Abstract
Deficits in central, subcortical dopamine (DA) signaling may underlie negative symptom severity, particularly anhedonia, in healthy individuals and in schizophrenia. To investigate these relationships, we assessed negative symptoms with the Schedule for the Assessment of Negative Symptoms and the Brief Negative Symptom Scale (BNSS) and self-reported anhedonia with the Scales for Physical and Social Anhedonia (SPSA), Temporal Experience of Pleasure Scale, and Snaith-Hamilton Pleasure Scale in 36 healthy controls (HC), 27 siblings (SIB) of individuals with schizophrenia, and 66 individuals with schizophrenia or schizoaffective disorder (SCZ). A subset of participants (N = 124) were genotyped for DA-related polymorphisms in genes for DRD4, DRD2/ANKK1, DAT1, and COMT, which were used to construct biologically-informed multi-locus genetic profile (MGP) scores reflective of subcortical dopaminergic signaling. DA receptor type 2 (D2R) binding was assessed among a second subset of participants (N = 23) using PET scans with the D2R-selective, non-displaceable radioligand (N-[11C]methyl)benperidol. Higher MGP scores, reflecting elevated subcortical dopaminergic signaling capacity, were associated with less negative symptom severity, as measured by the BNSS, across all participants. In addition, higher striatal D2R binding was associated with less physical and social anhedonia, as measured by the SPSA, across HC, SIB, and SCZ. The current preliminary findings support the hypothesis that subcortical DA function may contribute to negative symptom severity and self-reported anhedonia, independent of diagnostic status.
Keywords: schizophrenia, negative symptoms, anhedonia, genetic profile, dopamine, PET
1. Introduction
The etiology of schizophrenia remains unclear but central dopamine (DA) transmission likely plays a role in its manifestation. Positive symptoms may be due in part to mesostriatal DA dysfunction (Brisch et al., 2014; Howes and Nour, 2016; Kapur, 2003), characterized by elevated striatal DA synthesis capacity and synaptic DA availability (Howes et al., 2012; Laruelle et al., 1999; Reith et al., 1994) which may in turn confer aberrant incentive salience onto non-relevant stimuli (Heinz, 2002; Robinson and Berridge, 1993; Schultz et al., 1997) . By contrast, negative symptoms are posited to arise in part from hypodopaminergic transmission in prefrontal cortex (Davis et al., 1991; Goghari et al., 2010; Heinz, 2002; Knable and Weinberger, 1997). In addition, fMRI studies have shown that negative symptoms, especially those in the motivational dimension such as anhedonia, relate to blunted blood oxygen level-dependent (BOLD) reward-related activation in striatum among patients (Dowd and Barch, 2010, 2012; Juckel et al., 2006b; Simon et al., 2010; Waltz et al., 2009; Waltz et al., 2010) and healthy individuals (Corral-Frías et al., 2015). Negative symptoms may also be risk factors for depression (Kupferberg et al., 2016) and substance abuse (Leventhal et al., 2010) in healthy individuals. The role of striatal DA transmission in negative symptom severity therefore merits further investigation.
The study of DA-related genetic polymorphisms may aid in understanding the role of DA signaling in both positive and negative symptom severity in schizophrenia. The disease is 80–85% heritable (Cardno and Gottesman, 2000) and common DA-related polymorphisms have been associated with variability in DA signaling (Asghari et al., 1995; Gluskin and Mickey, 2016; Heinz et al., 2000; Meyer-Lindenberg et al., 2005; Thompson et al., 1997). DA-related multilocus genetic profiles (MGPs) are composites of several DA-related polymorphisms and putatively predict ‘net’ DA function. Such DA-related MGPs have been associated with striatal BOLD responsivity to reward (Nikolova et al., 2011; Stice et al., 2012), addictive behavior and personality (Davis and Loxton, 2013), food addiction, emotional and hedonic eating (Davis et al., 2013), and depression (Pearson-Fuhrhop et al., 2014). To our knowledge, MGPs have not yet been used to study the role of subcortical DA signaling in symptom severity in schizophrenia or in healthy individuals.
Investigation of striatal D2 receptors (D2R) may yield further insight into the link between symptom severity and striatal DA function. Some studies have found increased striatal D2/D3 receptor (D2/D3R) availability in schizophrenia compared to non-diseased individuals but many others have not (Howes et al., 2012). Alternatively, symptom severity may more strongly relate to D2/D3R availability than patient status. Low striatal D2/D3R availability in patients, either at baseline or due to receptor occupancy by antipsychotics, has been associated with greater negative symptom severity (de Haan et al., 2000; Heinz et al., 1998; Lataster et al., 2011; Martinot et al., 1994; Pickar et al., 1996; Uchida et al., 2009) and dysphoria (Mizrahi et al., 2007). However, it has also been associated with greater positive and less negative symptom severity (Pogarell et al., 2012) and some studies did not show any relationship (Agid et al., 2007; Graff-Guerrero et al., 2009; Kegeles et al., 2008; Klemm et al., 1996; Talvik et al., 2006). Some of the variability in these findings may be due to certain properties of the D2/D3R radioligands used (i.e. [11C]raclopride, [123I]iodobenzamide). First, these radiligands do not distinguish between D2R and D3R (Elsinga et al., 2006; Mukherjee et al., 1999; Videbaek et al., 2000). While their distribution overlaps, these receptor subtypes are preferentially localized to different brain regions. Therefore, D2R and D3R may have distinct functional roles in symptom severity. Second, these radioligands are vulnerable to displacement by endogenous DA (Dewey et al., 1992; Laruelle et al., 1995; Riccardi et al., 2006), confounding interpretation of PET measures of in vivo D2/D3R availability. The relationship between symptom severity and striatal D2R using a non-displaceable, D2R-selective PET radioligand has not yet been studied in individuals with schizophrenia or in healthy individuals.
Here, we studied positive and negative symptom severity, with an emphasis on self-reported anhedonia, in healthy controls (HC), siblings of individuals with schizophrenia (SIB), and individuals with schizophrenia or schizoaffective disorder (SCZ). Unaffected siblings provide the opportunity to study relationships among variables of interest in individuals who share genes with their affected siblings but are medication-naïve. First, we tested the hypothesis that MGP scores, which were composites of DA-related genotypes and formulated to reflect genetically regulated subcortical DA signaling capacity (Stice et al., 2012), would relate to symptom severity and self-reported anhedonia. Specifically, based on evidence of striatal hyperdopaminergic signaling in schizophrenia (Howes et al., 2012; Laruelle et al., 1999; Reith et al., 1994) and a lack of direct evidence for striatal hypodopaminergic signaling in association with symptom severity, we hypothesized that lower MGP scores that reflect lower subcortical DA signaling capacity would relate to lower levels of both positive and negative symptom severity and less self-reported anhedonia across HC, SIB, and SCZ. Second, using a novel D2R-selective PET radioligand that is not displaceable by endogenous DA, (N-[11C]methyl)benperidol ([11C]NMB), we tested the hypothesis that lower striatal D2R specific binding would relate to lower symptom severity and less self-reported anhedonia in a subset of HC, SIB, and unmedicated SCZ.
2. Material and Methods
2.1 Participants
Participants (N =129) aged 18–50 years, including HC (n = 36), SIB (n = 27), and SCZ (n = 66), were recruited by word of mouth, flyers, and during visits to clinics and mental health centers in St. Louis, MO (Table 1). In the whole sample, there were 12 pairs of related SIB and SCZ and 2 pair of SIB who were siblings of each other. All participants provided symptom severity and self-reported anhedonia data. Most participants provided saliva samples for genotyping (34 HC, 25 SIB, 65 SCZ; Table 2) and some completed [11C]NMB PET scans (10 HC, 10 SIB, 3 SCZ (unmedicated for ≥9 months at time of PET and unmedicated during clinical interview); 5 of whom who were not genotyped (2 HC, 2 SIB, and 1 SCZ). Of the individuals who were genotyped, there were 12 pairs of related SIB and SCZ and 1 pair of SIB who were siblings of each other. Two of the SIB who underwent PET scans were siblings of each other. Fifty five SCZ were taking antipsychotic medications at the time of screening. We converted antipsychotic medication doses to 100 mg chlorpromazine equivalents (Lehman et al., 2004 (for Loxapine calculation only); Leucht et al., 2014).
Table 1.
