Abstract
Objective:
To review population-based studies of the prevalence and incidence of epilepsy worldwide and use meta-analytic techniques to explore factors that may explain heterogeneity between estimates.
Methods:
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses standards were followed. We searched MEDLINE and EMBASE for articles published on the prevalence or incidence of epilepsy since 1985. Abstract, full-text review, and data abstraction were conducted in duplicate. Meta-analyses and meta-regressions were used to explore the association between prevalence or incidence, age group, sex, country level income, and study quality.
Results:
A total of 222 studies were included (197 on prevalence, 48 on incidence). The point prevalence of active epilepsy was 6.38 per 1,000 persons (95% confidence interval [95% CI] 5.57–7.30), while the lifetime prevalence was 7.60 per 1,000 persons (95% CI 6.17–9.38). The annual cumulative incidence of epilepsy was 67.77 per 100,000 persons (95% CI 56.69–81.03) while the incidence rate was 61.44 per 100,000 person-years (95% CI 50.75–74.38). The prevalence of epilepsy did not differ by age group, sex, or study quality. The active annual period prevalence, lifetime prevalence, and incidence rate of epilepsy were higher in low to middle income countries. Epilepsies of unknown etiology and those with generalized seizures had the highest prevalence.
Conclusions:
This study provides a comprehensive synthesis of the prevalence and incidence of epilepsy from published international studies and offers insight into factors that contribute to heterogeneity between estimates. Significant gaps (e.g., lack of incidence studies, stratification by age groups) were identified. Standardized reporting of future epidemiologic studies of epilepsy is needed.
Epilepsy is a serious neurologic condition associated with stigma,1 psychiatric comorbidity,2 and high economic costs.3 The WHO's 2010 Global Burden of Disease study ranks epilepsy as the second most burdensome neurologic disorder worldwide in terms of disability-adjusted life years.4
Studies investigating the prevalence and incidence of epilepsy are increasingly common, particularly in low- and middle-income countries. Estimates of the prevalence and incidence of epilepsy worldwide vary considerably, likely reflecting differences in measurement and reporting, along with clinical characteristics such as etiology and seizure type. Previous systematic reviews of the prevalence of epilepsy focused on specific regions (China,5 Europe,6 Latin America,7 and Arab countries8) and prior reviews on the incidence of epilepsy did not use meta-analyses to explore associated factors.9,10 Few of these studies explored potential sources of heterogeneity between estimates or they examined both prevalence and incidence globally.
Our aim was to estimate the prevalence and incidence of epilepsy from international studies, and to quantify the burden of epilepsy using meta-analytic techniques. We also explore the sources of heterogeneity between estimates, assessing factors such as age, sex, country income level, epilepsy syndrome, seizure type, epilepsy etiology, and study quality.
METHODS
Search strategy.
The systematic review was conducted according to a predetermined protocol and adhered to the Preferred Reporting Items for Systematic review and Meta-Analysis.11 The search strategy (appendix e-1 at Neurology.org) was developed by experts in epilepsy and epidemiology and an academic research librarian (D.L.L.). Search terms included prevalence, incidence, and epidemiology in conjunction with epilepsy, seizure, and convulsion. The search was conducted from 1985 to October 22, 2013, in MEDLINE and EMBASE. Articles in English or French were included. The reference lists of included articles were also hand searched. References were managed using EndNote X5.12
Study selection.
Abstracts and titles of all references were screened in duplicate by 2 independent reviewers to identify original, population-based studies on the prevalence or incidence of epilepsy. Two independent reviewers screened the full-text articles of abstracts identified in the first stage of review. Articles were included if they met the following criteria: (1) original research, (2) population-based (selecting the entire population or using probability-based sampling methods), (3) reported a prevalence or incidence of epilepsy (or raw numbers that allowed the calculation of an estimate). Disagreements pertaining to the inclusion of articles were resolved by consensus or involvement of a third author as necessary.
Data extraction and study quality.
Data abstraction was completed in duplicate by 2 independent reviewers using a standardized data collection form. When multiple articles reporting data from the same study population were identified, the most comprehensive data were used. When studies reported on different data collection years or subgroups (sex, age), all nonoverlapping data were included. Age, sex, study location, sources of ascertainment, and definitions/diagnostic criteria for epilepsy were extracted. Epilepsy prevalence or incidence estimates, raw numbers, and confidence intervals (CIs) (when provided) were recorded along with any stratified results by age, sex, or year of data collection.
The quality of included studies was evaluated using standard assessment tools13,14 (appendix e-2), and included sample representativeness, condition assessment, and statistical methods. Each study was given a quality score of 0 to 8 based on fulfillment of the quality criteria.
Data synthesis and analysis.
Prevalence estimates were divided into 2 groups: point prevalence and annual period prevalence. Point prevalence is the number of existing cases of epilepsy in a population, over the total population at one specific point in time (e.g., on June 30, 2013). Period prevalence includes both existing and new cases of epilepsy in a population over the total population over a defined period of time (e.g., between January 1 and December 31, 2013).
