Abstract
The incidence of plasmodesmata in the minor vein phloem of leaves varies widely between species. On this basis, two pathways of phloem loading have been proposed: symplastic where frequencies are high, and apoplastic where they are low. However, putative symplastic-loading species fall into at least two categories. In one, the plants translocate raffinose-family oligosaccharides (RFOs). In the other, the primary sugar in the phloem sap is sucrose (Suc). While a thermodynamically feasible mechanism of symplastic loading has been postulated for species that transport RFOs, no such mechanism is known for Suc transporters. We used p-chloromercuribenzenesulfonic acid inhibition of apoplastic loading to distinguish between the two pathways in three species that have abundant minor vein plasmodesmata and are therefore putative symplastic loaders. Clethra barbinervis and Liquidambar styraciflua transport Suc, while Catalpa speciosa transports RFOs. The results indicate that, contrary to the hypothesis that all species with abundant minor vein plasmodesmata load symplastically, C. barbinervis and L. styraciflua load from the apoplast. C. speciosa, being an RFO transporter, loads from the symplast, as expected. Data from these three species, and from the literature, also indicate that plants with abundant plasmodesmata in the minor vein phloem have abundant plasmodesmata between mesophyll cells. Thus, plasmodesmatal frequencies in the minor veins may be a reflection of overall frequencies in the lamina and may have limited relevance to phloem loading. We suggest that symplastic loading is restricted to plants that translocate oligosaccharides larger than Suc, such as RFOs, and that other plants, no matter how many plasmodesmata they have in the minor vein phloem, load via the apoplast.
Phloem loading is an energy-requiring process that elevates the solute content of sieve elements (SEs) and companion cells (CCs) to levels far above those of surrounding cells (Geiger et al., 1973; van Bel, 1993; Grusak et al., 1996; Turgeon, 1996, 2000; Beebe and Russin, 1999; Hellmann et al., 2000; Komor, 2000; Lalonde et al., 2003). The pressure gradient between source and sink organs drives long-distance transport. It has been suggested that there are two loading strategies—apoplastic and symplastic. This hypothesis derived originally from the observation that there is great variation in the number of plasmodesmata in the phloem of minor veins (Turgeon et al., 1975; Gamalei, 1989, 1990, 1991, 2000; van Bel, 1993). Gamalei has classified over 1,000 species on this basis and finds that it is a relatively consistent feature at the family level. He terms those with the highest number of minor vein plasmodesmata as type 1.
The physical basis for phloem loading via the apoplast is reasonably well understood; Suc enters the cell wall space and is driven across the plasma membranes of CCs and/or SEs by cotransport with protons (Lalonde et al., 2003). The physical basis for symplastic loading is conceptually more problematic, since, by definition, it involves movement from one cell to another through plasmodesmata. Active transport of small molecules through plasmodesmata is unknown, and diffusion against a concentration gradient is impossible.
A thermodynamically feasible mechanism of symplastic loading—the polymer trap—has been described (Turgeon, 1991), but it pertains only to plants that translocate high concentrations of raffinose-family oligosaccharides (RFOs) or possibly other molecules larger than Suc (see “Discussion”). The minor vein CCs of RFO-transporting plants have specialized plasmodesmata and are known as intermediary cells.
Although the polymer trap mechanism may account for symplastic loading in many species, others with high plasmodesmatal counts do not fall into this category; they do not transport high levels of RFOs, and they do not have intermediary cells. When surveys of plasmodesmatal numbers (Gamalei, 1989, 1991) and phloem sugars (Zimmermann and Ziegler, 1975) are compared, such plants account for almost one-quarter of all dicotyledonous families surveyed. If such plants load symplastically, they do so by an unknown mechanism.
In the experiments reported here, we studied phloem-loading characteristics in three species: Clethra barbinervis, Liquidambar styraciflua, and Catalpa speciosa. All three are type 1 according to Gamalei. The first two translocate Suc with only traces of RFO, while C. speciosa transports RFOs and has intermediary cells. We analyzed the effect of p-chloromercuribenzenesulfonic acid (PCMBS) on loading. PCMBS is a potent inhibitor of Suc transport proteins and efficiently blocks apoplastic (Giaquinta, 1983), but not symplastic (Weisberg et al., 1988), loading.
The results indicate that C. barbinervis and L. styraciflua load via the apoplast. C. speciosa loads via the symplast, as expected. Furthermore, we present evidence that Suc-transporting species such as C. barbinervis and L. styraciflua, with high plasmodesmatal counts in the minor veins, also have high plasmodesmatal counts between mesophyll cells, indicating that this feature of leaf anatomy may not be directly related to phloem loading.
In sum, our results do not support the hypothesis that plasmodesmatal frequency alone is a predictor of phloem-loading strategy. Rather, they suggest that symplastic loading is restricted to species that translocate polymers, such as RFOs, and that other plants, no matter how numerous their minor vein plasmodesmata, load via the apoplast.
RESULTS
C. barbinervis
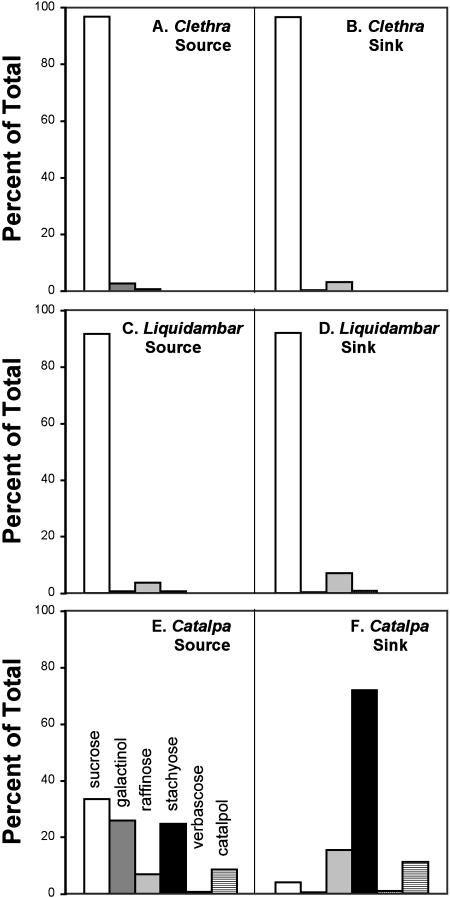
Following exposure to 14CO2, mature leaves of C. barbinervis produced radiolabeled Suc, with small amounts of galactinol and raffinose (Fig. 1A). Neither stachyose nor verbascose were detected. Radiolabeled sugar transported to sink tissue (immature leaves; Fig. 1B) consisted primarily of Suc, with a much smaller amount of raffinose and a trace of galactinol. These experiments were conducted over two growing seasons with the same results.
