Key Points
Viruses exploit the functions of the endoplasmic reticulum (ER) to promote both early and later stages of their life cycle, including entry, translation, replication, assembly, morphogenesis and egress. This observation reveals a shared principle that underlies virus–host cell relationships.
Viral entry often requires disassembly of the incoming virus particle. This is best exemplified in the case of polyomavirus entry, in which ER-associated machineries are hijacked to disassemble the virus and promote entry to the cytosol en route to the nucleus.
Many enveloped viruses, such as HIV and influenza virus, co-opt the ER-associated protein biosynthetic machinery to translate their genome and produce structural proteins that are necessary for the formation of virus particles and non-structural proteins that are essential during genome replication.
Replication of the viral genome, particularly for positive-sense RNA ((+)RNA) viruses including hepatitis C virus (HCV), dengue virus (DENV) and West Nile virus (WNV), occurs in virus-induced membranous structures that are most often derived from the ER. The formation of these structures requires morphological changes to the ER membrane, involving membrane rearrangements that are induced by viral non-structural proteins that are targeted to the ER.
As virus assembly is often coupled to genome replication, the assembly process frequently relies on the ER membrane. This strategy is seen for both RNA and DNA viruses.
Morphogenesis of assembled virus particles can also take advantage of the ER. This is best observed in the non-enveloped rotavirus, for which a transient enveloped intermediate is converted to the mature and infectious particle in the lumen of the ER.
After maturation in the ER, progeny virus particles egress the host through the ER-dependent secretory pathway, which provides a physical conduit to the extracellular environment.
The overall observations that the ER actively promotes all steps of viral infection have therapeutic implications. The development of chemical inhibitors of selective ER-associated components is emerging as a potential avenue of antiviral therapy, provided that these inhibitors have minimal toxicity to the host cell.
Supplementary information
The online version of this article (doi:10.1038/nrmicro.2016.60) contains supplementary material, which is available to authorized users.
Subject terms: Virus-host interactions, Endoplasmic reticulum, Viral infection
Many host structures are vital for viral infection and the endoplasmic reticulum (ER), in particular, is essential. In this Review, Tsai and colleagues highlight examples of subversion of the ER by diverse viruses to promote all stages of their life cycle, from entry to egress.
Supplementary information
The online version of this article (doi:10.1038/nrmicro.2016.60) contains supplementary material, which is available to authorized users.
Abstract
Viruses subvert the functions of their host cells to replicate and form new viral progeny. The endoplasmic reticulum (ER) has been identified as a central organelle that governs the intracellular interplay between viruses and hosts. In this Review, we analyse how viruses from vastly different families converge on this unique intracellular organelle during infection, co-opting some of the endogenous functions of the ER to promote distinct steps of the viral life cycle from entry and replication to assembly and egress. The ER can act as the common denominator during infection for diverse virus families, thereby providing a shared principle that underlies the apparent complexity of relationships between viruses and host cells. As a plethora of information illuminating the molecular and cellular basis of virus–ER interactions has become available, these insights may lead to the development of crucial therapeutic agents.
Supplementary information
The online version of this article (doi:10.1038/nrmicro.2016.60) contains supplementary material, which is available to authorized users.
Main
Viruses have evolved sophisticated strategies to establish infection. Some viruses bind to cellular receptors and initiate entry, whereas others hijack cellular factors that disassemble the virus particle to facilitate entry. After delivering the viral genetic material into the host cell and the translation of the viral genes, the resulting proteins either become part of a new virus particle (or particles) or promote genome replication in a process that can be tightly coupled to virus assembly. The newly assembled progeny, in turn, undergo morphogenesis, which produces mature virions that are poised to exit the host cell. Viruses can also co-opt cellular components to suppress the innate immune system of the host to further promote infection. To accomplish these distinct steps in a typical virus life cycle — entry, translation, replication, assembly, morphogenesis and egress — viruses have evolved the extraordinary ability to usurp the endogenous functions of every intracellular organelle. Remarkably, whereas most organelles can support only one or a few of the steps in viral infection, one organelle — the endoplasmic reticulum (ER) — is involved throughout the whole viral life cycle, dependent on the specific virus. This is owing to the numerous ER-resident channels, enzymes, chaperones and sensors, as well as the physical properties of the ER lipid bilayer, including its expansive surface area and its ability to undergo constant membrane rearrangements. The large surface area of the ER also enables it to connect with several other organelles, which is probably another reason why it is used by many viruses. Therefore, the ER is not only essential in maintaining cellular homeostasis; it also renders cells particularly susceptible to viral infection.
The ER is a membranous system consisting of the outer nuclear envelope that is contiguous with an intricate network of tubules and sheets1, which are shaped by resident factors in the ER2,3,4. The morphology of the ER is highly dynamic and experiences constant structural rearrangements, enabling the ER to carry out a myriad of functions5. In the rough, tubular ER, active protein biosynthesis occurs, whereas the sheet-like smooth ER functions in lipid metabolism and harbours detoxifying enzymes6,7,8.
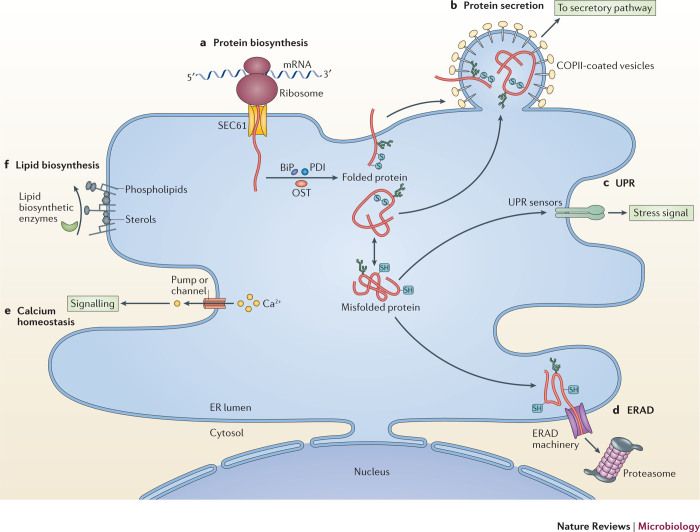
A major role of the ER is the biosynthesis of membrane and luminal proteins. Approximately one-third of all cellular proteins are synthesized in this organelle. During synthesis, nascent polypeptide chains are co-translationally translocated through the SEC61 translocation channel9 in the ER membrane (Fig. 1a). Luminal or secretory proteins are released from the channel into the lumen of the ER, whereas membrane proteins partition into the ER lipid bilayer. Once the newly synthesized client protein is disengaged, ER-resident enzymes carry out post-translational modifications to assist in protein folding and assembly. For example, the oligosaccharyl transferase (OST) complex attaches glycan moieties to proteins, and oxidoreductases form, break and isomerize disulfide bonds10,11. Molecular ER chaperones, such as the binding immunoglobulin protein (BiP; also known as GRP78), which is a heat shock protein 70 (HSP70) ATPase, also interact with the folding client protein to prevent aggregation and render it soluble12. Folded and assembled proteins are then packaged into COPII-coated vesicles and exit the ER13 (Fig. 1b), transiting through the classical secretory pathway en route to other cellular destinations or to the plasma membrane for secretion. Misfolded or misassembled proteins accumulate in the ER, which triggers a stress signalling cascade — the unfolded protein response (UPR) — that activates UPR sensors located on the ER membrane14 (Fig. 1c). Activation of these sensors causes an increase in the synthesis of ER-resident molecular chaperones and temporarily halts translation. If the UPR cannot remediate the misfolding of the protein, the misfolded client is retro-translocated to the cytosol where it is degraded by the proteasome in a pathway termed ER-associated degradation (ERAD)15,16 (Box 1; Fig. 1d). The ER also controls calcium homeostasis, through different calcium channels and pumps located in the ER membrane that directly communicate with calcium channels in the plasma membrane17 (Fig. 1e). In the ER, calcium is a cofactor for chaperones, such as calnexin and calreticulin, that control glycoprotein folding, and thus has a crucial role in protein quality control18. Furthermore, the ER also functions in the synthesis of lipids, including fatty acids, phospholipids, sphingolipids and cholesterol (Fig. 1f), through strategically localized enzymes that generate lipids that become part of the membrane or signalling molecules19.
