Abstract
Sulfate substituents naturally occurring in biomolecules, such as oligosaccharides and polysaccharides, can play a critical role in major physiological functions in plants and animals. We show that laminarin, a β-1,3 glucan with elicitor activity in tobacco (Nicotiana tabacum), becomes, after chemical sulfation, an inducer of the salicylic acid (SA) signaling pathway in tobacco and Arabidopsis thaliana. In tobacco cell suspensions, the oxidative burst induced by the laminarin sulfate PS3 was Ca2+ dependent but partially kinase independent, whereas laminarin triggered a strickly kinase-dependent oxidative burst. Cells treated with PS3 or laminarin remained fully responsive to a second application of laminarin or PS3, respectively, suggesting two distinct perception systems. In tobacco leaves, PS3, but not laminarin, caused electrolyte leakage and triggered scopoletin and SA accumulation. Expression of different families of Pathogenesis-Related (PR) proteins was analyzed in wild-type and mutant tobacco as well as in Arabidopsis. Laminarin induced expression of ethylene-dependent PR proteins, whereas PS3 triggered expression of ethylene- and SA-dependent PR proteins. In Arabidopsis, PS3-induced PR1 expression was also NPR1 (for nonexpressor of PR genes1) dependent. Structure-activity analysis revealed that (1) a minimum chain length is essential for biological activity of unsulfated as well as sulfated laminarin, (2) the sulfate residues are essential and cannot be replaced by other anionic groups, and (3) moderately sulfated β-1,3 glucans are active. In tobacco, PS3 and curdlan sulfate induced immunity against Tobacco mosaic virus infection, whereas laminarin induced only a weak resistance. The results open new routes to work out new molecules suitable for crop protection.
INTRODUCTION
Oligosaccharins are naturally occurring complex carbohydrates with biological regulatory functions (Albersheim et al., 1983). In plants and animals, they act as molecular signals that regulate growth, development, and survival in the environment (Côté and Hahn, 1994; Bakkers et al., 1999; Alban and Franz, 2001; Shibuya and Minami, 2001). Oligosaccharins active in plants derive from microbe, plant, or marine macroalgae cell wall structures. Only a few are fully characterized in terms of their structure and spectrum of biological activities. Different structural motifs have been described, including microbial β-1,6-1,3 glucans and chitin-derived oligomers, plant oligogalacturonides and xyloglucans, as well as marine macroalgae laminarin and fucans (Côté and Hahn, 1994; Klarzynski et al., 2000, 2003).
The relevance of some of these oligosaccharins as in vivo actors in defense systems is supported by their possible natural occurrence during plant–microbe interactions (Fritig et al., 1998). Evidence has also been provided that oligosaccharins enhance plant resistance against pathogens (Joubert et al., 1998), and it is thought that mimicking pathogen attack with such nonspecific elicitors might prove useful in the development of alternative strategies for crop protection.
Marine macroalgae constitute an inexpensive possible source of oligosaccharins because they contain a diversity of unique polysaccharides (Kloareg and Quatrano, 1988). For example, laminarin, a reserve carbohydrate of the brown alga Laminaria digitata, is a water-soluble β-1,3 glucan with an average degree of polymerization (DP) of 25 glucose units and with up to three single β-glucose branches at position 6 (Read et al., 1996). Laminarin stimulates defense responses in cell suspensions of tobacco (Nicotiana tabacum) (Klarzynski et al., 2000), grapevine (Vitis vinifera) (Aziz et al., 2003), alfalfa (Medicago sativa) (Cardinale et al., 2000), and rice (Oryza sativa) (Inui et al., 1997). The defense responses include activation of mitogen-activated protein kinases, Ca2+ influx, oxidative burst, and alkalinization of the extracellular medium. Applied to tobacco or grapevine plants, laminarin induces the accumulation of phytoalexins and expression of a set of Pathogenesis-Related (PR) proteins (Klarzynski et al., 2000; Aziz et al., 2003). However, laminarin does not trigger the hypersensitive response (HR).
The biological activity of β-glucans is thought to result from their binding to receptors. A putative receptor for a β-1,3, β-1,6 heptaglucan (β-1,6 glucose backbone with two β-1,3 glucose branches) has been cloned in soybean (Glycine max) and bean (Phaseolus vulgaris) (Umemoto et al., 1997; Mithofer et al., 2000). Furthermore, plant cells can discriminate between closely related glucans. For example, rice responds to a Pyricularia oryzae pentaglucan (β-1,3 glucose backbone with one β-1,6 branch) but not to a hexaglucan (β-1,6 glucose backbone with two β-1,3 branches) (Yamaguchi et al., 2000), and tobacco reacts to laminarin but not to the β-1,3, β-1,6 heptaglucan (Klarzynski et al., 2000).
Oligosaccharins can carry decorations that are important for their biological function. It is well established that the sulfate groups of some oligosaccharins mediate recognition in biochemical and physiological processes occurring in algae, land plants, and animals. The pattern of sulfate substitution in carrageenans is crucial for recognition between Chondrus crispus, a marine red algae, and its green algal pathogenic endophyte, Acrochaete operculata (Bouarab et al., 1999). The sulfate decoration of Nod factors, which are signaling compounds for the symbiosis between legumes and rhizobacteria, is a determinant of host specificity in the Sinorhizobium meliloti–alfalfa interaction (Roche et al., 1991). In mammals, basic fibroblast growth factor, a well-characterized signal molecule, is only biologically active and binds to its receptor in the presence of a specific sulfated oligosaccharide fragment of heparan sulfate that binds to both the growth factor and its receptor (Pye et al., 1998). Thus, there is growing evidence that biomolecules substituted with sulfate groups are involved in major physiological functions in plants and animals.
Because sulfate groups can modulate the biological activities of oligosaccharides, we chemically sulfated laminarin from L. digitata. The elicitor activities of sulfated and unmodified laminarin were investigated in tobacco and Arabidopsis thaliana both in tissue culture and in whole plants. We also report on studies of structure-activity relationships to evaluate the importance of the sulfation pattern, of the chain length, and of the sugar backbone on elicitor activity. Finally, using the tobacco–Tobacco mosaic virus (TMV) interaction as a model system, we compared the resistance inducing activity of sulfated and unmodified β-1,3 glucans. Our results indicate that naturally occurring carbohydrates can be chemically modified to alter their biological activities, thereby opening new routes for crop protection strategies.
RESULTS
Synthesis and Characterization of the Laminarin Sulfate PS3
The structure, size, and purity of the laminarin used for chemical sulfation were previously characterized by 13C NMR spectroscopy and high-pressure anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) (Lepagnol-Descamps et al., 1998). Laminarin consists of a β-1,3 glucan backbone with an average DP of 25 and one or two C6 glucose branches.
The chemical sulfation of laminarin was performed according to the method described by Alban et al. (1992), which had been optimized to obtain regular β-1,3 glucan sulfation without any polysaccharide degradation and to control the resulting degree of sulfation (DS). A DS is mainly dependent on the molar ratio of SO3/pyridine complex to glucose but is also influenced by other parameters, such as temperature and reaction time. Methylation analysis revealed that the primary OH group in position 6 is ∼10 times more accessible to sulfation than the secondary OH groups in positions 2 and 4 (Alban and Franz, 1994). One of the resulting laminarin sulfates was called PS3. PS3 has a DS of 2.4, as determined by elemental analysis, by conductivity detected ion chromatography of the sulfate ions released by trifluoroacetic acid hydrolysis, and by conductimetric NaOH titration of PS3 transformed into the acid form by ion exchange. The latter method also demonstrated that the sodium salt of PS3 is stable for several years. HPAEC-PAD analysis of PS3 showed no degradation of the glucan chain (i.e., the DP and the polydispersity remained unchanged). This was confirmed by gel permeation chromatography. The apparent molecular mass of PS3 (i.e., its hydrodynamic volume) is 18 kD as determined by gel permeation chromatography using neutral pullulans as standards. According to the methylation analysis, the C6-OH of PS3 is nearly completely sulfated, and the substitution of both C2-OH and C4-OH amounts to ∼70%.
Various analyses proved the purity of PS3. The absence of nitrogen according to elemental analysis indicated that the product is free of pyridine and N,N-dimethylformamide (DMF) used for the chemical sulfation. This was confirmed by 13C NMR spectroscopy and UV spectroscopy. As expected, after dialysis for 7 d against flowing deionized water, no contamination with salts or other low molecular weight compounds, such as oligosaccharides, was observed in gel permeation chromatograms.