Estimates of prevalence were additionally categorized into 2 mutually exclusive groups based on the definitions provided within individual articles: active and lifetime. Lifetime prevalence was conceptualized differently than active epilepsy prevalence, as it can be considered a type of period prevalence, conditional on survival, where the period is the time between birth and assessment. We reported on the point prevalence of active epilepsy, the annual period prevalence of active epilepsy, and the lifetime prevalence of epilepsy. These different categories take into account (1) the clinical differences of those with active vs inactive epilepsy and (2) how the time period of assessment may influence reported estimates.
Incidence estimates were stratified into cumulative incidence and incidence rate. Cumulative incidence is the number of new cases of epilepsy over the total number of people in the population at risk for developing epilepsy during a specified period of time (e.g., 64.90 persons with epilepsy per 100,000 persons during 1 year). We used the following formula to calculate annual cumulative incidence based on estimates provided in the articles: cumulative incidencen years = 1 − exp (− annual rate × n). The incidence rate of epilepsy is the number of new cases of epilepsy over the total amount of person-time at risk for developing epilepsy during a specified period of time (e.g., 68.40 persons with epilepsy per 100,000 person-years). In both cases the numerator is the number of new cases, while the denominator will differ as the incidence rate also incorporates time as a unit.
Seizure type and epilepsy etiology were categorized according to the most recent International League Against Epilepsy classification15; these subgroup analyses were only available for estimates of active point prevalence of epilepsy. Country income level was dichotomized into low–middle and high based on the World Bank's classification.16
Age was stratified into 2 broad groups for age-specific analysis: (1) those younger than 18 years and those 18 years and older and (2) in 10-year groups: 0–9, 10–19, 20–29, 30–39, 40–49, 50–59, and 60 and above, if available, and using only studies that reported on all 7 categories.
Studies were included in the meta-analysis if they reported the number of cases and sample denominator, the estimate with 95% CI, or the information with which to calculate the estimated prevalence or incidence. Age, sex, country income level, seizure type, and epilepsy etiology were examined categorically. Only estimates that included persons of all ages were included in the pooled analyses (except for age-specific analyses). The association of age, sex, country income level, and study quality with prevalence and incidence estimates was assessed using meta-regression if there were 2 studies or more per grouping. Boxplots assessed the presence of outliers (defined as an estimate more than 1.5 times the interquartile range beyond the first [p25] or third [p75] quartiles).17 We reported overall median prevalence or incidence and accompanying first and third quartiles (p25–p75).
The I2 was used to quantify the magnitude of between-study heterogeneity and the Cochrane Q statistic was calculated to determine significance. A priori, we decided to report the pooled, weighted estimate generated by random effects models, because we hypothesized a high degree of between-study heterogeneity. Publication bias was investigated visually using funnel plots and statistically using Begg18 and Egger19 tests.
R version 2.14 was used for all meta-analyses and meta-regressions.20 The meta package was used to generate the forest plots, pooled estimates, and to assess for publication bias.21 Meta-regression using restricted maximum likelihood estimation was conducted using the metafor package.22 Boxplots were generated using STATA v12.1.23 A p value <0.05 was deemed statistically significant.
RESULTS
Identification and description of studies.
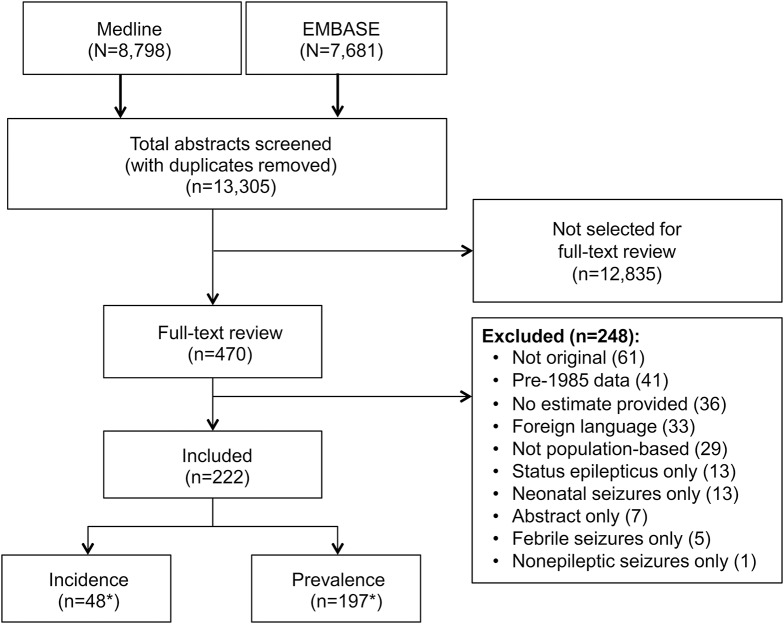
The search strategy yielded a total of 16,479 abstracts: 8,798 from MEDLINE and 7,681 from EMBASE (figure 1). We screened 13,305 unique abstracts and 470 articles met the criteria for full-text review, of which 248 were excluded. Hand searching did not contribute additional articles. A total of 197 articles reported on prevalence of epilepsy, 48 on incidence, and 24 on both (tables e-1 and e-2, and appendix e-3 for reference list).