Figure 1.
Radiolabel in the neutral fraction of extracts following exposure of the laminas of mature leaves to 14CO2. Extracts were made 1.5 h after the beginning of the exposure period either from the radiolabeled lamina itself (A, C, and E) or from sink tissue (B, D, and F). Data are expressed as the percentage of radioactivity in the neutral fraction. A and B, C. barbinervis; C and D, L. styraciflua; E and F, C. speciosa.
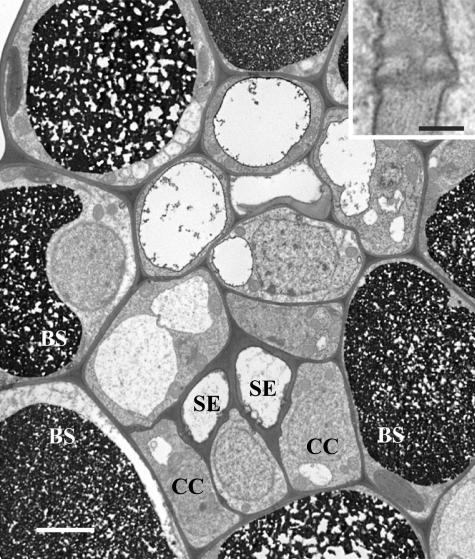
The cell arrangement in minor veins of C. barbinervis is relatively simple (Fig. 2). Commonly, a pair of SEs in the interior of the vein is flanked by CCs that directly abut the bundle sheath. Parenchyma cells are also present, although they appear similar enough to CCs that they often cannot be distinguished from the latter cell type if they abut a SE. Bundle sheath and mesophyll cells have heavy deposits of electron-dense material in the vacuoles. Bundle sheath cells and minor vein CCs are connected by numerous plasmodesmata. While these plasmodesmata are apparently clustered, they do not occur in dense fields, as in species that transport RFOs. The plasmodesmata are symmetrically branched in the characteristic H shape (Fig. 2, insert) described in the phloem of many Suc- and polyol-translocating plants (Roberts and Oparka, 2003).
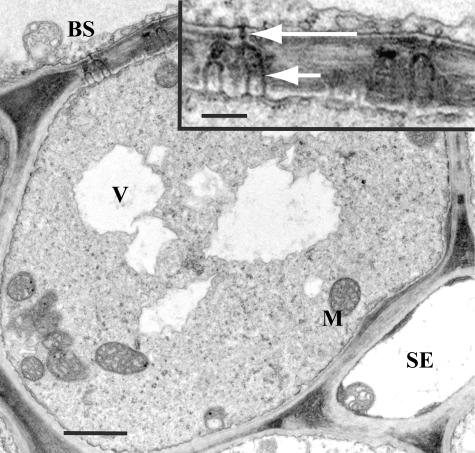
Figure 2.
Electron micrograph of a C. barbinervis minor vein. Two SEs are flanked by two CCs that directly abut bundle sheath (BS) cells on the other side. The cell between the two CCs may be phloem parenchyma, but the distinction between these cell types is often difficult to make. Bar = 3 μm. Plasmodesmata between BS cells and CCs are shown in the inset. They are H shaped and equally branched on both sides. Inset bar = 0.15 μm.
To determine the effect of the Suc transport inhibitor PCMBS on loading, leaves of C. barbinervis were excised and the inhibitor was introduced by transpiration. This has been shown to be an effective means of delivering PCMBS to minor veins (van Bel et al., 1994; Goggin et al., 2001). The leaves were then labeled with 14CO2 and the cut ends of the petioles were placed in a vial containing a solution of EDTA, which facilitates phloem exudation (King and Zeevaart, 1974; Costello et al., 1982; Fellows and Zeevaart, 1983). PCMBS was administered before the leaves were exposed to 14CO2 to ensure that it was present in the apoplast of the minor veins at the time of arrival of radiolabeled Suc (see “Discussion”). To determine whether the PCMBS effect could have been due to an inhibition of photosynthesis, a β-scintillation tube was pressed to the adaxial surface of each leaf immediately after 14CO2 was administered. The amount of radiolabel recorded in the water-treated (98.9 Bq; se = 5.1 Bq; n = 12) and PCMBS-treated (97.2 Bq; se = 3.8 Bq; n = 12) leaves was not statistically different.
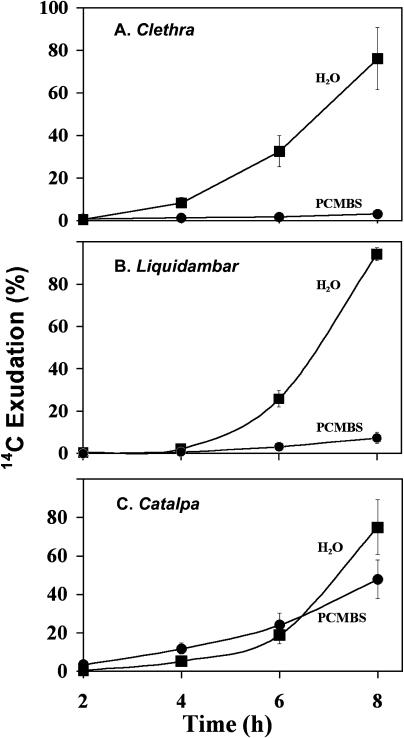
In controls, little radiolabel was detected in the first sample of exudate (Fig. 3A), 2 h after the start of the exudation period (4 h after the initial exposure to 14CO2). Thereafter, the amount of label in the exudate increased steadily. PCMBS treatment almost entirely prevented exudation (Fig. 3A).
Figure 3.
Exudation of 14C from leaves from C. barbinervis (A), L. styraciflua (B), and C. speciosa (C). Cut leaves were allowed to transpire either water or PCMBS for 30 min (C. barbinervis and C. speciosa) or 60 min (L. styraciflua) and were then exposed to 14CO2 for 1 h. Following another 1-h period in the dark to close the stomata, the petioles were recut and submerged in a solution of EDTA to facilitate exudation. Zero time refers to the beginning of the exudation period, 2 h after initial exposure to 14CO2. Exudation amounts are cumulative and are expressed as a percentage of the highest number of counts in replicate 8-h samples. Each experiment was replicated six times.
