Abstract
Surface glandular trichomes distributed throughout the aerial parts of sweet basil (Ocimum basilicum) produce and store monoterpene, sesquiterpene, and phenylpropene volatiles. Three distinct basil chemotypes were used to examine the molecular mechanisms underlying the divergence in their monoterpene and sesquiterpene content. The relative levels of specific terpenes in the glandular trichomes of each cultivar were correlated with the levels of transcripts for eight genes encoding distinct terpene synthases. In a cultivar that produces mostly (R)-linalool, transcripts of (R)-linalool synthase (LIS) were the most abundant of these eight. In a cultivar that synthesizes mostly geraniol, transcripts of geraniol synthase were the most abundant, but the glands of this cultivar also contained a transcript of an (R)-LIS gene with a 1-base insertion that caused a frameshift mutation. A geraniol synthase-LIS hybrid gene was constructed and expressed in Escherichia coli, and the protein catalyzed the formation of both geraniol and (R)-linalool from geranyl diphosphate. The total amounts of terpenes were correlated with total levels of terpene synthase activities, and negatively correlated with levels of phenylpropanoids and phenylalanine ammonia lyase activity. The relative levels of geranyl diphosphate synthase and farnesyl diphosphate synthase activities did not correlate with the total amount of terpenes produced, but showed some correlation with the ratio of monoterpenes to sesquiterpenes.
Plants produce a large number of secondary metabolites that function in a variety of ecological contexts. Many specialized compounds are toxic and can therefore serve as defense agents against microbial pathogens and insect and animal herbivores (Wittstock and Gershenzon, 2002; Theis and Lerdau, 2003; Wink, 2003). Other compounds are volatile and serve to attract pollinators or even insects that prey on the plant's enemies or repel harmful organisms (Pare and Tumlinson, 1999; Kessler and Baldwin, 2001; Baldwin et al., 2002; Pichersky and Gershenzon, 2002).
Secondary compounds with roles in defense are often sequestered in specialized cells or structures, presumably to protect the plant itself from its own toxicity (Gershenzon et al., 1989; Pare and Tumlinson, 1997; Duke et al., 2000; Dussourd and Hoyle, 2000; Hallahan, 2000; Martin et al., 2002). A common mechanism of sequestration has been the evolution of anatomical structures, termed glandular trichomes, on the surface of the aerial parts of the plants. Such structures typically contain gland cells (or a single cell) that synthesize these compounds and a cuticular sac covering the gland cells into which large amounts of the synthesized compounds are secreted. Upon damage to the tissue, or even upon mere physical pressure, the sacs rupture and release their contents. Once on the surface, secondary compounds with high vapor pressure will easily evaporate into the atmosphere.
The Lamiaceae is a large plant family that includes the mints, sages, and basils and is well recognized for the diversity of secondary compounds synthesized and stored in glands found on the surface of leaves, stems, and flowers. The glands of sweet basil (Ocimum basilicum) in particular are rich in phenylpropenes as well as monoterpenes and sesquiterpenes (Werker et al., 1993; Gang et al., 2001; Iijima et al., 2004), compounds that, individually and in combination, impart distinct flavor and aroma as judged by the human sensory systems. Because of consumer demand, many cultivars have been bred for particular gland constituencies. For example, Sweet Dani (SD) is a basil cultivar that is rich in citral, a mixture of the monoterpene aldehydes geranial and neral, which together impart a lemony aroma (Morales and Simon, 1997; Iijima et al., 2004). Some cultivars are rich in phenylpropenes such as methylchavicol or eugenol, while others contain more than one major compound (Simon et al., 1999; Gang et al., 2001).
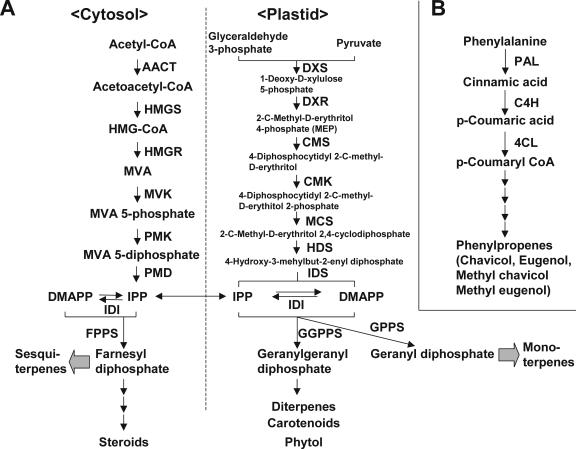
The phenylpropenes are derived from Phe and share the first few biosynthetic steps with the phenylpropanoids, although the entire biosynthetic pathway has not yet been elucidated (Hahlbrock and Grisebach, 1979; Jones, 1984; Gang et al., 2001). The C10 monoterpenes are known to be synthesized by the condensation of the two 5-carbon interconvertible isomers, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). The C15 sesquiterpenes, in turn, are synthesized by the sequential condensations of one DMAPP and two IPP molecules. In plants, both IPP and DMAPP are synthesized via two parallel pathways, the mevalonate (MVA) pathway localized in the cytosol and the methylerythritol 4-phosphate (MEP) pathway localized in the plastid (Fig. 1; Bochar et al., 1999; Lichtenthaler, 1999; Rodriguez-Concepcion and Boronat, 2002; Rohdich et al., 2003; Rohmer, 2003). These two pathways have been recently elucidated in plants and microorganisms, and the genes encoding all the enzymes in both pathways have now been identified (Rodriguez-Concepcion and Boronat, 2002; Lange and Ghassemian, 2003).
Figure 1.
Pathways leading to the generation of terpenes and phenylpropenes. A, Overview of the MVA and MEP pathways localized in the cytosol and the plastids, respectively. DXS, DXP synthase; DXR, DXP reductoisomerase; CMS, CDP-ME synthase; CMK, CDP-ME kinase; MCS, CMEPP synthase; HDS, HMBPP synthase; IDS, IPP/DMAPP synthase; IDI, IPP isomerase; AACT (acetoacetyl-CoA thiolase), HMGS (HMG synthase), HMGR (HMG reductase), MVK, MVA kinase; PMK, phosphomevalonate kinase; PMD, MVA diphosphate decarboxylase; and GGPPS, GGPP synthase. B, Overview of the phenylpropene pathway. The first few steps are shared with the phenylpropanoid pathways. Not all later steps are known.
The existence of distinct basil chemotypes is both the result of natural evolution and selective breeding (Darrah, 1974; Morales and Simon, 1996; Simon et al., 1999). This observation suggests that most basil lineages have the genetic potential to synthesize most, if not all, of the range of compounds that have been found collectively in this species. However, the molecular mechanisms by which such diversity is generated—gene silencing, gene duplications and modifications, differential gene regulation, and posttranslational modification—are not clear.
Previously, we reported the characterization of a geraniol synthase (GES) gene isolated from the basil cultivar SD (Iijima et al., 2004). Here we report the characterization of eight additional terpene synthases (TPSs) from the three basil cultivars SD, SW, and EMX. We examined the activities of these TPSs as well as the activities of the enzymes catalyzing the formation of geranyl diphosphate (GPP) and farnesyl diphosphate (FPP), the substrates of monoterpene and sesquiterpene synthase, respectively. We also examined the expression of genes encoding these enzymes and earlier enzymes in the MEP and MVA pathways to better understand the basis of the variation in volatile terpene profiles among these three basil cultivars. Finally, we examined the activity of Phe ammonia lyase (PAL), the key regulatory enzyme of the phenylpropanoid pathway, and the expression of PAL and several other phenylpropanoid genes to examine the interaction between the phenylpropanoid and terpenoid pathways.