Figure 1. Study flow diagram.
*Incidence and prevalence studies equal greater than 222 because 24 articles reported both incidence and prevalence.
Prevalence of active epilepsy.
Seventy-three studies reported on the point prevalence of active epilepsy and of those, 67 estimates (63 unique studies) were eligible for inclusion in the meta-analysis. The pooled point prevalence of active epilepsy was 6.38 per 1,000 persons (95% CI 5.57–7.30) (table 1, figure e-1). Heterogeneity existed between estimates (I2 = 99.6%, Q p value <0.0001). The median point prevalence of active epilepsy was 5.40 per 1,000 persons (p25–p75, 3.90–9.99). Four outliers were identified: 104.97 per 1,000 persons (95% CI 68.60–160.63) from Cameroon,24 57.23 per 1,000 persons (95% CI 36.98–88.56) from Panama,25 29.46 per 1,000 persons (95% CI 21.16–41.03) from Ethiopia,26 and 22.62 per 1,000 persons (95% CI 9.51–53.82) from Ecuador.27
Table 1.
Pooled estimates for each type of prevalence (per 1,000)
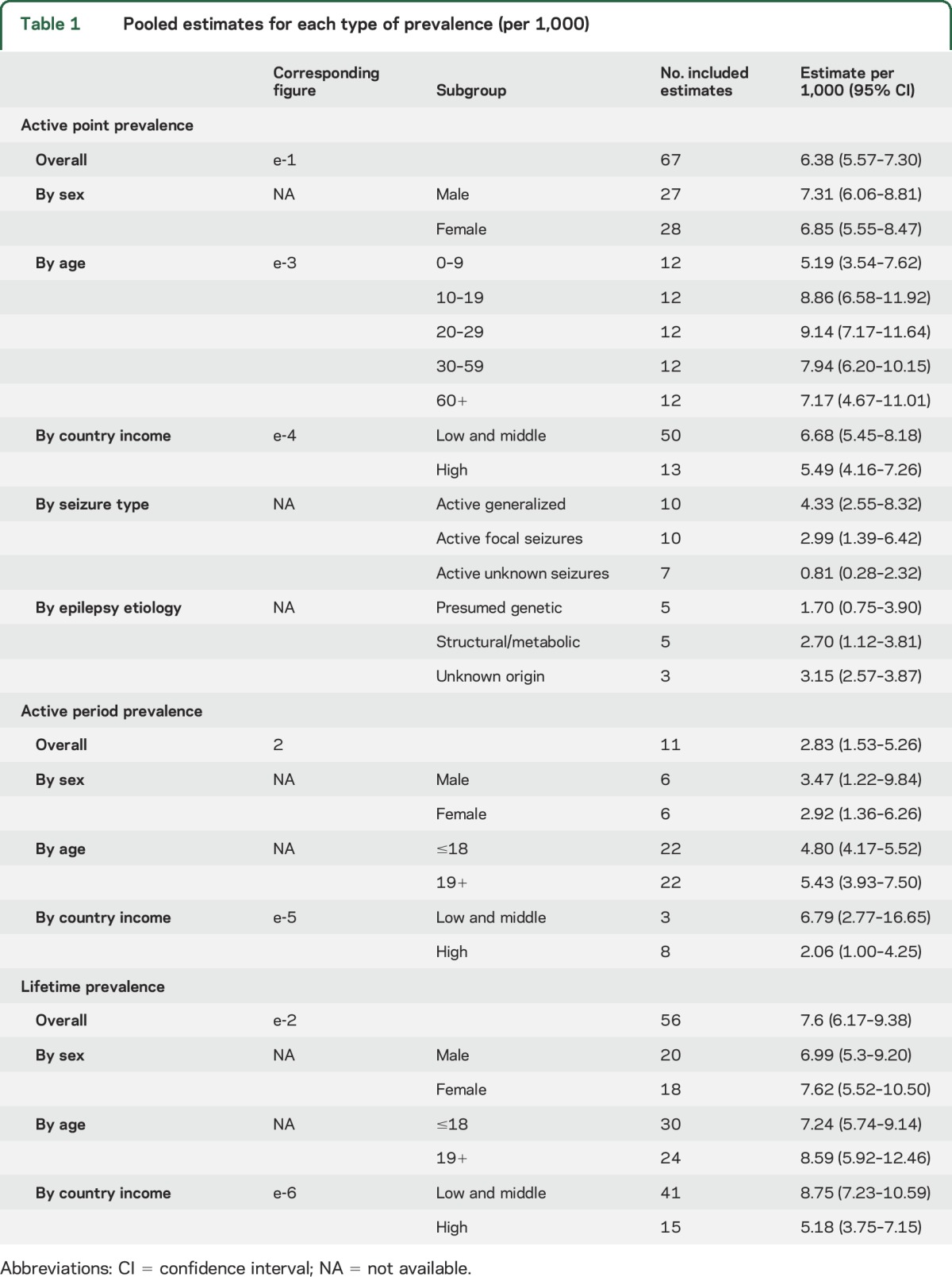
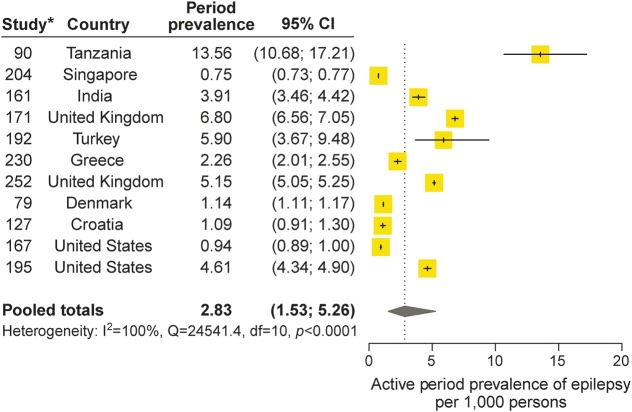
Twelve studies reported on the annual period prevalence of active epilepsy, and 11 were eligible for inclusion in the meta-analysis. The pooled annual period prevalence of active epilepsy was 2.83 per 1,000 persons (95% CI 1.53–5.26) (table 1, figure 2). Heterogeneity existed between estimates (I2 = 100%, Q p value <0.0001). The median annual period prevalence of active epilepsy was 3.91 per 1,000 persons (p25–p75, 1.14–5.15). One outlier from Tanzania (13.56 per 1,000 persons [95% CI 10.68–17.21]) was identified.28
Figure 2. Active period prevalence of epilepsy.
