippocampal damage than fentanyl as well as fewer behavioral deficits [61]. Another study found that preinjury isoflurane had neuroprotective effects [62]. It is hypothesized that fentanyl contributes to neural suppression, whereas isoflurane reduces excitotoxicity and promotes blood flow [54]. Ketamine also demonstrates neuroprotective properties via antagonism of N-methyl-D-aspartate (NMDA) receptors [63]. Halothane has also been reported to have neuroprotective properties after contusion injuries [64]. Use of neuroprotective anesthesia can obscure deficits in performance in all anesthetized groups. For fentanyl, the concern is that the resulting neural suppression could worsen performance and may obscure treatment effects in a drug study.
Despite these concerns, the overwhelming majority of CCI studies use anesthesia, commonly isoflurane. Volatile gases (e.g., isoflurane, halothane) are often preferred due to their relatively short half-life compared to long-acting options (e.g., pentobarbital), facilitating evaluation of righting reflex shortly after anesthesia discontinuation [16]. Typically a high dose (e.g., 4 %) is used to induce anesthesia followed by a reduced maintenance dose (see Subheading 4). Anesthetized animals should be monitored to ensure consciousness is not regained during surgery. Assessments like the toe pinch can be used to assess sufficiency of anesthesia in accordance with institutional and national policies. One recent study of repetitive closed head injury used unanaesthetized mice that were instead comfortably restrained to avoid the confounding effects of anesthesia on TBI outcomes and promote clinical relevance [19].
Commonly encountered problems surrounding anesthesia are summarized later. First, if animals are intubated and the animal is fighting the ventilator, changes in tube placement may alleviate the problem. Also, if consciousness is regained, then the anesthesia induction system should be checked to ensure that the anesthesia is set to the appropriate level and there are no leaks in the tubing. It is also important to consider that if gaseous anesthesia is used, specialized laboratory equipment is required including appropriate ventilation and scavenging systems to ensure the safety of personnel; isoflurane detection systems are available to monitor exposure.
1.5.3 Craniectomy and Sham Procedure
Researchers typically produce craniectomy using a pneumatic or electric drill, although a handheld trephine is sometimes used. Efforts to reduce heat production during the craniectomy can help reduce potential confounders, and sterile saline can be applied to the craniectomy site to reduce the temperature if the procedure is prolonged. Since anesthesia and craniectomy can result in behavioral and functional changes it is important that when invasive CCI is used control animals be exposed to sham. Empirical evidence shows that craniectomy results in inflammation and lesions regardless of whether a trephine or electric drill is used, as compared to naïve rats (anesthesia only). However, lesions were largest and behavioral deficits were most severe in animals that received their craniectomies using a drill [65]. Craniectomy location also affects outcome with midline craniectomy leading to more sagittal bleeding [16] than parasagittal craniectomy [66, 67]. Bilateral craniectomies have been used to enhance lateral movement of tissue and produce subsequent bilateral cortical contusions [68, 69]; producing bilateral craniectomies is also a good way to train individuals on the procedure. Details regarding the control group should be provided in publications [65], including any bleeding, mortality, inconsistency across animals, etc.
2 Materials
2.1 Animals
A strength of the CCI model is that it can be used in many species. In much of our work, adult male Sprague Dawley rats (280–320 g; Charles River Labs, Raleigh, VA, USA) are used; thus, this protocol is specific to Sprague Dawley rats. Our animals are routinely housed in a climate-controlled room with a 12 h light/dark cycle and are regularly monitored by the Department of Laboratory Animal Research.
2.2 Anesthesia
Induction Dose: 4.0 % isoflurane in 2:1 N2O:O2.
Maintenance Dose: 2 % delivered in 2:1 N2O:O2.
2.3 Supplies and Equipment
Homeothermic heating system (Harvard Apparatus, MA, USA).
Stereotaxic frame.
Isoflurane.
Cylinders of compressed N2O and O2 for isoflurane delivery.
Cylinder of compressed N2 to drive pneumatic tip.
Anesthesia chamber.
Gas scavenging system.
Cannula for intubation.
Laryngoscope to assist with intubation, if necessary.
Animal trimmers.
Pneumatic drill with drill bits.
Compressed air for drill.
Betadine.
Sterile drape.
Cotton-tipped applicators.
Gauze.
Saline-filled syringe.