RESULTS
Characterization of Monoterpenes and Sesquiterpenes Produced by Basil Cultivars EMX, SD, and SW
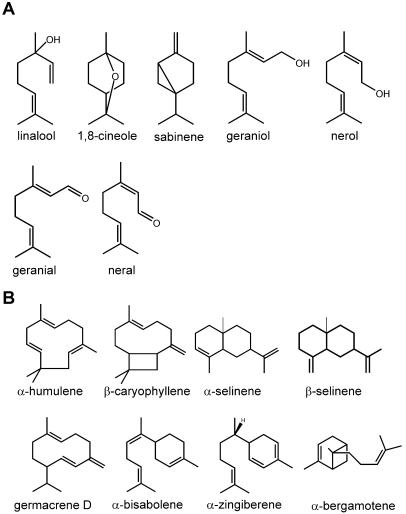
Terpene and phenylpropene constituents (Fig. 2) in leaves of basil cultivars EMX, SD, and SW were extracted and analyzed (Tables I and II). The major volatile constituent of EMX leaves was the phenylpropene methylchavicol (4.5 mg g−1 fresh weight [FW]), and this cultivar contained very low amounts of terpenes distributed almost evenly between monoterpenes and sesquiterpenes. On the other hand, the SD and SW cultivars contain lower amounts of phenylpropenes than cultivar EMX, and severalfold more terpenes than EMX, with monoterpenes predominating. The SD cultivar was particularly rich in geranial (6.5 mg g−1 FW) and neral (3.1 mg g−1 FW), both of which are derived from the oxidation of geraniol (Iijima et al., 2004), and it also had several sesquiterpenes in concentrations ranging from 90 to 537 μg g−1 FW, with β-caryophyllene and germacrene D being the most abundant. The main monoterpene in cultivar SW was (R)-linalool (2.1 mg g−1 FW) followed by 1,8-cineole (728 μg g−1 FW), and the main sesquiterpene was α-bergamotene (453 μg g−1 FW). When total volatile terpene content of each cultivar is plotted against the total phenylpropene content, a clear negative correlation is observed (Table II).
Figure 2.
Structures of the main monoterpenes and sesquiterpenes found in the basil glands. A, Monoterpenes. B, Sesquiterpenes.
Table I.
Quantification of volatiles in three basil cultivars (μg g−1 FW leaf tissue)
| Compounds | EMX | SD | SW |
|---|---|---|---|
| Monoterpenes | |||
| α-Pinene | 22 (4)a | 0 | 39 (10) |
| β-Pinene | 36 (9) | 0 | 75 (2) |
| β-Myrcene | 24 (6) | 0 | 88 (13) |
| Limonene | 46 (9) | 21 (13) | 32 (7) |
| 1,8-Cineole | 352 (88) | 0 | 728 (37) |
| Ocimene | 0 | 0 | 109 (11) |
| Linalool | 0 | 0 | 2,101 (418) |
| Sabinene | 279 (13) | 336 (39) | 296 (22) |
| Fenchol | 150 (85) | 0 | 0 |
| Camphor | 0 | 0 | 41 (2) |
| α-Terpineol | 0 | 0 | 68 (7) |
| Geranial | 0 | 6,541 (1,361) | 0 |
| Neral | 0 | 3,091 (728) | 0 |
| Geraniol | 0 | 325 (55) | 0 |
| Nerol | 0 | 138 (27) | 0 |
| Sesquiterpenes | |||
| β-Caryophyllene | 289 (78) | 537 (75) | 0 |
| α-Bergamotene | 0 | 0 | 453 (84) |
| β-Farnesene | 64 (19) | 90 (20) | 84 (4) |
| α-Caryophyllene | 77 (13) | 171 (24) | 18 (2) |
| α-Zingiberene | 39 (12) | 93 (13) | 43 (8) |
| Germacrene D | 86 (22) | 513 (80) | 123 (19) |
| γ-Cadinene | 0 | 0 | 88 (10) |
| β-Selinene | 0 | 207 (30) | 0 |
| α-Selinene | 0 | 171 (26) | 0 |
| α-Bisabolene | 207 (51) | 353 (54) | 0 |
| α-Cadinol | 0 | 0 | 98 (8) |
| Phenyl Propanoids | |||
| Methyl chavicol | 4,468 (957) | 142 (63) | 0 |
| Methyl eugenol | 42 (1) | 0 | 69 (23) |
| Eugenol | 0 | 41 (9) | 2,302 (13) |
| Other | |||
| cis-3-hexenol | 87 (54) | 152 (28) | 251 (6) |
Average values and sd (in parentheses) of triplicate for each plant line are given.
Table II.
The amounts of terpenes and phenylpropenes and the glandular enzymatic activities related to their formation
| Basil Cultivar | Mono + Sesqui = Total Terpenesa | Mono + Sesqui = Total TPS Activityb | GPPS + FPPS = Total Prenyltransferase Activityb | PAL Activityb | Phenylpropene Contenta |
|---|---|---|---|---|---|
| EMX | 0.9 + 0.8 = 1.7 (1.3:1)c | 9.4 + 13.3 = 22.7 (0.7:1)d | 23.4 + 10.0 = 33.4 (2.3:1)e | 27.2 | 5.3 |
| SD | 10.5 + 2.1 = 12.6 (4.9:1) | 144.8 + 22.7 = 167.5 (6.4:1) | 68.3 + 17.3 = 85.6 (3.9:1) | 9.8 | 0.19 |
| SW | 3.6 + 0.8 = 4.4 (4.4:1) | 28.8 + 22.9 = 51.7 (1.3:1) | 54.9 + 30.5 = 85.4 (1.8:1) | 17.4 | 2.4 |
Microgram per gram FW.
pkat per milligram protein.
Ratio of monoterpenes to sesquiterpenes.
Ratio of the activities of monoterpene synthases to sesquiterpene synthases.
Ratio of the activities of GPPS to FPPS.
Overall, EMX had 0.9 mg of monoterpenes g−1 FW, SD had 10.5 mg of monoterpenes g−1 FW, and SW had 3.6 mg monoterpenes g−1 FW. Cultivar SD also had the most sesquiterpenes, at 2.1 mg g−1 FW, SW had approximately one-third of this concentration, and EMX had only 0.8 mg sesquiterpenes g−1 FW.
Characterization of Terpene-Synthesizing Activities in Glands of the Three Cultivars
It has been previously established that, in the Lamiaceae in general and in basil specifically, the leaf terpenoids and phenylpropenes are synthesized almost exclusively in the peltate glands (Hallahan, 2000; Gang et al., 2001; an exception has been reported for patchouli, where terpenes are apparently stored in mesophyll glands [Maeda and Miyake, 1997]). We therefore isolated the basil peltate glands and examined the level of TPS activity capable of synthesizing monoterpenes and sesquiterpenes from the respective substrates GPP and FPP (Table II). Cultivar SD had the highest levels of activity of monoterpene synthases, about 5-fold higher than in SW and 15-fold higher than in EMX. The levels of sesquiterpene synthase activities in the glands of SD were 1.7-fold higher than in EMX, but similar to the levels of the corresponding enzymatic activities in SW.
Characterization of Prenyltransferase Activities in Glands of the Three Cultivars
GPP, the substrate of monoterpene synthases, is synthesized from IPP and DMAPP by the enzyme GPP synthase (GPPS), which has been found to be a heterodimer in several angiosperms (Burke et al., 1999, 2004; Tholl et al., 2004). FPP, the precursor of all sesquiterpenes, is synthesized from two molecules of IPP and one molecule of DMAPP by the homodimeric enzyme FPP synthase (FPPS). GPPS activity levels were highest in SD and lowest in EMX, and FPPS activity levels were highest in SW and lowest in EMX (Table II). However, FPPS activity levels were always lower than those of GPPS activity levels, ranging from only 25% in SD to 55% in SW. Geranylgeranyl diphosphate (GGPP) synthase activity was undetectable in glandular trichome extracts of these three cultivars (GGPP is the substrate of diterpene synthases; see Fig. 1).