Perception of Laminarin and PS3 by Cultured Tobacco Cells
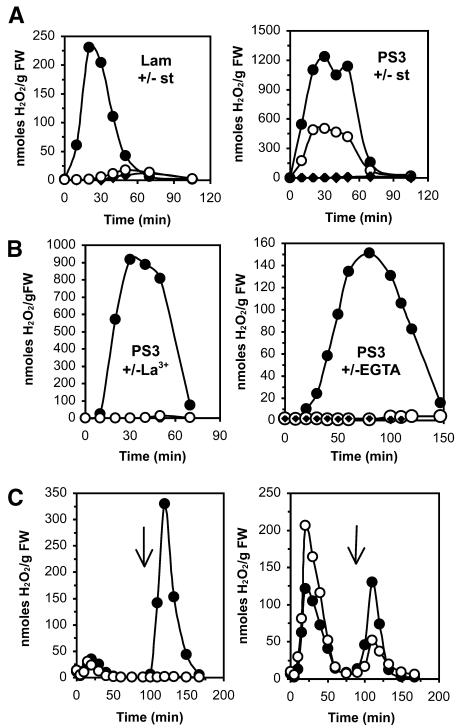
Cultured plant cells have been widely used as model systems to rapidly investigate the perception of molecules. To answer the question whether sulfate substitution of laminarin modifies the perception of this glucan by plant cells, we analyzed the extracellular oxidative burst induced in tobacco cell suspensions upon treatment with laminarin or PS3. Because laminarin showed maximal activity at 200 μg/mL (Klarzynski et al., 2000), we compared the effects of laminarin and PS3 at this concentration. Compared with laminarin, PS3 induced a fourfold to sixfold higher oxidative burst (Figure 1A, closed circles). Pretreatment with staurosporine, a kinase inhibitor, inhibited the laminarin and PS3-induced oxidative bursts (Figure 1A, open circles). Of note, the PS3-induced oxidative burst was inhibited by only 50% as observed in four independent experiments using different concentrations of PS3 (20, 40, 100, and 200 μg/mL). This partial inhibition was not because of the application of not enough staurosporine. Indeed, the same concentrations of staurosporine were able to totally suppress an elicitin-induced oxidative burst of similar extent (data not shown).
Figure 1.
Oxidative Burst Induced by Laminarin and PS3 in Tobacco Cell Suspensions.
Laminarin (Lam) and PS3 were applied to 1 g of tobacco cell suspensions. The oxidative burst was measured using luminol. Values are expressed as H2O2 equivalents calculated from a standard H2O2 curve. Each experiment was repeated up to four times. The graphs illustrate the results from a representative experiment. FW, fresh weight.
(A) Effect of staurosporine on the Lam- and PS3-induced oxidative burst. Staurosporine (10 μM) was applied 10 min before addition of Lam (200 μg/mL) or PS3 (200 μg/mL). Closed circles, elicitor alone; open circles, elicitor + staurosporine (st); closed diamonds, untreated control.
(B) Effect of La3+ and EGTA on the PS3-induced oxidative burst. LaCl3 (2 mM) was applied 10 min before addition of PS3 (200 μg/mL). EGTA (2 mM) was applied 10 min before addition of PS3 (50 μg/mL). Closed circles, PS3 alone; open circles, PS3 + La3+ or EGTA; closed diamonds, untreated control.
(C) Oxidative burst induced by successive addition of Lam or PS3. Lam (200 μg/mL) or PS3 (200 μg/mL) were applied at time 0, and the oxidative burst was measured. When the oxidative burst returned to basal values, the same or the second elicitor was added (arrows). The left graph shows the treatments Lam/Lam (open circles) and Lam/PS3 (closed circles). The right graph shows the treatments PS3/PS3 (open circles) and PS3/Lam (closed circles).
We further investigated whether the PS3-induced oxidative burst is Ca2+ dependent. Pretreatment of the cells with LaCl3 (2 mM), a calcium surrogate, or with EGTA (2 mM), a calcium chelator, prevented the oxidative burst (Figure 1B). The observation that the PS3-induced oxidative burst was partially kinase independent suggested a different mode of perception of laminarin and PS3 by the tobacco cells. To confirm this hypothesis, we analyzed whether successive addition of laminarin and PS3 would result in a refractory state. The establishment of a refractory state (i.e., the failure of cells to respond to a second dose of the same elicitor) is a convenient method to differentiate between different chemical stimuli (Felix et al., 1993; Rouet-Mayer et al., 1997; Binet et al., 1998). Cells pretreated with laminarin were refractory to a second application of laminarin, which confirms previous results measuring medium alkalinization (Klarzynski et al., 2000). However, they were not refractory to an application of PS3 (Figure 1C). After initial treatment with PS3, a second application of PS3 induced a very low oxidative burst, whereas the cells remained fully responsive to a second laminarin exposure (Figure 1C). This points to a different mode of perception of PS3 and laminarin by tobacco cells.
Symptoms Induced in Tobacco Plants by PS3
Next, we analyzed the eliciting activities of PS3 and laminarin on whole plants. Tobacco leaves infiltrated with laminarin (200 μg/mL) did not develop any symptoms. In contrast with this, tissues treated with PS3 (200 μg/mL) became shiny after a few hours followed by the development of a slight chlorosis after 2 d. These symptoms were strictly limited to the infiltrated tissue areas and never evolved to visible necrosis. Trypan blue and Evans blue staining revealed no dead cells in PS3-treated tissues. Furthermore, microscopic observations showed no cell alterations in tissues treated with either laminarin or PS3.
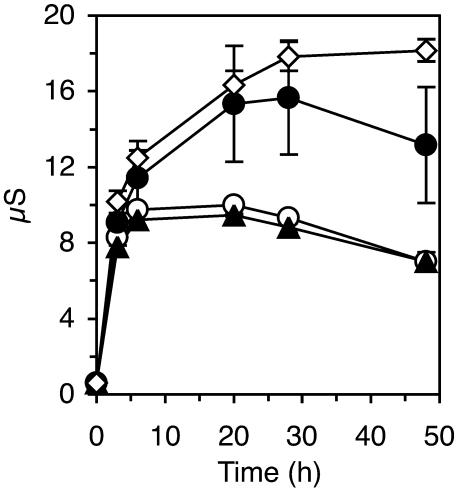
We also measured electrolyte leakage (Figure 2) to check the possibility of membrane damage that did not lead to cell death. Treatment with laminarin or water resulted in identical kinetics of electrolyte leakage. PS3 induced a significantly higher release of electrolytes. Compared with a treatment with the elicitin β-megaspermin, which induces a typical HR (Baillieul et al., 2003), electrolyte leakage induced by PS3 was comparable at early stages but still significantly lower at the end of the experiment. Thus, PS3, but not laminarin, induced electrolyte leakage.
Figure 2.
Monitoring of Electrolyte Leakage after Treatment of Tobacco Leaf Tissue with PS3 or Laminarin.
Leaves of tobacco plants were infiltrated with 200 μg/mL of PS3 (closed circles), laminarin (open circles), 50 nM β-megaspermin (open diamonds), water (closed triangles), or left untreated. Electrolyte leakage was measured from leaf discs punched out 3 h after treatment and placed on water. Results were expressed as the means ± sd calculated from three independent experiments. Because the untreated tissues gave the same values of conductivity as the water control, only the latter curve is shown.
Induction of Defense Responses in Tobacco and Arabidopsis Plants by PS3
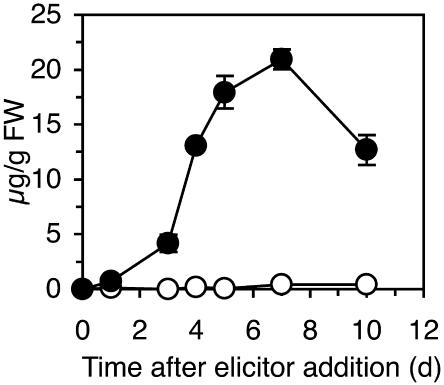
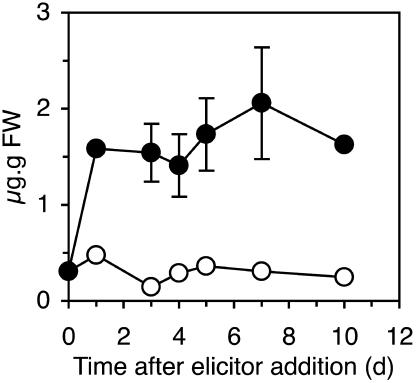
Two days after treatment, PS3-treated tobacco leaves revealed under UV light a blue fluorescence, which was limited to the infiltrated tissues. No fluorescence was detected in laminarin-treated plants. Tissues surrounding a HR lesion caused by elicitin infiltration or TMV infection showed a similar fluorescence as a result of scopoletin accumulation (Costet et al., 2002). As shown in Figure 3, scopoletin accumulated only in PS3-treated and not in laminarin-treated tissues, reaching 20 μg/g fresh weight 7 d after PS3 treatment.
Figure 3.
Scopoletin Accumulation in Tobacco Leaves Infiltrated with PS3 or Laminarin.
Leaves of tobacco plants were infiltrated with 200 μg/mL of PS3 (closed circles) or laminarin (open circles). Scopoletin levels were quantified by HPLC. Results are representative of a typical experiment and are expressed as the means ± sd calculated from two HPLC runs.