Figure 1. Endogenous functions of the ER.
a | Protein biosynthesis. The endoplasmic reticulum (ER) is the site for the biosynthesis of membrane and luminal proteins that function in the ER and in the classical secretory pathway, as well as for the biosynthesis of secreted proteins. In this process, a nascent polypeptide client is co-translationally translocated through the SEC61 translocon into the lumen of the ER (for luminal proteins) or laterally into the ER bilayer (for membrane proteins). The client protein then undergoes post-translational modifications to assist in its folding and assembly — tasks that are carried out by dedicated ER-localized enzymes or chaperones (oligosaccharyl transferase (OST), protein disulfide isomerase (PDI) family members and binding immunoglobulin protein (BiP)). b | Protein secretion. After folding and assembly, the client is packaged into a coat protein complex II (COPII)-coated vesicle that buds out of the ER. The client protein is transported through the classical secretory pathway en route to other cellular destinations or to the cell surface for secretion. c | Unfolded protein response (UPR). When a client protein misfolds or misassembles, it triggers the UPR, which stimulates a stress signalling cascade (through the activation of ER membrane sensors) that is intended to rectify the misfolding of proteins. d | ER-associated degradation (ERAD). Despite this effort to rectify protein misfolding, if the client remains terminally misfolded, it is then subjected to degradation by a process known as ERAD. During ERAD, a misfolded substrate is processed and retro-translocated into the cytosol for proteasome-mediated degradation. e | Calcium homeostasis. The ER also stores Ca2+ and controls its homeostasis — a process that is regulated by different calcium channels and pumps in the ER membrane that directly communicate with calcium channels in the plasma membrane. f | Lipid biogenesis. The ER is also the centre for lipid biogenesis, in which different lipid biosynthetic enzymes that are embedded and/or associated with the ER membrane generate lipids (sphingolipids, phospholipids and sterols) that are used for structural or signalling purposes. SH, sulfhydryl group.
In this Review, we explore how these diverse functions and unique physical properties of the ER are exploited by viruses from various families, including, for example, the Polyomaviridae, Flaviviridae and Reoviridae, to promote specific steps of their life cycle (Table 1). For clarity, the multistep infection cycle will be divided into two stages: an early stage (entry-associated virus disassembly and genome translation) and a later stage (viral genome replication, assembly, morphogenesis and egress).
Table 1. Viruses that exploit endogenous ER functions to promote infection.
| Family | Strains | ER functions | Mechanisms of ER exploitation | Refs |
|---|---|---|---|---|
| Non-enveloped RNA viruses | ||||
| Hepeviridae | Hepatitis E virus | Protein biosynthesis, folding and assembly | Synthesis of ORF2 | 62 |
| ERAD | Retro-translocation of ORF2 to the cytosol | 63 | ||
| Picornaviridae | Poliovirus | Membrane properties and lipid biosynthesis | Support of replication | 60 |
| Calcium homeostasis | Viroporin 2B releases Ca2+ | 167 | ||
| Enterovirus 71 | Membrane properties and lipid biosynthesis | Support of replication | 59 | |
| Reoviridae | Rotavirus | Calcium homeostasis | Non-structural protein 4 (NSP4) releases Ca2+ to recruit viral proteins to the site of assembly | 122 |
| Membrane properties and lipid biosynthesis | Support of assembly and morphogenesis | 124 | ||
| Protein secretion | Mature virion uses classical secretory pathway for egress | 133 | ||
| Enveloped RNA viruses | ||||
| Arteriviridae | Equine arteritis virus | Membrane properties and lipid biosynthesis | Support of replication | 90 |
| Coronaviridae | SARS coronavirus | Membrane properties and lipid biosynthesis | Support of replication and assembly | 88 |
| Mouse hepatitis virus | Membrane properties and lipid biosynthesis | Support of replication | ||
| Flaviviridae | Hepatitis C virus | Protein biosynthesis, folding and assembly | Synthesis of the glycoproteins E1 and E2 | 52, 53 |
| Membrane properties and lipid biosynthesis | Support of replication, assembly and morphogenesis | 53, 67 | ||
| UPR | Stimulation of the UPR to suppress immune responses and promote replication | 98 | ||
| Protein secretion | Mature virion uses classical secretory pathway for egress | 131 | ||
| Dengue virus | Protein biosynthesis, folding and assembly | Synthesis of preM and E glycoproteins | 50 | |
| Membrane properties and lipid biosynthesis | Support of replication and assembly | 91 | ||
| UPR | Stimulation of the UPR to suppress immune responses and promote replication | 98 | ||
| Protein secretion | Mature virion uses ER–Golgi recycling and the KDEL receptor for egress | 140 | ||
| Japanese encephalitis virus | Protein biosynthesis, folding and assembly | Synthesis of envelope protein | 89 | |
| Membrane properties and lipid biosynthesis | Support of replication | |||
| UPR | Activation of IRE1-dependent decay pathway to promote replication | 99 | ||
| West Nile virus | Membrane properties and lipid biosynthesis | Support of replication | 92 | |
| UPR | NS4A and NS4B induce the UPR to promote replication | 168 | ||
| Pestivirus | Membrane properties and lipid biosynthesis | Support of assembly and morphogenesis | 120 | |
| Orthomyxoviridae | Influenza A virus | Protein biosynthesis, folding and assembly | Synthesis of the haemagglutinin glycoprotein | 47 |
| UPR | Activation of IRE1 to promote replication | 100 | ||
| Calcium homeostasis | Increases IP3 to activate IP3R-mediated Ca2+ signalling to promote apotosis | 169 | ||
| Retroviridae | HIV-1 | Protein biosynthesis, folding and assembly | Synthesis of the envelope glycoprotein | 46 |
| Calcium homeostasis | Tat interacts with IP3R to release ER Ca2+ to regulate the production of TNF | 129 | ||
| Mouse mammary tumour virus | Protein biosynthesis, folding and assembly | Viral protein Rem is processed by ER-resident signal peptidase | 65 | |
| ERAD | Retro-translocation of Rem to the cytosol | |||
| Non-enveloped DNA viruses | ||||
| Parvoviridae | Parvovirus | Protein secretion | Mature virion uses classical secretory pathway for egress | 137 |
| Papillomaviridae | Human papillomavirus 16 | ERAD | ER-resident PDI family members are important for infection | 41 |
| Polyomaviridae |
Simian virus 40 Murine polyomavirus BK virus |
ERAD | ER-resident PDI family members partially disassemble the viruses and ER-associated cytosolic extraction machinery extracts and disassembles the viruses | 26, 27, 28, 29, 35, 37 |
| Enveloped DNA viruses | ||||
| Poxviridae | Vaccinia virus | Membrane properties and lipid biosynthesis | Support of replication and assembly | 115 |
E, envelope protein; ER, endoplasmic reticulum; ERAD, ER-associated degradation; IP3, inositol trisphosphate; IP3R, IP3 receptor; IRE1, inositol-requiring enzyme 1; NS, non-structural; PDI, protein disulfide isomerase; preM, pre-membrane protein; SARS, severe acute respiratory syndrome; TNF, tumour necrosis factor; UPR, unfolded protein response.
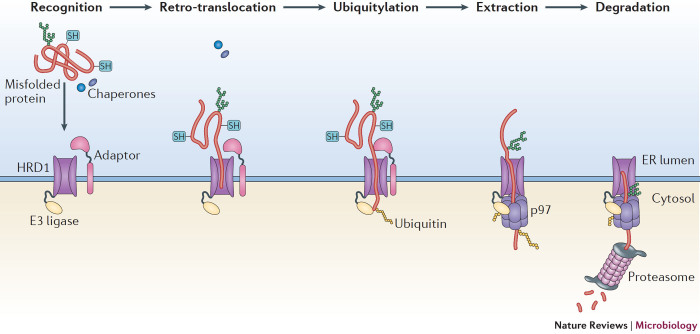
Box 1: ER-associated degradation.
Once a nascent polypeptide is translocated into the endoplasmic reticulum (ER), a dedicated network of cellular factors ensures that it folds and matures correctly before being transported to other cellular destinations. However, protein folding is an imperfect process and when a client protein in the ER misfolds, it is ejected into the cytosol and degraded through ER-associated degradation (ERAD, see the figure). This quality control pathway relies on a cascade of enzymes and chaperones that operate in a coordinated multistep manner, which can be spatially divided into client recognition, retro-translocation, ubiquitylation, extraction and degradation. To initiate ERAD, components in the ER lumen recognize and modify the misfolded client protein and target it to the membrane-bound ERAD machinery. Next, the client protein is retro-translocated across the ER lipid bilayer through a membrane channel the central component of which is the E3 ubiquitin ligase HRD1 (also known as SYVN1). When the misfolded client protein emerges on the cytosolic side of the ER membrane, it becomes ubiquitylated by HRD1. The ubiquitylated client protein is then extracted to the cytosol by the p97-dependent extraction machinery and finally delivered to the proteasome for degradation.
SH, sulfhydryl group.
Exploiting the ER during early stages of infection
Entry-associated virus disassembly. The first step in viral infection is entry into the host cell. For many viruses, entry requires disassembly of the incoming virus particle. Hijacking the functions of the ER for virus disassembly during entry is best described in the Polyomaviridae family. Polyomaviruses are non-enveloped double-stranded DNA (dsDNA) viruses that are responsible for many human diseases, including nephropathy, progressive multifocal leukoencephalopathy and Merkel cell carcinoma20. The two archetypical polyomaviruses, murine polyomavirus and simian virus 40, exhibit structural and genetic similarities to human polyomaviruses and have a similar entry mechanism to their human counterparts20. Not surprisingly, studies on these two non-human polyomaviruses have provided crucial insights into the cellular basis of polyomavirus infection. Structurally, polyomaviruses are composed of 72 pentamers of the major capsid protein VP1 that enclose the DNA genome, with each pentamer encasing an internal hydrophobic protein VP2 or VP3 (Refs 21,22). The carboxyl terminus of VP1 contacts a neighbouring VP1 pentamer to provide inter-pentamer support, with intra-pentamer and inter-pentamer disulfide bonds further reinforcing the viral architecture23.