L. styraciflua
Following exposure of mature leaves to 14CO2, radiolabel in the neutral fraction was present almost entirely as Suc, with small but measurable amounts of galactinol, raffinose, and stachyose (Fig. 1C). Verbascose was not detected. In sink tissues (petioles) the relative proportion of raffinose in the translocated material was elevated but in general reflected the distribution of radiolabel in the lamina (Fig. 1D).
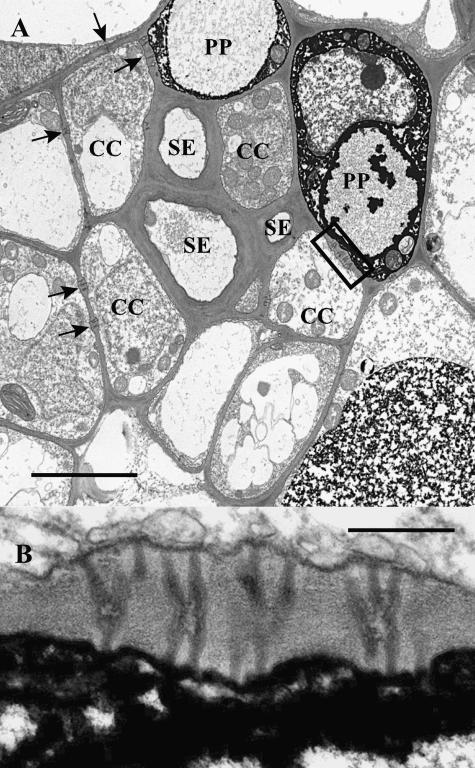
The phloem of sweet gum minor veins is arranged in a loosely concentric fashion, with clustered SEs surrounded in rings by CCs, phloem parenchyma cells, and bundle sheath cells (Fig. 4A). No phloem is present in the smallest veins. In larger minor veins, the phloem may be represented by as many as four SE/CC complexes. In order to reach the SE/CC complexes, in most instances photoassimilate must pass through phloem parenchyma cells or around them.
Figure 4.
Electron micrograph of an L. styraciflua minor vein. A, Low magnification micrograph illustrating the arrangement of cell types. The adaxial surface of the leaf is toward the lower left of the figure. SEs and CCs are surrounded by phloem parenchyma (PP) cells. Arrows indicate clusters of plasmodesmata between PP cells and CCs. Bar = 3 μm. B, High magnification of the cluster of plasmodesmata delimited by the rectangle in A. The plasmodesmata are equally branched on both sides. Bar = 0.3 μm.
The SEs of L. styraciflua minor veins have thick walls (Fig. 4A). Phloem parenchyma cells may contain electron-dense material (Fig. 4A), especially in larger veins. In small veins, electron-dense material is absent. When electron-dense material is absent, it is often difficult to distinguish CCs from phloem parenchyma cells unless the characteristic plasmodesmata between CCs and SEs are visible.
Plasmodesmata between phloem parenchyma cells and CCs are equally branched on both sides, forming the typical H shape seen at this interface in C. barbinervis and many other species (Fig. 4B). Branches on both sides of these plasmodesmata appear to be the same width.
In EDTA exudation studies, there was a lag period of several hours before radiolabel was detected in the collection fluid of control leaves (Fig. 3B). When leaves were treated with PCMBS, exudation was severely inhibited. To test for possible inhibition of photosynthetic uptake of 14CO2 by PCMBS, two 6-mm discs were removed from each leaf immediately prior to the first exudation measurement, and the amount of 14C in the discs was determined by scintillation counting. There was no statistically significant difference in the amount of 14C in discs from water-treated (149 Bq/disc; se = 21 Bq; n = 12) and PCMBS-treated (143 Bq/disc; se = 18 Bq; n =12) leaves.
C. speciosa
For comparison with C. barbinervis and L. styraciflua, we included the RFO-translocating species C. speciosa in our study. We chose this species because it is woody, as are the other two plants. Intermediary cells are present in C. speciosa minor veins. As described in other species, intermediary cells, a specialized type of CC, are linked to the bundle sheath by large numbers of clustered plasmodesmata that have more branches on the intermediary cell side than on the bundle sheath cell side (Fig. 5). Mature C. speciosa leaves synthesized radiolabeled Suc, galactinol, raffinose, stachyose, and verbascose from 14CO2 (Fig. 1E). The amount of labeled stachyose exceeded that of raffinose, which is typical of leaves with intermediary cells. Also typical of plants with intermediary cells, stachyose was the major labeled sugar exported, with a lesser amount of Suc and raffinose and only a small amount of galactinol (Fig. 1F). Limited galactinol transport is a consistent feature of plants that export RFOs (Turgeon, 1996; Ayre et al., 2003). Another compound in the neutral fraction of both source and sink tissues was labeled to approximately the same extent as raffinose and was identified by two-dimensional thin-layer chromatography as catalpol, an iridoid glycoside that acts as an insect deterrent (Marak et al., 2002).
Figure 5.
Electron micrograph of a portion of a C. speciosa minor vein. A, Low magnification micrograph illustrating an intermediary cell flanked by a BS cell and an SE. Intermediary cells have dense cytoplasm, small vacuoles (V), and numerous mitochondria (M). Bar = 1.0 μm. Plasmodesmata between BS cells and CCs are shown in the inset. They are highly branched on the intermediary cell side (short arrow) but much less so on the bundle sheath side (long arrow). Inset bar = 0.3 μm.
When mature leaves of C. speciosa were labeled with 14CO2, exudation into an EDTA solution persisted in the presence of PCMBS (Fig. 3C). This result was expected because phloem loading in species with intermediary cells has often been shown to be insensitive to PCMBS (Weisberg et al., 1988; Flora and Madore, 1993, 1996; van Bel et al., 1994). Accumulation of radiolabel in the exudate was reduced by PCMBS after 8 h, in comparison with water-treated control leaves. However, this may have been a nonspecific, toxic response to PCMBS since the PCMBS-treated, but not the water-treated, leaves showed marked browning in the intercostal regions by the 8-h time point. No toxic response to PCMBS was noted in either C. barbinervis or L. styraciflua.