Sterile surgical instruments (scalpel; scissors; periosteal elevator; microdissecting forceps; rongeurs; bulldog clips, etc.).
Temperature probe and associated readout (Harvard Apparatus, MA, USA).
MouseOx Plus, blood oxygenation monitoring system, and associated readout (Starr Life Sciences Corp., PA, USA).
Suture kit.
Pneumatic CCI device (Pittsburgh Precision Instruments, PA, USA).
Tip of desired size and shape.
3 Methods
Before starting the surgeries, ensure the CCI device is in good working order. Does the piston fire freely? Is the impact velocity and dwell time consistent with what is set?
Place the rat in an anesthesia chamber and induce anesthesia with 4% isoflurane in a 2:1 mixture of N2O:O2; ensure the animal is sufficiently anesthetized using a toe pinch test.
Intubate the rat.
Place the anesthetized animal in a stereotaxic frame and secure the incisor and ear bars to keep the animal secure throughout the surgery.
Adjust the anesthesia to the maintenance dose of 2 % isoflurane (see Note 1).
Assess the animal’s level of alertness using the toe pinch test for suppression of pedal response (or another similar test) to ensure sufficient anesthesia is being delivered.
Use the hair trimmers to shave the rat’s scalp moving both with and against the grain.
Use a sterile drape to cover the animal such that the only opening in the drape is directly over the exposed scalp.
Use gauze and antiseptic solution (e.g., Betadine) to scrub the scalp and prepare the surgical site.
Use a scalpel to make a midline incision (see Note 2).
Separate the muscle from the skull using the periosteal elevator and microdissecting forceps.
Reflect the skin and fascia to expose the underlying skull and scrub the surface of the skull with a cotton-tipped applicator.
Use pneumatic drill (hooked up to a compressed air cylinder) to create a craniectomy. Center the craniectomy between the sagittal suture and coronal ridge with the borders near the lambda and bregma for unobstructed tip clearance (see Note 3).
If necessary, use rongeurs to elongate the craniectomy until it is large enough to accommodate the impactor tip; carefully lift away the resulting bone flap, so as to avoid dura breech (see Note 4).
To ensure that the tip is centered over the craniectomy site, manually extend the shaft on the CCI device and gently lower the impactor tip so that it lightly and briefly touches the exposed dura mater.
With the piston statically pressurized and in the full stroke position, zero the tip to the cortical surface.
Carefully withdraw the tip and adjust the piston assembly to the desired impact depth based on the research goals and study protocol (see Note 5).
Induce injury by actuating the CCI device; discontinue the anesthesia (see Note 6).
Close the surgical site using sutures or another method. Apply topical anesthetic (e.g., lidocaine) to the surgical site to minimize discomfort.
Remove the rat from the stereotaxic frame and extubate.
Complete any assessments desired in the immediate postinjury period (e.g., righting reflex latency) and postsurgical monitoring.
Keep the test animal in a holding cage until it is able to fully recover from anesthesia, as evidenced by the return of spontaneous locomotion.
Once the animal has fully recovered, return it to the animal housing room and resume normal husbandry.
Continue to administer analgesic in accordance with institutional and government guidelines for pain management in laboratory animals.
4 Common Data Elements
The National Institute for Neurological Diseases and Stroke (NINDS) has published a set of common data elements (CDEs) for experimental TBI research including details surrounding the animals used (e.g., species, strain, commercial supplier), demographics (e.g., age, sex), metrics (e.g., weight), animal husbandry, and outcome assessment(s) used (e.g., timing of assessment, measures). Beyond the CDE’s generic to all experimental TBI models, the NINDS recognizes a set of CDEs specific to CCI research. The CCI-specific CDEs include, but are not limited to craniectomy size, tip (size, shape, angle, rigidity), and injury parameter settings (depth, dwell time, velocity). Researchers are encouraged to review the current list of CDEs during study planning, grant writing, and dissemination of findings to promote comparison of studies and the conduct of high-quality research [70].
5 Conclusion
CCI is one of the best characterized models of experimental TBI and it remains a popular choice for studying the physiologic and functional deficits that occur acutely and chronically following TBI. Traditionally, CCI is an invasive model that is preceded by craniectomy, but recently the model has been applied to study concussion and other types of closed head injury. The original CCI model was pneumatically driven but more recently, an electromagnetic alternative has been introduced which provides increased portability.
