Abstract
The phytochrome (phy) family of sensory photoreceptors (phyA to phyE) in Arabidopsis thaliana control plant developmental transitions in response to informational light signals throughout the life cycle. The photoactivated conformer of the photoreceptor Pfr has been shown to translocate into the nucleus where it induces changes in gene expression by an unknown mechanism. Here, we have identified two basic helix-loop-helix (bHLH) transcription factors, designated PHYTOCHROME-INTERACTING FACTOR5 (PIF5) and PIF6, which interact specifically with the Pfr form of phyB. These two factors cluster tightly with PIF3 and two other phy-interacting bHLH proteins in a phylogenetic subfamily within the large Arabidopsis bHLH (AtbHLH) family. We have identified a novel sequence motif (designated the active phytochrome binding [APB] motif) that is conserved in these phy-interacting AtbHLHs but not in other noninteractors. Using the isolated domain and site-directed mutagenesis, we have shown that this motif is both necessary and sufficient for binding to phyB. Transgenic expression of the native APB-containing AtbHLH protein, PIF4, in a pif4 null mutant, rescued the photoresponse defect in this mutant, whereas mutated PIF4 constructs with site-directed substitutions in conserved APB residues did not. These data indicate that the APB motif is necessary for PIF4 function in light-regulated seedling development and suggest that conformer-specific binding of phyB to PIF4 via the APB motif is necessary for this function in vivo. Binding assays with the isolated APB domain detected interaction with phyB, but none of the other four Arabidopsis phys. Collectively, the data suggest that the APB domain provides a phyB-specific recognition module within the AtbHLH family, thereby conferring photoreceptor target specificity on a subset of these transcription factors and, thus, the potential for selective signal channeling to segments of the transcriptional network.
INTRODUCTION
After germination, young seedlings growing in the darkness of subterranean environments are etiolated, having elongated hypocotyls, small cotyledons, and a pale yellow color, until the light exposure above the soil induces a major redirection of development toward the fully green photosynthetically active state. Higher plants have developed complex photosensory systems that enable them to use these initial light signals as a trigger for suppressing hypocotyl cell elongation, along with apical hook opening, cotyledon expansion, and greening. The photosensitivity of the seedlings during this deetiolation process in response to light in the red/far-red region of the spectrum is facilitated by the phytochromes (Quail, 2002a). The Arabidopsis thaliana phytochromes (phyA to phyE) are well characterized and display some overlapping but differential photosensory and physiological functions. Whereas phyA is the primary photoreceptor in continuous monochromatic far-red light, phyB plays the major role in continuous monochromatic red (Rc) light (Quail et al., 1995; Whitelam and Devlin, 1997; Quail, 1998; Smith, 2000). Expression profile analysis using oligonucleotide microarrays has identified multiple transcription factor genes as early targets of phyA in response to continuous monochromatic far-red light (Tepperman et al., 2001). Similarly, phyB and one or more other phytochromes regulate a similar core set of early-response genes in Rc (Tepperman et al., 2004). These global analyses of phy-regulated early gene expression have identified several new loci potentially involved in early regulatory steps in the phy-signaling pathway. Reverse genetics studies have indicated that at least some of these early targets may indeed play functional roles downstream in phy-signaling pathways (Tepperman et al., 2001; Khanna et al., 2003). The data indicate further that different members of the phy family act with a partial organ specificity in Rc. Whereas the apical zone responsiveness (hook and cotyledon responsiveness) is mediated by phyB and one or more other phytochromes, the inhibition of hypocotyl cell elongation is predominantly, if not exclusively, regulated by phyB (Tepperman et al., 2004).
The phytochrome molecule is a soluble chromoprotein with a monomeric molecular mass of ∼125 kD. The N-terminal domain of the polypeptide is the photosensory domain with a single covalently attached tetrapyrrole chromophore (phytochromobilin), and the C-terminal domain is involved in dimerization and nuclear translocation (Matsushita et al., 2003). The ability of the phy molecule to function as a molecular switch is integral to its photosensory activity. Biologically inactive Pr is reversibly switched, by absorption of a photon, to biologically active Pfr, which then becomes localized into the nucleus and can form speckles (Kircher et al., 2002; Matsushita et al., 2003). The nuclear translocation of the Pfr form is necessary for phyB function (Kircher et al., 2002; Huq et al., 2003; Matsushita et al., 2003), but the biological relevance of the nuclear speckles is presently unclear. Evidently, the C-terminal domain of phyB is necessary for its nuclear accumulation and speckle formation, and it attenuates phyB activity (Matsushita et al., 2003). This conclusion is based on data indicating that the N-terminal domain of phyB when fused to β-glucuronidase and a nuclear localization signal (NLS) dimerizes and accumulates in the nucleus, with biological activity considerably greater than the full-length photoreceptor, despite a failure to form speckles (Matsushita et al., 2003).
The mechanism by which the activated phy molecules transduce signaling information to photoresponsive genes is unknown. However, there is evidence that after nuclear translocation, the Pfr form of the photoreceptor can interact with selected transcription factors. Two such factors, PHYTOCHROME-INTERACTING FACTOR3 (PIF3) and PIF4, have been identified as binding specifically and reversibly to the Pfr form of the phy molecule (Ni et al., 1998, 1999; Huq and Quail, 2002; Shimizu-Sato et al., 2002). Both proteins belong to subfamily 15 of the Arabidopsis basic helix-loop-helix (AtbHLH) superfamily of transcriptional regulators (Bailey et al., 2003; Toledo-Ortiz et al., 2003). Genetic and reverse genetics studies indicate that both PIF3 and PIF4 function in light-regulated seedling deetiolation. Based on a long-hypocotyl phenotype observed for antisense PIF3–expressing Arabidopsis seedlings grown under prolonged irradiation, it was initially concluded that PIF3 acts positively in phy-regulated seedling deetiolation (Ni et al., 1999). However, more recent studies with multiple mutant alleles at the PIF3 locus show that the pif3 mutants instead have a complex deetiolation phenotype that includes a short hypocotyl under prolonged, continuous irradiation (Kim et al., 2003; Bauer et al., 2004; E. Monte and P. Quail, unpublished data) as well as delayed greening and cotyledon separation upon initial exposure to light (E. Monte and P. Quail, unpublished data). These data suggest that PIF3 appears to function negatively in some facets of phy-induced deetiolation, such as hypocotyl elongation, but positively in others, such as early plastid development. The contradiction in hypocotyl phenotype between the earlier (Ni et al., 1999) and more recent (Kim et al., 2003; Bauer et al., 2004; E. Monte and P. Quail, unpublished data) studies appears to have arisen because the long-hypocotyl phenotype exhibited by the principal antisense line (A22) used in the earlier study appears to result from a mutation at a locus unrelated to PIF3 expression (E. Monte and P. Quail, unpublished data). PIF4 also functions negatively in phyB-mediated hypocotyl and cotyledon cell expansion under prolonged irradiation (Huq and Quail, 2002). Taken together, these data have been interpreted to suggest that PIF3 and PIF4 are direct signaling targets of the photoactivated, nuclear localized, phytochrome molecule. Recent evidence for PIF3 indicates that this intranuclear phy–PIF3 interaction induces rapid degradation of the PIF3 protein (Bauer et al., 2004).