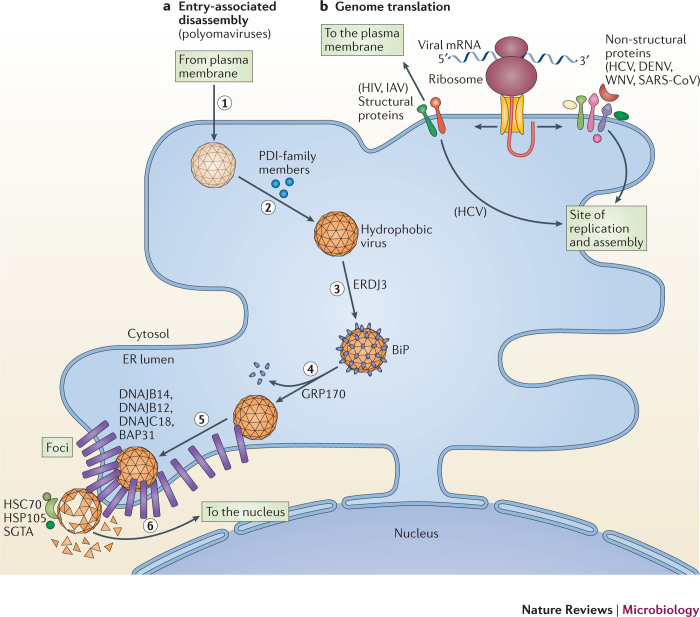
Polyomaviruses undergo receptor-mediated endocytosis and traffic to the endolysosomal system from which they travel through a lipid-dependent pathway to the ER24,25 (Fig. 2a, step 1). In the ER, polyomaviruses co-opt elements of the ERAD machinery to initiate penetration into the cytosol. Specifically, redox chaperones of the protein disulfide isomerase (PDI) family isomerize and reduce the disulfide bonds in VP1, as well as unfold its C-terminal arms26,27,28,29,30 (Fig. 2a, step 2). These reactions partially disassemble polyomaviruses, exposing VP2 and VP3 (Refs 27,31,32). This generates hydrophobic virus particles that recruit BiP through the luminal J protein ER DNAJ domain-containing protein 3 (ERDJ3, also known as DNAJB11)33 (Fig. 2a, step 3). The nucleotide exchange factor 170 kDa glucose-regulated protein (GRP170; also known as HYOU1), in turn, releases polyomaviruses from BiP, enabling the hydrophobic viruses to integrate into the ER membrane34 (Fig. 2a, step 4). Imaging analyses revealed that the membrane-integrated viruses induce the reorganization of selective ER membrane proteins, including B cell receptor-associated protein 31 (BAP31; also known as BCAP31) and the J proteins DNAJ homologue subfamily B member 12 (DNAJB12), DNAJB14 and DNAJC18 (Refs 32,35,36). This rearrangement is crucial for the penetration of the ER membrane and leads to the formation of discrete puncta called foci, which are proposed as cytosol entry sites for polyomaviruses (Fig. 2a, step 5). To complete the translocation process, the membrane-tethered cytosolic extraction machinery (composed of the heat shock cognate protein 70 (HSC70)–HSP105–small glutamine-rich tetratricopeptide repeat-containing protein-α (SGTA) complex (HSC70–HSP105–SGTA complex)) extracts the polyomaviruses into the cytosol35,37 (Fig. 2a, step 6). The extraction reaction is probably coupled to further disassembly of the virus particles, which is essential for their subsequent transport to the nucleus37. Therefore, the penetration of the ER membrane by polyomaviruses illustrates how luminal, membrane and cytosolic components of the ERAD apparatus are coordinately hijacked to accomplish this decisive entry step.
Figure 2. Exploiting the ER during the early stages of infection.
a | Entry-associated disassembly. Viruses must disassemble their capsid to release their genome. Members of the Polyomaviridae disassemble their capsid by co-opting components of the endoplasmic reticulum (ER)-associated degradation (ERAD) pathway. To cause infection, polyomaviruses undergo receptor-mediated endocytosis and traffic to the ER (step 1). Once at the ER, they use protein disulfide isomerase (PDI)-family members to isomerize and reduce viral disulfide bonds (step 2). These events partially disassemble the virus particles to form hydrophobic viruses that engage binding immunoglobulin protein (BiP) through the activity of ER DNAJ domain-containing protein 3 (ERDJ3; step 3). 170 kDa glucose-regulated protein (GRP170) then releases polyomavirus from BiP, enabling the hydrophobic virus to insert into the ER membrane (step 4). The membrane-inserted virus reorganizes selective ER membrane proteins (B cell receptor-associated protein 31 (BAP31) and the J proteins DNAJ homologue subfamily B member 12 (DNAJB12), DNAJB14 and DNAJ C18) in the lipid bilayer to form foci (step 5). The membrane-attached cytosolic extraction machinery (heat shock cognate protein 70 (HSC70)–heat shock protein 105 (HSP105)–small glutamine-rich tetratricopeptide repeat-containing protein-α (SGTA)) then ejects the virus from the foci into the cytosol in a reaction that simultaneously disassembles the virus (step 6). Cytosolic disassembly enables the resulting core virus particle to move into the nucleus to cause infection. b | Genome translation. Some viruses exploit the ER-associated biosynthetic machinery to translate their genetic code in two ways: the translation of viral structural proteins that are incorporated into virions (for example, the envelope glycoprotein of HIV and haemagglutinin of influenza A virus (IAV)) and the translation of viral non-structural proteins that promote the subsequent viral replication step. This is evident during translation of the positive-sense RNA ((+)RNA) genome of viruses in the Flaviviridae (hepatitis C virus (HCV), dengue virus (DENV) and West Nile virus WNV) and Coronaviridae (severe acute respiratory syndrome coronavirus (SARS-CoV)) families, in which the newly synthesized replication proteins target to sites of virus replication on the ER (or ER-derived) membrane compartments in preparation for replication. At these sites, the physical architecture of the ER membrane effectively acts as a scaffold to recruit the viral replication proteins.
The Papillomaviridae is the only other known virus family in which host cell entry may be associated with the ER. Although most infections that are caused by these non-enveloped DNA viruses are considered benign, some types of human papillomavirus (HPV), including HPV16 and HPV18, can lead to the formation of malignant tumours, for example cervical cancer38. To enter host cells, HPV traffics from the cell surface in endocytic vesicles to the Golgi apparatus from where it continues its retrograde journey to the ER39,40. Although the specific membrane penetration site for HPV remains controversial, the observation that proteins in the PDI family facilitate infection raises the possibility that papillomaviruses, similarly to polyomaviruses, hijack these redox chaperones to promote disassembly and membrane translocation41. The HSP70 chaperone system has been demonstrated to disassemble HPV in vitro42, analogous to the use of this chaperone system during the disassembly-mediated cytosol entry of polyomaviruses. After reaching the cytosol, HPV moves into the nucleus to establish infection. Intriguingly, hijacking the ERAD pathway is not limited to viruses. For example, many bacterial toxins also use this pathway during intoxication43,44,45.
Translating the viral genome. During entry and disassembly, the viral genome is exposed. This enables the genetic information to be transcribed and/or translated, depending on the nature of the genome (whether the genome is composed of DNA or RNA). For retroviruses, the RNA genome is first reverse transcribed to generate complementary DNA, which is the template for subsequent transcription and translation. Translation has two crucial purposes (Table 1). First, it produces viral structural proteins, such as viral envelope glycoproteins, which are necessary for the formation of virions (Fig. 2b). For example, the envelope glycoprotein of HIV-1 and haemagglutinin of influenza A virus (IAV) are synthesized by the ER-bound biosynthetic machinery46,47. Once inserted, folded and assembled, the structural proteins move to the surface of the host cell and are incorporated into new viral progeny during assembly. Other viral envelope glycoproteins (see below) are instead redirected to distinct regions of the ER membrane where virus assembly takes place. Regardless of the strategy that is used, translation, membrane insertion, folding and the assembly of enveloped proteins, arguably represent the most important ways in which all enveloped viruses exploit the functions of the ER. In fact, insights from the biosynthesis of viral envelope proteins have historically provided our earliest understanding of fundamental translocation mechanisms of the ER48,49.
Second, translation can also generate viral non-structural (NS) proteins that prepare and promote the ensuing replication of the viral genome (Fig. 2b). This is most prominently observed in positive-sense RNA ((+)RNA) viruses in which genome replication invariably occurs in association with virus-induced membranes, which are often derived from the ER. Associated with these membranes are numerous replication proteins that are translated from the viral genome. These proteins have the remarkable ability to rearrange the ER membrane, creating characteristic structures that define the virus replication sites that are referred to as 'replication factories' or 'replication complexes' (Ref. 50). Consequently, the physical architecture of the ER membrane is transformed into a docking station to recruit newly translated viral replication proteins that, in concert with host factors, drive and sustain replication of the viral genome.