Characterization of PAL Activity in Glands of the Three Cultivars
PAL catalyzes the first committed step in the phenylpropanoid and phenylpropene pathways. Because of the observed inverse correlation between terpene content and phenylpropene content in the basil glands, we examined the activity of PAL in the glands of the three basil cultivars (Table II). The EMX cultivar, with the highest content of phenylpropenes, had PAL activity of 27.2 pkat mg−1 protein, 2.8 times higher than that in SD, the basil cultivar with the lowest phenylpropene content. In SW, the cultivar with intermediate phenylpropene content, PAL activity was intermediate between EMX and SD.
Identification and Characterization of Monoterpene and Sesquiterpene Synthases Expressed in the Glands of Basil Cultivars EMX, SD, and SW
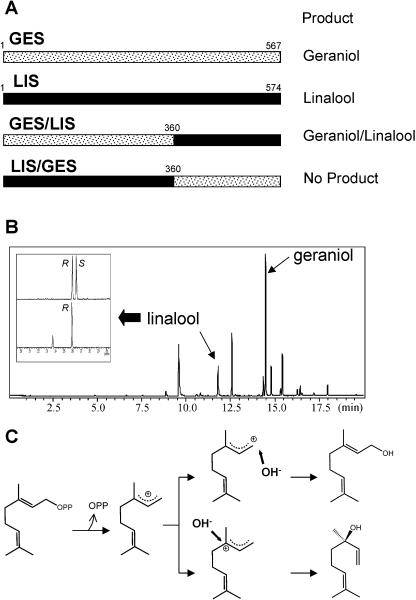
We had previously constructed annotated expressed sequence tag (EST) databases for SD, SW, and EMX peltate glandular trichomes that each contain approximately 3,500 ESTs (Gang et al., 2001; Iijima et al., 2004). An exhaustive search of these three databases revealed a total of nine contigs encoding proteins with sequence homology for known TPSs. After obtaining full-length cDNAs for all these contigs, the proteins encoded by these genes were aligned and compared (Fig. 3), and their phylogenetic relationships assessed (Fig. 4). One of these proteins, encoded by a cDNA found only in SD, has been previously identified as GES (the major monoterpenes in SD are geranial and neral, which are both derived from the oxidation of geraniol [Iijima et al., 2004]). A second cDNA, unique to SW, which showed 78% identity on the nucleotide sequence with GES, was expressed in E. coli and found to encode a monoterpene synthase that catalyzes the exclusive formation of (R)-linalool (Fig. 5A), which is the same linalool stereoisomer found in SW leaves. Interestingly, the SD EST database had several cDNAs that were almost identical in sequence to (R)-linalool synthase (LIS) from SW except that an insertion of one nucleotide (T) occurred between codon 228 and codon 229 (Fig. 3), causing a frameshift mutation and creating an aberrant open reading frame that encodes a truncated, nonfunctional enzyme.
Figure 3.
Comparison of the sequences of the proteins encoded by nine basil TPS cDNAs. SES (GenBank accession no. AY693643); GDS (GenBank accession no. AY693644); FES (GenBank accession no. AY693648); MYS (GenBank accession no. AY693649); TES (GenBank accession no. AY693650); ZIS (GenBank accession no. AY693646); GES (GenBank accession no. AY362553); LIS (GenBank accession no. AY693647); and CDS (GenBank accession no. AY693645). White text on black box indicates identical amino acids in five or more sequences. Arrowhead indicates the position of the insertion of a T nucleotide between codon 228 and codon 229 of the LIS gene expressed in the SD cultivar (see text). Also indicated is the conserved area centered around codon 360 (GES numbering) that constituted the junction of the GES/LIS and LIS/GES chimeric cDNA constructs described in the text and in Figure 6.
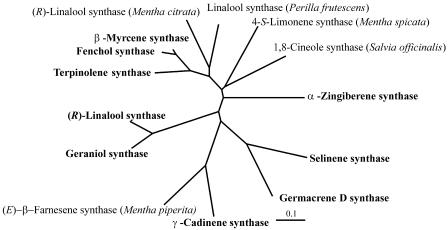
Figure 4.
Relatedness of basil monoterpene and sesquiterpene synthases to similar proteins in other species of Lamiaceae. Phylogenetic tree was constructed using the nearest neighbor-joining method. Sequences analyzed include the basil proteins in Figure 3, 1,8-cineole synthase from Salvia officinalis (Wise et al., 1998; GenBank accession no. AF051899), 4S-limonene synthase from Mentha spicata (Colby et al., 1993; GenBank accession no. L13459), (R)-LIS from Mentha citrata (Crowell et al., 2002; GenBank accession no. AY083653); LIS from Perilla frutescens (GenBank accession no. AF444798); and Mentha piperita (E)-β-farnesene synthase (Crock et al., 1997; GenBank accession no. AF024615).
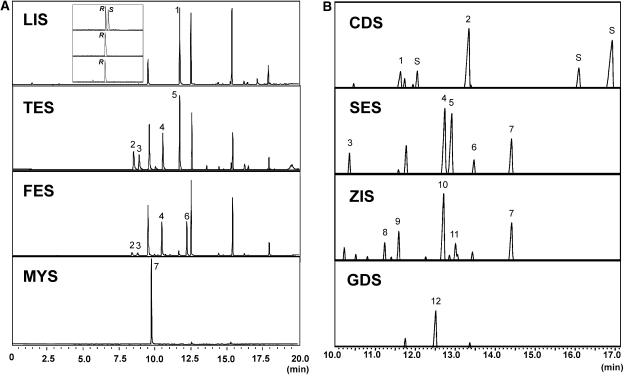
Figure 5.
Identification of the product of the eight basil TPSs. Gas chromatographic separation of products synthesized in vitro by monoterpene and sesquiterpene synthase overexpressed in E. coli. A, Monoterpene synthases LIS, TES, FES, and MYS. Reaction products were obtained by SPME from GPP as a substrate. Peak 1, Linalool; 2, α-pinene; 3, unidentified monoterpene; 4, limonene; 5, terpinolene; 6, fenchol; and 7, β-myrcene. Each compound was identified with comparisons of retention times and mass spectra of authentic standards. The unlabeled peaks are non-terpenoid volatiles also found in control E. coli extracts. Top (inset), GC analysis for (R)-linalool and (S)-linalool stereoisomers on a chiral column. The top trace shows separation of a racemic linalool standard mixture with the (R)-linalool peak labeled R and the (S)-linalool peak labeled S; the middle trace shows linalool extracted from basil glands; the bottom trace shows linalool extracted from the in vitro enzyme assay of E. coli-produced LIS. B, Sesquiterpene synthases CDS, SES, ZIS, and GDS. Reaction products were obtained by SPME from FPP as a substrate. Products were identified with comparisons of retention times and mass spectra of authentic standards. Peak 1, Muurola 3,5-diene; 2, γ-cadinene; 3, β-elemene; 4, β-selinene; 5, α-selinene; 6, epi-α-selinene; 7, nerolidol; 8, α-bergamotene; 9, β-farnesene; 10, α-zingiberene; 11, β-bisabolene; 12, germacrene D. S, Unidentified sesquiterpenes.
Monoterpene synthases are located in the plastids and are synthesized in the cytosol with an N-terminal transit peptide extension that is cleaved after transport into the organelles (Gavel and von Heijne, 1990; Williams et al., 1998). Both GES and LIS protein sequences appear to have transit peptides (Fig. 3). A third cDNA, found in SW only, was expressed in E. coli and found to encode for a protein that catalyzes the formation of terpinolene, limonene, β-pinene, and one additional unidentified monoterpene (Fig. 5A). Because its major product was terpinolene, it was designated terpinolene synthase (TES). Finally, two closely related cDNAs (95.1% identical to each other on the nucleotides level), designated fenchol synthase (FES) and β-myrcene synthase (MYS), were found in all three databases. FES catalyzes the formation of fenchol, α-pinene, limonene, and one additional unidentified monoterpene, and MYS catalyzes the exclusive formation of β-myrcene. Some of these monoterpene synthases catalyze the formation in vitro of some products that were not found in the glands (i.e. terpinolene). Similar apparent discrepancies between product profile of E. coli-produced plant TPSs and in vivo terpene profiles have been observed before (Jia et al., 1999). Metabolism of such products or lack of complete structural identity between the plant-derived and E. coli-produced enzymes has been implicated (Jia et al., 1999; Crowell et al. 2002).