Given the existence of other closely related AtbHLH family members, the possibility arises that some of these could also play similar roles in phy signaling pathways, forming a potential transcriptional network (Quail, 2002a, 2002b). To determine whether such other members of the AtbHLH family are capable of interacting with phyB, we have systematically screened the PIF3-related subgroup (subfamily 15) as well as selected AtbHLHs from other subfamilies for binding using in vitro pull-down assays. Here, we report that phyB does indeed interact selectively with two other AtbHLH proteins belonging to the PIF3 subgroup. Significantly, in addition, we have identified a novel motif conserved in members of this subgroup required for conformer-specific interaction with photoactivated phyB and for functional activity in vivo.
RESULTS
PIF3 and PIF4 have been previously reported to bind specifically to the biologically active Pfr form of phyB (Ni et al., 1998; Huq and Quail, 2002). The PIF3 group (AtbHLH subfamily number 15) consists of 15 members (Toledo-Ortiz et al., 2003). We tested eight other AtbHLHs for interaction with phyB and identified two new factors, designated here as PIF5 and PIF6, that specifically bind phyB in the Pfr form (Figure 1A). We propose this nomenclature to reflect the phy-interacting activity of these two proteins, which have been previously called PIL6 (for PIF3-LIKE6) and PIL2, respectively, based on their sequence relatedness to PIF3 (Yamashino et al., 2003). The six other AtbHLH proteins tested did not show any detectable interactions with phyB (Figure 1B). These included three from the PIF3 group (SPATULA [SPT], Heisler et al., 2001; PIL1, Yamashino et al., 2003; and BHLH023), as well as three from the neighboring AtbHLH subfamily 17 (BHLH058, BHLH059, and BHLH066). Additionally, previously reported LONG HYPOCOTYL IN FAR-RED1 (HFR1) was shown not to interact with phyB (Fairchild et al., 2000). Recently, we have identified still another member of AtbHLH subfamily 15, designated PIF1 that binds phyB (Pfr) (Huq et al., 2004).
Figure 1.
PIF5 and PIF6 Bind Specifically to phyB (Pfr), whereas Six Other AtbHLH Proteins Tested Do Not Bind phyB.
GAL4 activation domain (GAD) fusions (at N- or C-terminal domains) of full-length AtbHLH proteins were used as baits in in vitro coimmunoprecipitation assays. PhyB (Pfr) or phyB (Pfr) reconverted to Pr by a far-red light pulse (marked as Pr) were used as prey. Schematic diagram on the left shows the design of the experiments, and the SDS-PAGE separations of the pellet fractions are shown.
(A) GAD:PIF3, PIF4:GAD, GAD:PIF5, and GAD:PIF6 specifically bind phyB (Pfr). GAD alone was used as control.
(B) GAD:SPT, GAD:PIL1, GAD:BHLH023, GAD:BHLH058, GAD:BHLH059, and GAD:BHLH066 do not bind phyB.
(C) Quantification of in vitro coimmunoprecipitation assays. The percentage of phyB recovered was calculated per bait. PhyB input is shown in the inset.
Overall, 12 AtbHLH proteins have now been tested for interactions with phyB, including the four reported elsewhere and the eight tested here (Figure 1). Out of these 12 AtbHLH proteins, only five show detectable binding to phyB. These include the previously identified PIF3 (Ni et al., 1998, 1999), PIF4 (Huq and Quail, 2002), and PIF1 (Huq et al., 2004) and the two new members, PIF5 and PIF6 (summarized in Figure 2). Significantly, all five of these factors cluster tightly within subfamily 15 of the AtbHLH phylogenetic tree. We compared the phyB binding capacities of these two new interacting factors and found that PIF6 bound a similar percentage of phyB (Pfr) input to that of PIF3 (25 to 30%), whereas PIF5 and PIF4 bound lower percentages of phyB (Pfr) input (5 to 10%, see Figure 1C).
Figure 2.
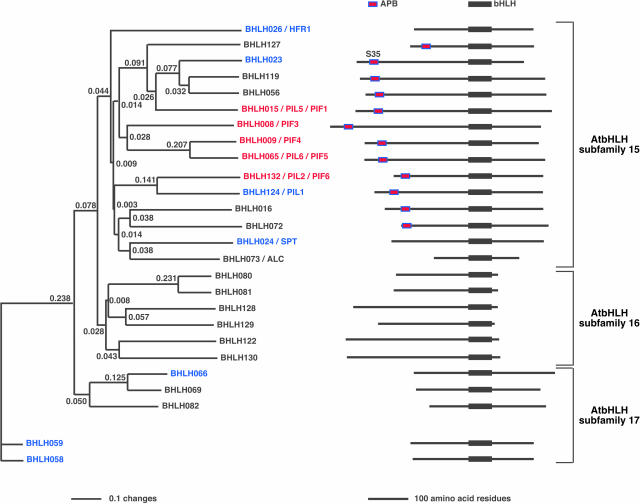
Members of the PIF3 Group of AtbHLH Proteins Contain a Conserved Sequence in their N-Terminal Domains.
Neighbor-joining phylogenetic tree using full-length amino acid sequences of proteins closely related to PIF3. The tree was constructed with PAUP 4.0 software using an alignment (MultiAlin; Corpet, 1988) of predicted full-length amino acid sequences for each protein. All proteins are identified by their generic names and grouped in their subfamilies (Bailey et al., 2003), and other synonym/s are indicated. Twelve of the full-length AtbHLH proteins from different subfamilies have been tested for interactions with phyB using in vitro coimmunoprecipitation assays. Seven of the tested proteins did not interact (in blue) with phyB, whereas five show interactions (in red) specifically with the active Pfr form of phyB. Four AtbHLH proteins previously tested for interactions with phyB are HFR1 (Fairchild et al., 2000), PIF3 (Ni et al., 1998), PIF4 (Huq and Quail, 2002), and PIF1 (also known as PIL5; Yamashino et al., 2003; Huq et al., 2004). We propose two new names, PIF5 (old name, PIL6; Yamashino et al., 2003) and PIF6 (old name, PIL2; Yamashino et al., 2003) for the new phyB interacting factors to reflect their molecular activity. On the right, we show stick diagrams of the full-length proteins aligned at their bHLH domains. The presence of the APB consensus sequence in 12 AtbHLH proteins in subfamily 15 is indicated, including the S35 present in BHLH023, instead of the invariant G. The branch lengths are proportional to the indicated distance values (changes) between sequences. SPT (Heisler et al., 2001); ALC, ALCATRAZ (Rajani and Sundaresan, 2001). Proteins that interact with phyB (Pfr) are in red, and those that were tested but do not interact are in blue.