Researchers employing the CCI model should give care and attention to study design and selection of injury parameters. Control over important confounding variables (e.g., hypothermia, hyperthermia) is critical to adequately address the research questions. The first step is to thoroughly explore the literature to consider how various injury parameters have panned out with respect to histopathological and functional consequences in the past. Researchers are also encouraged to conduct pilot work in order to tailor the experimental design (e.g., injury parameters, tip size, anesthesia type) and subsequently facilitate addressing the research goals. Pilot research also provides a valuable opportunity to ensure that the device is in proper working order and is calibrated.
This chapter introduced the CCI model including a brief overview of its development and extension to various species and research applications. A list of required supplies and equipment was provided as well as a detailed protocol for pneumatic CCI in rats. Discussion of confounding factors and troubleshooting methods were briefly discussed. Lastly, the importance of CDEs was extolled and exemplars of CDEs specific to CCI were noted. This introductory chapter will enable readers to be thoughtful consumers of publications describing CCI research and have the requisite knowledge needed to design and conduct a CCI study.
Acknowledgments
Support for this chapter comes from the following government funding sources: Department of Veterans Affairs grant RR&D B1127-I, NIH-NINDS grant R01-NS079061, and NIH-NINR grants 1F31NR014957 and T32NR009759. Additional support for this chapter comes from the following foundations and professional societies: The Pittsburgh Foundation, Sigma Theta Tau International Eta Chapter, the International Society for Nurses in Genetics, and the American Association of Neuroscience Nursing/Neuroscience Nursing Foundation. We would also like to acknowledge Mr. Michael D. Farmer for his time in generating the figures and Mrs. Marilyn K. Farmer for her continued editorial support.
Footnotes
Traditionally, the authors of this chapter use a maintenance dose of 2% isoflurane in a 2:1 mixture of N2O:O2 titrating up the dose if the animal is showing signs of regaining consciousness.
The incision made in our lab is approximately 20 mm long for rats (shorter for mice).
The authors strive to make consistent craniectomies that are approximately 6 mm in diameter to facilitate clearance of a 5 mm diameter tip.
It is common practice to discard the bone flap rather than attempt to reattach it, as this can lead to secondary injury (e.g., increased intracranial pressure).
In our lab, we induce moderate TBI using a 5 mm tip to deform the neural tissue of a rat to a depth of 2.8 mm at a velocity of 4 m/s.
Depending on the preference of the researchers and the method used to close the surgical site, anesthesia can be discontinued before or after wound site closure.
References
- 1.Kramer SP. A contribution to the theory of cerebral concussion. Anim Surg. 1896;23:163–173. doi: 10.1097/00000658-189601000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinder L, Olsson Y. Studies on vascular permeability changes in experimental brain concussion. I. Distribution of circulating fluorescent indicators in brain and cervical cord after sudden mechanical loading of the brain. Acta Neuropathol. 1968;11:183–200. doi: 10.1007/BF00692305. [DOI] [PubMed] [Google Scholar]
- 3.Denny-Brown D, Russell W. Experimental cerebral concussion. Brain. 1941;64:93. doi: 10.1113/jphysiol.1940.sp003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindgren S, Rinder L. Experimental studies in head injury. I. Some factors influencing results of model experiments. Biophysik. 1965;2:320–329. [PubMed] [Google Scholar]