A notable example is found in hepatitis C virus (HCV), a member of the Flaviviridae family. HCV is an important human pathogen that can cause chronic hepatitis, liver cirrhosis and hepatocellular carcinoma51. Once its (+)RNA genome is delivered into the host cell, it is recruited to the ER membrane for translation. Translation of the viral RNA produces a single polyprotein, which is processed by viral and cellular proteases to generate 10 distinct protein products: structural proteins that consist of the core protein and the envelope glycoproteins E1 and E2, as well as the p7 viroporin and the non-structural proteins NS2, NS3, NS4A, NS4B, NS5A and NS5B. Among these, NS3, NS4A, NS4B, NS5A and NS5B are necessary and sufficient for RNA replication, whereas p7 and NS2 are involved in the subsequent assembly step52,53. Importantly, the five replication proteins coordinately target to the cytosolic surface of the ER membrane, a process that is facilitated by the fact that NS4A, NS4B and NS5B are transmembrane proteins53. These replication proteins induce the formation of the replication site.
A similar process is observed during infection with dengue virus (DENV) and West Nile virus (WNV), two additional members of the Flaviviridae family. Infection with DENV can be associated with dengue haemorrhagic fever54, while infection with WNV can also cause severe human disease, including encephalitis and meningitis55. For both viruses, the ER-dependent translation of their (+)RNA genomes generates a single polyprotein56. Proteolytic cleavage of the polyprotein results in 10 mature viral proteins, comprising three structural proteins — capsid protein (C), pre-membrane protein (preM) and envelope protein (E) — and seven non-structural counterparts: NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5. As transmembrane proteins, NS2A, NS4A and NS4B are strategically positioned on the ER membrane where they establish the eventual viral replication factory, similar to HCV. The general strategy of targeting viral replication proteins to the ER membrane where they induce membrane rearrangements to form the viral replication site is also observed during infection with members of the Coronaviridae family57 (Table 1). There is evidence that members of the Picornaviridae family, such as poliovirus and enterovirus 71 (EV71), use a similar strategy58,59,60. Therefore, these examples elegantly demonstrate how diverse viruses have evolved conserved mechanisms to exploit the protein biosynthetic machinery and trafficking pathways that are associated with the ER, as well as the large surface area of the ER membrane, to recruit viral proteins and promote the formation of viral replication sites.
Intriguingly, although the typical function of the translocation machinery of the ER can be subverted to translate viral structural and non-structural proteins, more unconventional use of this apparatus may also be possible. For example, hepatitis E virus (HEV), a (+)RNA non-enveloped virus that causes inflammation of the liver61, uses the translocation machinery of the ER to deliver the capsid protein ORF2 into the ER62. However, after reaching the lumen of the ER, ORF2 seems to be glycosylated and retro-translocated back to the cytosol by co-opting the ERAD pathway63. The ORF2 capsid protein subsequently evades proteasomal degradation in the cytosol by an unknown mechanism, and is presumably available for virus assembly63. Why ORF2 is translocated into the lumen of the ER, as well as whether this 'in-and-out' transport pathway occurs during bona fide infection, remain to be addressed. The use of this unusual pathway is also observed during the synthesis of the Rem protein of mouse mammary tumour virus (MMTV), a retrovirus that is responsible for murine mammary carcinoma, which is used as a model to study breast cancer64. Rem is a virus-encoded, nuclear-localized protein that controls viral mRNA export from the nucleus to the cytosol. For Rem to reach the nucleus, it first co-translationally translocates into the ER, where it is processed by ER-resident signal peptidase before being retro-translocated back to the cytosol, from where it enters the nucleus65. To date, the physiological significance of this unconventional pathway in Rem function remains unclear. Nonetheless, these examples reveal novel ways by which viruses usurp ER translocation and retro-translocation, thereby coupling these two opposing membrane transport pathways, which is not observed during their normal cellular functions.
Co-opting the ER during later stages of infection
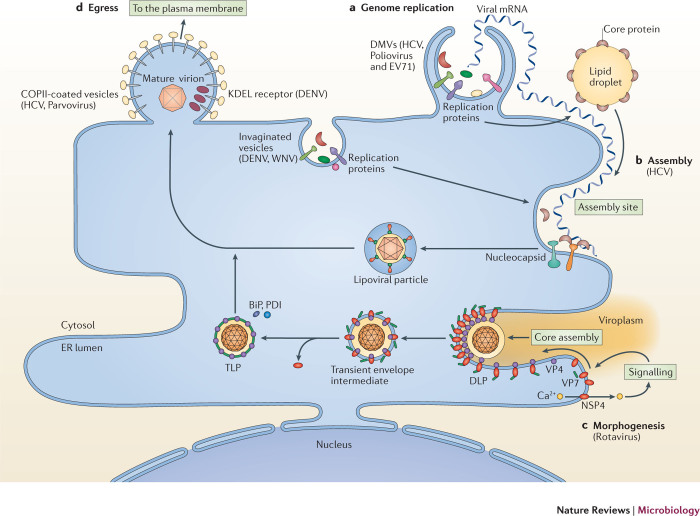
Viral genome replication. Once a virus enters the host cell, its genetic information is transcribed and translated to generate viral proteins, including replication proteins. When these replication proteins are strategically positioned on the ER membrane, the host cell is ready to support the replication of the viral genome and the subsequent assembly of new progeny virions, with assistance from cellular components. In many instances, viral replication and assembly are tightly coupled. Although these two events can occur in other intracellular organelles, depending on the virus, the ER is the most common site of replication and assembly66. As introduced above, numerous viruses in the (+)RNA Flaviviridae, Coronaviridae and Picornaviridae families exploit the ER to initiate and sustain viral replication and assembly (Table 1). With the exception of retroviruses, almost all RNA viruses use virus-encoded RNA-dependent RNA polymerases for RNA replication. Replication occurs in virus-induced membranous structures that are most often derived from the ER and have different morphologies and terminologies50 (Fig. 3a). Regardless of the classification, these replication sites act to increase the local concentrations of viral and host components that are essential for RNA replication, enable different steps of the replication reactions to be coordinated efficiently, and guard against the host innate immune system. Transcription of a negative-sense RNA ((−)RNA) intermediate at replication sites is then used for the synthesis of (+)RNA genomes, which are packaged into new virions during assembly. We will use ER-associated replication and assembly of HCV as the framework to understand these processes owing to the wealth of information that is available, although relevant examples from other viruses will be discussed.
Figure 3. Co-opting the ER to promote the later stages of infection.
Viruses can co-opt functions that are associated with the endoplasmic reticulum (ER) to achieve the four crucial later steps in infection — replication, assembly, morphogenesis and egress. a | Genome replication. During this process, the replication proteins of numerous viruses rearrange the ER membrane to generate membranous structures with different morphologies and terminologies, such as invaginated vesicles (for dengue virus (DENV) and West Nile virus (WNV)) and double-membrane vesicles (DMVs; for hepatitis C virus (HCV), poliovirus and enterovirus 71 (EV71)). These replication sites act to increase the local concentrations of viral and host components that are essential for RNA replication, and enable different steps of the replication process to be coordinated efficiently. b | Assembly. Virus assembly can be tightly coupled to, and coordinated with, genome replication, as exemplified in HCV. To initiate the assembly of viral progeny, a lipid droplet recruits viral core proteins to its surface and delivers them to the site of assembly. In one model of HCV virion assembly, core proteins capture the newly replicated positive-sense RNA ((+)RNA) that extrudes from the neighbouring DMV, forming the nucleocapsid. The nucleocapsid may then bud into the lumen of the ER, generating a newly assembled enveloped virus particle that contains the structural glycoproteins E1 and E2. c | Morphogenesis. Morphogenesis of the assembled HCV particle continues in the ER, with the acquisition of lipoproteins on its surface to generate the mature 'lipoviral' HCV particle that is poised to exit the host cell. For rotavirus, ER-dependent morphogenesis is initiated when its membrane protein non-structural protein 4 (NSP4) induces calcium release from the ER. This triggers a signalling cascade that delivers the structural proteins VP4 and VP7, with assistance from NSP4, to the ER membrane assembly site. The VP4–NSP4–VP7 complex recruits the double-layer particle (DLP) and deforms the membrane to form a transient enveloped intermediate in the lumen of the ER. Following the removal of the ER-derived lipid bilayer, VP7 correctly assembles on the surface of the mature infectious triple-layer particle (TLP), with the simultaneous release of NSP4. The morphologically matured virion then exits the host cell through lysis or secretion. d | Egress. The final step of infection is egress of the mature virion. Viruses that mature in the ER co-opt the ER-dependent secretory pathway to access the extracellular milieu. Examples of using this strategy can be found in egress of the mature HCV, rotavirus and parvovirus particles. Additionally, the ER could also have specific components, such as the KDEL receptor in the case of DENV, that are hijacked to promote exit. COPII, coat protein complex II.