These data suggested that the capability of the five members of the PIF3 group to specifically bind phyB (Pfr) might be because of a specific sequence or structural feature present in these AtbHLH proteins. Indeed, amino acid sequence alignments of the predicted full-length AtbHLH proteins revealed the presence of a conserved motif near the N terminus of 12 of the 15 members of the PIF3-related subfamily 15 (Figure 2). Further amino acid sequence alignments, along with Arabidopsis sequence database searches, showed that this conserved region is present exclusively in these 12 AtbHLH proteins (Figure 2). It was neither present in any other of the 162-member AtbHLH Arabidopsis family nor in any other proteins in the databases. This motif is conserved in all five of the AtbHLH proteins that we have found thus far to bind specifically to the active form of phyB. Conversely, the two out of three members of the PIF3-related subfamily 15 lacking this motif that we have tested for phyB interaction, HFR1 (Fairchild et al., 2000) and SPT (Rajani and Sundaresan, 2001), fail to bind to the photoreceptor (Fairchild et al., 2000; Figures 1B and 2). Similarly, the three members of AtbHLH subfamily 17 that we have tested for phyB binding, BHLH066, BHLH058, and BHLH059, do not interact detectably with the photoreceptor and do not contain the conserved sequence. Thus, the capacity of AtbHLH proteins to bind to phyB is strongly correlated with the presence of the conserved motif. For convenience, we have designated this motif the active phytochrome binding (APB) motif (Figure 3A). The two exceptions in the PIF3 cluster, BHLH023 and PIL1, that contain the APB motif, but do not bind detectably to phyB, are discussed below. Interestingly, phylogenetic tree analysis of the APB motif (Figure 3B) suggests very similar evolutionary relationships to those previously observed with the bHLH domains of these proteins (Toledo-Ortiz et al., 2003). The conservation of the evolutionary relationships between these two distinct domains (APB and bHLH) suggests conserved evolutionary relationships in the full-length context and possible functional relationships within subgroups. Notably, PIF4 and PIF5 cluster together and are closely related to PIF3 (Figures 2 and 3B).
Figure 3.
The APB Motif.
(A) Alignments of the predicted N-terminal (1 to 100) amino acid sequences of 12 members of the AtbHLH subfamily 15 containing amino acid sequence homologies to the APB motif. Conserved amino acid sequences (in red) of the APB motif (blue outlined) are shown. The invariant amino acid residues required for APB function (reverse red font) are E31, L32, G37, and Q38 (PIF3 and PIF5 positions). The APB consensus is in bold type.
(B) Phylogenetic neighbor-joining tree of the aligned APB sequences showing the putative evolutionary relationships of the APB motif between the family members. The distance values (changes) between sequences are proportional to the branch lengths (indicated). Proteins that interact with phyB (Pfr) are in red, and those that were tested but do not interact are in blue. The APB region of BHLH023 has a variation (S35 instead of invariant G).
The APB consensus present in the N-terminal domains of the 12 AtbHLH proteins contains four invariant residues (E31, L32, G37, and Q38; PIF3 and PIF5 positions; Figure 3A). To examine if these invariant residues are important for binding, we created point mutations for each separately in PIF5 (E31A, L31A, G37A, and Q38A). The data show that these point mutations eliminate detectable PIF5 binding to phyB (Figure 4A). These results indicate that the four invariant APB residues are individually necessary for PIF5 to selectively bind phyB (Pfr). The single point mutation (G35A) in PIF4 also eliminated its interaction with phyB (Figure 4B). The same mutation in PIF3 reduced its ability to bind phyB by ∼75% (data not shown), whereas the double mutation (E31A/G37A) eliminated its interaction with phyB (Figure 4B). These data further suggest that the APB motif activity is necessary for the capability of the PIFs to bind phyB (Pfr). This contradicts our previous report suggesting that a region in PIF3 with similarity to PER-ARNT-SIM (PAS) domains was required for its interaction with photoactivated phyB (Zhu et al., 2000). For unknown reasons, we have been unable to reproduce these earlier results. Using the same constructs and experimental conditions as Zhu et al. (2000), we observed no detectable binding of phyB to deletion mutants of PIF3 lacking the N-terminal APB-containing region, despite retention of the PAS-like domain. These repeat trials are instead consistent with the data presented here, indicating that the APB motif is required for phyB binding to PIF3.
Figure 4.
The APB Motif Is Necessary for Binding phyB (Pfr).
GAD fusions with full-length PIFs (containing wild-type APB, closed rectangles, or mutated APB, open rectangles) were used as baits in in vitro coimmunoprecipitation assays. PhyB (Pfr) or phyB (Pfr) reconverted to Pr by a far-red light pulse (marked as Pr) were used as prey. Schematic diagrams on the left show the design of the experiments, and the SDS-PAGE separations of the pellet fractions are shown on the right of each panel.
(A) GAD:PIF5 binds phyB (Pfr) but the point mutations (E31A, L32A, G37A, and Q38A) abrogate binding to phyB.
(B) APB mutations in PIF4:GAD (G35A) and GAD:PIF3 (E31A/G37A) eliminate their interactions with phyB (Pfr).
To determine whether the APB motif is sufficient to bind phyB (Pfr), we constructed GAL4 activation domain (GAD) fusions with the N-terminal 100–amino acid residues of PIF3, PIF4, and PIF6 containing the APB motif (Figure 3A) for use as bait in pull-down assays. The data show that all three APB-motif fusions are capable of binding specifically to phyB (Pfr) (Figures 5A and 5B). Furthermore, GAD:APB fusion proteins carrying point mutations of invariant residues in the APB motif lose their ability to show any detectable binding to phyB, verifying the specificity of the interaction (Figure 5A). We tested the APB motif for interactions with all five Arabidopsis phytochromes (phyA, phyB, phyC, phyD, and phyE) and found that the APB motif from PIF3 (GAD:APB) specifically binds phyB (Pfr). There was no detectable binding to other phytochromes (Figure 5C). Binding experiments performed under Rc irradiation provide evidence that this absence of detectable PIF3-APB motif binding to phyA, phyC, phyD, or phyE is not attributable to rapid dark reversion of these phytochromes (see Supplemental Figure 1 online). Together, then, these data provide compelling evidence that the APB motif is both necessary and sufficient for specific interactions of the PIF-bHLH transcription factors with the biologically active conformer of phyB (Figures 4 and 5). In addition, we have identified four amino acid residues in the APB consensus that are critical for APB function (Figures 4 and 5A). Quantitative comparison of the binding capacities of the various GAD:APB fusion proteins revealed that the APB motifs of PIF3 and PIF4 recovered 10 to 15% of the input phyB (Pfr) in the pellet, whereas the APB motif of PIF6 remarkably recovered ∼50% of the input phyB (Pfr) (Figure 5B), indicating a highly robust conformer-specific interaction.