Participant Demographics and Symptom Severity for Each Study
| Genotyping Study | ||||
|---|---|---|---|---|
| Healthy Control (n=34) |
Sibling (n=25) |
Schizophrenia (n=65) | Group Difference p- value |
|
| Mean (S.D.) | ||||
| Age at clinical interview |
34.4 (9.6) | 30.2 (8.6) | 37.4 (8.3)*** | <0.01 |
| Education (years) | 13.8 (2.2) † | 15.0 (2.2) | 13.2 (2.2)** | 0.01 |
| WAIS Scaled Score | 10.8 (2.9) | 12.2 (2.8) | 9.7 (3.1)+,*** | <0.01 |
| Cotinine levels over 4 study visits (ng/ml) |
1.5 (2.3) (n=28) |
0.8 (1.5) (n=23) |
1.4 (2.1) (n=50) | 0.26 |
| Brief Negative Symptom Scale Score |
2.4 (4.4) | 2.6 (4.8) | 18.5 (11.8)***,### | <0.001 |
| Schedule for Assessment of Negative Symptoms |
2.4 (2.7) | 2.8 (2.9) | 8.9 (3.8)***,### | <0.001 |
| Schedule for Assessment of Positive Symptoms |
0.2 (0.7) | 0.3 (0.7) | 5.8 (4.0)***,### | <0.001 |
| Scales for Physical and Social Anhedonia |
21.3 (11.4) | 16.6 (12.5) | 32.6 (14.6)***,### | <0.001 |
| Snaith-Hamilton Pleasure Scale |
52.9 (3.1) | 52.2 (4.7) | 48.8 (7.6)*,## | <0.01 |
| Temporal Experience of Pleasure Scale |
87.1 (9.2) | 88.0 (8.9) | 82.9 (18.4) | 0.22 |
| Multilocus Genetic Profile Score |
6.4 (1.0) | 6.6 (0.9) | 6.3 (0.9) | 0.26 |
| Distribution | ||||
| Gender | 16 F/18 M | 17 F/8 M | 37 F/28 M | 0.10 |
| Ethnicity | 20 AA, 13 EA (2 Hi), 1 Asian |
10 AA, 15 EA (1Hi) |
36 AA, 23 EA (0 Hi), 1 Asian, 2 Amer Ind/Alask Nat, 3 more than one race |
0.10 |
| PET D2R Binding Study | ||||
| Healthy Control (n=10) |
Sibling (n=10) |
Schizophrenia (n=3) | Group Difference p- value |
|
| Mean (S.D.) | ||||
| Age at PET scan | 34.6 (9.4) | 32.0 (7.6) | 40.3 (8.5) | 0.29 |
| Age at clinical interview |
33.1 (8.8) | 30.8 (8.1) | 40 (9) | 0.30 |
| Education (years) | 13.1 (1.7) † | 14.8 (1.6) | 12.3 (0.6)* | 0.04 |
| WAIS Scaled Score | 10.9 (2.8) | 12.2 (2.7) | 9.3 (4.0) | 0.44 |
| Cotinine levels at PET (ng/ml) |
1.4 (2.3) | 1.7 (2.3) | 1.1 (0.9) | 0.62 |
| Brief Negative Symptom Scale Score |
3.4 (6.1) | 2.6 (5.3) | 21.3 (15.5)**,## | 0.002 |
| Schedule for Assessment of Negative Symptoms |
3.2 (3.0) | 2.7 (2.6) | 10 (5.3)**,## | 0.006 |
| Schedule for Assessment of Positive Symptoms |
0.3 (0.7) | 0.4 (0.8) | 4.3 (4.2)***,### | 0.001 |
| Scales for Physical and Social Anhedonia |
22 (13.9) | 21.7 (15.6) |
35 (9.5) | 0.35 |
| Snaith-Hamilton Pleasure Scale |
52.7 (2.9) | 50.4 (6.1) | 51.7 (4.0) | 0.56 |
| Temporal Experience of Pleasure Scale |
89.9 (6.5) | 83.3 (11.8) |
112 (48.5) | 0.07† |
| Dorsal striatal D2R BPND |
7.7 (1.04) | 8.1 (1.1) | 7.6 (1.1) | 0.65 |
| Nucleus accumbens D2R BPND |
3.0 (0.4) | 3.2 (0.4) | 3.0 (0.2) | 0.54 |
| Distribution | ||||
| Gender | 4 F/6 M | 6 F/4 M | 1 F/2 M | 0.59 |
| Ethnicity | 5 AA, 5 EA (1 Hi) |
5 AA, 5 EA (0 Hi) |
2 AA, 1 EA (0 Hi) | 0.87 |
Abbreviations: WAIS, Wechsler Adult Intelligence Scale; F, female; M, male; AA, African American; EA, European American; Hi, Hispanic; As, Asian; Amer Ind/Alask Nat, American Indian/Alaskan Native; PET, positron emission tomography.
p ≤ 0.05, 0.01, 0.001 relative to sibling group;
p ≤ 0.01, 0.001 relative to healthy control group;
p < 0.10 relative to sibling group;
p = 0.09 relative to healthy control group. p-values shown in bold denote a statistically significant difference among groups.
Table 2.
Genotype scoring.
| Polymorphism | Allele Set |
N | Gender Distribution |
Ethnicity Distribution |
Group Distribution |
DA Signaling Capacity (score) |
|---|---|---|---|---|---|---|
| Taq1Aa | ||||||
| A1/A1 | 10 | 5 F/5 M | 0 EA, 9 AA, 1 multi |
1 HC, 1 Sib, 8 SCZ |
Low (1) | |
| A1/A2 | 47 | 26 F/21 M | 19 EA, 25 AA, 2 As, 1 AI* |
16 HC, 8 Sib, 23 SCZ |
Intermediate (1.5) |
|
| A2/A2 | 67 | 30 F/37 M | 32 EA, 32 AA, 2 multi, 1 AI*** |
17 HC, 16 Sib, 34 SCZ |
High (2) | |
| COMTval158meta | ||||||
| met/met | 27 | 13 F/16 M | 17 EA, 8 AA, 1As, 1 Multi |
5 HC, 6 Sib, 16 SCZ |
Low (1) | |
| met/val | 48 | 24 F/24 M | 16 EA, 30 AA, 1 AI, 1 multi## |
14 HC, 9 Sib, 25 SCZ |
Intermediate (1.5) |
|
| val/val | 49 | 24 F/25 M | 18 EA, 28 AA, 1 As, 1 AI, 1 multi# |
15 HC, 10 Sib, 24 SCZ |
High (2) | |
| DRD4 | ||||||
| repeats ≥ 7 |
51 | 26 F/25 M | 21 EA, 27 AA, 1 As, 1 AI, 1 Multi |
14 HC, 9 Sib, 28 SCZ |
Low (1) | |
| repeats < 7 |
73 | 35 F/38 M | 30 EA, 39 AA, 1 As, 1 AI, 2 Multi |
20 HC, 16 Sib, 37 SCZ |
High (2) | |
| SLC6A3 (DAT1) | ||||||
| 10R/10R | 68 | 35 F/33 M | 26 EA, 36 AA, 2 As, 1 AI, 3 Multi |
21 HC, 10 Sib, 37 SCZ |
Low (1) | |
| 9R/X | 56 | 26 F/30 M | 25 EA, 30 AA, 1 AI |
13 HC, 15 Sib, 28 SCZ |
High (2) | |
Abbreviations: DA, dopamine; F, female; M, male; EA, European American; AA, African American; multi, multiracial; As, Asian; AI, American Indian; HC, healthy control; SIB, sibling; SCZ, individual with schizophrenia. aResults of a univariate analysis of variance followed by Tukey’s LSD post hoc tests.
p ≤ 0.05, 0.001 relative to A1/A1.
p ≤ 0.05, 0.01 relative to met/met. Polymorphisms shown in bold denote a statistically significant difference in distributions between ethinicities.
After the study procedures were fully explained, all participants provided written informed consent prior to participation. The study protocol was approved by the Washington University School of Medicine Human Research Protection Office and the Radioactive Drug Research Committee, and was carried out in accordance with the principles expressed in the Declaration of Helsinki. Exclusionary criteria are described in Supplementary Information 1.