*Study numbers correspond to references in appendix e-3. CI = confidence interval.
Lifetime prevalence of epilepsy.
Sixty-seven studies reported on the lifetime prevalence of epilepsy, and 56 were eligible for inclusion in the meta-analysis. The pooled lifetime prevalence of epilepsy was 7.60 per 1,000 persons (95% CI 6.17–9.38) (table 1, figure e-2). Heterogeneity existed between estimates (I2 = 99.7%, Q p value <0.0001). The median lifetime prevalence of epilepsy was 7.06 per 1,000 persons (p25–p75, 4.74–11.23). Three studies were identified as outliers: from Panama (75.30 per 1,000 persons [95% CI 51.65–109.78]),25 Cameroon (49.00 per 1,000 persons [95% CI 40.19–59.74]),29 and Honduras (23.33 per 1,000 persons [95% CI 19.93–27.31]).30
Cumulative incidence of epilepsy.
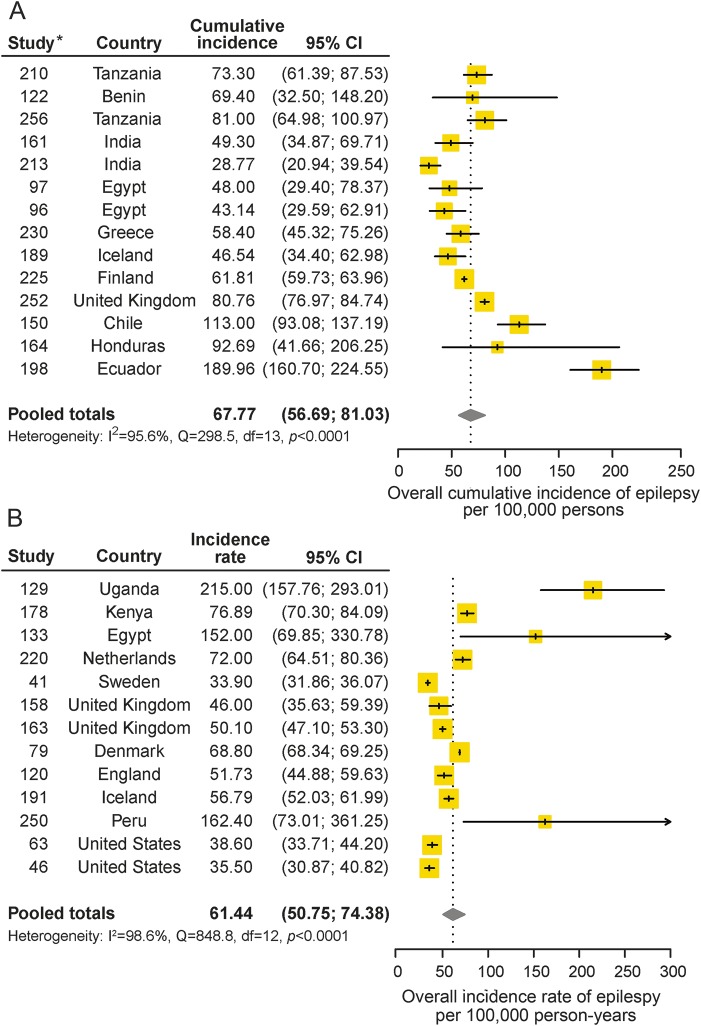
Thirty-one studies reported on the cumulative incidence of epilepsy, 14 of which were included in the meta-analysis. The pooled annual cumulative incidence of epilepsy was 67.77 per 100,000 persons (95% CI 56.69–81.03). Heterogeneity existed between estimates (I2 = 95.6%, Q p value <0.0001) (table 2, figure 3A).