Figure 5.
APB Motif Is Sufficient for Binding phyB (Pfr).
(A) GAD:APB fusion proteins used as baits are sufficient for interactions with phyB (Pfr). GAD fusions with N-terminal (1 to 100) amino acid sequences containing the wild-type APB (closed rectangles) from PIF3, PIF4, PIF6, PIL1, and mutated APB (open rectangles) from PIF3 (E31A/G37A), PIF4 (G35A), and PIF6 (E19A/G25A) were tested for interactions with phyB.
(B) Quantification of in vitro coimmunoprecipitation assays. PhyB input is shown in the inset.
(C) The APB motif of PIF3 (GAD:APB) was tested for interactions with phyA, phyB, phyC, phyD, and phyE. The prey input is shown in the left panels.
Despite the fact that they contain homologies to the APB motif in their N-terminal domains, two members of AtbHLH subfamily 15, BHLH023 and PIL1, do not interact with phyB (Figures 1B, 1C, 2, and 3). Notably, however, BHLH023 lacks the conserved G (S35 instead of G; as indicated in Figures 2 and 3A), which is invariant in all other members with the APB motif. This seems likely to account for its inability to bind to phyB because point mutations (G to A) of this conserved G residue eliminate binding in PIF5 and PIF4 (Figure 4). PIL1, on the other hand, shows no detectable binding to phyB but has all four of the invariant residues (Figures 1B and 3A). It is possible that the APB motif of PIL1 lacks one or more amino acid residues yet unknown to be important for binding. The GAD:APB fusion with the APB motif from PIL1, like those from PIF3, PIF4, and PIF6, can bind phyB (Pfr) photoreversibly (Figures 5A and 5B), though the interaction is weak. Quantitative binding curves indicate that the apparent affinity of the PIL1-APB domain for phyB is approximately fourfold lower than PIF3-APB (see Supplemental Figure 2 online). It is possible that the C-terminal domain of PIL1 inhibits APB activity, perhaps by masking it and making it unavailable for interaction with phyB. This effect may also be apparent in PIF6, the closest relative of PIL1 (Figures 1A, 2, 3B, 5A, and 5B), where the APB motif of PIF6 by itself shows a very strong interaction (recovered ∼50% of input phyB; Figure 5B), but the full-length PIF6 interacts with a relatively lower affinity (25 to 30% of input phyB recovered; Figure 1C).
To begin to evaluate the functional relevance of the APB-dependent phyB interactions to phyB signaling, we tested its contribution to PIF4 activity in vivo. We used the previously described pif4 mutant line, known as short under red-light2 (srl2), which is hypersensitive to Rc (Huq and Quail, 2002). The srl2 mutant line has a T-DNA insertional disruption upstream of the NLS in the bHLH region of PIF4 (Figure 6A). This insertion results in a detectable but truncated PIF4 transcript (Huq and Quail, 2002) that is not expected to encode a nuclear localized product capable of binding to DNA and is likely to be null for PIF4 transcriptional activity, if expressed. Initially, we demonstrated that expression of the native full-length PIF4 gene (PIF4 FL) under control of its own promoter provided phenotypic rescue of srl2 mutant seedlings (Figure 6). The transgenic line, designated PIF4 FL(2), which expresses the PIF4 mRNA at approximately threefold wild-type (Wassilewskija [Ws]) levels (Figure 7) is rescued for the srl2 phenotype (Figures 6B and 6C). By contrast, the transgenic line designated PIF4 FL(1), which is a high-level PIF4 overexpressor (25-fold relative to wild-type PIF4 levels; Figure 7), strongly overcompensates the srl2 phenotype, producing hypocotyl lengths similar to those of phyB (Columbia [Col]) in Rc (Figure 6B). By contrast, the short hypocotyl length phenotype of srl2 mutants is not, or only partially, rescued in lines carrying the G35A mutation (Figures 6B and 6C). This conserved residue G35 is required for APB-mediated interaction of PIF4 with phyB (Pfr) (Figures 4B and 5A). A second parameter of the seedling photomorphogenic phenotype observed in srl2 mutants is the enhanced expansion of cotyledon area (Huq and Quail, 2002), which is also rescued in PIF4 FL(2) transformants expressing functional APB-containing PIF4, but not rescued in PIF4 FL(G35A) transgenic lines (Figure 6D). The cotyledon area is greatly reduced in the overexpressing PIF4 FL(1) transformants (Figure 6D).
Figure 6.
APB Motif Activity Is Necessary for PIF4 Function.
The srl2 mutant lines transformed with the PIF4:PIF4 full-length gene containing either wild-type APB [shown in red; PIF4 FL(1) and PIF4 FL(2)] or mutated APB [G35A shown in blue; PIF4 FL(G35A-1), PIF4 FL(G35A-2), and PIF4 FL(G35A-3)].
(A) Stick diagram showing the PIF4 promoter (2 kb upstream of the ATG) and the PIF4 gene used for transformations of srl2 mutant lines (position of srl2 T-DNA insertion in relation to the NLS and the bHLH is indicated).
(B) Fluence rate response curves of mean hypocotyl lengths of wild-type (Ws), srl2 mutant, and indicated transgenic lines grown in Rc for 3 d. The phyB (Col) was used as a control.
(C) Seedling photomorphogenic phenotypes of srl2 mutant seedlings grown in Rc (17.8 μmol m−2 s−1) for 3 d are rescued by the PIF4 FL(2) transgene, are overcompensated in PIF4 overexpressing transgenic line PIF4 FL(1), and are not rescued in PIF4 FL(G35A) transgenic lines.
(D) Measurements of cotyledon area expansion in transgenic seedlings. The srl2 mutant seedlings grown for 3 d under Rc (17.8 μmol m−2 s−1) have enlarged cotyledon areas compared with the Ws (wild type), and this response is rescued (cotyledon expansion similar to the wild type) in the PIF4 FL(2) transgenic seedlings. The PIF4 FL(1) overexpressors show reduced cotyledon expansion; by contrast, all of the lines expressing mutated APB (G35A) have enlarged cotyledons compared with Ws. PhyB (Col) is shown for comparison. Approximately 20 to 25 seedlings for each line were used for measurements (40 to 50 cotyledons), and the values were normalized to Ws.