2.2 Symptom Severity and Anhedonia Assessment
During the screening day, a trained research assistant administered the Structured Clinical Interview for the DSM-IV (First et al., 2002) to determine the lifetime and current history of Axis I disorders and obtain basic demographic information (Table 1). To assess symptom severity, the Schedule for the Assessment of Negative Symptoms (SANS) (Andreasen, 1983a), Schedule for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1983b), and the Brief Negative Symptom Scale (BNSS) (Kirkpatrick et al., 2011) were administered. Participants completed self-report scales including the Scales for Physical and Social Anhedonia (SPSA) (Chapman et al., 1976), Snaith-Hamilton Pleasure Scale (SHAPS) (Snaith et al., 1995) and Temporal Experience of Pleasure Scale (TEPS) (Gard et al., 2006) to assess hedonic capacity as well as the Wechsler Adult Intelligence Scale vocabulary and matrix reasoning subtests to assess intellectual functioning (WAIS 4th Edition (Wechsler, 2008)).
2.3 Genotyping
Participants provided saliva on a voluntary basis with an Oragene saliva kit (www.dnagenotek.com). Following DNA extraction and quantitation normalization, the following polymorphisms were genotyped by the Molecular Psychiatry Core at Washington University in St. Louis School of Medicine by pyrosequencing: DRD2/ANKK1 TaqIA (rs1800497), COMT val158met (rs4680), the 48 bp exon 3 variable tandem number repeat (VNTR) polymorphism in the DRD4 gene, and the 40 bp DAT1 VNTR of the SLC6A3 gene. Call rates were 99% for DRD4 and SLC6A3 DAT1 and 100% for DRD2/ANKK1 TaqIA (rs1800497) and COMT val158met (rs4680). Across the full sample and within each ethnic group, polymorphisms did not deviate from Hardy-Weinberg Equilibrium (HWE), with the exception of COMT val158met rs4680, which diverged in the full sample and the European American subsample (Supplementary Table 1). The divergence from HWE for rs4680 appears to be driven by low heterozygosity observations among SIB and SCZ (Table 2). Given that the level of deviation from HWE in the full sample was above a Bonferroni corrected p-value for HWE testing (i.e., 0.05/4 single nucleotide polymorphisms ((SNPs), α = 0.0125), we retained this SNP in our profile score. Further, we report MGP results excluding COMT val158met rs4680 from our MGP score in Supplementary Information 2.
2.4 Multilocus Genetic Profile Scoring
MGP scores were assigned to each individual by assigning scores to allele pairs for each of 4 polymorphisms similar to the method used by Stice et al. (2012). The DRD2/ANKK1 TaqIA (rs1800497) A2 allele is associated with greater striatal D2R binding relative to the A1 allele (Eisenstein et al., 2016; Gluskin and Mickey, 2016). The COMT (rs4680) val allele may be associated with lower amounts of tonic DA transmission in cortical regions due to greater COMT activity, but it is thought to confer greater phasic DA transmission in striatum and other subcortical regions due to reduced tonic auto-inhibition relative to met/met (Bilder et al., 2004). The DRD448 bp exon 3 7-repeat variant is associated with reduced dopamine-mediated inhibition of cyclic AMP formation relative to DRD4 repeats <7 (Asghari et al., 1995). Finally, individuals with the 9-repeat allele of SLC6A3 DAT1 have higher DAT expression compared to individuals homozygous for the 10-repeat allele (van de Giessen et al., 2009; van Dyck et al., 2005). In contrast to Stice et al. (2012), individuals homozygous or heterozygous for the SLC6A3 DAT1 9-repeat allele were assigned the high score as in Nikolova et al. (Nikolova et al., 2011) and in Davis et al. (Davis and Loxton, 2013; Davis et al., 2013). In addition, we did not genotype participants for the DRD2-141C Ins/Del polymorphism; therefore, the MGP formula did not include scores for these alleles. Scores were summed such that higher MGP scores indicated higher DA signaling capacity (Table 2).
2.5 MRI and PET Acquisition, Preprocessing, and Analyses
MRI and PET data were acquired a mean of 14 months (S.D. = 11.5) after completion of the symptom severity and anhedonia measures in HC, SIB, and unmedicated SCZ. Simultaneous radioligand [11C]NMB PET and structural MR T1-weighted anatomical images were obtained with a Siemens Biograph mMR PET/MR scanner (Delso et al., 2011) using a 3-D MP-RAGE sequence (sagittal orientation, TR=2400 ms, TE=2.67 ms, flip angle=7 degrees, slab thickness=192 mm, FOV=256×256 mm; voxel dimensions= 1×1×1 mm). [11C]NMB, was prepared using an automated system previously described (Moerlein et al., 2010; Moerlein et al., 2004). Radiochemical purity of [11C]NMB was ≥ 95% and specific activity was ≥ 961 Ci/mmol (36 TBq/mmol). Injected dose of unlabeled NMB was ≤ 17.9 µg. Participants received 5.9–18.8 mCi [11C]NMB intravenously. Emission data were collected in 3D mode for 30 frames: 3 × 1 min, 4 × 2 min, 3 × 3 min and 20 × 5 min. Attenuation correction was MR-based (Delso et al., 2011).
MR and PET image processing was previously described in detail (Eisenstein et al., 2013; Eisenstein et al., 2012). A priori regions of interest (ROIs) including dorsal (putamen + caudate) and ventral (nucleus accumbens (NAc)) areas of the striatum were identified using FreeSurfer (Fischl et al., 2002) on the MP-RAGE MR images for each participant. Dynamic PET images were aligned to each other and then co-registered to the MP-RAGE image for each individual as previously described (Eisenstein et al., 2012). ROIs and the cerebellar reference region were resampled in the same atlas space (Hershey et al., 2003). Decay-corrected tissue activity curves were obtained from the dynamic PET data. D2R non-displaceable binding potentials (BPNDs) were obtained for each ROI using the Logan graphical method with whole cerebellum as a reference region (Antenor-Dorsey et al., 2008). D2R BPNDs for each ROI were averaged across left and right hemispheres to reduce the number of comparisons for primary analyses. We recently published a report within this sample linking D2R binding to DRD2/ANKK1 TaqIA (rs1800497) genotype (Eisenstein et al., 2016).
2.6 Primary Data Analyses
Demographic variables were tested for differences among groups with Kruskal-Wallis H tests. Differences between groups in gender and ethnicity distributions, symptom severity, self-reported anhedonia, MGP scores, and D2R binding were assessed with univariate analyses of variance followed by Tukey’s LSD post hoc tests. To eliminate Type I error inflation due to relatedness, genetic analyses (those including MGP scores as predictor variables) were performed on a sample in which data from one sibling of each sibling pair (6 SIB, 7 SCZ) was randomly removed, resulting in a total sample size of N = 111 unrelated individuals (34 HC, 19 SIB, 58 SCZ). Prediction of outcome variables by centered MGP scores were then assessed with hierarchical multiple linear regression (HMLR) models; covariates (age, gender, ethnicity, scaled WAIS score, and education) were included in step 1, diagnostic group (HC vs. SIB vs. SCZ) was included in step 2, MGP scores were included in step 3, and the diagnostic group x MGP scores interaction was entered in step 4. In complementary analyses that included the full sample (N = 124), a random effects model (REM) implemented using PROC MIXED in SAS 9.4 (SAS Institute, Cary, NC) was applied to assess prediction of study variable outcomes by MGP scores accounting for correlation of outcome variables from the same family. Multivariate REMs with group and MGP score considering age, gender, ethnicity and number of years of education were constructed via a forward selection procedure. This procedure takes into account all factors where p < 0.3 in the univariate analyses and the significance level for entering factors was set at 0.1. Residual plots from the models were used to assess the normality assumption of errors. Predictions of study variables by D2R binding was assessed using HMLR; covariates (diagnostic group, age at time of PET, age at time of clinical interview, gender, ethnicity, WAIS scaled score, and education) were included in step 1, and D2R binding was included in step 2. REMs were not performed for analyses including D2R binding because these included data from only two related SIB individuals. When MGP scores or D2R binding significantly predicted total scores on a scale that served as a dependent variable (i.e. BNSS), follow-up HMLRs were performed to determine if MGP or D2R binding related to scores on subscales (i.e. alogia) of that scale. For primary HMLR analyses regarding symptom severity (3 scales) and anhedonia (3 scales), resulting p-values were corrected for multiple comparisons using the Bonferroni method such that p ≤ 0.008 was considered statistically significant (0.05/6 scales). Otherwise, results were considered significant at p ≤ 0.05.