- 5.Lighthall JW. Controlled cortical impact: a new experimental brain injury model. J Neurotrauma. 1988;5:1–15. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- 6.Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol. 1982;12:564–574. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- 7.Govons SR, Govons RB, VanHuss WD, Heusner WW. Brain concussion in the rat. Exp Neurol. 1972;34:121–128. doi: 10.1016/0014-4886(72)90193-8. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson B, Pontén U, Voigt G. Experimental head injury in the rat. Part 1: Mechanics, pathophysiology, and morphology in an impact acceleration trauma model. J Neurosurg. 1977;47:241–251. doi: 10.3171/jns.1977.47.2.0241. [DOI] [PubMed] [Google Scholar]
- 9.Ommaya AK, Geller A, Parsons LC. The effect of experimental head injury on one-trial learning in rats. Int J Neurosci. 1971;1:371–378. doi: 10.3109/00207457109146986. [DOI] [PubMed] [Google Scholar]
- 10.Ommaya AK, Gennarelli TA. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain. 1974;97:633–654. doi: 10.1093/brain/97.1.633. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan HG, Martinez J, Becker DP, Miller JD, Griffith R, Wist AO. Fluid-percussion model of mechanical brain injury in the cat. J Neurosurg. 1976;45:521–534. [PubMed] [Google Scholar]
- 12.Cannon WB. Cerebral pressure following trauma. Am J Physiol. 1901;6:91–121. [Google Scholar]
- 13.Parkinson D, West M, Pathiraja T. Concussion: comparison of humans and rats. Neurosurgery. 1978;3:176–180. doi: 10.1227/00006123-197809000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Onyszchuk G, Al-Hafez B, He Y-Y, Bilgen M, Berman NEJ, Brooks WM. A mouse model of sensorimotor controlled cortical impact: characterization using longitudinal magnetic resonance imaging, behavioral assessments and histology. J Neurosci Methods. 2007;160:187–196. doi: 10.1016/j.jneumeth.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lighthall JW, Goshgarian HG, Pinderski CR. Characterization of axonal injury produced by controlled cortical impact. J Neurotrauma. 1990;7:65–76. doi: 10.1089/neu.1990.7.65. [DOI] [PubMed] [Google Scholar]
- 16.Dixon C, Clifton G, Lighthall J, Yaghmai A, Hayes R. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 17.Shitaka Y, Tran HT, Bennett RE, Sanchez L, Levy MA, Dikranian K, Brody DL. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol. 2011;70:551–567. doi: 10.1097/NEN.0b013e31821f891f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klemenhagen KC, O’Brien SP, Brody DL. Repetitive concussive traumatic brain injury interacts with post-injury foot shock stress to worsen social and depression-like behavior in mice. PLoS One. 2013;8:e74510. doi: 10.1371/journal.pone.0074510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petraglia AL, Plog BA, Dayawansa S, Chen M, Dashnaw ML, Czerniecka K, Walker CT, Viterise T, Hyrien O, Iliff JJ, Deane R, Nedergaard M, Huang JH. The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J Neurotrauma. 2014;31:1211–1224. doi: 10.1089/neu.2013.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon C, Kochanek P, Yan H, Schiding J, Griffith R, Baum E, Marion D, DeKosky S. One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J Neurotrauma. 1999;16:109–122. doi: 10.1089/neu.1999.16.109. [DOI] [PubMed] [Google Scholar]
- 21.Xiong Y, Zhang Y, Mahmood A, Meng Y, Zhang ZG, Morris DC, Chopp M. Neuroprotective and neurorestorative effects of thymosin β4 treatment initiated 6 hours after traumatic brain injury in rats. J Neurosurg. 2012;116:1081–1092. doi: 10.3171/2012.1.JNS111729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng Y, Xiong Y, Mahmood A, Zhang Y, Qu C, Chopp M. Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats. J Neurosurg. 2011;115:550–560. doi: 10.3171/2011.3.JNS101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longhi L, Watson DJ, Saatman KE, Thompson HJ, Zhang C, Fujimoto S, Royo N, Castelbuono D, Raghupathi R, Trojanowski JQ, Lee VM-Y, Wolfe JH, Stocchetti N, McIntosh TK. Ex vivo gene therapy using targeted engraftment of NGF-expressing human NT2N neurons attenuates cognitive deficits following traumatic brain injury in mice. J Neurotrauma. 2004;21:1723–1736. doi: 10.1089/neu.2004.21.1723. [DOI] [PubMed] [Google Scholar]