Figure 7.
Relative PIF4 Transcript Levels Compared with Percentage of Mutant Phenotype Rescue in Transgenic Seedlings.
(A) RNA gel blots showing Rc-induced expression of PIF4 message in transformants under control of the native PIF4 promoter. The Rc fold-induction values are indicated. All transgenes show approximately twofold induction in light, except for much higher levels detected in PIF4 FL(1).
(B) Relative PIF4 transcript levels in Rc (top graph), normalized to the Ws levels, are shown with the percentage of recovery toward the wild-type phenotype (bottom graph; percentage of recovery is the percentage of mutant hypocotyl length rescue in transgenic lines).
(C) Correlation between percentage of recovery (y axis) and relative PIF4 transcript levels (x axis) derived from the data presented above (B) for the indicated transgenic lines. Relative transcript level and percentage of recovery in the PIF4 FL transgenic lines (closed circles) and PIF4 FL(G35A) transgenic lines (open circles).
To ensure that the absence of phenotypic rescue by the mutated PIF4 sequence was not because of lack of adequate expression of this transgene, we examined the relative PIF4 transcript levels in the transgenic lines. All of the transformants showed approximately twofold Rc light–dependant induction of the PIF4 transcript under control of the PIF4 promoter, similar to the wild-type Ws (Figure 7A). The relative levels of Rc-induced PIF4 transcripts in PIF4 FL(G35A) lines are similar to or higher than (threefold to sixfold Ws levels) the PIF4 FL(2) (threefold Ws levels) transformants that do rescue the srl2 mutant phenotype (Figures 6 and 7). We calculated the percentage of recovery in hypocotyl length in the transgenic seedlings relative to the wild type and found that the percentage of recovery of the srl2 phenotype is closely related to the PIF4 transcript levels in transformants carrying a functional wild-type APB sequence (Figures 7B and 7C). By contrast, although the PIF4 FL(G35A) transformants contain similar or higher levels of the PIF4 transcript, the srl2-mutant phenotype is not complemented by the mutated PIF4 (G35A) transgene (Figures 7B and 7C) to a comparable extent. The basis for the partial apparent rescue by the mutated APB-containing PIF4 FL (G35A) at high transcript levels (Figures 7B and 7C) is not known. One possibility is that it reflects weak residual activity of the mutated APB domain not detectable by in vitro interaction assays. Alternatively, it cannot be ruled out that the mutated APB-containing PIF4 FL (G35A) polypeptide might form a weakly active complex with a truncated PIF4 product potentially produced from the observed truncated transcript in the srl2 mutant (Huq and Quail, 2002). Regardless, these results suggest a strong correlation between APB-dependent interaction of PIF4 with phyB (Pfr) and PIF4 function in promoting hypocotyl cell expansion (Figures 4, 6, and 7). Together, these data provide evidence that APB activity is necessary for PIF4 function in phyB signaling.
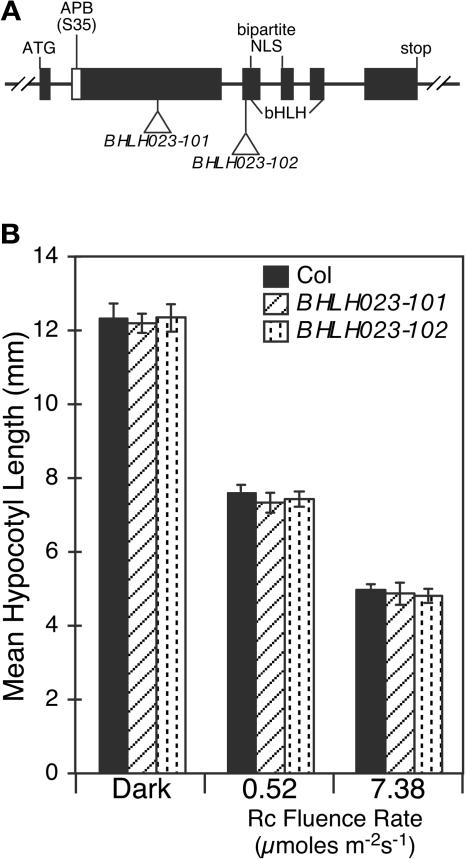
To examine whether the apparent natural, non-phyB binding variant BHLH023 (Figures 1B, 2, and 3) plays a role in phy-regulated seedling deetiolation, we identified two T-DNA insertional mutants, BHLH023-101 and BHLH023-102, and tested them for seedling photomorphogenesis phenotypes. Sequence analysis shows that the points of insertion are 456 bp (BHLH023-101) and 8 bp (BHLH023-102), respectively, upstream of the NLS and the bHLH domain (Figure 8A). Based on the positions of these insertions, the mutants would be predicted to be null for nuclear translocation and DNA binding ability if not protein null. Analysis of hypocotyl length in two different Rc fluence rates revealed no detectable light-responsiveness phenotypes in the BHLH023-101 and BHLH023-102 mutants (Figure 8B). These data thus provide further evidence of a negative correlation between lack of phyB binding capacity and functional involvement of AtbHLH proteins in phy signaling and strongly support the conclusion that APB activity is directly responsible for this functional activity in APB-containing AtbHLH proteins.
Figure 8.
AtbHLH023 Mutant Seedlings Do Not Show Detectable Seedling Photomorphogenic Phenotypes.
(A) Stick diagram showing the BHLH023 gene structure. Positions of the T-DNA insertions in BHLH023-101 and BHLH023-102 are indicated relative to the NLS and the bHLH domain. Based on the points of these insertions, the mutants would be predicted to be null for nuclear translocation and DNA binding if a stable truncated protein is made. The natural variation in the APB motif (S35) of BHLH023 is indicated.
(B) Mean hypocotyl lengths of wild-type (Col), BHLH023-101, and BHLH023-102 mutant seedlings grown in Rc (0.52 or 7.38 μmol m−2 s−1) for 3 d.