3. Results
3.1 Sample Description
See Table 1 for descriptive statistics. In the genotyped sample, diagnostic groups did not differ in mean urine cotinine levels or gender and ethnicity distributions. However, SIB were significantly younger, had more education, and higher WAIS scores than SCZ. Among the subset of individuals that underwent PET scans, SCZ were less educated than SIB but otherwise there were no significant group differences. SCZ antipsychotic medications included Arapiprazole, Clozapine, Haloperidol, Iloperidone, Loxipine, Olanzepine, Paliperidone, Risperidone, Quetiapine, and Ziprasidone. Dosage data was missing for 3 individuals. Eleven SCZ, including n = 3 who underwent PET, were not medicated. The mean (S.D.) 100 mg chlorpromazine equivalent was 723.5 mg (597.7) (range = 125–3333.3 mg).
Genotype distribution among ethnic groups differed for DRD2/ANKK1 TaqIA (rs1800497; F2,121 = 4.3, p = 0.02) and COMT val158met (rs4680; univariate ANOVA F2,121 = 3.6, p = 0.03, Table 2). Total MGP scores, however, did not differ between European American (mean (S.D.) = 6.4 (0.9)) and non-European American (6.3 (1.0)) individuals (Student’s between subjects t-test, t122 = −0.43, p = 0.67).
3.2 MGP Scores and Symptom Severity
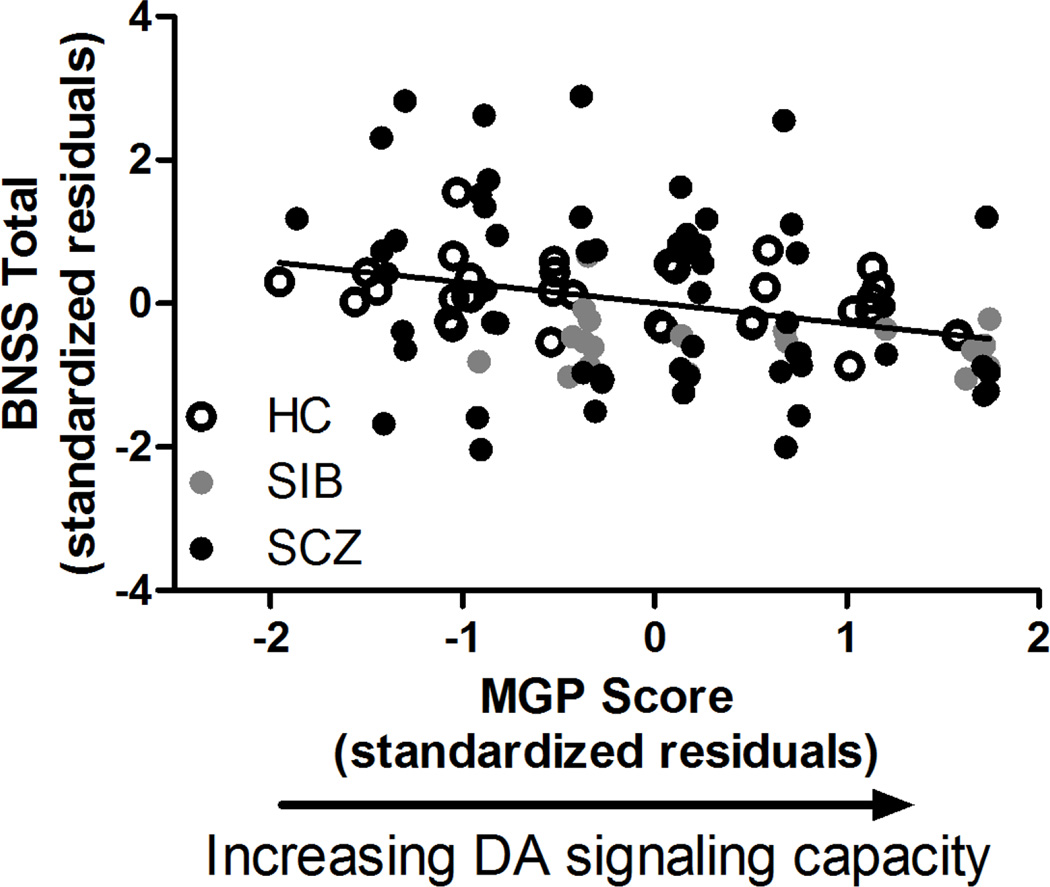
Unsurprisingly, SCZ reported higher symptom severity scores across measures (BNSS, SANS, SAPS) than HC and SIB (Table 1). MGP scores did not differ between groups (Table 1). Elevated MGP scores, reflecting higher subcortical DA signaling capacity, were associated with reduced negative symptom severity as measured by the BNSS across HC, SIB, and SCZ (r111 = −0.28, p = 0.004, ΔR2 = 0.04; Figure 1, Supplementary Table 2). The magnitude of this relationship was larger when only African Americans were included in the analysis (r62 = −0.33, p = 0.01, ΔR2 = 0.06) but smaller when only European Americans were included (r42 = −0.22, p = 0.19, ΔR2 = 0.03). Post-hoc analyses of BNSS subscales revealed that elevated MGP scores related to lower Anhedonia (r111 = −0.21, p = 0.04), Avolition (r111 = −0.20, p = 0.04), Blunted Affect (r111= −0.19, p = 0.05), Distress (r111 = −0.23, p = 0.02) and Alogia (r111 = −0.22, p = 0.03) but not Asociality (r111 = −0.17, p = 0.09) scores on subscales of the BNSS. MGP scores were more strongly associated with BNSS scores than individual DA-related SNPs or VNTRs (Supplementary Table 3). When separate analyses replaced the total MGP score with one calculated by summing allele scores and ‘leaving out’ contribution from one of the SNPs, results further indicated that no single loci drove the association between MGP and BNSS scores (associations remained significant or near-significant regardless of which SNP was ‘left out’; Supplementary Table 3). REM analyses that accounted for correlation of outcome variables from the same family also indicated that higher MGP scores predicted lower BNSS scores (Pearson r124 = −0.20, REM p = 0.05) across HC, SIB, and SCZ. MGP scores did not relate to negative or positive symptom severity as measured by the SANS (HMLR: r111 = −0.09, p = 0.35; Pearson r124 = −0.07, REM p = 0.87) or the SAPS (HMLR: r111 = −0.13, p = 0.20; Pearson r124 = −0.09, REM p = 0.77), respectively. There were no diagnostic group x MGP interactions for symptom severity (all p ≥ 0.27).
Figure 1.
Greater DA signaling capacity, as reflected by MGP scores, was related to lower negative symptom severity as measured by the Brief Negative Symptom scale (BNSS) across diagnostic groups. HC, healthy control; SIB, sibling of individual with schizophrenia; SCZ, individual diagnosed with schizophrenia or schizoaffective disorder; MGP, multilocus genetic profile.
3.3 MGP Scores and Self-reported Anhedonia
Overall, SCZ had significantly greater self-reported anhedonia than HC and SIB (Table 1). Elevated MGP scores related to lower self-reported anhedonia as measured by the SPSA but this finding did not survive multiple comparisons correction (HMLR: r111 = −0..20, p = 0.04, ΔR2 = 0.03; Pearson r124 = −0.16; REM p = 0.18). MGP scores did not correlate with hedonic capacity as measured by SHAPS (HMLR: r111 = −0.07, p = 0.51; Pearson r124 = −0.06, REM p = 0.30) or TEPS (HMLR: r111 = 0.08, p = 0.42; Pearson r124 = 0.09, REM p = 0.42). There were no diagnostic group x MGP interactions for anhedonia severity (all p ≥ 0.24).
Analyses including MGP scores not including COMT, which was not in HWE for the full sample, are presented in Supplementary Information 2. These results are similar in magnitude and direction to the results reported above.
3.4 Striatal D2R Binding and Symptom Severity
For each symptom severity measure (BNSS, SANS, SAPS), SCZ had significantly higher scores than HC and SIB (Table 1). D2R BPND did not differ between groups (Table 1). Dorsal striatal BPND was negatively correlated with SANS scores at trend-level significance (r23 = −0.45, p = 0.08; ΔR2 = 0.07). NAc D2R BPND did not relate to SANS scores (r23 = −0.38, p = 0.15). Neither dorsal striatal nor NAc D2R BPND was associated with negative symptom severity as measured by the BNSS (both ROIs: r23 ≥ −0.28, p ≥ 0.29), or positive symptom severity as measured by the SAPS (both ROIs: r23 ≥ −0.07, p ≥ 0.80).