Table 2.
Pooled estimates for each type of incidence of epilepsy
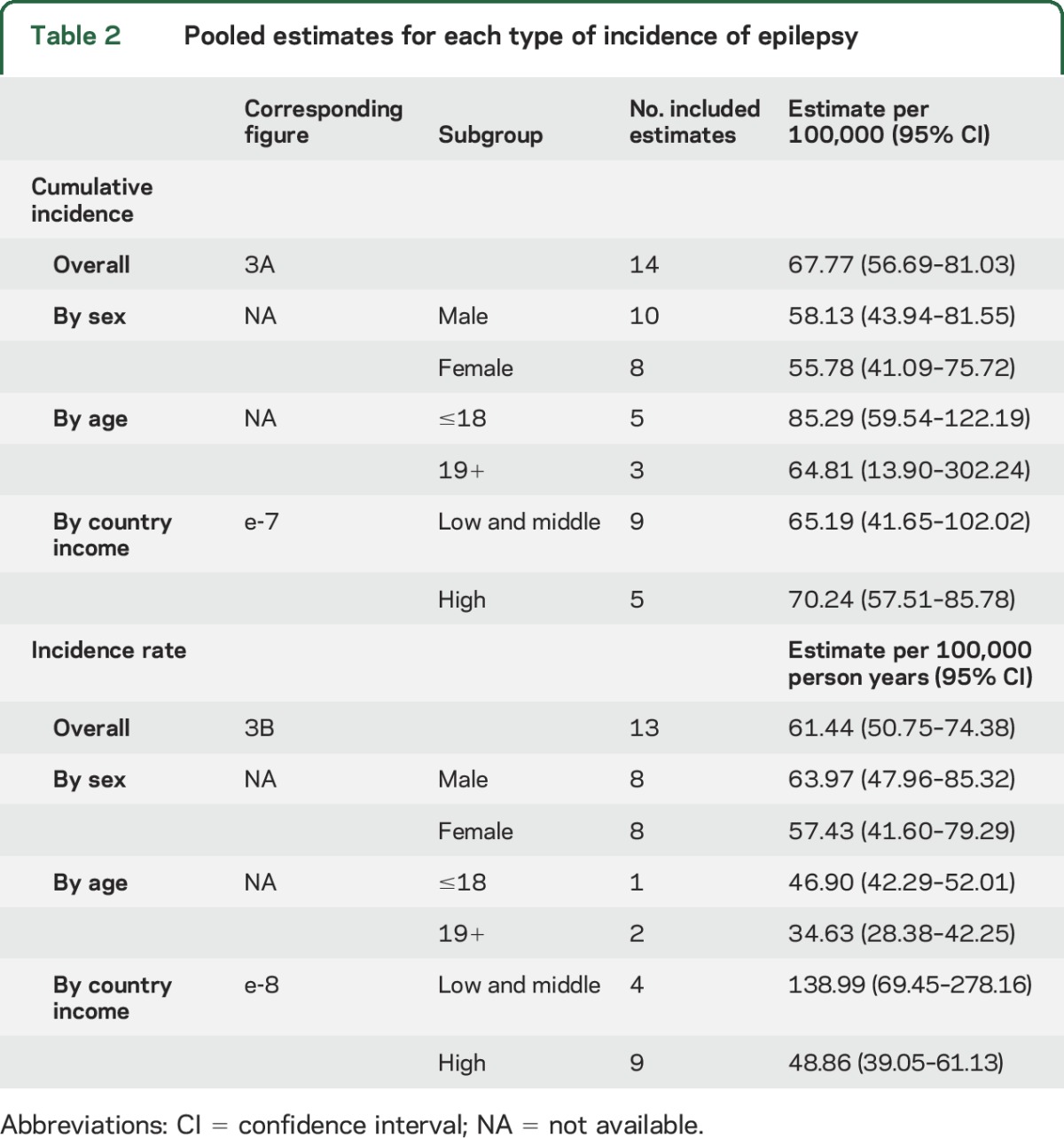
Figure 3. Incidence of epilepsy.
(A) Cumulative incidence of epilepsy. (B) Incidence rate of epilepsy. Study numbers correspond to references in appendix e-3. CI = confidence interval.
The median cumulative incidence of epilepsy was 65.61 per 100,000 persons (p25–p75, 48.00–81.00), and there was one outlier from Andean Ecuador (189.96 per 100,000 persons [95% CI 160.70–224.55]).31
Incidence rate of epilepsy.
Nineteen studies reported on the incidence rate of epilepsy, 13 of which were included in the meta-analysis. The pooled incidence rate of epilepsy was 61.44 per 100,000 person-years (95% CI 50.75–74.38). Heterogeneity existed between estimates (I2 = 98.6%, Q p value <0.0001) (table 2, figure 3B).
The median incidence rate of epilepsy was 56.79 per 100,000 person-years (p25–p75, 46.00–76.89), and there were 3 outliers (in the Assiut Governorate in Egypt,32 Chile,33 and West Uganda34).
Sources of heterogeneity.