DISCUSSION
The identification of PIF3 as a bHLH transcription factor capable of specific interaction with the Pfr form of phyB (Ni et al., 1998, 1999; Martinéz-García et al., 2000), together with evidence that phy molecules are induced to translocate into the nucleus as Pfr (Sakamoto and Nagatani, 1996; Kircher et al., 2002), suggested the existence of a direct signaling pathway from photoactivated phys to PIF3-regulated genes (Quail, 2002a, 2002b). Because the Arabidopsis bHLH proteins are encoded by a large multigene family (Bailey et al., 2003; Heim et al., 2003; Toledo-Ortiz et al., 2003), the possibility arose that other members of this family function in a similar manner to PIF3 in phy signaling. Accumulating evidence suggests that photoactivated phyB molecules can indeed interact with multiple AtbHLHs, potentially forming a transcriptional network. We previously reported that the AtbHLH protein PIF4 binds specifically to the Pfr form of phyB (Huq and Quail, 2002), and more recently we have identified another AtbHLH, PIF1, as being capable of interacting with both phyB and phyA (Huq et al., 2004). The evidence presented here identifies two additional AtbHLHs, PIF5 and PIF6, which also interact specifically with the Pfr form of phyB, and six other AtbHLHs that do not. Thus, together, the data so far define a core set of five phylogenetically related, phyB-interacting AtbHLHs, while also identifying another set of seven AtbHLHs, both from within the same subfamily, or from an adjacent subfamily, that do not bind detectably to phyB. These data are consistent with the proposal that the observed interactions are molecularly specific.
The recognition of multiple transcription factors by this single phy family member could at one extreme reflect complete redundancy, whereby all interacting AtbHLH factors are capable of regulating the same target gene set in response to phyB signaling. Alternatively, the multiple partners could indicate immediate channeling of phyB signaling to different subsets of genes, each of which is targeted separately by the different phy-interacting AtbHLHs. All AtbHLH factors tested so far can bind in vitro to the core G-box motif, CACGTG, in DNA fragments (Martinéz-García et al., 2000; Huq and Quail, 2002; Toledo-Ortiz et al., 2003), except HFR1, which appears to be a non-DNA binding variant (Fairchild et al., 2000). Theoretically, then, these multiple factors could target the same gene promoters. Alternatively, because the nucleotides flanking the core G-box motif appear to provide higher order binding sequence specificity (Hudson et al., 2003; Toledo-Ortiz et al., 2003), the different factors could be targeted to different genes. Currently, there are insufficient data to distinguish definitively between these extremes or intermediate possibilities. However, functional analysis of the roles of PIF1, PIF3, and PIF4 in light-regulated seedling deetiolation suggests at least partial channeling of phyB signaling into separate facets of the response by these three factors. Null mutants at each of these loci have differential phenotypes in light responsiveness. PIF1 appears to act primarily in precise regulation of the chlorophyll biosynthetic pathway to prevent lethal photo-oxidative damage caused by accumulation of excess free protochlorophyllide (Huq et al., 2004). PIF3 has a complex pattern of regulation, including being necessary for normal chlorophyll accumulation early in the deetiolation process immediately after exposure of seedlings to light (Kim et al., 2003; Bauer et al., 2004; E. Monte and P. Quail, unpublished data). PIF4 appears to act negatively in Rc-regulated hypocotyl and cotyledon cell expansion (Huq and Quail, 2002). It will be informative to compare the global light-regulated gene expression patterns in these AtbHLH mutants to identify the targets of each factor.
The data presented here provide evidence that the capacity of individual AtbHLH proteins to specifically recognize and bind to photoactivated phyB is conferred by the presence of a novel motif, designated APB, in the N-terminal region of the transcription factor. First, there is a strong positive correlation between the presence of the APB in the full-length, native AtbHLH and the capacity to bind to phyB. Of the 12 members of the AtbHLH family examined, the five that display phyB binding all contain the APB motif. Conversely, five of these 12 that do not bind to phyB do not contain the motif. Of the remaining two that do not bind phyB, BHLH023 contains a substitution in one of the otherwise invariant APB residues that we showed eliminates binding when mutated. This protein therefore appears likely to be a natural, noninteracting variant. The reason that full-length PIL1 (BHLH124) does not bind phyB is currently unclear but could be because of interference from other regions in the protein as the isolated APB region does bind, albeit more weakly, to phyB. Second, we provide direct evidence of the necessity of the APB motif for phyB binding in the context of the full-length AtbHLH protein by site-directed mutational analysis. Targeted substitution of invariant residues within the motif completely abrogated phyB binding. We were unable to reproduce our previous observation that a PAS-like domain within PIF3 was apparently involved in binding (Zhu et al., 2000). Third, direct evidence that the APB motif alone is sufficient for conformer-specific phyB binding was obtained. In some cases (PIF3, PIF4, and PIF5), this binding appeared to be somewhat reduced compared with the full-length protein, whereas a striking enhancement of binding was observed for the isolated PIF6 motif, and, as mentioned, a degree of binding by the isolated PIL1 APB motif was uncovered. These data provide evidence that the APB domain is both necessary and sufficient for conformer-specific binding of phyB to multiple AtbHLH proteins but that the apparent affinity of this domain for the photoreceptor may be subject to either positive or negative modulation by other regions of the protein.
Conversely, the APB domain appears to be specific to phyB binding within the phy family. No detectable interaction with the four other Arabidopsis phys was observed. It appears, therefore, that the APB motif provides a photoreceptor-specific recognition module within the AtbHLH family. The absence of this motif from all members of the AtbHLH family other than those specified here suggests that phyB signaling through this family may be confined to the subset of APB-containing members in subfamily 15. On the other hand, the interaction of PIF1 with both phyB and phyA (Huq et al., 2004) suggests the existence of other recognition motifs specific to other members of the phy family. This in turn suggests the possibility that individual members of the AtbHLH family, including divergent members not analyzed here, may carry single or multiple recognition motifs, which would define the interaction profile of each AtbHLH protein with the photoreceptor family. Such multiplicity of interactions would provide the potential for a complex signaling network at the interface between the photoreceptors and their primary reaction partners.
Evidence that the APB-mediated interactions between phyB and target AtbHLH proteins observed here by in vitro binding assays are functionally relevant to phyB signaling in the living cell is provided by our mutant rescue experiments. The data show that the native PIF4 gene, driven by its own promoter, can reverse the hypersensitive mutant phenotype of the pif4 mutant, srl2, when expressed transgenically, whereas an identical construct carrying a single site-directed amino acid substitution in the invariant G35 residue of the APB motif of PIF4 is ineffective. These data suggest that conformer-specific binding of phyB to PIF4 via the APB motif is necessary in vivo for PIF4 function in regulation of phyB-induced seedling deetiolation. Based on these data, we would predict that the functional activity of other APB-containing AtbHLH proteins in phyB signaling would likewise be dependent on an intact APB motif. Conversely, it may be predicted that non-APB-containing AtbHLH proteins will not be involved in phyB-regulated light responses. Consistent with this prediction, analysis of insertional mutants in the natural, non-phyB binding variant BHLH023 indicates the absence of a light-responsiveness phenotype. Another AtbHLH protein, SPT, belongs to the PIF3 group (subfamily 15) but does not contain an APB. Insertional mutants in SPT also exhibit no differential responsiveness to light compared with the wild type (data not shown). Our complete database searches using BLAST features indicate that the APB motif is found only in plants. We found four provisionally predicted rice (Oryza sativa) proteins containing homologies to the APB consensus, NP_912624, NP_911152 (designated, putative PIF4), NP_909897, and CAD32238 (designated, BP-5 protein). Interestingly, like in Arabidopsis, the rice homolog of SPT (AAK98706, putative SPT) lacks the APB motif.