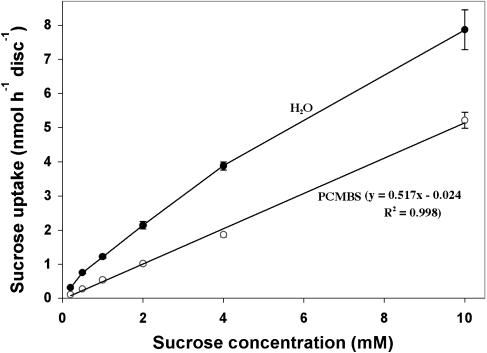
We considered the possibility that phloem loading is apoplastic in C. speciosa but that the Suc-proton cotransporters involved are insensitive to PCMBS. To test this hypothesis, abraded leaf discs were floated on [14C]Suc solutions of increasing concentration, in the presence and absence of PCMBS, and the amount of radiolabel taken up by the tissue was determined by scintillation counting. PCMBS reduced uptake to a linear function of Suc concentration (Fig. 6). There are two components to [14C]Suc uptake in leaf discs of all species studied to date: an apparently passive, linear component that is independent of concentration, and a saturable, carrier-mediated component that represents Suc-proton symport (Delrot and Bonnemain, 1981). Elimination of the saturable component by PCMBS indicates that the Suc carriers in C. speciosa are sensitive to PCMBS inhibition. Therefore, the lack of response to PCMBS in exudation studies cannot be attributed to ineffectiveness of this compound with respect to the inhibition of Suc transporters.
Figure 6.
[14C]Suc uptake by C. speciosa leaf discs incubated in different concentrations of Suc either with or without PCMBS for 1 h. PCMBS reduces uptake to a linear function of Suc concentration, indicating that carrier-mediated uptake has been inhibited. Where error bars are not shown, they are smaller than the data points.
Plasmodesmata in Minor Veins and Mesophyll Cells
We considered the possibility that plasmodesmatal frequencies in the minor vein phloem are a reflection of overall frequencies in the lamina, rather than a specific attribute of minor vein anatomy and loading mechanism. Plasmodesmata were counted in the common walls of mesophyll cells in mature leaves of the three species in this study. Frequencies were: C. barbinervis, 0.403 pd μm−1 (n = 176 interfaces); L. styraciflua, 0.548 pd μm−1 (n = 77); and C. speciosa, 0.433 pd μm−1 (n = 66).
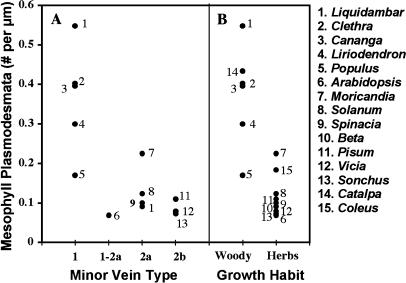
The data are shown in graphic form in Figure 7, with published values for 12 other species. Data from the literature were included only if they were gathered from mature leaves. In Figure 7A, values for 13 of the 15 species are grouped on the basis of minor vein type, according to Gamalei (1989, 1991). Values for C. speciosa and Coleus blumei were not included in Figure 7A, since these species have intermediary cells in the minor vein phloem and thus are not easily placed in any of Gamalei's types (see “Discussion”). As seen in Figure 7A, type 1 species have, in almost all cases, considerably more plasmodesmata in the minor vein phloem than any of the other types. Thus, species with high plasmodesmatal counts in the minor vein phloem (type 1) also have high plasmodesmatal counts in the mesophyll.
Figure 7.
Frequency of plasmodesmata between mesophyll cells. A, Data arranged according to minor vein type (Gamalei, 1989). Type 1 species have the most abundant plasmodesmata in the phloem of minor veins, type 2b the least. B, Data arranged according to growth habit. The data in A and B are from the following: 1 and 2, this study; 3, Fisher (1990); 4, Goggin et al. (2001); 5, Russin and Evert (1985); 6, Haritatos et al. (2000); 7, Beebe and Evert (1992); 8, McCauley and Evert (1989); 9, Warmbrodt and VanDerWoude (1990); 10, Evert and Mierzwa (1986); 11, Wimmers and Turgeon (1991); 12, Bourquin et al. (1990); 13, Fisher (1991); 14, this study; 15, Fisher (1986).
Since Gamalei's types are correlated with growth habit (Gamalei, 1991), the data are also expressed on this basis in Figure 7B. Data for C. speciosa and C. blumei are included in 7B. Expressed in this manner, the data indicate that plasmodesmatal frequencies in the mesophyll tend to be considerably higher in woody plants than in herbaceous species.
DISCUSSION
Given the importance of phloem loading in resource allocation and stress physiology, it is essential that we be able to predict its mechanism(s) in plants of interest. Using plasmodesmatal frequencies in this regard is an attractive idea, but it has not been tested adequately since most of the focus on putative symplastic loaders (type 1 according to Gamalei [1989, 1991, 2000]) has been on RFO-transporting plants (van Bel et al., 1992, 1994; Schrier et al., 2000; Voitsekhovskaja et al., 2000).
To date, the only detailed mechanism of symplastic loading that has been proposed—the polymer trap—applies to RFO species. This model is based on the correlation between the presence of intermediary cells in minor veins and the long-distance transport of RFOs (Turgeon et al., 1993, 2001). Intermediary cells always abut bundle sheath cells and are linked to them by extremely numerous plasmodesmata. The plasmodesmata are asymmetrically branched, with more numerous branches on the intermediary cell side.
According to the polymer trap hypothesis (Turgeon, 1996), Suc diffuses from bundle sheath cells into intermediary cells through the abundant plasmodesmata that connect the two cell types. In the intermediary cells, the Suc is converted to RFOs, primarily raffinose and stachyose. Since raffinose and stachyose molecules are larger than Suc, they are unable to permeate the plasmodesmata between intermediary cells and bundle sheath cells. The plasmodesmata therefore act as valves, preventing backflow. The RFOs and remaining Suc diffuse through presumably wider plasmodesmata into the SEs and are carried away to sink organs in the translocation stream.
The polymer trap mechanism cannot explain symplastic loading of Suc in the absence of polymer synthesis. Therefore, it is necessary to examine the phloem-loading characteristics of type 1 species that transport Suc exclusively, or with very limited RFO involvement, to determine if they are able to load via the symplast.
The PCMBS-14CO2-EDTA Method
The most widely used method to distinguish between apoplastic and symplastic loading is to inhibit Suc transporters with PCMBS, reasoning that symplastic loading, which does not involve transmembrane transport of Suc, should be insensitive to this compound. In this study we measured the effect of PCMBS on EDTA-facilitated exudation of phloem sap (King and Zeevaart, 1974). The EDTA method can be used to collect radiolabeled exudate following exposure of leaves to 14CO2 (Costello et al., 1982; Fellows and Zeevaart, 1983).