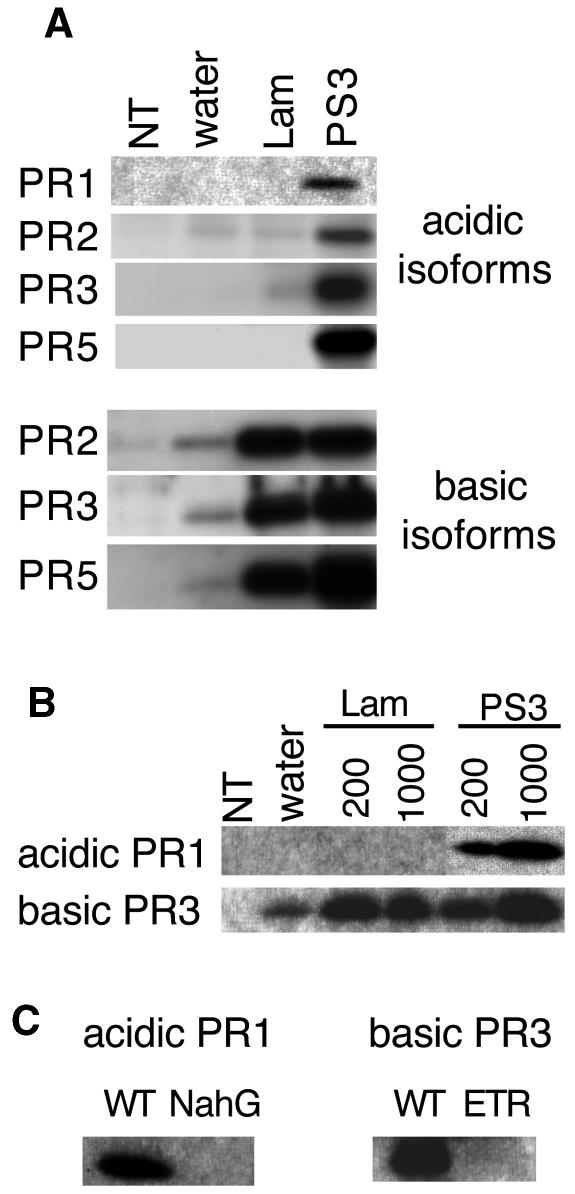
By protein gel blotting, we monitored the expression of acidic and basic PR1, PR2, PR3, and PR5 proteins in tobacco tissues after treatment with laminarin or PS3 (Figure 4). The basic isoforms of PR2, PR3, and PR5 accumulated in tissues treated with laminarin or PS3. By contrast, expression of the acidic isoforms of PR1, PR2, PR3, and PR5 occurred only in PS3-infiltrated tissues. Among the acidic isoforms, PR1 represents a typical marker of defense response in tobacco. Measuring acidic PR1 expression in seven independent experiments performed over a period of 36 months, we never observed PR1 protein accumulation in tissues treated with laminarin, but always in PS3-treated tissues. Increasing the concentration of laminarin to 1000 μg/mL also did not result in acidic PR1 accumulation (Figure 4B).
Figure 4.
Expression of PR Proteins in Tobacco upon Treatment with PS3 or Laminarin.
Leaves of tobacco plants were infiltrated with PS3 or laminarin (Lam), with water, or left untreated (NT). Proteins were extracted 3 d after treatments and submitted to protein gel blotting with specific antibodies.
(A) Analysis of acidic PR1, PR2, PR3, and PR5 proteins and basic PR2, PR3, and PR5 proteins in plants treated with 200 μg/mL of test compounds.
(B) Analysis of acidic PR1 and of basic PR3 in plants treated with 200 μg/mL and 1000 μg/mL of test compounds.
(C) Analysis of acidic PR1 in NahG tobacco plants and wild-type tobacco and of basic PR3 in ETR tobacco plants and wild-type tobacco treated with 200 μg/mL of PS3.
In tobacco, salicylic acid (SA) induces the expression of the acidic isoforms of PR1, PR2, PR3, and PR5 proteins (Ward et al., 1991; Yalpani et al., 1991; Cordelier et al., 2003), and ethylene induces the expression of the basic counterparts (Brederode et al., 1991; Knoester et al., 1998; Ohtsubo et al., 1999; Cordelier et al., 2003). Thus, our results suggest that laminarin induces an ethylene-dependent signaling pathway, whereas PS3 triggers both an ethylene-dependent and an SA-dependent signaling pathway. To test this hypothesis, we analyzed the expression of the acidic and basic PR proteins in NahG and ETR tobacco plants as well as SA levels. NahG plants catabolize SA into the inactive catechol (Delaney et al., 1994), whereas ETR plants are ethylene insensitive (Knoester et al., 1998). As shown in Figure 4C, expression of the acidic PR1 and of the basic PR3 proteins was suppressed in PS3-treated NahG and ETR plants, respectively. Expression of the basic PR3 protein was also suppressed in ETR tissues treated with laminarin (data not shown). SA accumulated in wild-type tobacco tissues treated with PS3, whereas no significant SA increase occurred in tissues treated with laminarin (Figure 5). These results support the hypothesis of a differential activation of signaling pathways by laminarin and PS3.
Figure 5.
SA Quantification in Tobacco Leaves Infiltrated with PS3 or Laminarin.
Leaves of tobacco plants were infiltrated with 200 μg/mL of PS3 (closed circles) or laminarin (open circles). SA was quantified by HPLC. Results are representative of a typical experiment and are expressed as the means ± sd calculated from two HPLC runs.
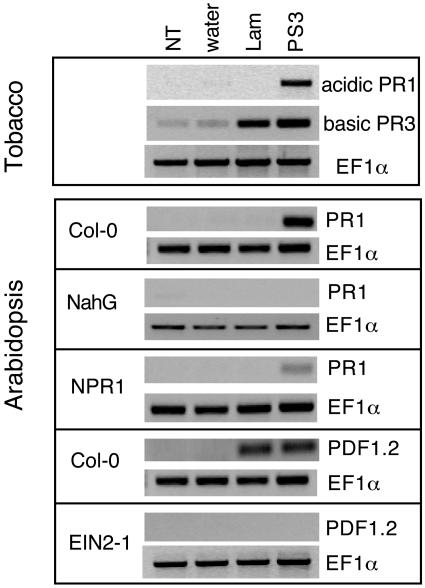
Because such a differential activation profile of the two compounds may be specific to tobacco, we additionally analyzed the induction of SA- and ethylene-dependent responses in Arabidopsis. PR1 and PDF1.2 are well-recognized markers for the SA and ethylene signaling pathways, respectively, in Arabidopsis (Penninckx et al., 1996, 1998). Expression of both genes was analyzed by semiquantitative RT-PCR (Figure 6). As a control of the method and to confirm the results of protein analysis, we first analyzed the expression of acidic PR1 and of basic PR3 genes in tobacco leaves. The results were similar to the protein gel blotting analysis. The acidic PR1 PCR product was detected after treatment with PS3, whereas the basic PR3 PCR product was detected after treatment with PS3 or laminarin. In Arabidopsis, PR gene expression corresponded to that observed in tobacco. In wild-type Columbia-0 (Col-0) plants, the PR1 gene was induced after PS3 treatment but not after laminarin treatment, whereas PDF1.2 was induced after both treatments. Furthermore, PR1 expression was suppressed in Arabidopsis NahG plants and strongly reduced in the nonexpressor of PR genes1 (npr1) mutant, whereas PDF1.2 expression was suppressed in the ein2 mutant. Thus, the differences between laminarin and PS3 in the induction of signaling pathways in Arabidopsis were similar to those found in tobacco.
Figure 6.
Expression of PR Transcripts in Tobacco and Arabidospis Tissues Treated with PS3 or Laminarin.
Tobacco plants were treated with 200 μg/mL of PS3 or laminarin (Lam), with water, or left untreated (NT). Tissues were harvested 24 h after treatments, and total RNA was isolated and used for semiquantitative RT-PCR analysis. Equal loading was monitored by amplification of EF1α PCR product. Expression of acidic PR1 and basic PR3 transcripts was analyzed in tobacco plants. Expression of PR1 and PDF1.2 transcripts was analyzed in Arabidopsis Col-0 (wild type), npr1 and ein2 mutants, and nahg transgenic plants.
Structure-Activity Analysis
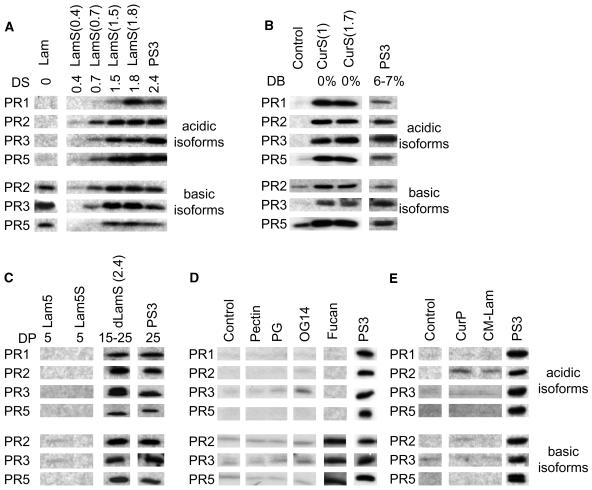
To establish structure-activity relationships, we analyzed the PR protein inducing activity of several oligosaccharides and polysaccharides in tobacco (Table 1). The activities of laminarin sulfates with DS of 0.4, 0.7, 1.5, 1.8, or 2.4 clearly demonstrated the dependency of the activities on the DS (Figure 7A). As shown by LamS(0.4) and LamS(0.7), a DS higher than 0.4 is required to trigger acidic and basic PR protein expression. The activity increases with increasing DS, but a DS of 1.5 seems to be sufficient to achieve maximal activity because LamS(1.5) and LamS(1.8) were as active as PS3 with a DS of 2.4. At first view, the lack of basic PR protein accumulation in tissues treated with LamS(0.4) observed in three independent experiments was surprising because laminarin (DS = 0) triggered the expression of these PR proteins. The same result, however, was observed with laminarin or curdlan substituted with other functional groups (see below).
Table 1.