Estimates of the prevalence and incidence of epilepsy by sex, age, country income level, and prevalence by seizure type and epilepsy etiology are presented in appendix e-4 and figures e-3 (pooled point prevalence of epilepsy in 10-year age groups), e-4 (active point prevalence of epilepsy by country income level), e-5 (active period prevalence of epilepsy by country income level), e-6 (lifetime prevalence of epilepsy by country income level), e-7 (cumulative incidence of epilepsy by country income level), and e-8 (incidence rate of epilepsy by country income level).
Publication bias.
There was no evidence of publication bias for any of the estimates of prevalence and incidence using visual inspection of funnel plots or Begg or Egger test (all p > 0.05).
Study quality.
The median study quality score was 6/8 (range 2–8) for the prevalence of epilepsy and 7/8 for studies of incidence (range 4–8) (tables e-3 and e-4). Meta-regression found no effect of study quality on the estimates of epilepsy prevalence or incidence, all p > 0.05.
DISCUSSION
This systematic review and meta-analysis of international studies on the prevalence and incidence of epilepsy used subgroups analyses to examine the relationship among socioeconomic, demographic, and clinical factors that may influence the prevalence and incidence of epilepsy.
Age has commonly been associated with the prevalence and incidence of epilepsy. Congruent with previous descriptive reports,35 we found that the incidence of epilepsy was generally higher in the youngest and oldest age groups; however, there were insufficient studies to perform a meta-analysis. The trend in the point prevalence of active epilepsy by 10-year age groups is consistent with previous reports.35 Prevalence is expectedly lowest early in life, increasing to its highest level during adolescence and early adulthood, decreases after age 30, and remains fairly constant for the remainder of life. The number of studies included in this pooled analysis was limited by the reporting of common age groupings in individual studies; only 12 of 63 eligible studies used common age groups, and analysis by 10-year age groups was not possible for estimates of incidence due to the small number of studies. The prevalence of epilepsy was slightly higher in studies of persons over the age of 18 compared to those under 18, while the reverse was true for the incidence of epilepsy. This finding is consistent with previous studies of the epidemiology of epilepsy in Europe.6 Elevated mortality could prevent the lifetime prevalence of epilepsy from increasing significantly with age (particularly in older age groups) when the incidence of epilepsy is not zero, as is the case here.
Sex, while not commonly thought to affect the occurrence of epilepsy, may contribute to differences in epilepsy incidence.35 The incidence of epilepsy tended to be higher in males than females. Some suggest that females may be more likely to conceal their epilepsy diagnosis if they live in a country where they would be considered unmarriageable or socially marginalized.35,36
There was no difference between high and low–middle income countries for the point prevalence of active epilepsy or cumulative incidence. However, the active annual period prevalence was significantly higher in low–middle income countries. Interestingly, more low–middle income countries (14/50; 28%) reported an active point prevalence of greater than 10 per 1,000 persons, compared to high-income countries (2/13; 15%). The incidence rate of epilepsy was also higher in low–middle income countries compared to high–middle income countries, which is corroborated by others.10 This trend was reversed for estimates of cumulative incidence. Factors such as premature mortality, etiology (e.g., CNS infections), variations in treatment, and study methodology (e.g., case ascertainment and case definition) may differ between high and low–middle income countries,37 which may partially explain our results. Regardless, the higher estimates in low- and middle-income countries are noteworthy from a public health standpoint as these low resource areas are also those with the highest treatment gap.38
Single pooled estimates of prevalence and incidence should be interpreted with caution, given the amount of heterogeneity between studies. Given this heterogeneity, we present median estimates along with the pooled estimates. Though we explored a number of factors that may partially explain high levels of heterogeneity, including age, sex, and country income level, we were unable to employ a single model to account for these factors together. A number of studies with high prevalence and incidence were identified as outliers (all from Latin America, Africa, or the Middle East), which may further influence heterogeneity. The authors of these studies commonly hypothesize that the presence of CNS infections or antibodies (e.g., neurocysticercosis, cysticercosis antibody),25,27–30 consanguinity or family history of epilepsy,24–26,29 and perinatal/prenatal risk factors28–30 may explain the higher prevalence and incidence.
The current study pooled over 124 million persons and over 655,000 persons with epilepsy, and used meta-regression to statistically examine the effect of many sources of heterogeneity. However, our study is not without limitations. There was heterogeneity between estimates of prevalence and incidence, which could be due to variable sampling methods, case ascertainment, and diagnostic methods. The quality of the included studies varied and some studies provided little information on sampling and data collection methodologies, though study quality was not associated with prevalence and incidence estimates. It was also impossible to conduct meta-analyses between some groups due to a smaller number of studies assessing those factors (e.g., under vs over age 65 years). Ideally, a multivariable meta-regression would have been employed to deal with the possible confounding effects of variables such as age and location, though this would have required a very large number of studies, and as such only stratified estimates are provided. Our finding that the annual period prevalence of epilepsy was lower than the point prevalence was unexpected and should be interpreted with caution. This finding was likely due to the large amount of heterogeneity (>99% for prevalence studies) that existed between these 2 groups of studies.