3.5 Striatal D2R Binding and Self-reported Anhedonia
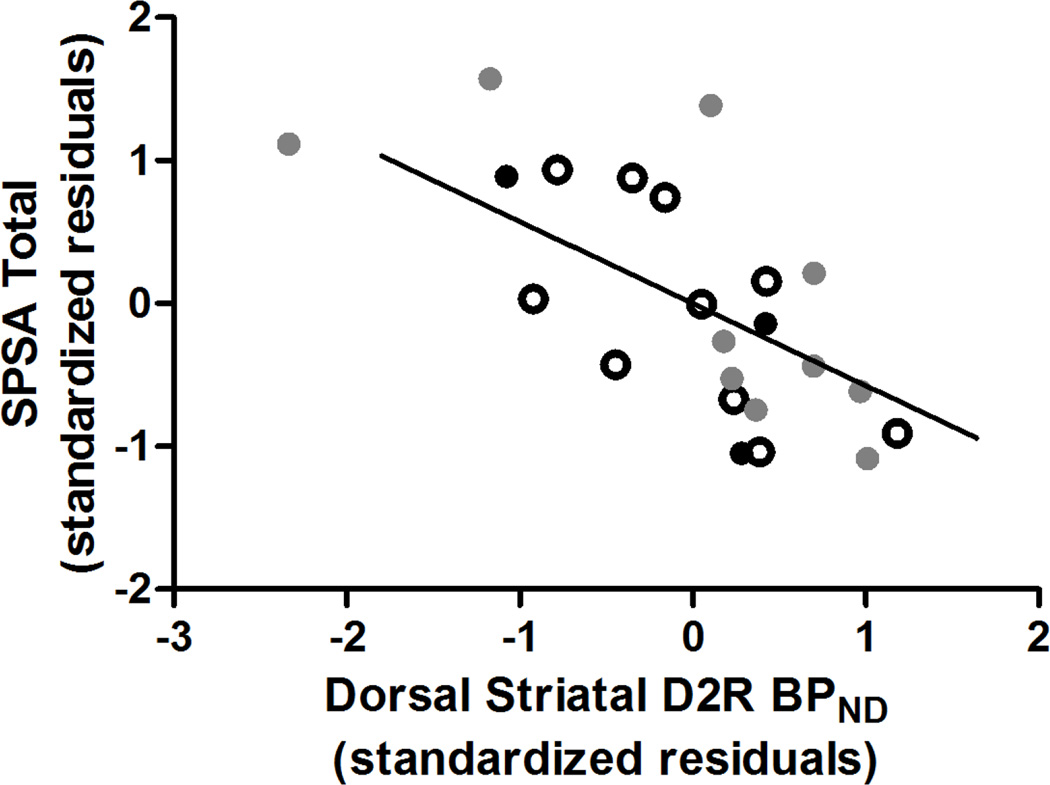
Self-reported anhedonia did not differ among groups (Table 1). Elevated dorsal striatal D2R BPND significantly predicted lower anhedonia as measured by the SPSA (r23 = −0.70, p = 0.003, ΔR2 = 0.28; Figure 2, Supplementary Table 4). This relationship remained even when excluding SCZ (r20 = −0.69, p = 0.009, ΔR2 = 0.26) and when excluding the related SIB (r21 = −0.64, p = 0.01, ΔR2 = 0.21) from the analyses. Specifically, in the whole sample, greater dorsal striatal D2R BPND related to both lower physical anhedonia (r23 = −0.56, p = 0.02, ΔR2 = 0.19) and social anhedonia (r23 = −0.69, p = 0.003, ΔR2 = 0.29). NAc D2R BPND predicted anhedonia as measured by the SPSA at a significance level that did not survive multiple comparisons correction (r23 = −0.57, p = 0.02, ΔR2 = 0.19). Neither dorsal striatal nor NAc D2R BPND were associated with SHAPS (both ROIs: r23 ≥ −0.30, p ≥ 0.26) or TEPS (r23≤ 0.21, p ≥ 0.43) scores.
Figure 2.
Lower D2R binding in dorsal striatum was associated with greater self-reported anhedonia as measured by the Scales for Physical and Social Anhedonia (SPSA) across diagnostic groups. HC, healthy control; SIB, sibling of individual with schizophrenia; SCZ, individual diagnosed with schizophrenia or schizoaffective disorder; D2R, dopamine D2 receptor; BPND, non-displaceable binding potential
3.6 Striatal D2R Binding and MGP Scores
Consistent with our previous report linking the DRD2/ANKK1 rs1800497 to D2R in this sample (Eisenstein et al., 2016), MGP scores predicted dorsal striatal (r18= 0.65, p = 0.02) but not NAc D2R BPND (r18 = 0.46, p = 0.11). At the individual SNP/VNTR level, the DRD2/ANKK1 rs1800497 polymorphism significantly related to dorsal striatal D2R BPND, as previously reported (Eisenstein et al., 2016). None of the other polymorphism tested (COMT rs4680, SLC6A3 DAT1, DRD4 exon 3 48 bp) predicted dorsal striatal D2R binding (all r ≤ 0.47, p ≥ 0.10). However, D2R binding was still predicted by the MGP score when the DRD2/ANKK1 rs1800497 polymorphism was excluded from the calculation (r18 = 0.55, p = 0.05).
4. Discussion
Contrary to our hypotheses, elevated subcortical DA signaling capacity, reflected by higher MGP scores, predicted lower negative symptom severity as measured by the BNSS, and, at a level that did not survive multiple comparisons correction, lower self-reported anhedonia as measured by the SPSA across HC, SIB, and SCZ. Our results add to existing PET/SPECT (de Haan et al., 2000; Heinz et al., 1998; Lataster et al., 2011; Martinot et al., 1994; Pickar et al., 1996; Uchida et al., 2009; Voruganti et al., 2001; Voruganti and Awad, 2006) and fMRI (Dowd and Barch, 2010, 2012; Galderisi et al., 2015; Goghari et al., 2010; Juckel et al., 2006a; Simon et al., 2010; Strauss et al., 2014; Waltz et al., 2009; Waltz et al., 2010) evidence that negative symptom severity relates to altered subcortical DA signaling and striatal reactivity, respectively. Our findings are also in line with those of other studies in which genetic profile scores reflecting high DA signaling capacity relate to increased brain responsivity to reward (Nikolova et al., 2011; Stice et al., 2012) and lower depressive symptom severity (Pearson-Fuhrhop et al., 2014). By contrast, we did not observe a relationship between MGP and SANS scores. The BNSS differs from the SANS in that, besides requiring shorter administration time, it differentiates consummatory from anticipatory pleasure, has a Distress subscale, and has separate items for measuring facial expression, vocal expression, and expressive gestures (Daniel, 2013). Given that, in our study, higher MGP scores related to anhedonia, as measured by the Anhedonia subscale, lack of normal distress, as measured by the Distress subscale, and poverty of speech, as measured by the Alogia subscale, these differences between scales are a potential reason for our limited ability to detect a relationship between DA signaling capacity and negative symptom severity as measured by the SANS. Future studies may aim to replicate our results, which should be viewed as preliminary due to small sample sizes, the small number of genes used in the MGP formula, and differences in genetic distributions between ethnic groups.