- 24.Longhi L, Gesuete R, Perego C, Ortolano F, Sacchi N, Villa P, Stocchetti N, De Simoni M-G. Long-lasting protection in brain trauma by endotoxin preconditioning. J Cereb Blood Flow Metab. 2011;31:1919–1929. doi: 10.1038/jcbfm.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng JP, Shaw KE, Monaco CM, Hoffman AN, Sozda CN, Olsen AS, Kline AE. A relatively brief exposure to environmental enrichment after experimental traumatic brain injury confers long-term cognitive benefits. J Neurotrauma. 2012;29:2684–2688. doi: 10.1089/neu.2012.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox GB, Faden AI. Traumatic brain injury causes delayed motor and cognitive impairment in a mutant mouse strain known to exhibit delayed Wallerian degeneration. J Neurosci Res. 1998;53:718–727. doi: 10.1002/(SICI)1097-4547(19980915)53:6<718::AID-JNR9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Dixon CE, Hamm RJ, Taft WC, Hayes RL. Increased anticholinergic sensitivity following closed skull impact and controlled cortical impact traumatic brain injury in the rat. J Neurotrauma. 1994;11:275–287. doi: 10.1089/neu.1994.11.275. [DOI] [PubMed] [Google Scholar]
- 28.Marklund N, Morales D, Clausen F, Hånell A, Kiwanuka O, Pitkänen A, Gimbel DA, Philipson O, Lannfelt L, Hillered L, Strittmatter SM, McIntosh TK. Functional outcome is impaired following traumatic brain injury in aging Nogo-A/B-deficient mice. Neuroscience. 2009;163:540–551. doi: 10.1016/j.neuroscience.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chauhan NB, Gatto R. Synergistic benefits of erythropoietin and simvastatin after traumatic brain injury. Brain Res. 2010;1360:177–192. doi: 10.1016/j.brainres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Chauhan NB, Gatto R. Restoration of cognitive deficits after statin feeding in TBI. Restor Neurol Neurosci. 2011;29:23–34. doi: 10.3233/RNN-2011-0573. [DOI] [PubMed] [Google Scholar]
- 31.Byrnes KR, Loane DJ, Stoica BA, Zhang J, Faden AI. Delayed mGluR5 activation limits neuroinflammation and neurodegeneration after traumatic brain injury. J Neuroinflammation. 2012;9:43. doi: 10.1186/1742-2094-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Chopp M, Mahmood A, Meng Y, Qu C, Xiong Y. Impact of inhibition of erythropoietin treatment-mediated neurogenesis in the dentate gyrus of the hippocampus on restoration of spatial learning after traumatic brain injury. Exp Neurol. 2012;235:336–344. doi: 10.1016/j.expneurol.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomasevic G, Laurer HL, Mattiasson G, van Steeg H, Wieloch T, McIntosh TK. Delayed neuromotor recovery and increased memory acquisition dysfunction following experimental brain trauma in mice lacking the DNA repair gene XPA. J Neurosurg. 2012;116:1368–1378. doi: 10.3171/2012.2.JNS11888. [DOI] [PubMed] [Google Scholar]
- 34.Xiong Y, Zhang Y, Mahmood A, Meng Y, Qu C, Chopp M. Erythropoietin mediates neurobehavioral recovery and neurovascular remodeling following traumatic brain injury in rats by increasing expression of vascular endothelial growth factor. Transl Stroke Res. 2011;2:619–632. doi: 10.1007/s12975-011-0120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han R-Z, Hu J-J, Weng Y-C, Li D-F, Huang Y. NMDA receptor antagonist MK-801 reduces neuronal damage and preserves learning and memory in a rat model of traumatic brain injury. Neurosci Bull. 2009;25:367–375. doi: 10.1007/s12264-009-0608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shultz SR, Bao F, Omana V, Chiu C, Brown A, Cain DP. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J Neurotrauma. 2012;29:281–294. doi: 10.1089/neu.2011.2123. [DOI] [PubMed] [Google Scholar]
- 37.Shultz SR, Bao F, Weaver LC, Cain DP, Brown A. Treatment with an anti-CD11d integrin antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion. J Neuroinflammation. 2013;10:26. doi: 10.1186/1742-2094-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamm RJ, Pike BR, Temple MD, O’Dell DM, Lyeth BG. The effect of postinjury kindled seizures on cognitive performance of traumatically brain-injured rats. Exp Neurol. 1995;136:143–148. doi: 10.1006/exnr.1995.1091. [DOI] [PubMed] [Google Scholar]
- 39.Hoane MR. Magnesium therapy and recovery of function in experimental models of brain injury and neurodegenerative disease. Clin Calcium. 2004;14:65–70. [PubMed] [Google Scholar]