The four other contigs encoded proteins with no apparent transit peptide (Fig. 3) and were therefore assigned as putative sesquiterpene synthases. Expression of their respective full-length cDNAs in E. coli followed by enzymatic assays identified these proteins as γ-cadinene synthase (CDS), selinene synthase (SES), α-zingiberene synthase (ZIS), and germacrene D synthase (GDS; Fig. 5B), named for the major compounds they produce in vitro, but all of these enzymes produced other sesquiterpene compounds as well from FPP. cDNAs for SES, ZIS, and GDS were found in the EST databases of all three varieties, but cDNAs encoding CDS were found only in the database of SW.
Construction of a Hybrid GES/LIS
The basil LIS is very different from other LIS sequences found in other species (Fig. 4). In contrast, basil LIS and GES are 81% identical on the protein level, suggesting that they evolved from a common ancestor relatively recently. The reaction mechanism of GES was previously determined (Iijima et al., 2004; Fig. 6C), and production of linalool could be very similar, except that the carbocation generated after removal of the pyrophosphate group will reside at the C3 position (Fig. 6C). In an attempt to identify areas in the proteins important for product specification, we constructed a hybrid protein by fusing the first 360 codons of GES with codons 361 to 574 of LIS (Fig. 6A). The hybrid protein that had the GES N terminus and the LIS C terminus (GES/LIS) catalyzed the formation of both geraniol and (R)-linalool (at an 8:2 ratio; Fig. 6B). On the other hand, the reciprocal hybrid protein (LIS/GES) did not have any activity under our experimental conditions.
Figure 6.
Products produced by a LIS/GES hybrid enzyme. A, Diagram of the hybrid cDNAs constructed. B, Gas chromatographic separation of products synthesized by the GES/LIS hybrid enzyme. Reaction products were obtained by SPME, and products were identified with comparisons of retention times and mass spectra of authentic standards. The unlabeled peaks are non-terpenoid volatiles also found in control E. coli extracts. Inset, GC analysis for (R)-linalool and (S)-linalool stereoisomers on a chiral column. The top trace shows separation of a racemic linalool standard mixture with the (R)-linalool peak labeled R and the (S)-linalool peak labeled S; the lower trace shows the linalool produced in the in vitro enzyme assay by the hybrid protein. C, The reaction mechanisms of GES and (R)-LIS.
Characterization of the Expression of Genes Encoding Terpenoid Biosynthetic Enzymes
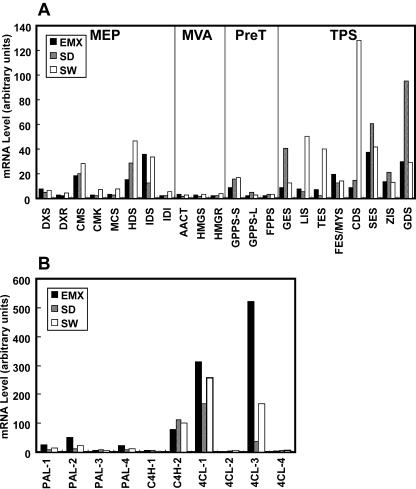
In addition to the nine TPSs described above, all the EST databases also contained cDNAs encoding both the large and small subunits of GPPS and FPPS, all the genes encoding the enzymes of the MEP pathway, and acetoacetyl CoA thiolase (AACT), HMG synthase (HMGS), and HMG reductase (HMGR), the first three enzymes of the MVA pathway (see Fig. 1). These cDNAs were bound to macroarray filters, which were then hybridized with labeled cDNAs derived from reverse transcription of whole glandular trichome RNA preparations from each of the three cultivars. Overall, steady-state levels of these monoterpene synthase mRNAs were the lowest in EMX glands, and the levels of expression of individual monoterpene synthases in the other two cultivars were also consistent with the observed levels of volatiles (e.g. relatively high levels of GES in the geraniol-producing SD cultivar, and high levels of LIS in the linalool-containing SW cultivar; Fig. 7). Similarly, CDS was more highly expressed in SW, which has the highest level of cadinene among the three cultivars, and SES and GDS had the highest expression levels in SD, which has the highest levels of α-selinene, β-selinene, and germacrene D. Because FES and MYS are 95.1% identical on the DNA level, this expression analysis method could not distinguish between them (Fig. 7). It is also possible that some as yet unidentified basil TPS genes are very similar to the genes on the blots; for example, the relatively high signal for TES in the SW glands (Fig. 7A) may be due to hybridization with a similar mRNA that encodes 1,8-cineole, a major component of SW volatile monoterpenes, and not only to hybridization to TES, which catalyzes the formation of limonene and β-pinene, which are only minor components of the SW volatile monoterpene fraction.
Figure 7.
Relative expression levels of genes encoding isoprenoid and phenylpropene biosynthetic enzymes. A, The relative expression of genes encoding MEP and MVA pathway enzymes, prenyl transferases, and TPSs. B, The relative expression of common genes encoding phenylpropene pathways PAL, C4H, and 4CL. The multiple related sequences encoding each gene were shown as numbers. The expression levels are shown in arbitrary units that are the same for both A and B.
The levels of expression of the earlier genes in the terpene biosynthetic pathways did not follow any clear pattern. In general, the levels of steady-state mRNAs of the three MVA pathway genes were uniformly low, whereas MEP genes showed variable levels of steady-state mRNA. Also noteworthy was the observation that mRNA levels for the small subunit of GPPS were higher than those of the large subunit of GPPS in all three cultivars.
Characterization of the Expression of Genes Encoding Phenylpropanoid Biosynthetic Enzymes
The expression levels of the first three genes in phenylpropanoid and phenylpropene pathways, including PAL as well as cinnamate 4-hydroxylase (C4H) and 4-coumarate:CoA ligase (4CL), were measured in the three basil cultivars (Fig. 7B). The basil EST databases contain several related sequences encoding each of these genes (four PALs, two C4Hs, four 4CLs). Although transcript levels varied widely among these genes, overall, levels of transcripts for PAL and 4CL genes were highest in EMX and lowest in SD. On the other hand, the levels of C4H transcripts were similar in all three cultivars.
DISCUSSION
Basil Cultivars Differ in the Expression of Specific TPSs
The multiplicity of terpenes produced by a single plant has been noted before for organisms as varied as grand fir trees (Bohlmann and Croteau, 1999) and Arabidopsis (Arabidopsis thaliana; Chen et al., 2003). This multiplicity is achieved both by the expression of multiple TPS genes and by the ability of some TPSs to catalyze the production of multiple products (Bohlmann et al., 1997; Aubourg et al., 2002; Chen et al., 2003; Martin et al., 2004). However, with the exception of Arabidopsis, the entire set of TPS genes in any plant species is not known. Consequently, the number of TPSs analyzed in most species has been small, and intraspecific variation in terpene gene expression has hardly been studied.
To better understand the molecular mechanisms that control chemical differentiation in basil, we have identified and characterized four monoterpene synthases and four sesquiterpene synthases expressed in the glandular trichomes of one or more of the three basil varieties, EMX, SD, and SW, while another basil monoterpene synthase, GES, had been previously reported (Iijima et al., 2004). While the majority of these nine TPSs make multiple products, they nevertheless still do not account for the entire spectrum of volatile terpenes found in these three varieties. It is possible that the basil genome contains additional monoterpene and sesquiterpene synthases, or that the expression of the above genes in planta leads to the synthesis of additional products not seen with the enzymes obtained in the prokaryotic expression system. Despite the incompleteness of the information on the TPS gene family in basil, our data indicate that the monoterpene and sesquiterpene synthases are differentially expressed in the glands of the three basil cultivars, and that the types of volatile terpenes produced in these glands are controlled by the expression of these TPSs. While this result is far from surprising, it nevertheless demonstrates that intraspecific differences in terpene gene expression exist in this largely cross-pollinating species and can be (and have been) exploited for breeding special chemotypes.