Structure and Characteristics of the Oligosaccharides and Polysaccharides Used in This Study
| Substituted (%)
|
|||||||
|---|---|---|---|---|---|---|---|
| Oligo- and Poly-β-1,3 Glucan | DP | MM (kD) | DB (%) | DS (Function) | C6-OH | C2-OH | C4-OH |
| Laminarin (Lam) | 25 | 5.8 | 6–7 | 0.0 | 0 | 0 | 0 |
| PS3 | 25 | 18.0 | 6–7 | 2.4 (-OSO3−) | 100 | 70 | 70 |
| dLamS(2.4)a | 15–25 | <18.0 | n.d. | 2.4 (-OSO3−) | 100 | 70 | 70 |
| Laminarin sulfate, LamS(0.4) | 25 | n.d. | 6–7 | 0.4 (-OSO3−) | 40 | 0 | 0 |
| Laminarin sulfate, LamS(0.7) | 25 | 15.4 | 6–7 | 0.7 (-OSO3−) | 60 | 5 | 5 |
| Laminarin sulfate, LamS(1.5) | 25 | 18.0 | 6–7 | 1.5 (-OSO3−) | 94 | 28 | 28 |
| Laminarin sulfate, LamS(1.8) | 25 | 18.0 | 6–7 | 1.8 (-OSO3−) | 97 | 42 | 41 |
| Laminaripentaose, Lam5b | 5 | n.d. | 0 | 0.0 | 0 | 0 | 0 |
| Laminaripentaose sulfate, Lam5S | 5 | n.d. | 0 | 2.6 (-OSO3−) | 100 | 80 | 80 |
| CM-Lam | 25 | 14.0 | 6–7 | 0.8 (CH3COO−) | 100 | 0 | 0 |
| Curdlan sulfate, CurS(1.7) | n.d. | 32.0 | 0 | 1.7 (-OSO3−) | 100 | 35 | 35 |
| Curdlan sulfate, CurS(1.0) | n.d. | 200.0 | 0 | 1.0 (-OSO3−) | 100 | 0 | 0 |
| CurP | n.d. | 170.0 | 0 | 2.3 (-OPO32−) | n.d. | n.d. | n.d. |
| Other Oligosaccharides and Polysaccharides | DP | Structure | |||||
| Pectinb | n.d. | Poly-α-1,4-galacturonic acid methyl ester | |||||
| Polygalacturonateb | n.d. | Poly-α-1,4-galacturonic acid | |||||
| Oligogalacturonatec | 14 | Oligo-α-1,4-galacturonic acid | |||||
| Fucan | 10 | Fivefold repetition of the disaccharide 1,2-α-l-Fucp-2(OSO3)-1,4-α-l-Fucp-2,3(diOSO3) | |||||
PS3 and Lam5S were synthesized for this study. LamS(0.4), LamS(0.7), LamS(1.5), LamS(1.8), dLamS(2.4), CM-Lam, CurS, CurP, and fucan were obtained and described previously (Alban and Franz, 1994, 2000, 2001; Stibich, 2000; Alban et al., 2001; Klarzynski et al., 2003). DP, mean degree of polymerization; MM, molecular mass determined by gel permeation chromatography; DB, degree of C6 branching; n.d., no data.
Obtained by sulfation of laminarin under degrading conditions.
Purchased from Sigma (St. Quentin Fallavier, France).
Provided by the Complex Carbohydrate Research Center (University of Georgia).
Figure 7.
Structure-Activity Analysis.
The compounds used for the treatment of the tobacco plants are described in Table 1. Leaves were infiltrated with 200 μg/mL of each compound, with water, or left untreated. Proteins were extracted 3 d after treatments and submitted to protein gel blotting with specific antibodies to analyze acidic PR1, PR2, PR3, and PR5 and basic PR2, PR3, and PR5.
(A) Comparing laminarin sulfate of different DS.
(B) Comparing β-1,6 branched β-1,3 glucan sulfate (PS3) and linear β-1,3 glucan sulfate (curdlan sulfate).
(C) Comparing sulfated β-1,3 glucans of different DPs.
(D) Comparing PS3 with different oligogalacturonates and polygalacturonates and with fucan.
(E) Comparing β-1,3 glucans carrying different substitution groups.
Next, we analyzed the PR-inducing activity of linear β-1,3 glucan sulfate molecules to evaluate the importance of β-1,6 side chains. Curdlan is a linear β-1,3 glucan isolated from the soil bacterium Alcaligenes faecalis. Curdlan was sulfated (Alban and Franz, 2001) to obtain CurS(1.7) with a DS of 1.7 and CurS(1.0) with a DS of 1.0, respectively. CurS(1.7), CurS(1.0), and PS3 induced the same symptoms on tobacco leaves (i.e., a slight chlorosis and, under UV light, a blue fluorescence limited to the infiltrated tissues). The three compounds exhibited the same PR protein–inducing activity (Figure 7B), indicating that the β-1,6 glucose side chains are not important for the biological activity.
The molecular weight of CurS(1.7) and of CurS(1.0) is about 2 and 10 times, respectively, higher than that of PS3 (Table 1). Although the lower DS of the two compounds allows no final conclusion yet, prolongation of the carbohydrate chain obviously does not modify the biological activity. To evaluate the influence of shortened chain length, we tested dLamS(2.4) with a DS of 2.4 and a DP of 15 to 25. PS3 and dLamS(2.4) displayed the same PR protein–inducing activity (Figure 7C), so that the activity seems to be independent of the chain length ranging from a DP of 15 to 25. In contrast with this, laminaripentaose sulfate (Lam5S) with a DS of 2.6 remained inactive (Figure 7C), indicating the requirement of a certain minimum chain length for any biological activity. This was confirmed by Lam5, the unsulfated control, which was also completely inactive, whereas laminarin triggered basic PR protein expression. Thus, above a certain minimum chain length, β-1,3 glucans trigger the basic PR protein expression in tobacco plants. For the additional acidic PR protein expression by such molecules, less than one sulfate per glucose unit appears to be sufficient, and for maximal activity 1 to 1.5 sulfate groups per glucose unit are apparently required.
To answer the question of whether sulfate residues can be replaced by other types of negatively charged groups, we examined carboxylmethylated laminarin (CM-Lam) and the three different galacturonans, pectin, polygalacturonic acid, and oligogalacturonic acid with a DP of 14. None of these molecules caused significant acidic and/or basic PR protein expression (Figures 7D and 7E). Because carboxylic acids are weaker acids than sulfuric acids and the degree of substitution of these compounds is lower than the DS of PS3, we also tested curdlan phosphate (CurP) with a degree of substitution of 2.3, which was similar to the DS of PS3. Because CurP was inactive as well (Figure 7E), we concluded that sulfate groups are essential for the induction of the acidic PR protein expression. However, in view of the induction of basic, but not acidic, PR protein expression by fucan (Figure 7D), sulfate groups do not seem to be sufficient for this activity. The tested fucan is a decasaccharide consisting of fucose units with a DS of 1.5. Its basic structure considerably differs from that of β-1,3 glucans. The activity profile found for fucan (induction of basic, but not acidic, PR protein expression) suggests that both a β-1,3 glucan backbone and sulfate residues are necessary to trigger acidic PR protein expression.
Induction of Resistance against TMV Infection in Tobacco by PS3
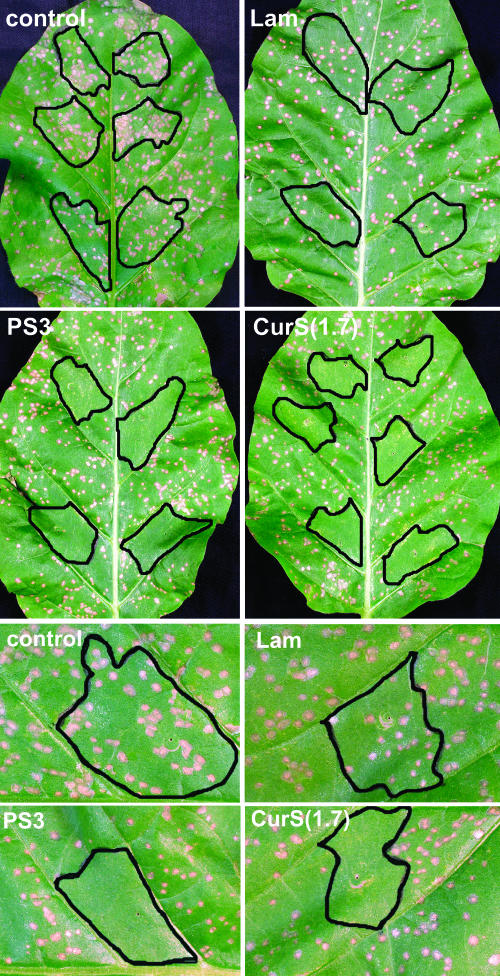
Because PS3 induced a larger spectrum of defense responses compared with laminarin, we tested whether PS3 would induce increased resistance against pathogen infection. Tobacco leaves were infiltrated at four to six spots per leaf with laminarin, PS3, CurS(1.7), or water. Three days later, the treated leaves were challenge inoculated with TMV. Figure 8 shows the symptoms after a period of another 7 d. PS3 and CurS(1.7) induced total immunity against TMV infection (i.e., no lesions developed in the infiltrated tissues). Total immunity was also observed when TMV was inoculated 8 d after PS3 treatment. The absence of lesions in PS3-treated tissues was observed in four independent experiments representing 58 infiltrated spots. In contrast with this, TMV lesions developed in tissues infiltrated with laminarin. The number of the lesions was lower, but lesions were not significantly smaller than those occurring in water-treated tissues. For a representative experiment, we measured 154 lesions in laminarin-infiltrated tissues and 446 lesions in water-infiltrated tissues. The mean lesion diameters were 2.43 ± 0.53 mm and 2.45 ± 0.50 mm, respectively.