The viral replication proteins of HCV induce extensive membrane rearrangements, forming ER-derived double-membrane vesicle (DMV) structures that are designed to support RNA replication67. These structures are embedded in a membranous matrix that is juxtaposed to the ER membrane and is known as the 'membranous web' (Refs 68,69). The identification of viral replicase components and replicase activity associated with purified DMVs argues strongly that these structures are responsible for the replication of the HCV genome, which is consistent with the finding that the kinetics of viral genome replication temporally coincide with the formation of DMVs53,70. Mechanistically, the biogenesis of DMVs requires substantial structural changes to the ER membrane, which involve membrane deformations, extensions and contractions to generate the appropriate topology. These morphological alterations are probably aided by the intrinsic plasticity of the ER membrane, which typically undergoes constant rearrangements. Topologically, it should be stressed that, as the viral replication proteins are associated with the cytoplasmic surface of the ER membrane, replication of the viral genome occurs in the cytoplasm.
The concerted action of all five viral replication proteins is essential for the formation of DMVs, with NS4B and NS5A probably having central roles71,72,73. However, an increasing number of host factors also contribute to the formation of DMVs53. For example, NS5A and NS5B target the host lipid kinase phosphatidylinositol 4-kinase-α (PI4Kα) to the ER-derived membrane, which stimulates its activity to locally produce phosphatidylinositol-4-phosphate (PI4P)74,75,76,77. PI4P is responsible for numerous cellular processes, including signal transduction, actin organization and membrane trafficking78, and can control membrane curvature79,80, which might enable it to directly regulate the formation of DMVs78. Alternatively, or additionally, PI4P recruits the cellular oxysterol-binding protein to sites of viral replication, which leads to cholesterol enrichment that is thought to be crucial for the replication of the viral genome through an undefined mechanism81,82. This is especially important as the ER membrane has a low concentration of cholesterol7. Another protein that is targeted to the DMV (by NS4B) is the proline-serine-threonine phosphatase-interacting protein 2 (PSTPIP2)83. This host protein is of interest because it contains a Bin–amphiphysin–Rvs domain (F-BAR domain) that has the ability to alter membrane curvature, which raises the possibility that PSTPIP2 shapes the ER membrane to generate the DMV84.
In the case of poliovirus, the viral replication proteins 2BC and 3A induce the necessary alterations to the ER membrane that trigger the formation of DMVs60, although the Golgi apparatus might contribute to the production of DMVs85. The 2C replication protein of EV71 acts in a similar capacity, in part, through the recruitment of the ER membrane protein reticulon 3 (RTN3), which is known to alter membrane curvature in the ER2,59. As both of these viruses form DMVs, it is perhaps not surprising that they also rely on the production of PI4P for their replication86,87. Intriguingly, the nsp3, nsp4 and nsp6 membrane proteins of murine hepatitis virus drive the formation of a modified DMV (known as EDEMsome) that contains a subset of ERAD regulators to support viral replication88. The formation of this modified DMV has also been reported for Japanese encephalitis virus (JEV) in the Flaviviridae family and the equine arteritis virus (EAV) in the Arteriviridae family89,90 (Table 1). Whether PI4P is essential during the replication of these viruses is unknown.
Other virus-induced, ER-derived membranous structures that are used to support viral replication but are morphologically distinct from the DMV, are invaginated vesicles (initially characterized as convoluted membranes and vesicle packets)67,91,92,93. DENV and WNV use their non-structural proteins NS4A and NS4B94,95 to trigger the formation of invaginated vesicles to promote their genome replication. Importantly, the direct visualization of double-stranded RNA (dsRNA; a replication marker) in the interior of the DENV-induced, ER-derived structure provides strong evidence that it acts as the site of RNA replication93. Topologically, both the DMV and invaginated vesicles are membranous structures that seem to remain mostly attached to the ER membrane71,93. Despite being closely related to HCV, the replication of DENV and WNV does not require PI4P96,97. Therefore, it is tempting to speculate that PI4P is essential for the replication of viruses that use the DMV, but not for viruses that use invaginated vesicles, as their site of replication. Regardless, the general idea of recruiting viral replication proteins to the ER membrane to create a replication factory (DMV or invaginated vesicles) to promote genome replication seems to be a broad principle that is shared across different virus families.
Apart from exploiting the propensity of the ER to undergo membrane rearrangements and its role in lipid biogenesis, ER-dependent UPR signalling is also hijacked to support viral replication. For example, HCV and DENV stimulate the UPR to promote viral replication through the impairment of the host antiviral innate immune response, whereas JEV activates the UPR to facilitate viral replication through controlling the inositol-requiring enzyme 1 (IRE1)-dependent decay pathway98,99. IAV also triggers the UPR to support viral replication through the activation of IRE1, although how the induction of IRE1 promotes viral replication remains unknown100. As there are numerous examples of viruses using the UPR to regulate their life cycle, we refer the reader to several recent reviews on this topic101,102,103,104,105.
Virus assembly. Although viral replication and assembly are often thought to be tightly coupled processes, how these two events are precisely interlinked is poorly understood. Nonetheless, a model has been developed for HCV. Although viral genome replication occurs at the ER-derived DMV, the site of virus assembly is less clear but could possibly involve distinct regions of an ER-derived membrane (Fig. 3b). An essential host component that couples viral replication and assembly is the lipid droplet, a cellular storage organelle of neutral lipids106. Owing, in part, to the small GTP-binding protein RAB18, lipid droplets can localize proximal to the DMV107. In addition, the amphipathic helices of the NS4B protein of HCV have been reported to bind to lipid droplets, which may contribute to their recruitment108. Situated next to the DMV, the key function of lipid droplets is to recruit the HCV core protein to its surface (Fig. 3b), assisted by cellular enzymes that maintain lipid homeostasis. Subsequently, core proteins are thought to be released from lipid droplets and delivered to an assembly site on what is presumed to be the ER membrane (or at least an ER-derived membranous structure; Fig. 3b). At the assembly site, core proteins encapsulate the newly replicated (+)RNA that is extruded from the nearby DMV, forming the nucleocapsid (Fig. 3b). These reactions are mediated by p7, NS2, NS3 and NS4A109,110,111,112,113. After formation, the nucleocapsid is thought to bud into the lumen of the ER, producing a newly assembled, enveloped virion that contains the surface glycoproteins E1 and E2 (Fig. 3b). In the Flaviviridae family, interaction between the capsid protein of DENV and lipid droplets in the host has also been reported to be important for virus replication and assembly114.
The coupling of replication and assembly that relies on the ER membrane is not a strategy that is exclusive to RNA viruses. For example, imaging studies have revealed that vaccinia virus, a large enveloped DNA virus in the Poxviridae family, uses the ER membrane during its replication and assembly115. Intriguingly, the membrane surface of the resulting viral progeny corresponds to the luminal side of the ER membrane without necessarily budding into the ER membrane. How this perplexing topology is achieved clearly requires further investigation, but may involve an unusual membrane rupture step116. PI4P was recently implicated in the replication and assembly of poxviruses, similar to the use of this lipid during the replication of HCV, poliovirus and EV71 (Ref. 117).
Viral morphogenesis. Once new virus particles are assembled, they typically undergo further morphogenesis to generate mature virions that are poised to egress from the host cell. Subverting the functions of the ER to support these pivotal steps in late infection has been well documented for certain viruses. For example, after assembly of the HCV particle in the ER (or ER-derived) membrane, HCV undergoes additional maturation steps to egress from the host, including the acquisition of lipoproteins, particularly apolipoprotein E, on its surface118 (Fig. 3b). Surface lipoproteins probably explain the rather heterogeneous morphology of mature infectious virions119. The ER has also been reported to regulate morphogenesis of pestiviruses in the Flaviviridae family104, and hepatitis B virus (HBV) in the Hepadnaviridae family120,121. During the propagation of HBV, a non-infectious HBV subviral envelope particle (SVP) is produced. Intriguingly, SVP morphogenesis from its initial filamentous form to mature spherical particles that are ready for secretion is thought to be initiated in the perinuclear ER121.
Perhaps the most remarkable case of ER-dependent viral morphogenesis is found during the maturation of rotavirus, a member of the Reoviridae family and a causative agent of severe infant and childhood diarrhoea. Structurally, this non-enveloped dsRNA virus contains three concentric layers, the inner (core shell), middle and outer layers, which are composed of VP2, VP6, VP7 and VP4, respectively. The combination of the core shell and middle layer is called the double-layer particle (DLP), whereas the addition of the outer layer to the DLP forms the mature infectious virus called the triple-layer particle (TLP). Morphogenesis of the DLP to the TLP is uniquely associated with the ER (Fig. 3c).