Additional Variability in Terpene Composition Is Achieved by Coding Sequence Evolution
The majority of the TPS proteins characterized in this study are fairly divergent from each other (<50%) and are therefore likely to have been present in the progenitor to the basil lineage. However, FES and MYS are 95% identical to each other, but they nevertheless produce different products. Likewise, GES and LIS are 81% identical to each other. Furthermore, the N-terminal transit peptides, which are usually not highly conserved, are almost identical to each other in these two pairs of genes. The relatively close similarity of these protein pairs indicates that further terpene biosynthetic diversity is continuing to be generated in the basil lineage by gene duplication and divergence. Particularly interesting is the observation of the close relatedness between basil GES and LIS, since geraniol and linalool are structurally similar monoterpene alcohols, differing only in the substitution position of the hydroxyl group. Furthermore, previously characterized LISs from many species as well as GES (which has so far been reported only from basil) were found to produce only a single product, either linalool or geraniol, but not both (Pichersky et al., 1995; Jia et al., 1999; Crowell et al., 2002; Chen et al., 2003; Iijima et al., 2004). This is also the case for the basil LIS and GES as well. However, the results of our domain-swapping experiments demonstrate that it is possible to generate a monoterpene synthase that can synthesize both geraniol and linalool, and that multiple amino acids must contribute to such a dual selectivity. Such an enzyme, however, has not yet been found in nature. Domain-swapping experiments with sesquiterpene synthases and other monoterpene synthases have obtained similar results, showing that chimeric enzymes often synthesize a combination of the products of the original enzymes (Back and Chappell, 1996; El Tamer et al., 2003; Katoh et al., 2003; Peters and Croteau, 2003).
The process of random mutations can lead to functional divergence in duplicated genes (Pichersky and Gang, 2000) and could also generate nonfunctional genes, such as the frameshift mutation observed in a linalool-like gene found in cultivar SD. Interestingly, this mutated gene, whose sequence is otherwise almost identical to that of LIS from SW, seems not to be highly expressed (Fig. 7); however, it must be expressed at some level since it was present in the EST database of SD. A similar situation has recently been reported in maize, where one variety has one locus encoding a functional TPS and another locus encoding a nonfunctional TPS due to a frameshift mutation, whereas in a second variety the functional and nonfunctional loci are reversed (Kollner et al., 2004). However, the expression of the genes in the two loci was not examined separately.
While gene duplications are considered a prerequisite for divergence when genes encoding essential functions are involved, in secondary metabolism this requirement is not absolute (Pichersky and Gang, 2000). It is possible that some of the different TPSs observed here are truly allelic. However, short of fully sequencing the basil genome or large-scale genomic cloning and sequencing efforts, only genetic crosses would allow the determination of allelism, and this task is further complicated by the fact that some of these genes encode enzymes with multiple and sometimes overlapping products.
Another intriguing observation is the closer similarity of the basil ZIS to the basil monoterpene synthases rather than to the other three sesquiterpene synthases or to sesquiterpene synthases from other species (Fig. 4). It is possible that ZIS evolved from a monoterpene synthase by a deletion of the coding region for a transit peptide. This hypothesis is plausible since it has been previously demonstrated that TPSs can use both FPP and GPP as substrates (Kollner et al., 2004), and we found this to be true for ZIS as well (although the activity of ZIS with GPP, which produced β-pinene, limonene, and α-terpineol, was 13-fold lower than with FPP). Previously, the sesquiterpene farnesene synthase has been proposed to have evolved from a monoterpene synthase in several plants (Martin et al., 2004; Pechous and Whitaker, 2004).
Enzyme Activity Levels Are Only Partially Correlated with mRNA Levels
Our results indicate that genes encoding the key enzymes in both the MVA and MEP pathways are active in the basil glands, although the steady-state levels of transcripts for some, but not all, MEP genes were higher than the transcript levels of the three MVA genes we tested (the other MVA genes were not present in the EST databases at all, suggesting even lower levels of expression). However, assessing the relative contribution of these two pathways to the synthesis of the final product is not straightforward because a linear correlation between transcript levels and protein levels cannot be assumed and, furthermore, the specific activity of each enzyme is unique and can vary greatly among enzymes.
With these caveats, our results indicate a loose correlation between transcript levels and enzymatic activity levels for GPPS, FPPS, and the TPSs in the three cultivars. EMX glands had the lowest levels of transcripts for each of these enzymes, and correspondingly lower levels of enzymatic activities. Transcript levels of the small subunit of GPPS are similar in SD and SW (and, as in snapdragon, higher than transcript levels for the large subunit [Tholl et al., 2004]), and GPPS activity levels in these two cultivars are similar. For FPPS, transcript levels in SD glands are similar to those in SW glands, but FPPS activity levels are about twice as high in SW compared to SD. The loose correlation between mRNA levels and enzyme activities means that post-translation regulation mechanisms may be operative.
The Overall Output of Terpenes and the Ratio of Monoterpene to Sesquiterpene Are Influenced by Different Factors
The total amount of terpenes produced correlated well with the total levels of TPS activities, but not with the total levels of GPPS and FPPS activities. For example, SD leaves contain roughly three times more total terpenes than do SW leaves, and the ratio of TPS activity levels between these cultivars is also close to 3:1 (Table II). Likewise, SD leaves have about 7.4 times more terpenes than EMX leaves, and the ratio of TPS activity levels between the two cultivars is similar. On the other hand, SD and SW leaves have similar levels of total prenyltransferase activities, but SD has 3-fold more terpenes. Total terpene content was also negatively correlated with total phenylpropene content and PAL activity (Table II). These observations suggest that high levels of the final biosynthetic enzymes in the terpene pathways, coupled with lower levels of PAL activity, tend to restrict the phenylpropene pathway and increase the flux in the terpene pathway.
The ratio of total monoterpenes to total sesquiterpenes produced by each cultivar was weakly correlated with both the ratio of the activity levels of monoterpene synthases to sesquiterpene synthases, and the ratio of GPPS and FPPS activities (Table II). It therefore appears that once the flux in the terpene pathways is increased, GPPS and FPPS may exert some control on the levels of precursors that are directed toward the specific synthesis of monoterpenes and sesquiterpenes, but do not greatly influence the total amount of terpenes produced. This scenario is only valid if the monoterpene and sesquiterpene pathways are not completely independent. In fact, it was previously shown that cross-talk between the MVA and MEP pathways can occur (Bick and Lange, 2003; Hemmerlin et al., 2003; Laule et al., 2003). For example, in tobacco (Nicotiana tabacum) and Arabidopsis, the overexpression of HMGR did not affect the formation of sesquiterpenes (Chappell et al., 1995; Re et al., 1995; Schaller et al., 1995), suggesting that these sesquiterpenes were synthesized from IPP exported from the plastid into the cytosol. It was also reported that sesquiterpenes in chamomile are partially derived from the MEP pathway (Adam et al., 1999), and Piel et al. (1998) showed that sesquiterpenes as well as monoterpenes are generated from deoxy-d-xylulose in several plants. A detailed flux analysis will be necessary to firmly establish the parameters of cross-talk between cytosolic and plastidic pathways of terpene biosynthesis in basil glandular trichomes.