Figure 8.
Induction of Resistance against TMV in Tobacco Treated with PS3, Curdlan Sulfate, or Laminarin.
Leaves of tobacco plants were infiltrated with PS3, curdlan sulfate [CurS(1.7)], or laminarin (Lam) at 200 μg/mL for each compound. Three days later, the treated leaves were challenge inoculated with TMV, and the symptoms were photographed 7 d later. Controls were leaves infiltrated with water.
DISCUSSION
Naturally occurring carbohydrates can be sulfated, and these sulfate groups often play a key role in major physiological processes in plants and animals. Here, we show that chemical sulfation of a β-1,3 glucan leads to a molecule with new and improved biological activities in tobacco and Arabidopsis.
The semisynthetic β-1,3 glucan sulfate PS3 triggered defense and resistance responses, which were not induced by the native unsulfated molecule. In tobacco, PS3, but not laminarin, caused accumulation of the phytoalexin scopoletin and of the acidic PR1, PR2, PR3, and PR5 proteins as well as induction of total immunity to TMV infection. The lack of acidic PR1, PR2, PR3, and PR5 protein induction by laminarin was at first unexpected. Indeed, Klarzynski et al. (2000) reported an accumulation of PR proteins of family 1, 2, 3, and 5 in tobacco leaves infiltrated with the same laminarin fraction. However, the acidic and basic isoforms were not discriminated. As for other tobacco/elicitor (Cordelier et al., 2003) and tobacco/pathogen (Friedrich et al., 1995) systems, induction of the acidic PR proteins by PS3 occurred via the SA signaling pathway and that of the basic counterparts by PS3 or laminarin via the ethylene signaling pathway. Upon PS3 treatment, NahG and ETR plants failed to produce acidic PR1 and basic PR3, respectively, and SA accumulated in response to PS3 but not to laminarin. In tobacco, SA and scopoletin are derived from l-Phe via the phenylpropanoid pathway (Murray et al., 1982; Dorey et al., 1997; Schalk et al., 1998; Chong et al., 2001). Accumulation of these two metabolites in tobacco upon PS3 but not laminarin treatment further highlights the specific activity of PS3. To our knowledge, this is the first report showing that by chemical sulfation a polysaccharidic elicitor becomes an inducer of the SA signaling pathway in tobacco.
The wider spectrum of defense responses induced by PS3 correlated with an increased resistance of tobacco to TMV infection. A branched and a linear β-1,3 glucan sulfate, PS3 and CurS(1.7), induced total immunity (i.e., no lesions developed), whereas the unsulfated β-1,3 glucan laminarin caused only partial immunity. Apparently, both the unsulfated and the sulfated β-1,3 glucans were acting on virus infection initiation rather than on virus development and/or spread because the number, but not the size, of the lesions was decreased. Increased resistance to TMV was described in tobacco plants with high SA (Verberne et al., 2000) or scopoletin levels (Ahl Goy et al., 1993). However, resistance was assessed by the size of the lesions and not by the number. So far, the mechanism of virus immunity induced by β-1,3 glucan sulfate is unknown.
Arabidopsis and tobacco responded similarly to laminarin and PS3 application. The use of Arabidopsis wild-type, transgenic, and mutant plants showed that PS3 induced an SA-dependent expression of PR1 as well as an ethylene-dependent expression of PDF1.2, whereas laminarin triggered only PDF1.2. Furthermore, PS3-induced PR1 expression was additionally NPR1 dependent. Other polysaccharides were shown to activate defense responses in Arabidopsis, such as chitin (Salinas-Mondragon et al., 1999; Ramonell et al., 2002; Zhang et al., 2002), galacturonides (Norman et al., 1999), and a Phytophthora cell wall preparation (Trezzini et al., 1993), which is known to contain highly branched β-1,3/β-1,6 glucans. Chitin signaling involved both SA- and jasmonate-dependent pathways (Ramonell et al., 2002; Zhang et al., 2002). Galacturonides induced the jasmonate signaling pathway in Arabidopsis (Norman et al., 1999) and tomato (Lycopersicon esculentum) (Doares et al., 1995). Our results show that β-1,3 glucans with rare β-1,6 glucosidic linkages activated the ethylene, but not the SA, signaling pathway in Arabidopsis.
One possible explanation for the differential activation of signaling pathways in Arabidopsis by sulfated and unsulfated glucans could be deduced from the recent analysis of the pmr4 Arabidopsis mutant. PMR4 is considered to be the main callose synthase responsible for callose deposition upon biotic and abiotic stresses. This pmr4 mutant is resistant to powdery mildew infection and is unable to produce a pathogen-induced callose response (Nishimura et al., 2003). The susceptibility to powdery mildew was restored in the pmr4 nahg double mutant, suggesting that callose, a linear β-1,3 glucan, or callose synthase negatively regulates the SA pathway. Because PR1 activation in Arabidopsis is regulated by the SA signaling pathway, the lack of PR1 expression in laminarin-treated Arabidopsis plants could be explained by a negative feedback of the glucan. Consequently, sulfation of the glucan would counteract the negative feedback effect. However, this remains to be demonstrated.
Structure-activity relationship analysis highlighted some features of the β-1,3 glucan sulfate relevant for its unique activation profile. Independent of the presence or absence of sulfate groups, only β-1,3 glucans above a minimum chain length (DP > 5) exhibit PR protein–inducing activity. In addition to the basic PR protein induction by the unsulfated laminarin, β-1,3 glucan sulfates with a DS above 0.4 induced acidic PR protein expression. This activity is DS dependent but did not further improve at DS ≥ 1.5. One possible explanation for the lack of Lam0.4 activity could be that the addition of a sulfate residue to about each second glucose in the chain is sufficient to change the three-dimensional structure so that the typical structure of laminarin is broken and, consequently, the affinity to the assumed receptor. But the charge density is too low to enable efficient binding to those receptor molecules that bind the higher charged laminarin sulfates. Branching of the glucan backbone did not appear to be critical for the biological activity because the linear curdlan sulfate molecules were as active as PS3 in inducing PR protein expression and resistance against TMV. A nonspecific effect attributable to the negative charges along the polysaccharide molecule could be excluded because CurP and CM-Lam as well as other uronic acid–containing polymers were inactive. This result underlines the important function of the sulfate residues for the biological activity. However, the presence of sulfate residues in an oligosaccharide or polysaccharide is not always associated with the same activity as that of β-1,3 glucan sulfates. Indeed, the naturally sulfated fucan (DS of 1.5) strongly induced expression of basic PR proteins but not of the acidic isoforms. This observation highlights the importance of the glucan backbone. To further clarify the role of the β-1,3 glucan backbone, experiments with other sulfated oligosaccharides and polysaccharides are necessary. It should be noted that β-1,3 glucan molecules carrying functional groups [carboxymethyl, phosphate, or even rarely occurring sulfate groups, such as LamS(0.4)] failed to induce basic PR protein expression. Hence, modification of the β-1,3 glucan backbone with negatively charged groups other than sulfate residues (at low DS for the latter) seems to suppress biological activity. This suggests that the biological activity of the β-1,3 glucan sulfates does not result from a higher eliciting efficiency but rather from a different way of sensing β-1,3 glucan sulfates by the tobacco cells.
Analysis of the oxidative burst in tobacco cell suspensions provides two arguments for a different mode of perception of PS3 and laminarin by tobacco cells. The oxidative burst as well as Ca2+ influx, phosphorylation events, and extracellular alkalinization have been described as events occurring after elicitor treatments and preceding defense response activation (Dixon and Lamb, 1990). Staurosporine, a kinase inhibitor, blocked the oxidative burst induced by either PS3 or laminarin, indicating the recruitment of a signaling pathway involving protein phosphorylation for both elicitors. However, the 50% suppression of the PS3-induced oxidative burst points to the involvement of a kinase-independent mechanism. Both the kinase-dependent and -independent mechanisms were shown to be Ca2+ dependent because the oxidative burst was blocked by either a Ca2+ surrogate or a Ca2+ chelator. Therefore, PS3 is suggested to activate another signaling pathway in addition to that triggered by the unsulfated laminarin. A possible kinase-independent mechanism of action would be the direct interaction of PS3 with plasma membranes. Yeast elicitor, a mixture of glucan, mannan, and chitin, was shown to exhibit pore-forming properties resulting in ion fluxes (Klusener and Weiler, 1999). This pore-forming activity would apply to PS3 but not to laminarin, and it could explain the electrolyte leakage caused by PS3. However, no pore formation by PS3 was observed in human cells, as no haemolysis was observed (S. Alban, unpublished data).
A second argument is the fact that cells pretreated with laminarin or PS3 were not refractory to a second addition of PS3 or laminarin, respectively. The establishment of a refractory state has been used as a convenient method to differentiate between different qualities of chemical stimuli (Felix et al., 1993; Rouet-Mayer et al., 1997; Binet et al., 1998). Klarzynski et al. (2000) have shown that tobacco cells first treated with laminarin failed to produce an alkalinization response after a second application of laminarin. This refractory state was confirmed here by measuring the oxidative burst. PS3-treated cells, however, responded only partially to a second PS3. The biological significance of this observation remains to be elucidated. It could be compared with the kinase-independent mechanism discussed above.