Recent intracellular localization studies using fluorescent protein–tagged phys and PIF3 have provided evidence of rapid colocalization of phyB and PIF3 in subnuclear speckles within minutes of light-induced photoreceptor translocation into the nucleus, suggestive of direct phyB–PIF3 interaction in vivo (Bauer et al., 2004). Moreover, the formation of these early speckles appears to be correlated with rapid, light-induced degradation of the PIF3 protein. It will be of interest to determine whether a native APB sequence motif in PIF3 is necessary for speckle formation and associated PIF3 degradation. Likewise, it will be interesting to examine whether other APB-containing proteins also form light-induced early speckles and become destabilized.
Collectively, the data presented here indicate that the APB domain we have identified provides a phyB-specific recognition module within the AtbHLH family of transcription factors, thereby conferring photoreceptor target specificity on a subset of these factors. Extrapolation of this concept to other phy and AtbHLH family members raises the possibility of a flexible array of combinatorial interactions, ranging from the specific to the redundant, at the interface between the photoreceptors and target transcriptional regulators. Such a configuration would provide the potential for both specific and convergent, overlapping signaling from the individual phys, as well as amplification and diversification of the light signal into multiple pathways mediated by the downstream transcriptional networks regulated by the different target AtbHLHs.
METHODS
Seedling Growth Conditions and Measurements
Seeds were surface sterilized, plated on growth medium without sucrose as described (Hoecker et al., 1999), and exposed to red or far-red light treatments as described (Wagner et al., 1991). Fluence rates were measured using a spectroradiometer (model L1-1800; LI-COR, Lincoln, NE). Hypocotyl length measurements were performed using digital images taken with a Nikon Coolpix 990 (Tokyo, Japan) digital camera and NIH imaging software (National Institutes of Health, Bethesda, MD).
Arabidopsis thaliana Transformations and Transgenic Analysis
The PIF4:PIF4 full length gene (5.5-kb fragment, including ∼2 kb PIF4 promoter sequence and the 5′- and 3′-untranslated regions) was amplified from genomic DNA isolated from wild-type Col-0 using PFU Turbo Polymerase (Stratagene, La Jolla, CA). Site-directed mutagenesis was performed to create the APB (G35A) mutations using the Quick Change site-directed mutagenesis kit (Stratagene). These constructs were cloned into the pZp-121 vector (Hajdukiewicz et al., 1994), introduced into GV3101 (MP90) Agrobacterium tumefaciens, and then used to transform srl2 mutant plants using the floral dip method as described by Clough and Bent (1998). Transgenic seed were selected from screening for resistance on growth medium–Suc plates containing 100 μg/mL of gentamycin, and the resistant plants were transplanted to soil and grown in greenhouse conditions.
In Vitro Coimmunoprecipitation Assay
The AtbHLH full-length cDNAs (for PIF5, PIF6, SPT, BHLH007, BHLH059, and BHLH066) were obtained from the ABRC (Columbus, OH), and BHLH023 and PIL1 cDNAs were amplified from an Arabidopsis cDNA library by PCR. The bait cDNAs were cloned into pET17b in vitro expression vector (Invitrogen, Carlsbad, CA) and sequenced. Bait proteins were expressed as fusions with the GAD sequence at either the N or the C terminus of the AtbHLH sequences as specified. The in vitro expression construct used for phyB was the same as described (Ni et al., 1999). All proteins were expressed in vitro using the TnT transcription/translation system (Promega, Madison, WI) in the presence of [35S] Met. The bait was prepared by incubating the in vitro–expressed fusion proteins with monoclonal anti-GAD antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and Protein A attached to agarose beads for 2 h at 4°C. The bait was washed three times in 1× PBS wash buffer, pH 7.2, containing 0.1% (v/v) Tergitol Nonidet P-40 (Sigma, St. Louis, MO) and one capsule of complete protease inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany). The prey (phyB) was prepared by incubating with phycocyanobilin for 1 h at 4°C in the dark, followed by a saturating 10-min red light pulse of 350 μmol m−2 s−1 (for Pfr conformer). One-half of the red light–treated prey was further treated with a saturating 5-min far-red light pulse of 297 μmol m−2 s−1 (for reconverted Pr). The prepared bait and prey were incubated together in 1× PBS binding buffer (1× PBS wash buffer including 0.1% BSA) for 2 h at 4°C, and the pellets were washed three times in the dark with the wash buffer and separated on SDS-PAGE. Quantifications were performed using phosphor imaging software.
RNA Isolation and Analysis
Total RNA was isolate using the Qiagen RNeasy plant mini kit (Valencia, CA) from 4-d-old seedlings either grown in the dark or exposed to Rc for 3 h. The full-length PIF4 open reading frame was used as a probe for RNA gel blots containing total RNA from the PIF4FL transgenic seedlings.
Supplementary Material
Acknowledgments
We thank Karen Kaczorowski for providing phycocyanobilin, the ABRC (Columbus, OH) for providing the AtbHLH EST clones, and Jim Tepperman, Henrik Johanneson, and Katherine Krolikowski for critical discussions. This work was supported by grants from the National Institutes of Health (GM47475), the Tory Mesa Research Institute, San Diego, CA, Department of Energy Basic Energy Sciences (DE-FG03-87ER13742), and the USDA Current Research Information Service (5335-21000-010-00D).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Peter H. Quail (quail@nature.berkeley.edu).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.025643.