MATERIALS AND METHODS
Plant Material
Seeds for SD (Sweet Dani) were obtained from a local nursery. The source of the seeds of EMX and SW cultivars is described in Gang et al. (2001). Seeds were planted in small pots, covered with plastic wrap, and put in the growth chamber for 2 nights. After germination, they were grown in the greenhouse for 1 week and transferred into 500-mL pots. Sunshine Mix no. 1 potting soil was used for planting and plants were grown under constant illumination.
Volatile Oil Extraction from Leaves
Basil (Ocimum basilicum) young leaves (0.5–1 cm, 50 mg) of each cultivar were added to liquid N2 and ground by mortar and pestle. The powder was soaked in 2 mL methyl tert-butyl ether (MTBE) containing an internal standard (toluene, 0.02 mg) and extracted for 2 h at room temperature in 5-mL glass vials with tightly sealed rubber septa caps. The MTBE upper layer, which included the volatile oil, was removed and placed into another vial and concentrated to 200 μL under gentle N2 gas flow for gas chromatography-mass spectrometry (GC-MS) analysis. Data points were obtained in triplicate.
GC-MS Analysis of Plant Volatiles
A Shimadzu QP-5000 system (Shimadzu, Columbia, MD) equipped with Shimadzu GC-17 gas chromatograph was used for GC-MS analysis of volatile compounds. Separation was performed on a CP-5 column (30 m × 0.32 mm i.d. × 1-μm film thickness; Alltech Associates, Deerfield, IL). The GC condition was the same as reported previously. Ultrapure helium was used as the carrier gas at a rate of 1.3 mL min−1. Samples (2 μL) were injected by the Shimadzu AOC-17 autoinjector. Eluted compounds were identified by comparing their retention time and mass fragmentation patterns with standard compounds. Linalool optical isomers were analyzed by a CyclosilB column (30 m × 0.32 mm i.d. × 0.25-μm film thickness; J&W Scientific, Folsom, CA). The conditions were set for 55°C as the initial temperature for 2-min hold, and gradient to 220°C by 2°C min−1. Injection and detector temperatures were set at 220°C and 250°C, respectively.
Crude Enzyme Extraction from Glands
Glands were isolated following the procedures previously described by Gang et al. (2001). The crude enzyme extracts from these glands were prepared as previously described (Iijima et al., 2004).
TPS Enzyme Assays
TPS assay was measured as previously described (Iijima et al., 2004). TPS activity was assayed by incubating 5 μL of the enzyme sample in a final volume of 50 μL buffer containing 50 mm HEPES-KOH, pH 8.0, 1 mm dithioerythritol, 0.5 mm MnCl2, 20 mm MgCl2, 10% glycerol, and 54 μm of [1-3H]GPP or [1-3H]FPP (final specific activity 20 mCi mmol−1; American Radiolabeled Chemicals, St. Louis). After incubation for 30 min at 32°C, 160 μL hexane were added to the tube, vortexed briefly, and centrifuged to separate the phases. The hexane layer was directly placed into a scintillation vial containing 2 mL of nonaqueous scintillation fluid (Econo-Safe Research Products International, Mount Prospect, IL). This extraction procedure was repeated twice and the total hexane phase was counted in a liquid scintillation counter (LS-6500 model; Beckman Instruments, Fullerton, CA). Extracts containing heat-inactivated enzymes were used as controls.
The generated compounds were isolated by solid phase microextraction (SPME) as previously described (Iijima et al., 2004). The same GC conditions used for the analysis of plant extracts were also used for the identification of monoterpene products in the in vitro assays. Sesquiterpenes were analyzed with an Rtx-5SIL (30 m × 0.25 mm) column. Helium (1 mL min−1) was used as a carrier gas. The injector and detector temperatures were 250°C and 280°C, respectively. The conditions used were as follows: initial temperature was 50°C min−1 followed to 130°C at a rate of 20°C min−1, to 170°C at a rate of 3°C, and, finally, to 260°C at a rate of 50°C min−1.
Prenyltransferase Assays
Prenyltransferase activity was measured according to Tholl et al. (2001). The reaction was initiated by adding 5 μL of enzyme to the assay solution containing 40 μm [1-14C]IPP (final specific activity, 22.5 mCi mmol−1) and 40 μm DMAPP in a final volume of 50 μL buffer (25 mm MOPSO, pH 7.0, 10% [v/v] glycerol, 2 mm dithiothreitol, and 10 mm MgCl2). After 250 μL hexane were immediately overlaid on the assay mixtures, they were incubated for 40 min at 30°C. Assays were stopped by adding 5 μL of 3 m HCl and incubated for an additional 20 min at 30°C to hydrolyze the acid-labile allylic diphosphates. After hydrolysis, products were extracted by vortexing for 15 s and centrifuging for 1 min. The hexane phase (180 μL) was counted in a liquid scintillation counter. For product identification, the reaction was scaled up 2-fold and, after HCl hydrolysis, cold standard compounds (geraniol, linalool, nerolidol, farnesol, and geranylgeraniol) were added and the whole mixture extracted two times with 100 μL of MTBE, concentrated to 30 μL by gentle nitrogen gas flow, and 7 μL were injected into radio GC (Shimadzu GC 17) connected to a thermal conductivity detector and equipped with a radio detector (IN/US Systems, Tampa, FL). Helium was used as the carrier gas, and injection and detection ports were set at 250°C and 300°C, respectively. The separation was achieved with an XTI-5 column (30 m × 0.25 mm with 0.25-μm phase coating; Restek, Bellefonte, PA) and a gradient from 50°C (2-min hold) to 300°C at 10°C min−1, with an increase to 330°C at 30°C min−1 afterward.
PAL Assay
PAL activity was measured according to the method of Gang et al. (2001). Five microliters of the enzyme sample were reacted with 51 μm of [U-14C]Phe (final specific activity 9.2 mCi mmol−1) in 50 μL of assay buffer (100 mm Na-borate buffer, pH 8.8). After incubation for 1 h at 28°C, 5 μL of 6 n HCl were added and the mixture extracted with 160 μL of ethyl acetate. The ethyl acetate layer (130 μL) was used for scintillation counting.
Isolation of TPS cDNAs and Expression in Escherichia coli
The construction of EST databases from the peltate glands of three basil cultivars EMX, SD, and SW was previously reported (Iijima et al., 2004). BLAST searches revealed numerous ESTs with sequence similarity to TPSs. Potential TPS cDNAs were assembled into contigs. Full-length cDNA from each contig (obtained by 5′ RACE when necessary) were cloned into the pCRT7/CT-TOPO TA vector (Invitrogen, Carlsbad, CA) and expressed in the E. coli expression system. These plasmids were transformed to Codon Plus cells (Invitrogen). E. coli cultures carrying TPS expression constructs were induced by adding 0.5 mm isopropylthio-β-galactoside and grown at 18°C for 18 h. Cells were then harvested by centrifugation, resuspended in lysis buffer, and sonicated as previously described (Chen et al., 2003).
Sequence Analysis
Alignment of multiple protein sequences was performed using the ClustalX program (Thompson et al., 1997). Sequence relatedness by the neighbor-joining method was determined using the protocol included in the ClustalX package. The phylogenic tree was drawn using the TREEVIEW program (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html; Page, 1996).