We did not observe a relationship between positive SAPS-measured symptom severity and subcortical DA signaling capacity as reflected by MGP scores. This is surprising since hyperdopaminergic function in the mesolimbic pathway may underlie positive symptoms in schizophrenia (Davis et al., 1991). However, most SCZ were medicated and variability in positive symptom severity may have been masked in these individuals. It is also possible that SNPs related to presynaptic aspects of DA signaling would better predict positive symptom severity than an MGP score that may reflect overall subcortical DA signaling capacity
Also contrary to our hypotheses, higher dorsal striatal D2R binding, as measured by PET with [11C]NMB, was associated with less self-reported anhedonia, as measured by the SPSA, and, at a near-significant level, less negative symptom severity as measured by the SANS across diagnostic groups. This relationship remained intact when SCZ were removed from the analysis. Our finding is in line with those in which D2R pharmacological blockade or genetic knockout caused reward-related behavior deficits (Elmer et al., 2005; Wise, 1978) and D2/D3R agonism reversed anhedonia (Willner et al., 1994) in rodents. Also consistent with our results, human pharmacological studies show that anhedonia is attenuated by chronic D2/D3R agonism in Parkinson disease (Lemke et al., 2006) and chronic, partial D2R agonism in schizophrenia (Liemburg et al., 2011). Finally, greater striatal D2/D3R occupancy with antipsychotics relates to dysphoria in patients (Mizrahi et al., 2007) and antipsychotic use induces dysphoria in healthy individuals (King et al., 1995). Of note, DA receptor sensitivity is positively related to affective flattening, but not anhedonia per se, in depressed patients (Schmidt et al., 2001). However, unlike the current and previous human studies described, the Schmidt et al. (2001) study did not distinguish between D1- and D2-type receptors. In summary, the current preliminary result, which must be confirmed by future replication studies, supports the hypothesis that anhedonia is in part due to low D2R function (Heinz et al., 1994; Wise, 1982).
MGP scores predicted dorsal striatal D2R binding as measured by PET. We have previously shown, in this same sample, that DRD2/ANKK1 TaqIA A1 allele carriers had lower dorsal striatal D2R specific binding relative to individuals homozygous for A2 (Eisenstein et al., 2016) . The presence of this relationship in this sample lends support to the use of [11C]NMB as a valid PET radioligand to measure D2R binding. Unfortunately, we did not have the statistical power (n = 15 participants with PET and genetic data) to test whether D2R binding mediated the relationship between MGP scores and self-reported anhedonia.
The current study has limitations. First, the sample size was small and precluded reliable analysis of genotype frequencies in small ethnic subgroups and reported associations should be interpreted with caution until replicated. Second, the PET radioligand used, [11C]NMB, due to its selectivity for D2R over D3R, is not optimal for measuring D2R in NAc since it contains fewer D2R than dorsal striatal regions, causing increased variability in D2R BPND measurements (Eisenstein et al., 2012). We were likely unable to detect strong relationships between ventral striatum and other variables for this reason. However, greater NAc D2R did relate to less self-rated anhedonia, as measured by the SPSA, at a significance level that did not pass multiple comparisons correction. For similar reasons, we did not examine D2R binding in the prefrontal cortex, which would have been interesting given its association with negative symptoms (Davis et al., 1991; Goghari et al., 2010; Knable and Weinberger, 1997). Third, we did not have statistical power to investigate the impact of smoking severity or antipsychotic medication use on the relationships between MGP scores or striatal D2R binding and negative symptoms. Fourth, for some participants, there was a considerable length of time between completion of anhedonia self-report questionnaires and PET measurement of striatal D2R binding. However, previous research indicates high test-retest reliability for trait anhedonia in patients and controls over 90 days (Blanchard et al., 1998) , 1 year (Blanchard et al., 2001), and 10 years (Herbener and Harrow, 2002). Fifth, the relationships between MGP scores or D2R binding and negative symptom severity did not hold across all measures. Although moderate to high correlations in the expected directions were observed between SANS and BNSS scores and between SPSA, SHAPS, and TEPS scores (data not shown), subtle differences in aspects of negative symptom severity and self-reported anhedonia that are measured by each scale and/or low statistical power to detect these relationships may have contributed to our null findings. Future studies should include larger samples and control for ethnicity to avoid these kinds of limitations. Finally, MR-based attenuation correction may bias PET activity measurements such that it is underestimated in most of the brain and overestimated in deep brain regions compared to CT-based attenuation maps (Su et al., 2016). Since the bias does not appear to affect clinical assessment and PET activities after both MR- and CT-based attenuation methods are closely correlated (Su et al., 2016), it should not have affected our results in a way that changes their interpretation. In the future, CT-based attenuation correction may be implemented to obtain more accurate PET activity measurements from hybrid PET/MR scanners.
In summary, the current study shows that subcortical DA signaling capacity, as reflected by MGP scores, and dorsal striatal D2R binding correlated with clinician-rated and self-reported negative symptom severity across HC, SIB and SCZ. These preliminary results support the notion that striatal DA signaling contributes to negative symptoms, including anhedonia, thereby serving as a potential pharmacotherapeutic target in psychiatric diseases including schizophrenia and depression.
Supplementary Material
Acknowledgments
The studies presented in this work were conducted using the Center for Clinical Imaging Research located at the Washington University Medical Center. We thank Jonathan M. Koller, B.S. for his help with PET/MR processing. We thank Melissa Cornejo, Arthur Schaffer, and Danielle Kelly for their help with recruiting participants.
Role of funding source. This research was funded by a NARSAD Young Investigator Award (SAE); a McDonnell Center for Systems Neuroscience New Resource Proposal (SAE); Gregory B. Couch Award (DMB); Barnes-Jewish Hospital Foundation (Elliot Stein Family Fund) (JSP); and the National Institutes of Health [UL1 TR000448, R01 MH066031 (DMB), R01 AG045231 (RB)]. RB receives support from the Klingenstein Third Generation Foundation. This study was supported in part by the Neuroimaging Informatics and Analysis Center (1P30NS098577). This publication was made possible by Grant Number UL1 RR024992 (LC) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. These funding sources had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions. SAE, RB, and DMB wrote the manuscript. SAE and LC analyzed data. SAE, RB, DMB, KJB, JSP, and TH contributed to study design and methods. SMM contributed reagents. All authors reviewed and edited the manuscript.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington D.C: American Psychiatric Association; 2000. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, Iowa: The University of Iowa; 1983a. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, Iowa: The University of Iowa; 1983b. [Google Scholar]
- Agid O, Mamo D, Ginovart N, Vitcu I, Wilson AA, Zipursky RB, Kapur S. Striatal vs extrastriatal dopamine D2 receptors in antipsychotic response--a double-blind PET study in schizophrenia. Neuropsychopharmacology. 2007;32(6):1209–1215. doi: 10.1038/sj.npp.1301242. [DOI] [PubMed] [Google Scholar]
- Antenor-Dorsey JA, Markham J, Moerlein SM, Videen TO, Perlmutter JS. Validation of the reference tissue model for estimation of dopaminergic D2-like receptor binding with [18F](N-methyl)benperidol in humans. Nucl Med Biol. 2008;35(3):335–341. doi: 10.1016/j.nucmedbio.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65(3):1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Horan WP, Brown SA. Diagnostic differences in social anhedonia: a longitudinal study of schizophrenia and major depressive disorder. J Abnorm Psychol. 2001;110(3):363–371. doi: 10.1037//0021-843x.110.3.363. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophrenia bulletin. 1998;24(3):413–424. doi: 10.1093/oxfordjournals.schbul.a033336. [DOI] [PubMed] [Google Scholar]
- Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, Bogerts B, Braun K, Jankowski Z, Kumaratilake J, Henneberg M, Gos T. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry. 2014;5:47. doi: 10.3389/fpsyt.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardno AG, Gottesman II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet. 2000;97(1):12–17. [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for Physical and Social Anhedonia. Journal of Abnormal Psychology. 1976;85(4):374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Corral-Frías NS, Nikolova YS, Michalski LJ, Baranger DA, Hariri AR, Bogdan R. Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychol Med. 2015;45(12):2605–2617. doi: 10.1017/S0033291715000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence. Scale-Fourth. San Antonio, TX: Pearson; 2008. [Google Scholar]
- Daniel DG. Issues in selection of instruments to measure negative symptoms. Schizophrenia research. 2013;150(2–3):343–345. doi: 10.1016/j.schres.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Davis C, Loxton NJ. Addictive behaviors and addiction-prone personality traits: associations with a dopamine multilocus genetic profile. Addict Behav. 2013;38(7):2306–2312. doi: 10.1016/j.addbeh.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Davis C, Loxton NJ, Levitan RD, Kaplan AS, Carter JC, Kennedy JL. ‘Food addiction’ and its association with a dopaminergic multilocus genetic profile. Physiol Behav. 2013;118:63–69. doi: 10.1016/j.physbeh.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148(11):1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- de Haan L, Lavalaye J, Linszen D, Dingemans PM, Booij J. Subjective experience and striatal dopamine D(2) receptor occupancy in patients with schizophrenia stabilized by olanzapine or risperidone. Am J Psychiatry. 2000;157(6):1019–1020. doi: 10.1176/appi.ajp.157.6.1019. [DOI] [PubMed] [Google Scholar]
- Delso G, Furst S, Jakoby B, Ladebeck R, Ganter C, Nekolla SG, Schwaiger M, Ziegler SI. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med. 2011;52(12):1914–1922. doi: 10.2967/jnumed.111.092726. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Yu DW, Ferrieri RA, King PT, MacGregor RR, Martin TP, Wolf AP, et al. GABAergic inhibition of endogenous dopamine release measured in vivo with 11C–raclopride and positron emission tomography. J Neurosci. 1992;12(10):3773–3780. doi: 10.1523/JNEUROSCI.12-10-03773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd EC, Barch DM. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biological psychiatry. 2010;67(10):902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd EC, Barch DM. Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PLoS One. 2012;7(5):e35622. doi: 10.1371/journal.pone.0035622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein SA, Antenor-Dorsey JA, Gredysa DM, Koller JM, Bihun EC, Ranck SA, Arbelaez AM, Klein S, Perlmutter JS, Moerlein SM, Black KJ, Hershey T. A comparison of D2 receptor specific binding in obese and normal-weight individuals using PET with (N-[(11)C]methyl)benperidol. Synapse. 2013;67(11):748–756. doi: 10.1002/syn.21680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein SA, Bogdan R, Love-Gregory L, Corral-Frias NS, Koller JM, Black KJ, Moerlein SM, Perlmutter JS, Barch DM, Hershey T. Prediction of striatal D2 receptor binding by DRD2/ANKK1 TaqIA allele status. Synapse. 2016;70(10):418–431. doi: 10.1002/syn.21916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein SA, Koller JM, Piccirillo M, Kim A, Antenor-Dorsey JA, Videen TO, Snyder AZ, Karimi M, Moerlein SM, Black KJ, Perlmutter JS, Hershey T. Characterization of extrastriatal D2 in vivo specific binding of [(1)(8)F](N-methyl)benperidol using PET. Synapse. 2012;66(9):770–780. doi: 10.1002/syn.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Levy J, Rubinstein M, Low MJ, Grandy DK, Wise RA. Brain stimulation and morphine reward deficits in dopamine D2 receptor-deficient mice. Psychopharmacology. 2005;182(1):33–44. doi: 10.1007/s00213-005-0051-2. [DOI] [PubMed] [Google Scholar]
- Elsinga PH, Hatano K, Ishiwata K. PET tracers for imaging of the dopaminergic system. Curr Med Chem. 2006;13(18):2139–2153. doi: 10.2174/092986706777935258. [DOI] [PubMed] [Google Scholar]
- First MBSR, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Galderisi S, Merlotti E, Mucci A. Neurobiological background of negative symptoms. Eur Arch Psychiatry Clin Neurosci. 2015;265(7):543–558. doi: 10.1007/s00406-015-0590-4. [DOI] [PubMed] [Google Scholar]
- Gard DEG-GM, Kring AM, Oliver JP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality. 2006;40:1086–1102. [Google Scholar]
- Gluskin BS, Mickey BJ. Genetic variation and dopamine D2 receptor availability: a systematic review and meta-analysis of human in vivo molecular imaging studies. Transl Psychiatry. 2016;6:e747. doi: 10.1038/tp.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari VM, Sponheim SR, MacDonald AW., 3rd The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci Biobehav Rev. 2010;34(3):468–486. doi: 10.1016/j.neubiorev.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Guerrero A, Mizrahi R, Agid O, Marcon H, Barsoum P, Rusjan P, Wilson AA, Zipursky R, Kapur S. The dopamine D2 receptors in high-affinity state and D3 receptors in schizophrenia: a clinical [11C]-(+)-PHNO PET study. Neuropsychopharmacology. 2009;34(4):1078–1086. doi: 10.1038/npp.2008.199. [DOI] [PubMed] [Google Scholar]
- Heinz A. Dopaminergic dysfunction in alcoholism and schizophrenia--psychopathological and behavioral correlates. Eur Psychiatry. 2002;17(1):9–16. doi: 10.1016/s0924-9338(02)00628-4. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22(2):133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Heinz A, Knable MB, Coppola R, Gorey JG, Jones DW, Lee KS, Weinberger DR. Psychomotor slowing, negative symptoms and dopamine receptor availability--an IBZM SPECT study in neuroleptic-treated and drug-free schizophrenic patients. Schizophrenia research. 1998;31(1):19–26. doi: 10.1016/s0920-9964(98)00003-6. [DOI] [PubMed] [Google Scholar]
- Heinz A, Schmidt LG, Reischies FM. Anhedonia in schizophrenic, depressed, or alcohol-dependent patients--neurobiological correlates. Pharmacopsychiatry. 1994;27(Suppl 1):7–10. doi: 10.1055/s-2007-1014317. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Harrow M. The course of anhedonia during 10 years of schizophrenic illness. J Abnorm Psychol. 2002;111(2):237–248. [PubMed] [Google Scholar]
- Hershey T, Black KJ, Carl JL, McGee-Minnich L, Snyder AZ, Perlmutter JS. Long term treatment and disease severity change brain responses to levodopa in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74(7):844–851. doi: 10.1136/jnnp.74.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Nour MM. Dopamine and the aberrant salience hypothesis of schizophrenia. World Psychiatry. 2016;15(1):3–4. doi: 10.1002/wps.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, Knutson B, Kienast T, Gallinat J, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology. 2006a;187(2):222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage. 2006b;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Slifstein M, Frankle WG, Xu X, Hackett E, Bae SA, Gonzales R, Kim JH, Alvarez B, Gil R, Laruelle M, Abi-Dargham A. Dose-occupancy study of striatal and extrastriatal dopamine D2 receptors by aripiprazole in schizophrenia with PET and [18F]fallypride. Neuropsychopharmacology. 2008;33(13):3111–3125. doi: 10.1038/npp.2008.33. [DOI] [PubMed] [Google Scholar]
- King DJ, Burke M, Lucas RA. Antipsychotic drug-induced dysphoria. Br J Psychiatry. 1995;167(4):480–482. doi: 10.1192/bjp.167.4.480. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Linh N, Fischer BA, Daniel DG, Cienfuegos A, Marder SR. The Brief Negative Symptom Scale: Psychometric Properties. Schizophrenia bulletin. 2011;37(2):300–305. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm E, Grunwald F, Kasper S, Menzel C, Broich K, Danos P, Reichmann K, Krappel C, Rieker O, Briele B, Hotze AL, Moller HJ, Biersack HJ. [123I]IBZM SPECT for imaging of striatal D2 dopamine receptors in 56 schizophrenic patients taking various neuroleptics. Am J Psychiatry. 1996;153(2):183–190. doi: 10.1176/ajp.153.2.183. [DOI] [PubMed] [Google Scholar]
- Knable MB, Weinberger DR. Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol. 1997;11(2):123–131. doi: 10.1177/026988119701100205. [DOI] [PubMed] [Google Scholar]
- Kupferberg A, Bicks L, Hasler G. Social functioning in major depressive disorder. Neurosci Biobehav Rev. 2016;69:313–332. doi: 10.1016/j.neubiorev.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biological psychiatry. 1999;46(1):56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Rosenblatt W, Zea-Ponce Y, Zoghbi SS, Baldwin RM, Charney DS, Hoffer PB, Kung HF, et al. SPECT imaging of striatal dopamine release after amphetamine challenge. J Nucl Med. 1995;36(7):1182–1190. [PubMed] [Google Scholar]