This systematic review and meta-analysis presents information on the burden of epilepsy from international studies. The focus on population-based studies allows for the results to be more applicable to primary care settings, where much of epilepsy care is provided. Few studies reported on the prevalence and incidence of epilepsy by seizure type or etiology, and there were surprisingly no studies from high-income regions such as Australia that were identified or met our eligibility criteria. These are considerable gaps that must be addressed in future work. The possible effect of stigma and cultural differences in epilepsy reporting (or in seeking medical attention) also needs to be explored in future studies as it may explain some of the lower estimates reported in certain regions, where the burden of epilepsy would be expected to be much higher. Methodologic factors contributing to study heterogeneity should also be explored, including data collection methods, sources of case ascertainment, and criteria used for assessment. Future epilepsy epidemiologic studies should consider following the Standards of Reporting of Neurological Disorders checklist39 and published Standards for Epidemiologic Studies and Surveillance of Epilepsy40 to enhance the quality of reporting of such studies and decrease the heterogeneity between studies to facilitate international comparisons.
Supplementary Material
ACKNOWLEDGMENT
This study is part of the National Population Health Study of Neurological Conditions. The authors thank the membership of the Neurological Health Charities Canada and the Public Health Agency of Canada for their contributions and Dr. Emilio Perucca and Dr. Solomon L. Moshé for feedback.
GLOSSARY
- CI
confidence interval
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
K.M. Fiest: substantial contribution to acquisition, analysis, and interpretation of data, drafting the work, and revising it critically for intellectual content. K.M. Sauro: substantial contribution to acquisition, analysis, and interpretation of data, drafting the work, and revising it critically for intellectual content. S. Wiebe: substantial contribution to conception or design of the work, interpretation of data, revising the work critically for intellectual content. S.B. Patten: substantial contribution to conception or design of the work, interpretation of data, revising the work critically for intellectual content. C.S. Kwon: substantial contribution to acquisition of data and revising the work critically for intellectual content. J. Dykeman: substantial contribution to acquisition, analysis, and interpretation of data and revising the work critically for intellectual content. T. Pringsheim: obtained study funding and provided substantial contribution to conception or design of the work, interpretation of data, revising the work critically for intellectual content. D.L. Lorenzetti: substantial contribution to conception or design of the work, interpretation of data, revising the work critically for intellectual content. N. Jette: obtained study funding and provided substantial contribution to conception or design of the work, acquisition, analysis, and interpretation of data, drafting the work, and revising it critically for intellectual content.
STUDY FUNDING
Funding provided by the Public Health Agency of Canada to N. Jetté and T. Pringsheim. The opinions expressed in this publication are those of the authors/researchers and do not necessarily reflect the official views of the Public Health Agency of Canada.
DISCLOSURE
K. Fiest held a studentship from Alberta Innovates Health Solutions during the study period. K. Sauro held a studentship from Alberta Innovates Health Solutions during the study period. S. Wiebe holds the Hopewell Professorship of Clinical Neurosciences Research from the Hotchkiss Brain Institute. S. Patten is a Senior Health Scholar with Alberta Innovates Health Solutions. C. Kwon, J. Dykeman, T. Pringsheim, and D. Lorenzetti report no disclosures relevant to the manuscript. N. Jette holds a Canada Research Chair in Neurologic Health Services Research and held an Alberta Innovates Health Solutions Population Health Investigator Award during the study period. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Fiest KM, Birbeck GL, Jacoby A, Jette N. Stigma in epilepsy. Curr Neurol Neurosci Rep 2014;14:444. [DOI] [PubMed] [Google Scholar]
- 2.Tellez-Zenteno JF, Patten SB, Jette N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia 2007;48:2336–2344. [DOI] [PubMed] [Google Scholar]
- 3.Begley CE, Beghi E. The economic cost of epilepsy: a review of the literature. Epilepsia 2002;43(suppl 4):3–9. [DOI] [PubMed] [Google Scholar]
- 4.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–2223. [DOI] [PubMed] [Google Scholar]
- 5.Gu L, Liang B, Chen Q, et al. Prevalence of epilepsy in the People's Republic of China: a systematic review. Epilepsy Res 2013;105:195–205. [DOI] [PubMed] [Google Scholar]
- 6.Forsgren L, Beghi E, Oun A, Sillanpaa M. The epidemiology of epilepsy in Europe: a systematic review. Eur J Neurol 2005;12:245–253. [DOI] [PubMed] [Google Scholar]
- 7.Burneo JG, Tellez-Zenteno J, Wiebe S. Understanding the burden of epilepsy in Latin America: a systematic review of its prevalence and incidence. Epilepsy Res 2005;66:63–74. [DOI] [PubMed] [Google Scholar]