References
- Bailey, P.C., Martin, C., Toledo-Ortiz, G., Quail, P.H., Huq, E., Heim, M.A., Jakoby, J., Werber, M., and Weisshaar, B. (2003). Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana. Plant Cell 15, 2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, D., Viczián, A., Kircher, S., Nobis, T., Nitschke, R., Kunkel, T., Panigrahi, K., Ádám, E., Fejes, E., Schäfer, E., and Nagy, F. (2004). COP1 and multiple photoreceptors control degradation of PIF3, a transcription factor, required for light signalling in Arabidopsis. Plant Cell 16, 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Corpet, F. (1988). Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16, 10881–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild, C.D., Schumaker, M.A., and Quail, P.H. (2000). HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev. 14, 2371–2399. [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz, P., Svab, Z., and Maliga, P. (1994). The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25, 989–994. [DOI] [PubMed] [Google Scholar]
- Heim, M.A., Jakoby, M., Werber, M., Martin, C., Weisshaar, B., and Bailey, P.C. (2003). The basic helix-loop-helix transcription factor family in plants: A genome-wide study of protein structure and functional diversity. Mol. Biol. Evol. 20, 735–747. [DOI] [PubMed] [Google Scholar]
- Heisler, M.G.B., Atkinson, A., Bylstra, Y.H., Walsh, R., and Smyth, D.R. (2001). SPATULA, a gene that controls development of carpel margin tissues in Arabidopsis, encodes a bHLH protein. Development 128, 1089–1098. [DOI] [PubMed] [Google Scholar]
- Hoecker, U., Tepperman, J.M., and Quail, P.H. (1999). SPA1: A WD-repeat protein specific to phytochrome A signal transduction. Science 284, 496–499. [DOI] [PubMed] [Google Scholar]
- Hudson, M.E., Lisch, D.R., and Quail, P.H. (2003). The FHY3 and FAR1 genes encode transposase related proteins involved in regulation of gene expression by the phytochrome A-signaling pathway. Plant J. 34, 453–471. [DOI] [PubMed] [Google Scholar]
- Huq, E., Al-Sady, B., Hudson, M.E., Kim, C., Apel, K., and Quail, P.H. (2004). PHYTOCHROME-INTERACTING FACTOR 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305, 1937–1941. [DOI] [PubMed] [Google Scholar]
- Huq, E., Al-Sady, B., and Quail, P.H. (2003). Nuclear translocation of the photoreceptor phytochrome B is necessary for its biological function in seedling photomorphogenesis. Plant J. 35, 660–664. [DOI] [PubMed] [Google Scholar]
- Huq, E., and Quail, P.H. (2002). PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling pathway. EMBO J. 21, 2441–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, R., Kikis, E.A., and Quail, P.H. (2003). EARLY FLOWERING 4 functions in phytochrome B-regulated seedling de-etiolation. Plant Physiol. 133, 1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J., Yi, H., Choi, G., Shin, B., Song, P.-S., and Choi, G. (2003). Functional characterization of PIF3 in phytochrome-mediated light signal transduction. Plant Cell 15, 2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher, S., Gil, P., Kozma-Bognár, L., Fejes, E., Speth, V., Husselstein-Muller, T., Bauer, D., Ádám, E., Schäfer, E., and Nagy, N. (2002). Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A, B, C, D, and E is regulated differentially by light and exhibits a diurnal rhythm. Plant Cell 14, 1541–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinéz-García, J.F., Huq, E., and Quail, P.H. (2000). Direct targeting of light signals to a promoter element-bound transcription factor. Science 288, 859–863. [DOI] [PubMed] [Google Scholar]
- Matsushita, T., Mochizuki, N., and Nagatani, A. (2003). Dimers of the N-terminal domain of phytochrome B are functional in the nucleus. Nature 424, 571–574. [DOI] [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1998). PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95, 657–667. [DOI] [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1999). Binding of phytochrome B to its nuclear signaling partner PIF3 is reversibly induced by light. Nature 400, 781–784. [DOI] [PubMed] [Google Scholar]
- Quail, P.H. (1998). The phytochrome family: Dissection of functional roles and signalling pathways among family members. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 1399–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail, P.H. (2002. a). Phytochrome photosensory signaling networks. Nat. Rev. Mol. Cell Biol. 3, 85–93. [DOI] [PubMed] [Google Scholar]
- Quail, P.H. (2002. b). Photosensory perception and signaling in plant cells: New paradigms? Curr. Opin. Cell Biol. 14, 180–188. [DOI] [PubMed] [Google Scholar]
- Quail, P.H., Boylan, M.T., Parks, B.M., Short, T.W., Xu, Y., and Wagner, D. (1995). Phytochromes: Photosensory perception and signal transduction. Science 268, 675–680. [DOI] [PubMed] [Google Scholar]
- Rajani, S., and Sundaresan, V. (2001). The Arabidopsis myc/bHLH gene ALCATRAZ enables cell separation in fruit dehescence. Curr. Biol. 11, 1914–1922. [DOI] [PubMed] [Google Scholar]
- Sakamoto, K., and Nagatani, A. (1996). Nuclear localization activity of phytochrome B. Plant J. 10, 859–868. [DOI] [PubMed] [Google Scholar]
- Shimizu-Sato, S., Huq, E., Tepperman, J.M., and Quail, P.H. (2002). A light-switchable gene promoter system. Nat. Biotechnol. 10, 1041–1044. [DOI] [PubMed] [Google Scholar]
- Smith, H. (2000). Phytochromes and light signal perception by plants: An emerging synthesis. Nature 407, 585–591. [DOI] [PubMed] [Google Scholar]
- Tepperman, J.M., Hudson, M.E., Khanna, R., Zhu, T., Chang, S.H., Wang, X., and Quail, P.H. (2004). Expression profiling of phyB mutant demonstrates substantial contribution of other phytochromes to red-light-regulated gene expression during seedling de-etiolation. Plant J. 38, 725–739. [DOI] [PubMed] [Google Scholar]
- Tepperman, J.M., Zhu, T., Chang, H.-S., Wand, X., and Quail, P.H. (2001). Multiple transcription-factor genes are early targets of phytochrome A signaling. Proc. Natl. Acad. Sci. USA 98, 9437–9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Ortiz, G., Huq, E., and Quail, P.H. (2003). The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 15, 1749–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, D.R., Tepperman, J.M., and Quail, P.H. (1991). Overexpression of phytochrome B induces a short hypocotyl phenotype in transgenic Arabidopsis. Plant Cell 3, 1275–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam, G.C., and Devlin, P.F. (1997). Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Environ. 20, 752–758. [Google Scholar]
- Yamashino, T., Matsushika, A., Fujimori, T., Sato, S., Kato, T., Tabata, S., and Mizuno, T. (2003). A link between circadian-controlled bHLH factors and the APRR1/TOC1 quintet in Arabidopsis thaliana. Plant Cell Physiol. 44, 619–629. [DOI] [PubMed] [Google Scholar]
- Zhu, Y., Tepperman, J.M., Fairchild, C.D., and Quail, P.H. (2000). Phytochrome B binds with greater apparent affinity than phytochrome A to the basic helix-loop-helix factor PIF3 in a reaction requiring the PAS domain of PIF3. Proc. Natl. Acad. Sci. USA 97, 13419–13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.