- Lataster J, van Os J, de Haan L, Thewissen V, Bak M, Lataster T, Lardinois M, Delespaul PA, Myin-Germeys I. Emotional experience and estimates of D2 receptor occupancy in psychotic patients treated with haloperidol, risperidone, or olanzapine: an experience sampling study. J Clin Psychiatry. 2011;72(10):1397–1404. doi: 10.4088/JCP.09m05466yel. [DOI] [PubMed] [Google Scholar]
- Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J. American Psychiatric Association, Steering Committee on Practice Guidelines. Am J Psychiatry. 2004;161(2):1–56. [PubMed] [Google Scholar]
- Lemke MR, Brecht HM, Koester J, Reichmann H. Effects of the dopamine agonist pramipexole on depression, anhedonia and motor functioning in Parkinson’s disease. J Neurol Sci. 2006;248(1–2):266–270. doi: 10.1016/j.jns.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophrenia bulletin. 2014;40(2):314–326. doi: 10.1093/schbul/sbu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Brightman M, Ameringer KJ, Greenberg J, Mickens L, Ray LA, Sun P, Sussman S. Anhedonia associated with stimulant use and dependence in a population-based sample of American adults. Exp Clin Psychopharmacol. 2010;18(6):562–569. doi: 10.1037/a0021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liemburg E, Aleman A, Bous J, Hollander K, Knegtering H. An open randomized pilot trial on the differential effects of aripiprazole versus risperidone on anhedonia and subjective well-being. Pharmacopsychiatry. 2011;44(3):109–113. doi: 10.1055/s-0031-1271688. [DOI] [PubMed] [Google Scholar]
- Martinot JL, Paillere-Martinot ML, Loc’h C, Lecrubier Y, Dao-Castellana MH, Aubin F, Allilaire JF, Mazoyer B, Maziere B, Syrota A. Central D2 receptors and negative symptoms of schizophrenia. Br J Psychiatry. 1994;164(1):27–34. doi: 10.1192/bjp.164.1.27. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8(5):594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Rusjan P, Agid O, Graff A, Mamo DC, Zipursky RB, Kapur S. Adverse subjective experience with antipsychotics and its relationship to striatal and extrastriatal D2 receptors: a PET study in schizophrenia. Am J Psychiatry. 2007;164(4):630–637. doi: 10.1176/ajp.2007.164.4.630. [DOI] [PubMed] [Google Scholar]
- Moerlein SM, LaVenture J, Gaehle GG, Robben J, Perlmutter JS, Mach RH. Automated Production of N-([C-11]Methyl)benperidol ([C-11]NMB) for Clinical Application. Eur J Nucl Med Mol I. 2010;37:S366–S366. [Google Scholar]
- Moerlein SM, Perlmutter JS, Welch MJ. Radiosynthesis of (N-[C-11]methyl)benperidol for PET investigation of D2 receptor binding. Radiochim Acta. 2004;92(4–6):333–339. [Google Scholar]
- Mukherjee J, Yang ZY, Brown T, Lew R, Wernick M, Ouyang X, Yasillo N, Chen CT, Mintzer R, Cooper M. Preliminary assessment of extrastriatal dopamine D-2 receptor binding in the rodent and nonhuman primate brains using the high affinity radioligand, 18F–fallypride. Nucl Med Biol. 1999;26(5):519–527. doi: 10.1016/s0969-8051(99)00012-8. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(9):1940–1947. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson-Fuhrhop KM, Dunn EC, Mortero S, Devan WJ, Falcone GJ, Lee P, Holmes AJ, Hollinshead MO, Roffman JL, Smoller JW, Rosand J, Cramer SC. Dopamine genetic risk score predicts depressive symptoms in healthy adults and adults with depression. PLoS One. 2014;9(5):e93772. doi: 10.1371/journal.pone.0093772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickar D, Su TP, Weinberger DR, Coppola R, Malhotra AK, Knable MB, Lee KS, Gorey J, Bartko JJ, Breier A, Hsiao J. Individual variation in D2 dopamine receptor occupancy in clozapine-treated patients. Am J Psychiatry. 1996;153(12):1571–1578. doi: 10.1176/ajp.153.12.1571. [DOI] [PubMed] [Google Scholar]
- Pogarell O, Koch W, Karch S, Dehning S, Muller N, Tatsch K, Poepperl G, Moller HJ. Dopaminergic neurotransmission in patients with schizophrenia in relation to positive and negative symptoms. Pharmacopsychiatry. 2012;45(Suppl 1):S36–S41. doi: 10.1055/s-0032-1306313. [DOI] [PubMed] [Google Scholar]
- Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F, Bachneff S, Cumming P, Diksic M, Dyve SE, Etienne P, Evans AC, Lal S, Shevell M, Savard G, Wong DF, Chouinard G, Gjedde A. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(24):11651–11654. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B, Anderson S, Doop M, Woodward N, Schoenberg E, Schmidt D, Baldwin R, Kessler R. Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology. 2006;31(5):1016–1026. doi: 10.1038/sj.npp.1300916. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain research. Brain research reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Nolte-Zenker B, Patzer J, Bauer M, Schmidt LG, Heinz A. Psychopathological correlates of reduced dopamine receptor sensitivity in depression, schizophrenia, and opiate and alcohol dependence. Pharmacopsychiatry. 2001;34(2):66–72. doi: 10.1055/s-2001-15184. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Biller A, Walther S, Roesch-Ely D, Stippich C, Weisbrod M, Kaiser S. Neural correlates of reward processing in schizophrenia--relationship to apathy and depression. Schizophrenia research. 2010;118(1–3):154–161. doi: 10.1016/j.schres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger K, Epstein L, Smolen A. Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. J Neurosci. 2012;32(29):10093–10100. doi: 10.1523/JNEUROSCI.1506-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophrenia bulletin. 2014;40(Suppl 2):S107–S116. doi: 10.1093/schbul/sbt197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Rubin B, McConathy J, Laforest R, Qi J, Sharma A, Priatna A, Benzinger T. Impact of MR based attenuation correction on neurological PET studies. J Nucl Med. 2016 doi: 10.2967/jnumed.115.164822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talvik M, Nordstrom AL, Okubo Y, Olsson H, Borg J, Halldin C, Farde L. Dopamine D2 receptor binding in drug-naive patients with schizophrenia examined with raclopride-C11 and positron emission tomography. Psychiatry Res. 2006;148(2–3):165–173. doi: 10.1016/j.pscychresns.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Thompson J, Thomas N, Singleton A, Piggott M, Lloyd S, Perry EK, Morris CM, Perry RH, Ferrier IN, Court JA. D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7(6):479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Uchida H, Rajji TK, Mulsant BH, Kapur S, Pollock BG, Graff-Guerrero A, Menon M, Mamo DC. D2 receptor blockade by risperidone correlates with attention deficits in late-life schizophrenia. J Clin Psychopharmacol. 2009;29(6):571–575. doi: 10.1097/JCP.0b013e3181bf4ea3. [DOI] [PubMed] [Google Scholar]
- van de Giessen E, de Win MM, Tanck MW, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J Nucl Med. 2009;50(1):45–52. doi: 10.2967/jnumed.108.053652. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med. 2005;46(5):745–751. [PubMed] [Google Scholar]
- Videbaek C, Toska K, Scheideler MA, Paulson OB, Moos Knudsen G. SPECT tracer [(123)I]IBZM has similar affinity to dopamine D2 and D3 receptors. Synapse. 2000;38(3):338–342. doi: 10.1002/1098-2396(20001201)38:3<338::AID-SYN13>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Voruganti L, Slomka P, Zabel P, Costa G, So A, Mattar A, Awad AG. Subjective effects of AMPT-induced dopamine depletion in schizophrenia: correlation between dysphoric responses and striatal D(2) binding ratios on SPECT imaging. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;25(5):642–650. doi: 10.1016/S0893-133X(01)00263-9. [DOI] [PubMed] [Google Scholar]
- Voruganti LN, Awad AG. Subjective and behavioural consequences of striatal dopamine depletion in schizophrenia--findings from an in vivo SPECT study. Schizophrenia research. 2006;88(1–3):179–186. doi: 10.1016/j.schres.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Schweitzer JB, Gold JM, Kurup PK, Ross TJ, Salmeron BJ, Rose EJ, McClure SM, Stein EA. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34(6):1567–1577. doi: 10.1038/npp.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Schweitzer JB, Ross TJ, Kurup PK, Salmeron BJ, Rose EJ, Gold JM, Stein EA. Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(12):2427–2439. doi: 10.1038/npp.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Lappas S, Cheeta S, Muscat R. Reversal of stress-induced anhedonia by the dopamine receptor agonist, pramipexole. Psychopharmacology. 1994;115(4):454–462. doi: 10.1007/BF02245568. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neuroleptic attenuation of intracranial self-stimulation: reward or performance deficits? Life Sci. 1978;22(7):535–542. doi: 10.1016/0024-3205(78)90331-4. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neuroleptics and Operant-Behavior - the Anhedonia Hypothesis. Behav Brain Sci. 1982;5(1):39–53. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.