The morphogenesis of the DLP to the TLP is initiated when the rotavirus non-structural membrane protein NSP4, which acts as a viroporin, binds to the ER membrane and induces the release of calcium into the cytosol122 (Fig. 3c). The subsequent rise in cytosolic calcium activates a kinase-dependent signalling cascade that triggers autophagy (Fig. 3c). This membrane trafficking pathway is then used to deliver rotavirus structural proteins VP4 and VP7 to the site of virus assembly on the ER membrane (Fig. 3c). At this juncture, NSP4 carries out several crucial functions, including interactions with VP4 on the cytosolic side of the ER membrane, and VP7 on the luminal side that remains loosely associated with NSP4. NSP4 also binds to the DLP, targeting it to the cytosolic side of the ER membrane. Next, the newly formed DLP–VP4–NSP4–VP7 complex drives membrane deformation, which generates a transient enveloped intermediate in the lumen of the ER (Fig. 3c). As a consequence of this inward budding event, VP7 is displayed on the surface of the enveloped intermediate. Removal of the ER-derived lipid bilayer then enables the assembly of VP7 on the surface of the mature infectious TLP, with the concomitant release of NSP4 from the DLP (Fig. 3c). Finally, the TLP exits the host cell by lysis or secretion. Perhaps the most enigmatic step during these ER-dependent remodelling events is how the lipid bilayer that encompasses the transient enveloped intermediate becomes 'solubilized'. One possibility is the use of the viral structural protein VP7, which has membrane lytic activity123. In this scenario, the conformational change that accompanies the assembly of VP7 might expose its lytic domain, enabling VP7 to disrupt the integrity of the membrane. Whether components of the ER are exploited to facilitate the removal of the membrane is also unclear. In this regard, there is evidence that the ER-resident chaperone BiP and the oxidoreductase PDI support rotavirus morphogenesis in the ER124, although their roles may be indirect and instead be co-opted to assist in the conformational changes of VP7. Therefore, rotavirus morphogenesis seems to exploit three distinct functions and characteristics that are associated with the ER: intraluminal calcium efflux (to trigger the cytosolic signalling cascade), active rearrangement of the ER membrane (ideally suited for the inward budding reaction), and the use of ER-resident folding chaperones and enzymes (to promote the formation of the TLP).
Similar to NSP4 from rotavirus, other viral proteins also disrupt the ER membrane to stimulate the release of calcium from the ER, including the 2B and 2BC proteins of EV71 and rhinovirus, as well as the pUL37×1 protein of human cytomegalovirus125,126,127. Furthermore, the Tat protein of HIV-1 can activate the ryanodine and inositol trisphosphate (IP3) receptors to mobilize calcium from the ER into the cytosol128,129,130. Although calcium release does not seem to be directly linked to facilitating viral morphogenesis in these examples, they nonetheless highlight the widespread use of viral components to cause calcium efflux from the ER to regulate aspects of the infection process.
Viral egress. Egress of the mature virion represents the final step in the viral life cycle. Essentially all viruses exit the host cell by being released into the extracellular milieu. Viruses that assemble and mature in the ER take advantage of the ER-associated biosynthetic machinery that enables the synthesis of viral structural proteins. Moreover, they use the ER-dependent secretory pathway to reach the plasma membrane for the release of viruses into the extracellular environment (Fig. 3d). For example, after envelopment in the ER, the new HCV particles are thought to use components of the classical secretory pathway for egress131, although a recent report implicated the clathrin-dependent pathway as another possibility for HCV egress132. In addition, there is evidence that rotavirus, following ER-dependent morphogenesis, uses the classical secretory pathway for exit118; a non-conventional egress strategy has also been proposed133,134. Although non-enveloped viruses are typically released through cell lysis, there are examples in which active vesicular transport is involved135,136. The progeny of parvovirus are packaged into COPII-coated vesicles in the ER before being transported to the Golgi apparatus en route to the plasma membrane for egress137. Interestingly, COPII-dependent vesicular budding was reported to be essential during infection with poliovirus, although this pathway is more likely to be involved in the formation of its genome replication site than in egress138,139.
In addition to providing a simple conduit to the cell surface, the ER could harbour specific factors that directly promote exit from the host cell. In fact, the discovery that the ER-resident KDEL receptor, a receptor that normally cycles cellular client proteins between the ER and the Golgi apparatus, is hijacked by DENV for host cell exit represents an excellent example of this possibility140 (Fig. 3d). Future studies will undoubtedly reveal other host components in the ER-dependent secretory pathway that guide viral egress. It is interesting to note that apart from the ER-dependent secretory pathway, autophagosomes that are derived from the ER can also be exploited for viral egress141,142.
Antiviral strategies targeting the ER
As viruses exploit the ER during infection, pharmacological strategies that are aimed at disrupting the function of the ER should, in principle, lead to the generation of broad-spectrum antiviral agents. Indeed, drugs that impair the ER-resident glycan-trimming enzymes α-glucosidase I and α-glucosidase II have been shown to block infection with DNA viruses, including herpes simplex virus 2, cytomegalovirus and HBV, as well as RNA viruses, such as HIV, HCV, JEV and WNV143. As these enzymes remove glucose residues in N-linked glycans that are attached to newly synthesized proteins and are important for correct folding, the inhibition of α-glucosidase is expected to disrupt the function of the newly synthesized viral glycoproteins. Targeting the UPR pathway could also prove useful103,144,145,146. Another possibility is to manipulate the ERAD system, as numerous viruses are known to exploit ERAD to evade the host innate immune system and sustain infection (Box 2). In this situation, impairing ERAD may help to boost host immunity during infection. An orally active inhibitor against a key ERAD regulator — p97—was recently identified147,148; whether this inhibitor can limit virus infection remains to be seen. The cytosolic chaperone HSP70 and its cohort of co-chaperones also control ERAD149,150. Because of the connection that flaviviruses have to the ERAD pathway89,151,152, it is perhaps not surprising that a recent report identified a chemical inhibitor of HSP70 that blocked infection by members of this family153. Finally, drugs that target lipid synthesis and metabolism in the host have also been demonstrated to inhibit infection with DENV and HCV, most likely by affecting functions that are associated with the ER154. Clearly the most important criterion in the effort to develop effective antiviral therapies by targeting endogenous functions of the ER will be to minimize toxicity against the host cell.
Box 2: Viruses co-opt ER functions to evade host immunity.
The host immune system can activate an antiviral response. However, several viruses have evolved strategies to dampen this immune response by manipulating functions of the endoplasmic reticulum (ER), thereby promoting their infection155,156. One well-characterized example comes from the Herpesviridae family, in which viruses evade host immunity by suppressing or eliminating host immune factors. For example, the human cytomegalovirus transmembrane proteins US2 and US11 trigger ER-associated degradation (ERAD)-dependent proteasomal degradation of the host antigen-presenting major histocompatibility complex (MHC) class I molecule157,158. Similarly, the mK3 (also known as MIR1) E3 ligase of murine gammaherpesvirus 68 and the pK3 E3 ligase of rodent herpesvirus Peru ubiquitylate MHC class I to promote its ERAD-mediated degradation159,160. The absence of MHC class I impedes viral antigens from being correctly displayed on the surface of infected cells, thereby preventing the host from mounting an effective immune response. Interestingly, other viruses in the Herpesviridae family (herpes simplex virus, Epstein–Barr virus and varicella virus) evade the host immune response by blocking transporter associated with antigen processing (TAP)-mediated peptide transport into the ER, which consequently prevents peptide assembly with MHC class I161,162,163. During infection with HIV-1, the viral Vpu protein co-opts the ERAD pathway to degrade the CD4 host cell entry receptor164,165, which surprisingly results in more vigorous HIV infection. For example, the degradation of CD4 has been shown to stimulate the release of new viral progeny from the host cell surface166.
Conclusion
Efforts to develop therapies against viruses that cause debilitating human diseases have uncovered unparalleled insights into the dynamic relationship between viruses and host cells. This interplay is evident in the ER, where viruses have evolved elegant strategies to exploit intrinsic functions of the ER to promote every stage of infection. For example, members of the Polyomaviridae co-opt ERAD to disassemble and to access the cytosol of the host cell. Members of the Flaviviridae, Coronaviridae and Picornaviridae take advantage of the propensity for structural rearrangements in the ER membrane, sculpting this membrane to create ER-derived replication and assembly sites. The non-enveloped rotavirus exploits the physical properties of the ER and the presence of pro-folding chaperones to facilitate morphogenesis in the lumen of the ER. Despite these detailed understandings, many crucial questions remain. During virus entry, do polyomavirus-induced structures on the ER membrane act as the site of entry to the cytosol? Do new HCV particles assemble directly on the ER membrane or instead on an ER-derived membranous structure? During morphogenesis, how does rotavirus lose its transient lipid bilayer in the lumen of the ER to generate the infectious TLP virion? What is required to address these and other outstanding questions is a comprehensive strategy that combines the use of rigorous classical biochemical and cell-based approaches with state-of-the-art imaging methods, including live-cell single-particle tracking and cryo-electron tomography. These efforts will surely reveal fascinating insights into the ever-changing landscape of interactions between viruses and host cells. More importantly, they should also provide practical information that better positions the virology community to combat unresolved or new viral diseases.
Acknowledgements
The authors thank A. Ono and A. Tai for carefully reading this manuscript. The authors are also grateful to the members of their laboratory for insightful discussions in relation to this manuscript. This work is supported by the US National Institutes of Health (NIH; grants RO1AI064296 and RO1GM113722 to B.T.). C.N.C is supported by the Cellular and Molecular Biology Program NIH Training Grant (T32-GM007315).
Glossary
- Morphogenesis
The process by which a virus particle changes its shape and structure.
- SEC61 translocation channel
An endoplasmic reticulum (ER) membrane protein complex that translocates nascent polypeptide chains from the cytosol into the lumen of the ER.
- Post-translational modifications
Enzyme-mediated, covalent additions to proteins that often occur after biosynthesis.