It is important in such studies to expose the leaves to 14CO2 after PCMBS is administered. If leaves are exposed to 14CO2 first, radiolabel will enter the translocation stream before the PCMBS exerts its inhibitory effect. Therefore, a species that loads via the apoplast may appear to be insensitive to PCMBS and be misinterpreted as a symplastic loader. This can be a substantial problem since, in EDTA-facilitated exudation experiments, it takes hours for sugars in transit in the phloem to be cleared from the transport stream, giving the false impression that phloem loading is taking place during this time period. The long transit time is apparent in the lag between the time when the leaves are exposed to 14CO2 and when the label is first detected in exudate (Hoffmann-Thoma et al., 2001; Fig. 3, this work).
Loading Characteristics of Suc-Transporting, Type 1 Species
Studies on Liriodendron tulipifera, a type 1 plant that transports Suc, indicated that this species loads via the apoplast (Goggin et al., 2001). More recently, Hoffmann-Thoma et al. (2001) studied loading in three species: Ajuga reptans (Lamiaceae; type 1-RFO transport; Bachmann et al., 1994), Aucuba japonica (Aucubaceae; type 1-Suc transport; Gamalei, 1989), and Hedera helix (Araliaceae; type 2; Gamalei, 1989). Surprisingly, they were unable to detect an inhibitory effect of PCMBS on exudation in any of these plants. It is not clear why PCMBS was ineffective; however, it should be noted that 14CO2 was not employed. Rather, unlabeled exudate was used to measure transport following exposure of the leaves to PCMBS. A disadvantage of this approach is that the collection fluid could be contaminated by sugar that is already in the phloem prior to administration of PCMBS, as discussed above.
To extend our understanding of the distribution of loading strategies, we report here studies on three additional species. On the basis of the surveys of Gamalei (1989) and Zimmermann and Ziegler (1975), we expected one of them, C. speciosa, to be an RFO-transporting plant with intermediary cells. This proved to be true. Also as expected, exudation of 14C-photoassimilate was not impeded by PCMBS until the compound became toxic, again validating the experimental approach.
In addition to Suc and RFOs, C. speciosa translocates catalpol, an iridoid glycoside. Iridoid glycosides are secondary compounds that deter feeding by generalist and nonadapted insect herbivores (Marak et al., 2002). Antirrhinoside, another iridoid glycoside, is transported in the phloem of Asarina scandens (Gowan et al., 1995). It is possible that these compounds inhibit phloem-feeding insects.
Although PCMBS did not inhibit loading in C. speciosa, it severely compromised exudation of 14C-photoassimilate in C. barbinervis and L. styraciflua. In C. barbinervis, Suc is the major transport sugar. This was an unexpected result, since Zimmermann and Ziegler (1975) detected large amounts of raffinose and stachyose in samples of phloem sap in Clethra acuminata. The minor veins of C. barbinervis do not contain true intermediary cells, based on plasmodesmata structure. Nonetheless, the overall architecture of the minor veins, with a pair of large CCs abutting the bundle sheath, is like that of plants with intermediary cells.
L. styraciflua translocates Suc almost to the exclusion of other sugars. As in many Suc-translocating species, the SE/CC complexes do not have direct symplastic contact with the bundle sheath but instead are buried within relatively large minor veins. Thus, the minor vein phloem is distinctly different from that of RFO-transporting plants that have much more direct symplastic continuity between the mesophyll and phloem, even though the number of plasmodesmata is high. As in C. barbinervis, the plasmodesmata in Liriodendron styraciflua minor vein phloem are the typical H shape common in mature leaf tissues (Roberts and Oparka, 2003). They are not as extensively branched as the plasmodesmata in intermediary cells nor are the branches asymmetric.
The results of exudation experiments on C. barbinervis and Liquidambar styraciflua were distinctly different from those on C. speciosa in that PCMBS had a strong inhibitory effect. From the combined results of the anatomical, exudation, and 14C-transport studies, it appears that C. speciosa loads via the symplast by the polymer trap mechanism while C. barbinervis and L. styraciflua load from the apoplast.
It is necessary then to ask how phloem loading from the apoplast can occur when the CCs have appreciable symplastic connection to the surrounding parenchyma cells. Why do the accumulated sugars not leak back to the mesophyll through the plasmodesmata? One possibility is that the plasmodesmata close, or partially close, when the pressure in the CC is higher than in surrounding cells, thus preventing Suc escape. Pressure sensitivity has not been shown for this cell type, but it has been documented in trichomes (Oparka and Prior, 1992).
If plasmodesmata in the CCs of Suc-transporting plants are pressure sensitive, they must react differently from those of intermediary cells, since the latter are open, even though the pressure difference between the intermediary cells and bundle sheath cells is extreme (Turgeon and Hepler, 1989). It seems reasonable to suggest differences in function since the plasmodesmata between intermediary cells and bundle sheath cells are unusual and, apparently, specialized; they are always asymmetrically branched and, in at least some species, visibly narrowed on the intermediary cell side.
Could apoplastic and symplastic loading mechanisms operate at the same time in the same cells or at least in the same veins? There appears to be no theoretical reason why Suc could not load apoplastically into intermediary cells, although plasmodesmata offer a low-resistance pathway. Symplastic and apoplastic phloem-loading mechanisms could also work concurrently in different cells in the same vein, or in different vein orders. Structural correlates, such as the presence of ordinary CCs in the minor vein phloem of polymer-trap species, suggest that this may occur (Turgeon et al., 1975; Fisher, 1986; van Bel and Gamalei, 1991; Turgeon, 1996; Knop et al., 2001, 2004). Nonetheless, attempts to demonstrate the presence of both mechanisms by inhibiting the apoplastic component with PCMBS have either been negative (Flora and Madore, 1993, 1996) or have indicated that the apoplastic component is small (Knop et al., 2004).
Why do type 1 Suc-transporting species, such as C. barbinervis and L. styraciflua, have so many plasmodesmata in the minor vein phloem if they are not used for phloem loading? Data on plasmodesmatal counts in the mesophyll, from this study and from the literature (Fig. 7), indicate that a conceptual focus on loading may be overly narrow; species with high counts in the minor veins also have large numbers of plasmodesmata between mesophyll cells. This suggests that the purpose of such extensive symplastic continuity throughout the leaf should not be sought exclusively within the framework of phloem transport. Note that type 1 species are predominately woody, whereas type 2 species are more often herbaceous (Gamalei, 1989, 1991). Therefore, the difference in plasmodesmatal frequencies between the two types may be related to growth habit rather than the strategy of phloem loading. In support of this interpretation, consider that Arabidopsis (Arabidopsis thaliana) is a type 1-2a species, with moderately high numbers of plasmodesmata in the minor vein phloem (Haritatos et al., 2000). As such, one might expect it to have moderately high numbers of mesophyll plasmodesmata. However, it has the lowest number on record (Fig. 7), which correlates better with its herbaceous nature.