- 8.Benamer HT, Grosset DG. A systematic review of the epidemiology of epilepsy in Arab countries. Epilepsia 2009;50:2301–2304. [DOI] [PubMed] [Google Scholar]
- 9.Kotsopoulos IA, van Merode T, Kessels FG, de Krom MC, Knottnerus JA. Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia 2002;43:1402–1409. [DOI] [PubMed] [Google Scholar]
- 10.Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Incidence of epilepsy: a systematic review and meta-analysis. Neurology 2011;77:1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EndNote [computer program], version X5. San Francisco, CA: Clarivate Analytics; 2011. [Google Scholar]
- 13.Boyle MH. Guidelines for evaluating prevalence studies. Evid Based Ment Health 1998;1:37–39. [Google Scholar]
- 14.Loney PL, Chambers LW, Bennett KJ, Roberts JG, Strafford PW. Critical appraisal of health literature: prevalence or incidence of a health problem. Chronic Dis Can 1998;19:170–176. [PubMed] [Google Scholar]
- 15.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010;51:676–685. [DOI] [PubMed] [Google Scholar]
- 16.World Bank. Countries and Economies [online]. Available at: http://data.worldbank.org/country. Accessed August 5, 2016. [Google Scholar]
- 17.Hamilton L. Graphs: statistics with STATA: updated for version 10. Belmont, CA: Brooks/Cole, Cengage Learning; 2009: 98. [Google Scholar]
- 18.Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101. [PubMed] [Google Scholar]
- 19.Egger M, Smith G. Bias in meta-analysis detected by simple, graphical test. Br Med J 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R: A Language and Environment for Statistical Computing [Computer Program]. Vienna: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 21.Schwarzer G. Meta: an R package for meta-analysis. R News 2007;7:40–45. [Google Scholar]
- 22.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. [Google Scholar]
- 23.STATA [computer program], version 12.1. College Station, TX: StataCorp; 2011. [Google Scholar]
- 24.Prischich F, De Rinaldis M, Bruno F, et al. High prevalence of epilepsy in a village in the Littoral Province of Cameroon. Epilepsy Res 2008;82:200–210. [DOI] [PubMed] [Google Scholar]
- 25.Gracia F, de Lao SL, Castillo L, et al. Epidemiology of epilepsy in Guaymi Indians from Bocas del Toro Province, Republic of Panama. Epilepsia 1990;31:718–723. [DOI] [PubMed] [Google Scholar]
- 26.Almu S, Tadesse Z, Cooper P, Hackett R. The prevalence of epilepsy in the Zay Society, Ethiopia: an area of high prevalence. Seizure 2006;15:211–213. [DOI] [PubMed] [Google Scholar]
- 27.Basch EM, Cruz ME, Tapia D, Cruz A. Prevalence of epilepsy in a migrant population near Quito, Ecuador. Neuroepidemiology 1997;16:94–98. [DOI] [PubMed] [Google Scholar]
- 28.Dent W, Helbok R, Matuja WB, Scheunemann S, Schmutzhard E. Prevalence of active epilepsy in a rural area in South Tanzania: a door-to-door survey. Epilepsia 2005;46:1963–1969. [DOI] [PubMed] [Google Scholar]
- 29.Njamnshi A, Sini V, Djientcheu V, et al. Risk factors associated with epilepsy in a rural area in Cameroon: a preliminary study. Afr J Neurol Sci 2007;26:18–26. [Google Scholar]
- 30.Medina MT, Duron RM, Martinez L, et al. Prevalence, incidence, and etiology of epilepsies in rural Honduras: the Salama Study. Epilepsia 2005;46:124–131. [DOI] [PubMed] [Google Scholar]
- 31.Placencia M, Shorvon SD, Paredes V, et al. Epileptic seizures in an Andean region of Ecuador: incidence and prevalence and regional variation. Brain 1992;115:771–782. [DOI] [PubMed] [Google Scholar]
- 32.Khedr EM, Shawky OA, Ahmed MA, et al. A community based epidemiological study of epilepsy in Assiut Governorate/Egypt. Epilepsy Res 2013;103:294–302. [DOI] [PubMed] [Google Scholar]
- 33.Lavados J, Germain L, Morales A, Campero M, Lavados P. A descriptive study of epilepsy in the district of El Salvador, Chile, 1984–1988. Acta Neurol Scand 1992;85:249–256. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser C, Asaba G, Leichsenring M, Kabagambe G. High incidence of epilepsy related to onchocerciasis in West Uganda. Epilepsy Res 1998;30:247–251. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee PN, Filippi D, Allen Hauser W. The descriptive epidemiology of epilepsy-a review. Epilepsy Res 2009;85:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bharucha NE, Bharucha EP, Bharucha AE, Bhise AV, Schoenberg BS. Prevalence of epilepsy in the Parsi community of Bombay. Epilepsia 1988;29:111–115. [DOI] [PubMed] [Google Scholar]
- 37.Beghi E, Hesdorffer D. Prevalence of epilepsy: an unknown quantity. Epilepsia 2014;55:963–967. [DOI] [PubMed] [Google Scholar]
- 38.Meyer AC, Dua T, Ma J, Saxena S, Birbeck G. Global disparities in the epilepsy treatment gap: a systematic review. Bull World Health Organ 2010;88:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett DA, Brayne C, Feigin VL, et al. Development of the Standards of Reporting of Neurological Disorders (STROND) checklist: a guideline for the reporting of incidence and prevalence studies in neuroepidemiology. Neurology 2015;85:821–828. [DOI] [PubMed] [Google Scholar]
- 40.Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 2011;52(Suppl 7):2–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.