Our results further highlight the observation that different plants have developed the ability to react to structurally different, but related, β-glucans: soybean and rice recognize branched β-glucans, and tobacco reacts to linear β-1,3 glucans. The well-known β-1,3 β-1,6 heptaglucoside (β-1,6 backbone with β-1,3 side chains) elicits phytoalexin accumulation in soybean (Sharp et al., 1984) and other plants in Fabacae (Cosio et al., 1996), but it is inactive in tobacco (Klarzynski et al., 2000). A β-1,6 β-1,3 glucan (β-1,3 backbone with a β-1,6 side chain) induces phytoalexin production in rice but not in soybean (Yamaguchi et al., 2000). In rice, linear β-1,3 glucans are inactive (Yamaguchi et al., 2000), and in soybean, a linear β-1,6 glucan is inactive as well (Cheong et al., 1991), emphasizing the importance of the side chains for biological activity in these plants. In soybean, laminaribiose (DP of 2) shows very poor activity at a very high concentration (>7 mM, the β-1,3 β-1,6 heptaglucoside shows activity at 8 nM), whereas linear β-1,3 glucans of higher DP were not tested (Cheong et al., 1991). Plasma membrane–located proteins binding with high affinity to the β-1,3 β-1,6 heptaglucoside have been purified (Cheong et al., 1993; Mithofer et al., 1996, 1999) and cloned from soybean (Umemoto et al., 1997) and bean (Mithofer et al., 2000). Membranes prepared from Arabidopsis or tobacco plants do not bind this β-1,3-1,6 heptaglucoside (Côté et al., 2000).
In conclusion, our results show that chemical sulfation of naturally occurring polysaccharides can dramatically change their biological activities in plants to increase their defense and resistance eliciting activities. This opens new routes for the development of new compounds suitable for crop protection. Interestingly, in animal and human cell test systems, PS3 exhibits several biological effects, such as strong anti-inflammatory and antimetastatic activities (Alban and Franz, 2001; Becker et al., 2003). Thus, PS3 and more generally sulfated β-1,3 glucans seem to have a distinct structure that enables interactions with molecules involved in both plant and animal/human biological processes.
METHODS
Synthesis and Characterization of Sulfated β-1,3 Glucans
Synthesis
Laminarin was extracted and purified from the marine brown algae Laminaria digitata as described (Klarzynski et al., 2000). Laminaripentaose (Lam5), a purified pentasaccharide consisting of five β-1,3 linked glucose units, was purchased from Sigma. Sulfation of laminarin and Lam5 resulted in a laminarin sulfate fraction called PS3 and Lam5S. To produce PS3, 1 g of laminarin was dissolved in a mixture of 20 mL of DMF and 2.8 mL of pyridine, and the mixture was heated to 60°C. Sulfation was performed by continuous addition of 5.9 g of SO3-pyridine complex for 2 h at 60°C. Stirring was continued for another 2 h. After cooling to room temperature, the supernatant was removed by pipetting from the slippery brown precipitate formed during the reaction. The latter was dissolved in a 2.5 M NaOH solution, and the resulting solution was then poured onto 99% EtOH to obtain a final concentration equal to 90% EtOH. After 12 h stirring at 4°C, the white precipitate was isolated by centrifugation and dissolved in distilled water. The solution of PS3 was dialyzed (molecular weight cut off 1000 D) for 7 d at room temperature against flowing deionized water. The solution was then frozen at −70°C and freeze-dried. A similar procedure was used to obtain Lam5S. Lam5 (100 mg) was stirred for 3 h at 60°C with 653 mg of SO3-pyridine complex in 5 mL of dry DMF containing 350 μL of pyridine. The supernatant was then removed, and the precipitate was dissolved in 2.5 M NaOH solution. Water was then coevaporated with 99% ethanol, and the resulting product was dialyzed 4 d against flowing deionized water with a 500 D cut-off membrane. The curdlan sulfate (CurS) was obtained by activation and sulfation of the bacterial β-1,3 glucan curdlan (Wako Pure Chemical Industries, Osaka, Japan) as described previously (Alban and Franz, 2000).
Sulfate Content Determination
The principle is based on the hydrolysis of the glucan sulfate by trifluoroacetic acid and the quantification of the resulting ions by ion chromatography with conductivity detection as described (Alban et al., 2001).
Molecular Weight Determination
The molecular weight was determined by gel permeation chromatography (Alban et al., 2001) on a Sephadex S75 column using a fast protein liquid chromatography system (Amersham Biosciences Europe, Freiburg, Germany) and a refractive index detector (Waters, Eschborn, Germany). For calibration, neutral pullulans, stachyose, and glucose were used. The samples were dissolved in water to give a final concentration of 1 mg/mL. The eluant was 0.1 M sodium chloride in water.
HPAEC-PAD
HPAEC-PAD was performed using a Dionex chromatograph in the configuration DX500 (Dionex, Sunnyvale, CA), a 50 μL injection loop, a CarboPac PA100 column (4 × 250 mm), and an electrochemical detector with a gold working electrode. Chromatography was performed as described in Lepagnol-Descamps et al. (1998).
Methylation Analysis
The sulfation pattern was evaluated by a modified methylation procedure (Alban and Franz, 1994). The resulting methylated, ethylated, alditol acetates were analyzed by combined gas–liquid chromatography mass spectrometry, providing qualitative and quantitative information about the carbohydrate structure and the distribution of the sulfate groups.
Plant Material and Treatments
Tobacco cell suspensions (Nicotiana tabacum cv Xanthi) were grown, maintained, and used for experiments as described (Binet et al., 1998). Tobacco plants, N. tabacum cv Samsun H (seeds obtained from Altadis, Institut du Tabac, Bergerac, France), N. tabacum cv Xanthi nc, N. tabacum cv Samsun NN transgenic for the ETR gene and N. tabacum cv Xanthi nc transgenic for the NAHG bacterial gene, were grown in a greenhouse under controlled conditions. Two- to three-month-old plants were placed 2 to 3 d before treatments in a growth chamber at 22 ± 1°C with a photoperiod of 16 h. Arabidopsis thaliana plants were of ecotype Col-0. Seeds of ethylene response mutant ein2-1 (Guzman and Ecker, 1990) and nonexpresser of PR genes NPR1 (Cao et al., 1994) were obtained from the Nottingham Arabidopsis Stock Centre Biological Resource Center (accession numbers CS3071 and CS3726). Seeds of nahg transgenic Arabidopsis Col-0 plants were a gift from R. Dietrich (Syngenta, Research Triangle Park, NC). Plants were grown with a 12-h light period at 20°C, and 6-week-old plants were used for experiments. Plant treatments were performed by infiltration of aqueous solutions of the elicitors with a syringe into the mesophyll of fully developed leaves. The infiltrated tissue was immediately delineated with a felt-tip marker when necessary. β-Megaspermin was obtained as described (Baillieul et al., 2003). For virus infection, tobacco plants were first infiltrated with water, laminarin, PS3, or curdlan sulfate [CurS(1.7)] at four to six spots per leaf and were challenge inoculated with TMV 3 or 8 d later by rubbing the upper leaf surface with a suspension of purified virus at a 0.1 μg·mL−1 concentration in the presence of abrasive celite. The number and the size of TMV lesions were then determined 6 d after virus inoculation using an ocular micrometer.
Defense Response Analyses
The oxidative burst was measured as described (Binet et al., 1998). Staurosporine, LaCl3, and EGTA were purchased from Sigma. For electrolyte leakage measurements, tobacco leaf discs were punched out using a 5-mm-diameter cork borer 3 h after treatments. Fifteen leaf discs from each treated area were placed in 20 mL of water to lower the background signal derived from the wounded cells. Disks were then placed in Petri dishes containing 10 mL of fresh water under continuous light at 24°C. Measurements were taken using a conductivity meter (Conductometer E518; Metrohm, Herisau, Switzerland). Results were expressed as the means and standard deviation calculated from three independent experiments.
Protein extraction was performed from 100 to 200 mg of leaf tissues by grinding in 100 mM Mes buffer (ratio buffer:tissue of 3:1), pH 7.5, containing 15 mM β-mercaptoethanol and charcoal. The crude extract was centrifuged at 13,000 rpm for 20 min, and the supernatant was used to perform acidic and basic PR protein analysis by protein gel blotting as described (Cordelier et al., 2003).
Total scopoletin and SA (free and conjugated forms) were analyzed. Procedures for scopoletin and SA extraction and quantification have been described (Klarzynski et al., 2000; Costet et al., 2002).
RT-PCR Gene Expression Analysis
Total RNA was extracted from leaf tissues using Trizol (Invitrogen, Carlsbad, CA) according to the protocol supplied by the manufacturer. First-strand cDNA synthesis was made from 2 μg of RNA. The synthesized cDNA was used as a matrix for PCR. PCR was performed with denaturing, annealing, and extension temperatures of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, respectively, for 25 to 30 cycles. Gene-specific primers were as follows: tobacco acidic PR-1 (forward, 5′-GATGCCCATAACACAGCTCG-3′; reverse, 5′-TTTACAGATCCAGTTCTTCAGAGG-3′), tobacco basic PR-3 (forward, 5′-GCCATAGGAGTGGACCTGCTAAAC-3′; reverse, 5′-AAAAGACCTCTGGTTGCCGC-3′), Arabidopsis PR-1 (forward, 5′-CTACGCAGAACAACTAAGAGGCAAC-3′; reverse, 5′-TTGGCACATCCGAGTCTCACTG-3′), Arabidopsis PDF1.2 (forward, 5′-ATGGCTAAGTTTGCTTCC-3′; reverse, 5′-TTAACATGGGACGTAACAGATACAC-3′). As control for equal cDNA amounts in each reaction, PCR was performed with primers for tobacco EF-1α (forward, 5′-TCGCCTTGTGGAAGTTTGAGAC-3′; reverse, 5′-CACCAACAGCAACAGTTTGACG-3′), Arabidopsis EF-1α (forward, 5′-TTGCTCCCACAGGATTGACCACTG -3′; reverse, 5′-TCACTTCGCACCCTTCTTGACG-3′). The gene-specific primers of tobacco acidic PR-1, tobacco basic PR-3, Arabidopsis PR-1, Arabidopsis PDF1.2, tobacco EF-1α, and Arabidopsis EF-1α generate expected product sizes of 535, 336, 222, 243, 1050, and 548 bp, respectively. PCR products were separated on a 1% agarose gel and visualized after ethidium bromide staining. Quantification of the PCR products was made in gel using the Bio-Rad GelDoc apparatus together with the Bio-Rad Quantity One software (Hercules, CA). Control reactions to normalize RT-PCR amplification were run with the EF-1α specific primers and four serial dilutions of each first-strand cDNA. PCR were performed through 25 cycles and resulted in amplification linearly related to RNA amounts.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers CS3071 and CS3726.