Construction of Hybrid GES-LIS Protein
Construction of GES/LIS chimeric cDNAs was carried out by PCR. A conserved region centered around codon 360 (using the GES numbering) was chosen as the junction. For the GES/LIS cDNA, the N-terminal encoding part of the GES cDNA was amplified by PCR with the sense primer 5′-AATGTCTTGTGCACGGATCACCGTAAC-3′ and the antisense primer 5′-AACGCCATGTAGCATATTTTCATGTA-3′, and the C-terminal encoding part of the LIS cDNA was amplified with the sense primer 5′-TACATGAAAATATGCTACATGGCGTT-3′ and the antisense primer 5′-TGAGCTAAGAAGAAAGAAGAGGAGTGAAG-3′. The two amplified fragments were mixed and the complete hybrid cDNA was obtained by PCR with primers 5′-AATGTCTTGTGCACGGATCACCGTAAC-3′ and 5′-TGAGCTAAGAAGAAAGAAGAGGAGTGAAG-3′. To make a LIS/GES cDNA, the N-terminal encoding part of the LIS cDNA was amplified by PCR with the sense primer 5′-AATGTCTTGTGCACGGATCACCGTAAC-3′ and the antisense primer 5′-AACGCCATGTAGCATATTTTCATGTA-3′, and the C-terminal encoding part of GES cDNA was amplified with the sense primer 5′-TACATGAAAATATGCTACATGGCGTT-3′ and the antisense primer 5′-TATTTATTGAGTGAAGAAGAGGGCATCCAC-3′. The two amplified fragments were mixed and the complete hybrid cDNA was obtained by PCR with primers 5′-AATGTCTTGTGCACGGATCACCGTAAC-3′ and 5′-TATTTATTGAGTGAAGAAGAGGGCATCCAC-3′.
Analysis of the Expression of the Genes Encoding the Terpene and Phenylpropene Biosynthesis Pathway
Full-length cDNAs of the nine TPSs were amplified by PCR using each plasmid as a template with T7 and V5 primers. Other genes encoding MEP and MVA pathways (Fig. 1) and phenylpropene pathways were identified from the basil EST databases by BLAST search, and those partial fragments were prepared by PCR with T7 and T3 primers from each EST clone as the template. Ten microliters of each PCR product were diluted with 100 μL of 0.4 m NaOH and 25 mm Na2EDTA, and incubated at 94°C for 10 min. Slot blot was prepared on Hybond-N+ membrane (Amersham-Pharmacia Biotech, Piscataway, NJ). The PCR products (95 μL) were added to sample wells of the apparatus and vacuum was applied. Each well was rinsed with 200 μL of 20 × SSPE (3.6 m NaCl, 20 mm phosphate buffer, pH 7.4, 20 mm EDTA). The wells were evacuated again and the vacuum was applied for a couple of minutes. Membrane was air dried and cross-linked by UV and oven. A ubiquitin cDNA was used as control. Two microliters of RNA prepared from three basil glands were reverse transcribed by M-MuLV reverse transcriptase (Roche, Mannheim, Germany) with dNTPs including 0.4 mm dCT32P (0.5 mCi). After purification, these reverse transcripts were used as probes for hybridization.
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AY693643, AY693644, AY693648, AY693649, AY693650, AY693646, AY362553, AY693647, and AY693645.
This work was supported by the U.S. Department of Agriculture-Binational Agricultural Research and Development Fund (grant no. IS–3332–02C to E.P. and E.L.), by the National Research Initiative Competitive Grants Program-U.S. Department of Agriculture (grant no. 2001–35318–10006 to E.P.), by the Vaadia-Binational Agricultural Research and Development Fund postdoctoral fellowship (FI–328–2002 to E.F.), and by the National Science Foundation (grant no. MCB–0210170 to D.R.G.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.051318.
References
- Adam KP, Thiel R, Zapp J (1999) Incorporation of 1-[1-13C]Deoxy-D-xylulose in chamomile sesquiterpenes. Arch Biochem Biophys 369: 127–132 [DOI] [PubMed] [Google Scholar]
- Aubourg S, Lecharny A, Bohlmann J (2002) Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Genet Genomics 267: 730–745 [DOI] [PubMed] [Google Scholar]
- Back K, Chappell J (1996) Identifying functional domains within terpene cyclases using a domain-swapping strategy. Proc Natl Acad Sci USA 93: 6841–6845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Kessler A, Halitschke R (2002) Volatile signaling in plant-plant-herbivore interactions: What is real? Curr Opin Plant Biol 5: 351–354 [DOI] [PubMed] [Google Scholar]
- Bick JA, Lange BM (2003) Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch Biochem Biophys 415: 146–154 [DOI] [PubMed] [Google Scholar]
- Bochar DA, Friesen JA, Stauffacher CV, Rodwell VW (1999) Comprehensive Natural Product Chemistry, Isoprenoids Including Steroids and Carotenoids, Vol 2. Pergamon Press, Tarrytown, NY, pp 15–44
- Bohlmann J, Croteau R (1999) Diversity and Variability of Terpenoid Defenses in Conifers: Molecular Genetics, Biochemistry and Evolution of the Terpene Synthase Gene Family in Grand Fir (Abies grandis), Vols 132–146. John Wiley and Sons, West Sussex, UK, pp 132–146 [DOI] [PubMed]
- Bohlmann J, Steele CL, Croteau R (1997) Monoterpene synthases from grand fir (Abies grandis). cDNA isolation, characterization, and functional expression of myrcene synthase, (-)-(4S)-limonene synthase, and (-)-(1S,5S)-pinene synthase. J Biol Chem 272: 21784–21792 [DOI] [PubMed] [Google Scholar]
- Burke C, Klettke K, Croteau R (2004) Heteromeric geranyl diphosphate synthase from mint: construction of a functional fusion protein and inhibition by bisphosphonate substrate analogs. Arch Biochem Biophys 422: 52–60 [DOI] [PubMed] [Google Scholar]
- Burke CC, Wildung MR, Croteau R (1999) Geranyl diphosphate synthase: cloning, expression, and characterization of this prenyltransferase as a heterodimer. Proc Natl Acad Sci USA 96: 13062–13067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J, Wolf F, Proulx J, Cuellar R, Saunders C (1995) Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase a rate-limiting step for isoprenoid biosynthesis in plants? Plant Physiol 109: 1337–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Tholl D, D'Auria JC, Farooq A, Pichersky E, Gershenzon J (2003) Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell 15: 481–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby SM, Alonso WR, Katahira EJ, Mcgarvey DJ, Croteau R (1993) 4S-limonene synthase from the oil glands of spearmint (Mentha spicata): cDNA isolation, characterization, and bacterial expression of the catalytically active monoterpene cyclase. J Biol Chem 268: 23016–23024 [PubMed] [Google Scholar]
- Crock J, Wildung M, Croteau R (1997) Isolation and bacterial expression of a sesquiterpene synthase cDNA clone from peppermint (Mentha × piperita L.) that produces the aphid alarm pheromone (E)-beta-farnesene. Proc Natl Acad Sci USA 94: 12833–12838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell AL, Williams DC, Davis EM, Wildung MR, Croteau R (2002) Molecular cloning and characterization of a new linalool synthase. Arch Biochem Biophys 405: 112–121 [DOI] [PubMed] [Google Scholar]
- Darrah HH (1974) Investigation of the cultivars of basils (Ocimum). Econ Bot 28: 63–67 [Google Scholar]
- Duke SO, Canel C, Rimondo AM, Tellez MR, Duke MV, Paul RN (2000) Current and Potential Exploitation of Plant Glandular Trichome Productivity, Vol 31. Academic Press, San Diego, pp 121–151
- Dussourd DE, Hoyle AM (2000) Poisoned plusiines: toxicity of milkweed latex and cardenolides to some generalist caterpillars. Chemoecology 10: 11–16 [Google Scholar]
- El Tamer MK, Lucker J, Bosch D, Verhoeven HA, Verstappen FW, Schwab W, van Tunen AJ, Voragen AG, de Maagd RA, Bouwmeester HJ (2003) Domain swapping of Citrus limon monoterpene synthases: impact on enzymatic activity and product specificity. Arch Biochem Biophys 411: 196–203 [DOI] [PubMed] [Google Scholar]
- Gang DR, Wang JH, Dudareva N, Nam KH, Simon JE, Lewinsohn E, Pichersky E (2001) An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol 125: 539–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavel Y, von Heijne G (1990) A conserved cleavage-site motif in chloroplast transit peptides. FEBS Lett 261: 455–458 [DOI] [PubMed] [Google Scholar]