- 40.Xiong Y, Mahmood A, Zhang Y, Meng Y, Zhang ZG, Qu C, Sager TN, Chopp M. Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury. J Neurosurg. 2011;114:549–559. doi: 10.3171/2010.10.JNS10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rau TF, Kothiwal AS, Rova AR, Brooks DM, Poulsen DJ. Treatment with low-dose methamphetamine improves behavioral and cognitive function after severe traumatic brain injury. J Trauma Acute Care Surg. 2012;73:S165–S172. doi: 10.1097/TA.0b013e318260896a. [DOI] [PubMed] [Google Scholar]
- 42.Hallam TM, Floyd CL, Folkerts MM, Lee LL, Gong Q-Z, Lyeth BG, Muizelaar JP, Berman RF. Comparison of behavioral deficits and acute neuronal degeneration in rat lateral fluid percussion and weight-drop brain injury models. J Neurotrauma. 2004;21:521–539. doi: 10.1089/089771504774129865. [DOI] [PubMed] [Google Scholar]
- 43.Thompson HJ, LeBold DG, Marklund N, Morales DM, Hagner AP, McIntosh TK. Cognitive evaluation of traumatically brain-injured rats using serial testing in the Morris water maze. Restor Neurol Neurosci. 2006;24:109–114. [PMC free article] [PubMed] [Google Scholar]
- 44.Osier ND, Carlson SW, DeSana A, Dixon CE. Chronic histopathological and behavioral outcomes of experimental traumatic brain injury in adult male animals. J Neurotrauma. 2015;32:1861. doi: 10.1089/neu.2014.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox GB, LeVasseur RA, Faden AI. Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to traumatic brain injury: implications for gene targeting approaches to neurotrauma. J Neurotrauma. 1999;16:377–389. doi: 10.1089/neu.1999.16.377. [DOI] [PubMed] [Google Scholar]
- 46.Hannay HJ, Feldman Z, Phan P, Keyani A, Panwar N, Goodman JC, Robertson CS. Validation of a controlled cortical impact model of head injury in mice. J Neurotrauma. 1999;16:1103–1114. doi: 10.1089/neu.1999.16.1103. [DOI] [PubMed] [Google Scholar]
- 47.Fox GB, Fan L, LeVasseur RA, Faden AI. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J Neurotrauma. 1998;15:599–614. doi: 10.1089/neu.1998.15.599. [DOI] [PubMed] [Google Scholar]
- 48.Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- 49.Han X, Tong J, Zhang J, Farahvar A, Wang E, Yang J, Samadani U, Smith DH, Huang JH. Imipramine treatment improves cognitive outcome associated with enhanced hippocampal neurogenesis after traumatic brain injury in mice. J Neurotrauma. 2011;28:995–1007. doi: 10.1089/neu.2010.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scafidi S, Racz J, Hazelton J, McKenna MC, Fiskum G. Neuroprotection by acetyl-L-carnitine after traumatic injury to the immature rat brain. Dev Neurosci. 2010;32:480–487. doi: 10.1159/000323178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duhaime AC, Margulies SS, Durham SR, O’Rourke MM, Golden JA, Marwaha S, Raghupathi R. Maturation-dependent response of the piglet brain to scaled cortical impact. J Neurosurg. 2000;93:455–462. doi: 10.3171/jns.2000.93.3.0455. [DOI] [PubMed] [Google Scholar]
- 52.Manley GT, Rosenthal G, Lam M, Morabito D, Yan D, Derugin N, Bollen A, Knudson MM, Panter SS. Controlled cortical impact in swine: pathophysiology and biomechanics. J Neurotrauma. 2006;23:128–139. doi: 10.1089/neu.2006.23.128. [DOI] [PubMed] [Google Scholar]
- 53.Costine BA, Quebeda-Clerkin PB, Dodge CP, Harris BT, Hillier SC, Duhaime A-C. Neuron-specific enolase, but not S100B or myelin basic protein, increases in peripheral blood corresponding to lesion volume after cortical impact in piglets. J Neurotrauma. 2012;29:2689–2695. doi: 10.1089/neu.2012.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kline AE, Dixon CE. Contemporary in vivo models of brain trauma and a comparison of injury responses. In: Miller LP, Hayes RL, editors. Head trauma: basic, preclinical, and clinical directions. John Wiley & Sons; New York, NY: 2001. pp. 65–84. [Google Scholar]
- 55.King C, Robinson T, Dixon CE, Rao GR, Larnard D, Nemoto CEM. Brain temperature profiles during epidural cooling with the ChillerPad in a monkey model of traumatic brain injury. J Neurotrauma. 2010;27:1895–1903. doi: 10.1089/neu.2009.1178. [DOI] [PubMed] [Google Scholar]