Acknowledgments
We thank A.G. Darvill and S. Eberhard (Complex Carbohydrate Research Center, Athens, GA) for providing purified oligogalacturonate of DP 14, J. Mütterer (Institut de Biologie Moléculaire des Plantes, Centre National de la Recherche Scientifique, Strasbourg, France) for the microscopy analysis, R. Dietrich (Syngenta) for providing seeds of transgenic nahg Arabidopsis plants, and Altadis (Bergerac, France) for providing seeds of N. tabacum cv Samsun H. We thank also M. Seemanpillai and B. Dobay for English corrections. Most of the experiments with cultured tobacco cells were performed at Dijon (France) at the Unité Mixte de Recherche Plante-Microbe-Environnement headed by A. Pugin. We are particularly grateful to A. Pugin for his advice.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Serge Kauffmann (serge.kauffmann@ibmp-ulp.u-strasbg.fr).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.024968.
References
- Ahl Goy, P., Sicher, H., Reich, R., Aichholz, R., Blum, W., Schmidt, E., and Kessmann, H. (1993). Accumulation of scopoletin is associated with the high disease resistance of the hybrid Nicotiana glutinosa X Nicotiana debneyi. Planta 191, 200–206. [Google Scholar]
- Alban, S., and Franz, G. (1994). Gas-liquid chromatography-mass spectrometry analysis of anticoagulant active curdlan sulfates. Semin. Thromb. Hemost. 20, 152–158. [DOI] [PubMed] [Google Scholar]
- Alban, S., and Franz, G. (2000). Characterization of the anticoagulant actions of a semisynthetic curdlan sulfate. Thromb. Res. 99, 377–388. [DOI] [PubMed] [Google Scholar]
- Alban, S., and Franz, G. (2001). Partial synthetic glucan sulfates as potential new antithrombotics: A review. Biomacromolecules 2, 354–361. [DOI] [PubMed] [Google Scholar]
- Alban, S., Kraus, J., and Franz, G. (1992). Synthesis of laminarin sulfates with anticoagulant activity. Arzneimittelforschung 42, 1005–1008. [PubMed] [Google Scholar]
- Alban, S., Schauerte, A., and Franz, G. (2001). Anticoagulant sulfated polysaccharides. I. Synthesis and structure-activity relationships of new pullulan sulfates. Carbohydr. Polym. 47, 267–276. [Google Scholar]
- Albersheim, P., et al. (1983). Oligosacchatins: Naturally occuring carbohydrates with biological regulatory functions. In Structure and Function of Plant Genomes, O. Ciffery and L. Dure III, eds (New York: Plenum Publishing), pp. 293–312.
- Aziz, A., Poinssot, B., Daire, X., Adrian, M., Bezier, A., Lambert, B., Joubert, J.M., and Pugin, A. (2003). Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant-Microbe Interact. 16, 1118–1128. [DOI] [PubMed] [Google Scholar]
- Baillieul, F., De Ruffray, P., and Kauffmann, S. (2003). Molecular cloning and biological activity of alpha-, beta-, and gamma-megaspermin, three elicitins secreted by phytophthora megasperma H20. Plant Physiol. 131, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkers, J., Kijne, J.W., and Spaink, H.P. (1999). Function of chitin oligosaccharides in plant and animal development. EXS 87, 71–83. [DOI] [PubMed] [Google Scholar]
- Becker, M., Franz, G., and Alban, S. (2003). Inhibition of PMN-elastase activity by semisynthetic glucan sulfates. Thromb. Haemost. 89, 915–925. [PubMed] [Google Scholar]
- Binet, M.-N., Bourque, S., Lebrun-Garcia, A., Chiltz, A., and Pugin, A. (1998). Comparison of the effects of cryptogein and oligogalacturonides on tobacco cells and evidence of different forms of desensitization induced by these elicitors. Plant Sci. 137, 33–41. [Google Scholar]
- Bouarab, K., Potin, P., Correa, J., and Kloareg, B. (1999). Sulfated oligosaccharides mediate the interaction between a marine red alga and its green algal pathogenic endophyte. Plant Cell 11, 1635–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brederode, F.T., Linthorst, H.J.M., and Bol, J.F. (1991). Differential induction of acquired resistance and PR gene expression in tobacco by virus infection, ethephon treatment, UV light and wounding. Plant Mol. Biol. 17, 1117–1125. [DOI] [PubMed] [Google Scholar]
- Cao, H., Bowling, S.A., Gordon, A.S., and Dong, X. (1994). Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6, 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale, F., Jonak, C., Ligterink, W., Niehaus, K., Boller, T., and Hirt, H. (2000). Differential activation of four specific MAPK pathways by distinct elicitors. J. Biol. Chem. 275, 36734–36740. [DOI] [PubMed] [Google Scholar]
- Cheong, J.J., Alba, R., Cote, F., Enkerli, J., and Hahn, M.G. (1993). Solubilization of functional plasma membrane-localized hepta-beta-glucoside elicitor-binding proteins from soybean. Plant Physiol. 103, 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong, J.J., Birberg, W., Fugedi, P., Pilotti, A., Garegg, P.J., Hong, N., Ogawa, T., and Hahn, M.G. (1991). Structure-activity relationships of oligo-beta-glucoside elicitors of phytoalexin accumulation in soybean. Plant Cell 3, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, J., Pierrel, M.A., Atanassova, R., Werck-Reichhart, D., Fritig, B., and Saindrenan, P. (2001). Free and conjugated benzoic acid in tobacco plants and cell cultures. Induced accumulation upon elicitation of defense responses and role as salicylic acid precursors. Plant Physiol. 125, 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordelier, S., de Ruffray, P., Fritig, B., and Kauffmann, S. (2003). Biological and molecular comparison between localized and systemic acquired resistance induced in tobacco by a Phytophthora megasperma glycoprotein elicitin. Plant Mol. Biol. 51, 109–118. [DOI] [PubMed] [Google Scholar]
- Cosio, E.G., Feger, M., Miller, C.J., Antelo, L., and Ebel, J. (1996). High-affinity binding of fungal β-glucan elicitors to cell membranes or species of the plant family Fabacae. Planta 200, 92–99. [Google Scholar]
- Costet, L., Fritig, B., and Kauffmann, S. (2002). Scopoletin expression in elicitor-treated and tobacco mosaic virus-infected tobacco plants. Physiol. Plant. 115, 228–235. [DOI] [PubMed] [Google Scholar]
- Côté, F., and Hahn, M. (1994). Oligosaccharins: Structures and signal transduction. Plant Mol. Biol. 26, 1379–1411. [DOI] [PubMed] [Google Scholar]
- Côté, F., Roberts, K.A., and Hahn, M.G. (2000). Identification of high-affinity binding sites for the hepta-beta-glucoside elicitor in membranes of the model legumes Medicago truncatula and Lotus japonicus. Planta 211, 596–605. [DOI] [PubMed] [Google Scholar]
- Delaney, T.P., Ukness, S., Vernooij, B., Friedrich, L., Weymann, K., Negrotto, N., Gaffney, T., Gut-Rella, M., Kessmann, H., Ward, E., and Ryals, J. (1994). A central role of salicylic acid in plant disease resistance. Science 266, 1247–1250. [DOI] [PubMed] [Google Scholar]
- Dixon, R.J., and Lamb, C.J. (1990). Molecular communication in interactions between plants and microbial pathogens. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41, 339–367. [Google Scholar]
- Doares, S.H., Syrovets, T., Weiler, E.W., and Ryan, C.A. (1995). Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proc. Natl. Acad. Sci. USA 92, 4095–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey, S., Baillieul, F., Pierrel, M.A., Saindrenan, P., Fritig, B., and Kauffmann, S. (1997). Spatial and temporal induction of cell death, defense genes, and accumulation of salicylic acid in tobacco leaves reacting hypersensitively to a fungal glycoprotein elicitor. Mol. Plant-Microbe Interact. 10, 646–655. [Google Scholar]
- Felix, G., Regenass, M., and Boller, T. (1993). Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: Induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 4, 307–316. [Google Scholar]
- Friedrich, L., Vernooij, B., Gaffney, T., Morse, A., and Ryals, J. (1995). Characterization of tobacco plants expressing a bacterial salicylate hydroxylase gene. Plant Mol. Biol. 29, 959–968. [DOI] [PubMed] [Google Scholar]
- Fritig, B., Heitz, T., and Legrand, M. (1998). Antimicrobial proteins in induced plant defense. Curr. Opin. Immunol. 10, 16–22. [DOI] [PubMed] [Google Scholar]
- Guzman, P., and Ecker, J.R. (1990). Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui, H., Yamaguchi, Y., and Hirano, S. (1997). Elicitor actions of N-acetylchitooligosaccharides and laminarioligosaccharides for chitinase and L-phenylalanine ammonia-lyase induction in rice suspension culture. Biosci. Biotechnol. Biochem. 61, 975–978. [DOI] [PubMed] [Google Scholar]
- Joubert, J.M., Yvin, J.C., Barchietto, T., Seng, J.M., Plesse, B., Klarzynski, O., Kopp, M., Fritig, B., and Kloareg, B. (1998). A β-1,3 glucan, specific to a marine alga, stimulates plant defence reactions and induces broad range resistance against pathogens. In The 1998 Brighton Conference: Pests and Diseases. (Farnham, UK: British Crop Protection Council), pp. 441–448.