Although data on type 1 Suc-transporting species are still limited, they do not support the prevailing hypothesis that all plants with abundant minor vein plasmodesmata load via the symplast. Rather, available evidence is consistent with the hypothesis that symplastic phloem loading is restricted to species that use the polymer trap mechanism and that other plants, no matter how many plasmodesmata they have in the minor vein phloem, load Suc via the apoplast. Assuming that this conclusion is correct, it will be possible to discriminate easily between apoplastic- and symplastic-loading plants on the basis of the sugars they export from leaves.
MATERIALS AND METHODS
Plant Material
Experiments were conducted on fully expanded leaves taken from mature trees and /or shrubs on the Cornell University campus in the months of June through August over the course of two growing seasons. Species were Catalpa speciosa Warder (Bignoniaceae), Clethra barbinervis Siebold & Zucc. (Clethraceae), and Liquidambar styraciflua L. (Hamamelidaceae).
Carbohydrate Synthesis and Transport
Leaves were exposed in a cuvette to 1.0 MBq of 14CO2 that was generated in a syringe by adding an excess of 80% lactic acid to Na214CO3 (6.6 × 105 MBq mmol−1; ICN Biomedicals, Irvine, CA). The leaf was allowed to fix 14CO2 for 15 min. The leaf and the sink were separately excised 1.5 h after the beginning of the fixation period and frozen in liquid N2. Sink tissues were covered with aluminum foil during the exposure and transport periods. Tissues were ground in liquid N2. Soluble carbohydrates were extracted in a mixture of methanol, chloroform, and water (12:5:3; v/v/v). Aqueous and nonaqueous phases were separated by addition of water; the aqueous phase was passed through a layer of cation (AG1-X4, carbonate form; Bio-Rad, Hercules, CA) exchange resin, a layer of polyvinylpolypyrrolidone, and a layer of anion (AG50-X4; Bio-Rad) exchange resin. The neutral fraction was taken to dryness in a heating block at 70°C under a stream of N2, dissolved in water, and spotted on silica plates. Radioactive compounds were identified by two-dimensional thin-layer chromatography and visualization with vanillin. Procedures are described in detail in Haritatos et al. (2000). Catalpol was identified in the same two-dimensional system by cochromatography with the authentic compound (Wako Pure Chemical Industries, Richmond, VA) and visualization with vanillin.
Electron Microscopy
Pieces of lamina approximately 1.5 mm long on a side were fixed in 2% (w/v) glutaraldehyde plus 2% (w/v) paraformaldehyde in 70 mm sodium cacodylate buffer, pH 6.8, for 4 h at 4°C. The samples were washed in the same buffer at 4°C, post fixed in 1% (w/v) osmium tetroxide in the same buffer at 4°C overnight, dehydrated in acetone at room temperature, and embedded in Spurr resin (Electron Microscopy Sciences, Ft. Washington, PA). Thin sections for electron microscopy were stained with uranyl acetate and lead citrate and photographed at 60 kV with a Philips (Eindhoven, The Netherlands) EM-300 transmission electron microscope. Plasmodesmata were counted on electron micrographs taken at 10,500× magnification. Regions of mesophyll tissue were selected randomly, and all interfaces in that region, from the upper to the lower epidermis, were photographed. Only those plasmodesmata extending at least halfway across the common walls of contiguous cells were counted. Where plasmodesmata were branched, each branch was counted as a single plasmodesma. The frequency of plasmodesmata per square micrometer was calculated by the method of Gunning, described in the open discussion following the paper of Robards (1976).
Phloem Exudation
Shoots were removed from trees, recut immediately under water, and brought to the laboratory within 5 min. Petioles were cut with a sharp razor blade, under the surface of water, and leaves were distributed to vials (two per vial for C. speciosa and three per vial for C. barbinervis and L. styraciflua) with the cut ends of the petioles immersed in either water or PCMBS solution in water (0.5 mm). The leaves were placed under water-filtered lights (metal halide; 350 μmol photons m−2 s−1) for 30 min (C. speciosa and C. barbinervis) or 60 min (L. styraciflua) to allow transpiration of the water or PCMBS solution into the lamina. The amount of uptake was measured by weighing the vials. L. styraciflua leaves were allowed to transpire longer because the rate of uptake was lower (0.05 mL h−1 g−1 lamina tissue) than in the leaves of the other two species (0.30 and 0.37 mL h−1 g−1 lamina tissue for C. speciosa and C. barbinervis, respectively). At the end of the uptake period, the leaves were transferred to vials containing water and exposed to 14CO2 (1.7 MBq) for 1 h in a 16.9-L photosynthesis chamber under the same light conditions. A battery-powered fan inside the chamber circulated the air efficiently. The 14CO2 was generated by addition of 80% lactic acid to Na214CO3. After the 14CO2 exposure period, leaves were transferred to the dark, with their the petioles immersed in water, for 1 h to close the stomata and prevent further transpiration. The petioles were then recut under water to a standard length, and the leaves were immediately transferred to plastic tubes containing EDTA solution (20 mm disodium ethylenediaminetetraacetic acid, pH 7.0, with KOH) to facilitate exudation (King and Zeevaart, 1974). The leaves were placed in a dark, humid chamber to reduce transpiration; this inhibited reflux of exuded label and uptake of EDTA, which is toxic. At intervals, the EDTA solution was entirely removed for scintillation counting and replaced.
Counts of radioactivity were added at each time point to provide cumulative data on the amount of label exuded. For each species, two replicate experiments, each with and without PCMBS, were conducted together, and the entire experiment was repeated three times on different days for a total of six replicates. Since the leaves fixed different amounts of 14CO2 on the different days, the results were normalized by expressing them as a percentage of the highest number of counts on a given day.