- ER chaperones
Endoplasmic reticulum (ER)-resident proteins that assist in the correct folding and unfolding of nascent polypeptide chains or macromolecular structures.
- COPII-coated vesicles
(Coat protein complex II (COPII)-coated vesicles) Vesicles covered with a protein coat that buds from the endoplasmic reticulum (ER) membrane that transport cargos to the cis-Golgi.
- Classical secretory pathway
An intracellular route that transports cargos from the endoplasmic reticulum (ER) to the Golgi apparatus and to the plasma membrane.
- Progressive multifocal leukoencephalopathy
A rare, demyelinating disease of the brain.
- Merkel cell carcinoma
A rare but aggressive type of neuroendocrine skin cancer.
- Receptor-mediated endocytosis
An intracellular transport process in which a ligand binds to a receptor on the plasma membrane, thereby inducing the internalization of the receptor–ligand complex.
- Endolysosomal system
A cellular membranous system that is composed of endosomes and lysosomes.
- J protein
A molecular co-chaperone that contains a conserved ∼70 amino acid DNAJ domain (J domain), which stimulates the activity of the heat shock protein 70 (HSP70) chaperone.
- Golgi apparatus
A cellular organelle that processes cargos and other macromolecules, packaging them into vesicles for delivery to the plasma membrane or to the endoplasmic reticulum.
- Viroporin
A viral hydrophobic protein that perforates and disrupts the integrity of membranes.
- ER-resident signal peptidase
An endoplasmic reticulum (ER)-localized enzyme that removes the amino-terminal signal sequence of a nascent polypeptide chain.
- F-BAR domain
(Bin–amphiphysin–Rvs domain). A protein domain that is used to promote membrane curvature.
- Inositol-requiring enzyme 1 (IRE1)-dependent decay pathway
A pathway that degrades mRNAs that encode endoplasmic reticulum (ER) membrane and secreted proteins through the cytoplasmic endonuclease domain of IRE1.
- Autophagy
A cellular degradative mechanism of recycling unwanted components to provide nutrients and maintain cellular survival.
Biographies
Madhu Sudhan Ravindran is a postdoctoral fellow in the laboratory of Billy Tsai at the University of Michigan Medical School, Ann Arbor, USA. He received his B.Sc. and his M.Sc. from University of Madras, Chennai, India, and conducted his Ph.D. research at the National University of Singapore under the guidance of Markus R. Wenk. During his Ph.D., he investigated mycobacterial lipidomics and also studied host lipids during viral entry. His current research focuses on the membrane penetration of polyomavirus using a combination of biochemical and imaging techniques.
Parikshit Bagchi received his B.Sc. in microbiology from the University of Calcutta, Kolkata, India, and his M.Sc. in microbiology and microbial technology from the University of Kalyani, India. He completed his Ph.D. in microbiology at the University of Calcutta under the guidance of Mamta Chawla-Sarkar. For his Ph.D. research, he investigated the role of rotavirus non-structural proteins in viral replication and pathogenesis. His current research as a postdoctoral fellow in the laboratory of Billy Tsai is focused on the cellular trafficking of polyomavirus, especially the ER membrane penetration step during viral infection.
Corey Nathaniel Cunningham received his B.Sc. from Westminster College, New Wilmington, Pennsylvania, USA. He is currently a third year graduate student in the cellular and molecular biology Ph.D. programme at the University of Michigan, Ann Arbor, USA. His research in the laboratory of Billy Tsai centres on elucidating ER protein quality control pathways that dispose of mutant proinsulins.
Billy Tsai is the Corydon Ford Professor of Cell and Developmental Biology at the University of Michigan Medical School, Ann Arbor, USA. He received his Ph.D. from Harvard University, Cambridge, Massachusetts, USA, and postdoctoral training at Harvard Medical School, Boston, Massachusetts, USA, under the supervision of Tom Rapoport. His laboratory is interested in understanding the molecular interplay between a DNA tumour virus (polyomavirus) and its host cell.
PowerPoint slides
Competing interests
The authors declare no competing financial interests.
Footnotes
Madhu Sudhan Ravindran and Parikshit Bagchi: These authors contributed equally to this work.
References
- 1.Shibata Y, Voeltz GK, Rapoport TA. Rough sheets and smooth tubules. Cell. 2006;126:435–439. doi: 10.1016/j.cell.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 3.Shibata Y, et al. Mechanisms determining the morphology of the peripheral ER. Cell. 2010;143:774–788. doi: 10.1016/j.cell.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borgese N, Francolini M, Snapp E. Endoplasmic reticulum architecture: structures in flux. Curr. Opin. Cell Biol. 2006;18:358–364. doi: 10.1016/j.ceb.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westrate LM, Lee JE, Prinz WA, Voeltz GK. Form follows function: the importance of endoplasmic reticulum shape. Annu. Rev. Biochem. 2015;84:791–811. doi: 10.1146/annurev-biochem-072711-163501. [DOI] [PubMed] [Google Scholar]
- 6.Voeltz GK, Rolls MM, Rapoport TA. Structural organization of the endoplasmic reticulum. EMBO Rep. 2002;3:944–950. doi: 10.1093/embo-reports/kvf202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu Y, Hendershot LM. Organization of the functions and components of the endoplasmic reticulum. Adv. Exp. Med. Biol. 2007;594:37–46. doi: 10.1007/978-0-387-39975-1_4. [DOI] [PubMed] [Google Scholar]
- 9.Park E, Rapoport TA. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu. Rev. Biophys. 2012;41:21–40. doi: 10.1146/annurev-biophys-050511-102312. [DOI] [PubMed] [Google Scholar]
- 10.Chavan M, Lennarz W. The molecular basis of coupling of translocation and N-glycosylation. Trends Biochem. Sci. 2006;31:17–20. doi: 10.1016/j.tibs.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Bulleid NJ. Disulfide bond formation in the mammalian endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2012;4:a013219. doi: 10.1101/cshperspect.a013219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behnke J, Feige MJ, Hendershot LM. BiP and its nucleotide exchange factors Grp170 and Sil1: mechanisms of action and biological functions. J. Mol. Biol. 2015;427:1589–1608. doi: 10.1016/j.jmb.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller EA, Schekman R. COPII — a flexible vesicle formation system. Curr. Opin. Cell Biol. 2013;25:420–427. doi: 10.1016/j.ceb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 2013;5:a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodsky JL. Cleaning up: ER-associated degradation to the rescue. Cell. 2012;151:1163–1167. doi: 10.1016/j.cell.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olzmann JA, Kopito RR, Christianson JC. The mammalian endoplasmic reticulum-associated degradation system. Cold Spring Harb. Perspect. Biol. 2013;5:a013185. doi: 10.1101/cshperspect.a013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam AK, Galione A. The endoplasmic reticulum and junctional membrane communication during calcium signaling. Biochim. Biophys. Acta. 2013;1833:2542–2559. doi: 10.1016/j.bbamcr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Tannous A, Pisoni GB, Hebert DN, Molinari M. N-linked sugar-regulated protein folding and quality control in the ER. Semin. Cell Dev. Biol. 2015;41:79–89. doi: 10.1016/j.semcdb.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natter K, et al. The spatial organization of lipid synthesis in the yeast Saccharomyces cerevisiae derived from large scale green fluorescent protein tagging and high resolution microscopy. Mol. Cell Proteomics. 2005;4:662–672. doi: 10.1074/mcp.M400123-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.DeCaprio JA, Garcea RL. A cornucopia of human polyomaviruses. Nat. Rev. Microbiol. 2013;11:264–276. doi: 10.1038/nrmicro2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liddington RC, et al. Structure of simian virus 40 at 3.8-Å resolution. Nature. 1991;354:278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- 22.Chen XS, Stehle T, Harrison SC. Interaction of polyomavirus internal protein VP2 with the major capsid protein VP1 and implications for participation of VP2 in viral entry. EMBO J. 1998;17:3233–3240. doi: 10.1093/emboj/17.12.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stehle T, Harrison SC. High-resolution structure of a polyomavirus VP1-oligosaccharide complex: implications for assembly and receptor binding. EMBO J. 1997;16:5139–5148. doi: 10.1093/emboj/16.16.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kartenbeck J, Stukenbrok H, Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J. Cell Biol. 1989;109:2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian M, Cai D, Verhey KJ, Tsai B. A lipid receptor sorts polyomavirus from the endolysosome to the endoplasmic reticulum to cause infection. PLoS Pathog. 2009;5:e1000465. doi: 10.1371/journal.ppat.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schelhaas M, et al. Simian virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell. 2007;131:516–529. doi: 10.1016/j.cell.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert J, Ou W, Silver J, Benjamin T. Downregulation of protein disulfide isomerase inhibits infection by the mouse polyomavirus. J. Virol. 2006;80:10868–10870. doi: 10.1128/JVI.01117-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue T, et al. ERdj5 reductase cooperates with protein disulfide isomerase to promote simian virus 40 endoplasmic reticulum membrane translocation. J. Virol. 2015;89:8897–8908. doi: 10.1128/JVI.00941-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magnuson B, et al. ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding. Mol. Cell. 2005;20:289–300. doi: 10.1016/j.molcel.