- Gershenzon J, Maffei M, Croteau R (1989) Biochemical and histochemical localization of monoterpene byosynthesis in the glandular trichomes of spearmint (Mentha spicata). Plant Physiol 89: 1351–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlbrock K, Grisebach H (1979) Enzymic controls in the biosynthesis of lignin and flavonoids. Annu Rev Plant Physiol 30: 105–130 [Google Scholar]
- Hallahan DL (2000) Monoterpenoid Biosynthesis in Glandular Trichomes of Labiate Plants, Vol 31. Academic Press, San Diego, pp 77–120
- Hemmerlin A, Hoeffler JF, Meyer O, Tritsch D, Kagan IA, Grosdemange-Billiard C, Rohmer M, Bach TJ (2003) Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow-2 cells. J Biol Chem 278: 26666–26676 [DOI] [PubMed] [Google Scholar]
- Iijima Y, Gang DR, Fridman E, Lewinsohn E, Pichersky E (2004) Characterization of geraniol synthase from the peltate glands of sweet basil. Plant Physiol 134: 370–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia JW, Crock J, Lu S, Croteau R, Chen XY (1999) (3R)-Linalool synthase from Artemisia annua L.: cDNA isolation, characterization, and wound induction. Arch Biochem Biophys 372: 143–149 [DOI] [PubMed] [Google Scholar]
- Jones H (1984) Phenylalanine ammonia-lyase: regulation of its induction, and its role in plant development. Phytochemistry 23: 1349–1359 [Google Scholar]
- Katoh S, Hyatt D, Croteau R (2003) Altering product outcome in Abies grandis (-)-limonene synthase and (-)-limonene/(-)-alpha-pinene synthase by domain swapping and directed mutagenesis. Arch Biochem Biophys 425: 65–76 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2104–2105 [DOI] [PubMed] [Google Scholar]
- Kollner TG, Schnee C, Gershenzon J, Degenhardt J (2004) The variability of sesquiterpenes emitted from two Zea mays cultivars is controlled by allelic variation of two terpene synthase genes encoding stereoselective multiple product enzymes. Plant Cell 16: 1115–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange BM, Ghassemian M (2003) Genome organization in Arabidopsis thaliana: a survey for genes involved in isoprenoid and chlorophyll metabolism. Plant Mol Biol 51: 925–948 [DOI] [PubMed] [Google Scholar]
- Laule O, Furholz A, Chang HS, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange M (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6866–6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK (1999) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50: 47–65 [DOI] [PubMed] [Google Scholar]
- Maeda E, Miyake H (1997) Leaf anatomy of patchouli with reference to the disposition of mesophyll glands. Jpn J Crop Sci 66: 307–317 [Google Scholar]
- Martin D, Fäldt J, Bohlmann J (2004) Functional characterization of a nine Norway spruce terpene synthase (TPS) genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily. Plant Physiol 135: 1908–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Tholl D, Gershenzon J, Bohlmann J (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129: 1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales MR, Simon JE (1996) New Basil Selections with Compact Inflorescence for the Ornamental Market. ASHS Press, Alexandria, VA, pp 543–546
- Morales MR, Simon JE (1997) ‘Sweet Dani’: a new culinary and ornamental lemon basil. HortScience 32: 148–149 [Google Scholar]
- Page RDM (1996) Tree view: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12: 357–358 [DOI] [PubMed] [Google Scholar]
- Pare PW, Tumlinson JH (1997) De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol 114: 1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121: 325–332 [PMC free article] [PubMed] [Google Scholar]
- Pechous SW, Whitaker BD (2004) Cloning and functional expression of an (E,E)-alpha-farnesene synthase cDNA from peel tissue of apple fruit. Planta 219: 84–94 [DOI] [PubMed] [Google Scholar]
- Peters RJ, Croteau RB (2003) Alternative termination chemistries utilized by monoterpene cyclases: chimeric analysis of bornyl diphosphate, 1,8-cineole, and sabinene synthases. Arch Biochem Biophys 417: 203–211 [DOI] [PubMed] [Google Scholar]
- Pichersky E, Gang DR (2000) Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective. Trends Plant Sci 5: 439–445 [DOI] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5: 237–243 [DOI] [PubMed] [Google Scholar]
- Pichersky E, Lewinsohn E, Cloteau R (1995) Purification and characterization of S-linalool synthase, an enzyme involved in the production of floral scent in Clarkia breweri. Arch Biochem Biophys 316: 803–807 [DOI] [PubMed] [Google Scholar]
- Piel J, Donath J, Bandemer K, Boland W (1998) Mevalonate-independent biosynthesis of terpenoid volatiles in plants: induced and constitutive emission of volatiles. Angew Chem Int Ed Engl 37: 2478–2481 [DOI] [PubMed] [Google Scholar]
- Re EB, Jones D, Learned RM (1995) Co-expression of native and introduced genes reveals cryptic regulation of HMG CoA reductase expression in Arabidopsis. Plant J 7: 771–784 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Concepcion M, Boronat A (2002) Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol 130: 1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohdich F, Hecht S, Bacher A, Eisenreich W (2003) The deoxyxylulose phosphate pathway of isoprenoid biosynthesis. Discovery and function of the ispDEFGH genes and their cognate enzyme. Pure Appl Chem 75: 393–405 [Google Scholar]
- Rohmer M (2003) Mevalonate-independent methylerythritol phosphate pathway for isoprenoid biosynthesis: elucidation and distribution. Pure Appl Chem 75: 375–388 [Google Scholar]
- Schaller H, Grausem B, Benveniste P, Chye ML, Tan CT, Song YH, Chua NH (1995) Expression of the Hevea brasiliensis (H.B.K.) Mull. Arg. 3-hydroxy-3-methylglutaryl-coenzyme A reductase 1 in tobacco results in sterol overproduction. Plant Physiol 109: 761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JE, Morales MR, Phippen WB, Vieira RF, Hao Z (1999) Basil: A Source of Aroma Compounds and a Popular Culinary and Ornamental Herb. ASHS Press, Alexandria, VA, pp 499–505
- Theis N, Lerdau M (2003) The evolution of function in plant secondary metabolites. Int J Plant Sci 164: S93–S102 [Google Scholar]
- Tholl D, Croteau R, Gershenzon J (2001) Partial purification and characterization of the short-chain prenyltransferases, gernayl diphosphate synthase and farnesyl diphosphate synthase, from Abies grandis (grand fir). Arch Biochem Biophys 386: 233–242 [DOI] [PubMed] [Google Scholar]
- Tholl D, Kish CM, Orlova I, Sherman D, Gershenzon J, Pichersky E, Dudareva N (2004) Formation of monoterpenes in Antirrhinum majus and Clarkia breweri flowers involves heterodimeric geranyl diphosphate synthases. Plant Cell 16: 977–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker E, Putievsky E, Ravid U, Dudai N, Katzir I (1993) Glandular hairs and essential oil in developing leaves of Ocimum basilicum L. (Lamiaceae). Ann Bot (Lond) 71: 43–50 [Google Scholar]
- Williams DC, Mcgarvey DJ, Katahira EJ, Croteau R (1998) Truncation of limonene synthase preprotein provides a fully active ‘pseudomature’ form of this monoterpene cyclase and reveals the function of the amino-terminal arginine pair. Biochemistry 37: 12213–12220 [DOI] [PubMed] [Google Scholar]
- Wink M (2003) Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64: 3–19 [DOI] [PubMed] [Google Scholar]
- Wise ML, Savage TJ, Katahira E, Croteau R (1998) Monoterpene synthases from common sage (Salvia officinalis)–cDNA isolation, characterization, and functional expression of (+)-sabinene synthase, 1,8-cineole synthase, and (+)-bornyl diphosphate synthase. J Biol Chem 273: 14891–14899 [DOI] [PubMed] [Google Scholar]
- Wittstock U, Gershenzon J (2002) Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr Opin Plant Biol 5: 300–307 [DOI] [PubMed] [Google Scholar]