Kinetics of [14C]Suc Uptake
The upper surfaces of C. speciosa leaves were abraded, first with carborundum and then with very fine sandpaper (320 grit). Leaf discs were removed, under water, with a cork borer (6.0-mm diameter) and floated, abraded side down, on 20 mm MES buffer (2-[N-morpholino]ethanesulfonic acid-NaOH, pH 5.5, plus 2 mm CaCl2). Discs were distributed randomly onto the surface of 2.0 mL of the same buffer in small (3.5-cm diameter) plastic petri dishes (20 discs/dish). The buffer was removed from the dishes and replaced with 2.0 mL of [14C]Suc (0.2–10 mm; 13 or 26 kBq mL−1) in the same buffer. Solutions contained either 2 mm PCMBS or the same amount of water as control, and sufficient sorbitol was included to bring all solutions to the same osmolality. The dishes were placed on a rotating shaker (100 rpm). Sixty minutes later the [14C]Suc solution was removed, and the leaf discs were washed with ice-cold buffer three times (20 min each) and placed in scintillation vials (4 discs/vial). The tissue was extracted in the vial with 0.5 mL of ethanol/acetic acid (3/1; v/v) for 1 h at 50°C followed by decolorization with 0.1 mL of commercial bleach (Sun et al., 1988). Counts were measured using Ecoscint (National Diagnostics, Atlanta) as the scintillation solution.
Acknowledgments
We thank Edwin Reidel and Ashlee McCaskill for reviewing the manuscript.
This work was supported by the National Science Foundation (grant no. IBN–0110638 to R.T.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.042036.
References
- Ayre BG, Keller F, Turgeon R (2003) Symplastic continuity between companion cells and the translocation stream: long-distance transport is controlled by retention and retrieval mechanisms in the phloem. Plant Physiol 131: 1518–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M, Matile P, Keller F (1994) Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L. Cold acclimation, translocation, and sink to source transition: discovery of chain elongation enzyme. Plant Physiol 105: 1335–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DU, Evert RF (1992) Photoassimilate pathways and phloem loading in the leaf of Moricandia arvensis L. Dc. Brassicaceae. Int J Plant Sci 153: 61–77 [Google Scholar]
- Beebe DU, Russin WA (1999) Plasmodesmata in the phloem-loading pathway. In AJE van Bel, WJP Van Kesteren, eds, Plasmodesmata: Structure, Function, Role in Cell Communication. Springer-Verlag, New York, pp 261–293
- Bourquin S, Bonnemain J-L, Delrot S (1990) Inhibition of loading of 14C assimilates by p-chloromercuribenzenesulfonic acid: localization of the apoplastic pathway in Vicia faba. Plant Physiol 92: 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LR, Bassham JA, Calvin M (1982) Enhancement of phloem exudation from Fraxinus uhdei Wenz. (evergreen ash) using ethylenediaminetetraacetic acid. Plant Physiol 69: 77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrot S, Bonnemain J-L (1981) Involvement of protons as a substrate for the sucrose carrier during phloem loading in Vicia faba leaves. Plant Physiol 67: 560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evert RF, Mierzwa RJ (1986) Pathway(s) of assimilate movement from mesophyll cells to sieve tubes in the Beta vulgaris leaf. In J Cronshaw, RT Giauinta, WJ Lucas, eds, Phloem Transport. Alan Liss, New York, pp 419–432
- Fellows RJ, Zeevaart JAD (1983) Comparison of EDTA enhanced exudation from detached, and translocation from attached, bean leaves (Phaseolus vulgaris cultivar Montcalm). Plant Physiol 71: 716–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DG (1986) Ultrastructure, plasmodesmatal frequency, and solute concentration in green areas of variegated Coleus blumei Benth. leaves. Planta 169: 141–152 [DOI] [PubMed] [Google Scholar]
- Fisher DG (1990) Distribution of plasmodesmata in leaves. A comparison of Cananga odorata with other species using different measures of plasmodesmatal frequency. In AW Robards, H Jongsma, WJ Lucas, J Pitts, D Spray, eds, Parallels in Cell to Cell Junctions in Plants and Animals. Springer-Verlag, Berlin, pp 199–221
- Fisher DG (1991) Plasmodesmatal frequency and other structural aspects of assimilate collection and phloem loading in leaves of Sonchus oleraceus (Asteraceae), a species with minor vein transfer cells. Am J Bot 78: 1549–1559 [Google Scholar]
- Flora LL, Madore MA (1993) Stachyose and mannitol transport in olive (Olea europaea L.). Planta 189: 484–490 [Google Scholar]
- Flora LL, Madore MA (1996) Significance of minor-vein anatomy to carbohydrate transport. Planta 198: 171–178 [Google Scholar]
- Gamalei Y (1989) Structure and function of leaf minor veins in trees and herbs. Trees (Berl) 3: 96–110 [Google Scholar]
- Gamalei Y (1990) Double-routed phloem loading and its development related to the plant evolution from trees to herbs. Physiol Plant 79: A94 [Google Scholar]
- Gamalei Y (1991) Phloem loading and its development related to plant evolution from trees to herbs. Trees (Berl) 5: 50–64 [Google Scholar]
- Gamalei Y (2000) Comparative anatomy and physiology of minor veins and paraveinal parenchyma in the leaves of dicots. Bot Z 85: 34–49 [Google Scholar]
- Geiger DR, Giaquinta RT, Sovonick SA, Fellows RJ (1973) Solute distribution in sugar beet leaves in relation to phloem loading and translocation. Plant Physiol 52: 585–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta RT (1983) Phloem loading of sucrose. Annu Rev Plant Physiol 34: 347–387 [Google Scholar]
- Goggin FL, Medville R, Turgeon R (2001) Phloem loading in the tulip tree. Mechanisms and evolutionary implications. Plant Physiol 125: 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowan E, Lewis BA, Turgeon R (1995) Phloem transport of antirrhinoside, an iridoid glycoside, in Asarina scandens (Scrophulariaceae). J Chem Ecol 21: 1781–1788 [DOI] [PubMed] [Google Scholar]
- Grusak MA, Beebe DU, Turgeon R (1996) Phloem loading. In E Zamski, AA Schaffer, eds, Photoassimilate Distribution in Plants and Crops. Marcel Dekker, New York, pp 209–277