- Klarzynski, O., Descamps, V., Plesse, B., Yvin, J.C., Kloareg, B., and Fritig, B. (2003). Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol. Plant-Microbe Interact. 16, 115–122. [DOI] [PubMed] [Google Scholar]
- Klarzynski, O., Plesse, B., Joubert, J.M., Yvin, J.C., Kopp, M., Kloareg, B., and Fritig, B. (2000). Linear beta-1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 124, 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloareg, B., and Quatrano, R.S. (1988). Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Mar. Biol. Annu. Rev. 26, 259–315. [Google Scholar]
- Klusener, B., and Weiler, E.W. (1999). Pore-forming properties of elicitors of plant defense reactions and cellulolytic enzymes. FEBS Lett. 459, 263–266. [DOI] [PubMed] [Google Scholar]
- Knoester, M., Van Loon, L.C., Van den Heuvel, J., Hennig, J., Bol, J.F., and Linthorst, H.J.M. (1998). Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc. Natl. Acad. Sci. USA 95, 1933–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepagnol-Descamps, V., Richard, C., Lahaye, M., Potin, P., Yvin, J.C., and Kloareg, B. (1998). Purification and determination of the action pattern of Haliotis tuberculata laminarinase. Carbohydr. Res. 310, 283–289. [DOI] [PubMed] [Google Scholar]
- Mithofer, A., Fliegmann, J., and Ebel, J. (1999). Isolation of a French bean (Phaseolus vulgaris L.) homolog to the beta-glucan elicitor-binding protein of soybean (Glycine max L.). Biochim. Biophys. Acta 1418, 127–132. [DOI] [PubMed] [Google Scholar]
- Mithofer, A., Fliegmann, J., Neuhaus-Url, G., Schwarz, H., and Ebel, J. (2000). The hepta-beta-glucoside elicitor-binding proteins from legumes represent a putative receptor family. Biol. Chem. 381, 705–713. [DOI] [PubMed] [Google Scholar]
- Mithofer, A., Lottspeich, F., and Ebel, J. (1996). One-step purification of the beta-glucan elicitor-binding protein from soybean (Glycine max L.) roots and characterization of an anti-peptide antiserum. FEBS Lett. 381, 203–207. [DOI] [PubMed] [Google Scholar]
- Murray, R.D.H., Méndez, J., and Brown, S.A. (1982). The Natural Coumarins: Occurence, Chemistry and Biochemistry. (New York: John Wiley & Sons).
- Nishimura, M.T., Stein, M., Hou, B.H., Vogel, J.P., Edwards, H., and Somerville, S.C. (2003). Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301, 969–972. [DOI] [PubMed] [Google Scholar]
- Norman, C., Vidal, S., and Palva, E.T. (1999). Oligogalacturonide-mediated induction of a gene involved in jasmonic acid synthesis in response to the cell-wall-degrading enzymes of the plant pathogen Erwinia carotovora. Mol. Plant-Microbe Interact. 12, 640–644. [DOI] [PubMed] [Google Scholar]
- Ohtsubo, N., Mitsuhara, I., Koga, M., Seo, S., and Ohashi, Y. (1999). Ethylene promotes the necrotic lesion formation and basic PR gene expression in TMV-infected tobacco. Plant Cell Physiol. 40, 808–817. [Google Scholar]
- Penninckx, I.A., Eggermont, K., Terras, F.R., Thomma, B.P., De Samblanx, G.W., Buchala, A., Metraux, J.P., Manners, J.M., and Broekaert, W.F. (1996). Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 8, 2309–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx, I.A., Thomma, B.P., Buchala, A., Metraux, J.P., and Broekaert, W.F. (1998). Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10, 2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pye, D.A., Vives, R.R., Turnbull, J.E., Hyde, P., and Gallagher, J.T. (1998). Heparan sulfate oligosaccharides require 6-O-sulfation for promotion of basic fibroblast growth factor mitogenic activity. J. Biol. Chem. 273, 22936–22942. [DOI] [PubMed] [Google Scholar]
- Ramonell, K.M., Zhang, B., Ewing, R.M., Chen, Y., Xu, D., Stacey, G., and Somerville, S. (2002). Microarray analysis of chitin elicitation in Arabidopsis thaliana. Mol. Plant Pathol. 3, 301–311. [DOI] [PubMed] [Google Scholar]
- Read, S.M., Currie, G., and Bacic, A. (1996). Analysis of the structural heterogeneity of laminarin by electrospray-ionisation-mass spectrometry. Carbohydr. Res. 281, 187–201. [DOI] [PubMed] [Google Scholar]
- Roche, P., Debelle, F., Maillet, F., Lerouge, P., Faucher, C., Truchet, G., Denarie, J., and Prome, J.C. (1991). Molecular basis of symbiotic host specificity in Rhizobium meliloti: nodH and nodPQ genes encode the sulfation of lipo-oligosaccharide signals. Cell 67, 1131–1143. [DOI] [PubMed] [Google Scholar]
- Rouet-Mayer, M.-A., Mathieu, Y., Cazalé, A.-C., Guern, J., and Laurière, C. (1997). Extracellular alkalinization and oxidative burst induced by fungal pectin lyase in tobacco cells are not due to the perception of oligogalacturonide fragments. Plant Physiol. Biochem. 35, 321–330. [Google Scholar]
- Salinas-Mondragon, R.E., Garciduenas-Pina, C., and Guzman, P. (1999). Early elicitor induction in members of a novel multigene family coding for highly related RING-H2 proteins in Arabidopsis thaliana. Plant Mol. Biol. 40, 579–590. [DOI] [PubMed] [Google Scholar]
- Schalk, M., Cabello-Hurtado, F., Pierrel, M.A., Atanossova, R., Saindrenan, P., and Werck-Reichhart, D. (1998). Piperonylic acid, a selective, mechanism-based inactivator of the trans-cinnamate 4-hydroxylase: A new tool to control the flux of metabolites in the phenylpropanoid pathway. Plant Physiol. 118, 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, J.K., McNeil, M., and Albersheim, P. (1984). The primary structures of one elicitor-active and seven elicitor-inactive hexa(beta-D-glucopyranosyl)-D-glucitols isolated from the mycelial walls of Phytophthora megasperma f. sp. glycinea. J. Biol. Chem. 259, 11321–11336. [PubMed] [Google Scholar]
- Shibuya, N., and Minami, E. (2001). Oligosaccharide signalling for defence responses in plant. Physiol. Mol. Plant Pathol. 59, 223–233. [Google Scholar]
- Stibich, H. (2000). Neuartige Antiphlogistisch Wirksame β-1,3 GlucanSulfate: Partialsynthese und Physiologische Testung. PhD dissertation (Regensburg, Germany: University of Regensburg).
- Trezzini, G.F., Horrichs, A., and Somssich, I.E. (1993). Isolation of putative defense-related genes from Arabidopsis thaliana and expression in fungal elicitor-treated cells. Plant Mol. Biol. 21, 385–389. [DOI] [PubMed] [Google Scholar]
- Umemoto, N., Kakitani, M., Iwamatsu, A., Yoshikawa, M., Yamaoka, N., and Ishida, I. (1997). The structure and function of a soybean beta-glucan-elicitor-binding protein. Proc. Natl. Acad. Sci. USA 94, 1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberne, M.C., Verpoorte, R., Bol, J.F., Mercado-Blanco, J., and Linthorst, H.J. (2000). Overproduction of salicylic acid in plants by bacterial transgenes enhances pathogen resistance. Nat. Biotechnol. 18, 779–783. [DOI] [PubMed] [Google Scholar]
- Ward, E., Uknes, S., Williams, S., Dincher, S., Wiederhold, D., Alexander, D., Ahl-Goy, P., Metraux, J.P., and Ryals, J. (1991). Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell 3, 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani, N., Silvermann, P., Wilson, T.M.A., Kleier, D.A., and Raskin, I. (1991). Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell 3, 809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, T., Yamada, A., Hong, N., Ogawa, T., Ishii, T., and Shibuya, N. (2000). Differences in the recognition of glucan elicitor signals between rice and soybean: Beta-glucan fragments from the rice blast disease fungus Pyricularia oryzae that elicit phytoalexin biosynthesis in suspension-cultured rice cells. Plant Cell 12, 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B., Ramonell, K., Somerville, S., and Stacey, G. (2002). Characterization of early, chitin-induced gene expression in Arabidopsis. Mol. Plant-Microbe Interact. 15, 963–970. [DOI] [PubMed] [Google Scholar]