- 56.Dennis AM, Haselkorn ML, Vagni VA, Garman RH, Janesko-Feldman K, Bayir H, Clark RSB, Jenkins LW, Dixon CE, Kochanek PM. Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death. J Neurotrauma. 2009;26:889–899. doi: 10.1089/neu.2008.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandhir R, Berman NEJ. Age-dependent response of CCAAT/enhancer binding proteins following traumatic brain injury in mice. Neurochem Int. 2010;56:188–193. doi: 10.1016/j.neuint.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hemerka JN, Wu X, Dixon CE, Garman RH, Exo JL, Shellington DK, Blasiole B, Vagni VA, Janesko-Feldman K, Xu M, Wisniewski SR, Bayır H, Jenkins LW, Clark RSB, Tisherman SA, Kochanek PM. Severe brief pressure- controlled hemorrhagic shock after traumatic brain injury exacerbates functional deficits and long-term neuropathological damage in mice. J Neurotrauma. 2012;29:2192–2208. doi: 10.1089/neu.2011.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monaco CM, Mattiola VV, Folweiler KA, Tay JK, Yelleswarapu NK, Curatolo LM, Matter AM, Cheng JP, Kline AE. Environmental enrichment promotes robust functional and histological benefits in female rats after controlled cortical impact injury. Exp Neurol. 2013;247:410–418. doi: 10.1016/j.expneurol.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pleasant JM, Carlson SW, Mao H, Scheff SW, Yang KH, Saatman KE. Rate of neurodegeneration in the mouse controlled cortical impact model is influenced by impactor tip shape: implications for mechanistic and therapeutic studies. J Neurotrauma. 2011;28:2245–2262. doi: 10.1089/neu.2010.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Statler KD, Kochanek PM, Dixon CE, Alexander HL, Warner DS, Clark RS, Wisniewski SR, Graham SH, Jenkins LW, Marion DW, Safar PJ. Isoflurane improves long-term neurologic outcome versus fentanyl after traumatic brain injury in rats. J Neurotrauma. 2000;17:1179–1189. doi: 10.1089/neu.2000.17.1179. [DOI] [PubMed] [Google Scholar]
- 62.Statler KD, Alexander H, Vagni V, Holubkov R, Dixon CE, Clark R, Jenkins L, Kochanek PM. Isoflurane exerts neuroprotective actions at or near the time of severe traumatic brain injury. Brain Res. 2006;1076:216–224. doi: 10.1016/j.brainres.2005.12.106. [DOI] [PubMed] [Google Scholar]
- 63.McDonald JW, Roeser NF, Silverstein FS, Johnston MV. Quantitative assessment of neuroprotection against NMDA-induced brain injury. Exp Neurol. 1989;106:289–296. doi: 10.1016/0014-4886(89)90162-3. [DOI] [PubMed] [Google Scholar]
- 64.McPherson RW, Kirsch JR, Salzman SK, Traystman RJ. The neurobiology of central nervous system trauma. Oxford University Press; New York, NY: 1994. [Google Scholar]
- 65.Cole JT, Yarnell A, Kean WS, Gold E, Lewis B, Ren M, McMullen DC, Jacobowitz DM, Pollard HB, O’Neill JT, Grunberg NE, Dalgard CL, Frank JA, Watson WD. Craniotomy: true sham for traumatic brain injury, or a sham of a sham? J Neurotrauma. 2011;28:359–369. doi: 10.1089/neu.2010.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin SS, Bray ER, Dixon CE. Effects of nicotine administration on striatal dopamine signaling after traumatic brain injury in rats. J Neurotrauma. 2012;29:843–850. doi: 10.1089/neu.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shin SS, Bales JW, Yan HQ, Kline AE, Wagner AK, Lyons-Weiler J, Dixon CE. The effect of environmental enrichment on substantia nigra gene expression after traumatic brain injury in rats. J Neurotrauma. 2013;30:259–270. doi: 10.1089/neu.2012.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meaney DF, Ross DT, Winkelstein BA, Brasko J, Goldstein D, Bilston LB, Thibault LE, Gennarelli TA. Modification of the cortical impact model to produce axonal injury in the rat cerebral cortex. J Neurotrauma. 1994;11:599–612. doi: 10.1089/neu.1994.11.599. [DOI] [PubMed] [Google Scholar]
- 69.He J, Evans C-O, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004;189:404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 70.RIGOR Improving the quality of NINDS-supported pre-clinical and clinical research through rigorous study design and transparent reporting