- Haritatos E, Medville R, Turgeon R (2000) Minor vein structure and sugar transport in Arabidopsis thaliana. Planta 211: 105–111 [DOI] [PubMed] [Google Scholar]
- Hellmann H, Barker L, Funck D, Frommer WB (2000) The regulation of assimilate allocation and transport. Aust J Plant Physiol 27: 583–594 [Google Scholar]
- Hoffmann-Thoma G, van Bel AJE, Ehlers K (2001) Ultrastructure of minor-vein phloem and assimilate export in summer and winter leaves of the symplasmically loading evergreens Ajuga reptans L., Aucuba japonica Thunb., and Hedera helix L. Planta 212: 231–242 [DOI] [PubMed] [Google Scholar]
- King RW, Zeevaart JAD (1974) Enhancement of phloem exudation from cut petioles by chelating agents. Plant Physiol 53: 96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop C, Stadler R, Sauer N, Lohaus G (2004) AmSUT1, a sucrose transporter in collection and transport phloem of the putative symplastic phloem loader Alonsoa meridionalis. Plant Physiol 134: 204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop C, Voitsekhovskaja O, Lohaus G (2001) Sucrose transporters in two members of the Scrophulariaceae with different types of transport sugar. Planta 213: 80–91 [DOI] [PubMed] [Google Scholar]
- Komor E (2000) Source physiology and assimilate transport: the interaction of sucrose metabolism, starch storage and phloem export in source leaves and the effects on sugar status in phloem. Aust J Plant Physiol 27: 497–505 [Google Scholar]
- Lalonde S, Tegeder M, Throne HM, Frommer WB, Patrick JW (2003) Phloem loading and unloading of sugars and amino acids. Plant Cell Environ 26: 37–56 [Google Scholar]
- Marak HB, Biere A, van Damme JMM (2002) Two herbivore-deterrent iridoid glycosides reduce the in-vitro growth of a specialist but not of a generalist fungus of Plantago lanceolata L. Chemoecology 12: 185–192 [Google Scholar]
- McCauley MM, Evert RF (1989) Minor veins of the potato (Solanum tuberosum L.) leaf: ultrastructure and plasmodesmatal frequency. Bot Gaz 150: 351–368 [Google Scholar]
- Oparka KJ, Prior DAM (1992) Direct evidence for pressure-generated closure of plasmodesmata. Plant J 2: 741–750 [Google Scholar]
- Robards AW (1976) Plasmodesmata in higher plants. In BES Gunning, AW Robards, eds, Intercellular Communication in Higher Plants: Studies on Plasmodesmata. Springer-Verlag, Berlin, pp 15–57
- Roberts AG, Oparka KJ (2003) Plasmodesmata and the control of symplastic transport. Plant Cell Environ 26: 103–124 [Google Scholar]
- Russin WA, Evert RF (1985) Studies on the leaf of Populus deltoides (Salicaceae): ultrastructure, plasmodesmatal frequency, and solute concentrations. Am J Bot 72: 1232–1247 [Google Scholar]
- Schrier AA, Hoffmann-Thoma G, van Bel AJE (2000) Temperature effects on symplasmic and apoplasmic phloem loading and loading-associated carbohydrate processing. Aust J Plant Physiol 27: 769–778 [Google Scholar]
- Sun D, Wimmers LE, Turgeon R (1988) Scintillation counting of 14C-labeled soluble and insoluble compounds in plant tissues. Anal Biochem 169: 424–427 [DOI] [PubMed] [Google Scholar]
- Turgeon R (1991) Symplastic phloem loading and the sink-source transition in leaves: a model. In J-L Bonnemain, S Delrot, J Dainty, WJ Lucas, eds, Recent Advances in Phloem Transport and Assimilate Compartmentation. Ouest Editions, Nantes, France, pp 18–22
- Turgeon R (1996) Phloem loading and plasmodesmata. Trends Plant Sci 1: 418–423 [Google Scholar]
- Turgeon R (2000) Plasmodesmata and solute exchange in the phloem. Aust J Plant Physiol 27: 521–529 [Google Scholar]
- Turgeon R, Beebe DU, Gowan E (1993) The intermediary cell: minor-vein anatomy and raffinose oligosaccharide synthesis in the Scrophulariaceae. Planta 191: 446–456 [Google Scholar]
- Turgeon R, Hepler PK (1989) Symplastic continuity between mesophyll and companion cells in minor veins of mature Cucurbita pepo L. leaves. Planta 179: 24–31 [DOI] [PubMed] [Google Scholar]
- Turgeon R, Medville R, Nixon KC (2001) The evolution of minor vein phloem and phloem loading. Am J Bot 88: 1331–1339 [PubMed] [Google Scholar]
- Turgeon R, Webb JA, Evert RF (1975) Ultrastructure of minor veins in Cucurbita pepo leaves. Protoplasma 83: 217–231 [Google Scholar]
- van Bel AJE (1993) Strategies of phloem loading. Annu Rev Plant Physiol Plant Mol Biol 44: 253–281 [Google Scholar]
- van Bel AJE, Ammerlaan A, van Dijk AA (1994) A three-step screening procedure to identify the mode of phloem loading in intact leaves: evidence for symplasmic and apoplasmic phloem loading associated with the type of companion cell. Planta 192: 31–39 [Google Scholar]
- van Bel AJE, Gamalei Y (1991) Multiprogrammed phloem loading. In J-L Bonnemain, S Delrot, J Dainty, WJ Lucas, eds, Proceedings of the 1990 International Conference on Phloem Transport and Assimilate Compartmentation. Ouest Editions, Nantes, France, pp 128–139
- van Bel AJE, Gamalei YV, Ammerlaan A, Bik LPM (1992) Dissimilar phloem loading in leaves with symplasmic or apoplasmic minor-vein configurations. Planta 186: 518–525 [DOI] [PubMed] [Google Scholar]
- Voitsekhovskaja OV, Pakhomova MV, Syutkina AV, Gamalei YV, Heber U (2000) Compartmentation of assimilate fluxes in leaves II: apoplastic sugar levels in leaves of plants with different companion cell types. Plant Biol 2: 107–112 [Google Scholar]
- Warmbrodt RD, VanDerWoude WJ (1990) Leaf of Spinacia oleracea (spinach): ultrastructure, and plasmodesmatal distribution and frequency, in relation to sieve-tube loading. Am J Bot 77: 1361–1377 [Google Scholar]
- Weisberg LA, Wimmers LE, Turgeon R (1988) Photoassimilate transport characteristics of nonchlorophyllous and green tissue in variegated leaves of Coleus blumei Benth. Planta 175: 1–8 [DOI] [PubMed] [Google Scholar]
- Wimmers LE, Turgeon R (1991) Transfer cells and solute uptake in the minor veins of Pisum sativum leaves. Planta 186: 2–12 [DOI] [PubMed] [Google Scholar]
- Zimmermann MH, Ziegler H (1975) List of sugars and sugar alcohols in sieve-tube exudates. In MH Zimmermann, JA Milburn, eds, Encyclopedia of Plant Physiology, N.S., Vol. 1, Transport in Plants 1: Phloem Transport. Springer, New York, pp 480–503