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 30.Rainey-Barger EK, Magnuson B, Tsai B. A chaperone-activated nonenveloped virus perforates the physiologically relevant endoplasmic reticulum membrane. J. Virol. 2007;81:12996–13004. doi: 10.1128/JVI.01037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue T, Tsai B. A large and intact viral particle penetrates the endoplasmic reticulum membrane to reach the cytosol. PLoS Pathog. 2011;7:e1002037. doi: 10.1371/journal.ppat.1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geiger R, et al. BAP31 and BiP are essential for dislocation of SV40 from the endoplasmic reticulum to the cytosol. Nat. Cell Biol. 2011;13:1305–1314. doi: 10.1038/ncb2339. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin EC, et al. BiP and multiple DNAJ molecular chaperones in the endoplasmic reticulum are required for efficient simian virus 40 infection. mBio. 2011;2:e00101-11. doi: 10.1128/mBio.00101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue T, Tsai B. A nucleotide exchange factor promotes endoplasmic reticulum-to-cytosol membrane penetration of the nonenveloped virus simian virus 40. J. Virol. 2015;89:4069–4079. doi: 10.1128/JVI.03552-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walczak CP, Ravindran MS, Inoue T, Tsai B. A cytosolic chaperone complexes with dynamic membrane J-proteins and mobilizes a nonenveloped virus out of the endoplasmic reticulum. PLoS Pathog. 2014;10:e1004007. doi: 10.1371/journal.ppat.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagchi P, Walczak CP, Tsai B. The endoplasmic reticulum membrane J protein C18 executes a distinct role in promoting simian virus 40 membrane penetration. J. Virol. 2015;89:4058–4068. doi: 10.1128/JVI.03574-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravindran MS, Bagchi P, Inoue T, Tsai BA. Non-enveloped virus hijacks host disaggregation machinery to translocate across the endoplasmic reticulum membrane. PLoS Pathog. 2015;11:e1005086. doi: 10.1371/journal.ppat.1005086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol. Oncol. 2010;117:S5–S10. doi: 10.1016/j.ygyno.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 39.Laniosz V, Dabydeen SA, Havens MA, Meneses PI. Human papillomavirus type 16 infection of human keratinocytes requires clathrin and caveolin-1 and is brefeldin a sensitive. J. Virol. 2009;83:8221–8232. doi: 10.1128/JVI.00576-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W, Kazakov T, Popa A, DiMaio D. Vesicular trafficking of incoming human papillomavirus 16 to the Golgi apparatus and endoplasmic reticulum requires γ-secretase activity. mBio. 2014;5:e01777–14. doi: 10.1128/mBio.01777-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campos SK, Chapman JA, Deymier MJ, Bronnimann MP, Ozbun MA. Opposing effects of bacitracin on human papillomavirus type 16 infection: enhancement of binding and entry and inhibition of endosomal penetration. J. Virol. 2012;86:4169–4181. doi: 10.1128/JVI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chromy LR, Oltman A, Estes PA, Garcea RL. Chaperone-mediated in vitro disassembly of polyoma- and papillomaviruses. J. Virol. 2006;80:5086–5091. doi: 10.1128/JVI.80.10.5086-5091.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hazes B, Read RJ. Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry. 1997;36:11051–11054. doi: 10.1021/bi971383p. [DOI] [PubMed] [Google Scholar]
- 44.He K, Ravindran MS, Tsai B. A bacterial toxin and a nonenveloped virus hijack ER-to-cytosol membrane translocation pathways to cause disease. Crit. Rev. Biochem. Mol. Biol. 2015;50:477–488. doi: 10.3109/10409238.2015.1085826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spooner RA, Lord JM. How ricin and Shiga toxin reach the cytosol of target cells: retrotranslocation from the endoplasmic reticulum. Curr. Top. Microbiol. Immunol. 2012;357:19–40. doi: 10.1007/82_2011_154. [DOI] [PubMed] [Google Scholar]
- 46.Checkley MA, Luttge BG, Freed EO. HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J. Mol. Biol. 2011;410:582–608. doi: 10.1016/j.jmb.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hebert DN, Zhang JX, Chen W, Foellmer B, Helenius A. The number and location of glycans on influenza hemagglutinin determine folding and association with calnexin and calreticulin. J. Cell Biol. 1997;139:613–623. doi: 10.1083/jcb.139.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doms RW, Lamb RA, Rose JK, Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993;193:545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- 49.Braakman I, van Anken E. Folding of viral envelope glycoproteins in the endoplasmic reticulum. Traffic. 2000;1:533–539. doi: 10.1034/j.1600-0854.2000.010702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reid CR, Airo AM, Hobman TC. The virus–host interplay: biogenesis of +RNA replication complexes. Viruses. 2015;7:4385–4413. doi: 10.3390/v7082825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamane D, McGivern DR, Masaki T, Lemon SM. Liver injury and disease pathogenesis in chronic hepatitis C. Curr. Top. Microbiol. Immunol. 2013;369:263–288. doi: 10.1007/978-3-642-27340-7_11. [DOI] [PubMed] [Google Scholar]
- 52.Lohmann V. Hepatitis C virus RNA replication. Curr. Top. Microbiol. Immunol. 2013;369:167–198. doi: 10.1007/978-3-642-27340-7_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paul D, Madan V, Bartenschlager R. Hepatitis C virus RNA replication and assembly: living on the fat of the land. Cell Host Microbe. 2014;16:569–579. doi: 10.1016/j.chom.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guzman MG, et al. Dengue: a continuing global threat. Nat. Rev. Microbiol. 2010;8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chancey C, Grinev A, Volkova E, Rios M. The global ecology and epidemiology of West Nile virus. Biomed. Res. Int. 2015;2015:376230. doi: 10.1155/2015/376230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 57.Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richards AL, Soares-Martins JA, Riddell GT, Jackson WT. Generation of unique poliovirus RNA replication organelles. mBio. 2014;5:e00833-13. doi: 10.1128/mBio.00833-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang WF, et al. Reticulon 3 binds the 2C protein of enterovirus 71 and is required for viral replication. J. Biol. Chem. 2007;282:5888–5898. doi: 10.1074/jbc.M611145200. [DOI] [PubMed] [Google Scholar]
- 60.Suhy DA, Giddings TH, Kirkegaard K. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles. J. Virol. 2000;74:8953–8965. doi: 10.1128/jvi.74.19.8953-8965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mast EE, Krawczynski K. Hepatitis E: an overview. Annu. Rev. Med. 1996;47:257–266. doi: 10.1146/annurev.med.47.1.257. [DOI] [PubMed] [Google Scholar]
- 62.Zafrullah M, Ozdener MH, Kumar R, Panda SK, Jameel S. Mutational analysis of glycosylation, membrane translocation, and cell surface expression of the hepatitis E virus ORF2 protein. J. Virol. 1999;73:4074–4082. doi: 10.1128/jvi.73.5.4074-4082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Surjit M, Jameel S, Lal SK. Cytoplasmic localization of the ORF2 protein of hepatitis E virus is dependent on its ability to undergo retrotranslocation from the endoplasmic reticulum. J. Virol. 2007;81:3339–3345. doi: 10.1128/JVI.02039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ross SR. Mouse mammary tumor virus molecular biology and oncogenesis. Viruses. 2010;2:2000–2012. doi: 10.3390/v2092000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Byun H, et al. Retroviral Rem protein requires processing by signal peptidase and retrotranslocation for nuclear function. Proc. Natl Acad. Sci. USA. 2010;107:12287–12292. doi: 10.1073/pnas.1004303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inoue T, Tsai B. How viruses use the endoplasmic reticulum for entry, replication, and assembly. Cold Spring Harb. Perspect. Biol. 2013;5:a013250. doi: 10.1101/cshperspect.a013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paul D, Bartenschlager R. Architecture and biogenesis of plus-strand RNA virus replication factories. World J. Virol. 2013;2:32–48. doi: 10.5501/wjv.v2.i2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Egger D, et al. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J. Virol. 2002;76:5974–5984. doi: 10.1128/JVI.76.12.5974-5984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gosert R, et al. Identification of the hepatitis C virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J. Virol. 2003;77:5487–5492. doi: 10.1128/JVI.77.9.5487-5492.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paul D, Hoppe S, Saher G, Krijnse-Locker J, Bartenschlager R. Morphological and biochemical characterization of the membranous hepatitis C virus replication compartment. J. Virol. 2013;87:10612–10627. doi: 10.1128/JVI.01370-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romero-Brey I, et al. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLoS Pathog. 2012;8:e1003056. doi: 10.1371/journal.ppat.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gouttenoire J, et al. Aminoterminal amphipathic α-helix AH1 of hepatitis C virus nonstructural protein 4B possesses a dual role in RNA replication and virus production. PLoS Pathog. 2014;10:e1004501. doi: 10.1371/journal.ppat.1004501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paul D, et al. NS4B self-interaction through conserved C-terminal elements is required for the establishment of functional hepatitis C virus replication complexes. J. Virol. 2011;85:6963–6976. doi: 10.1128/JVI.00502-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berger KL, et al. Roles for endocytic trafficking and phosphatidylinositol 4-kinase IIIα in hepatitis C virus replication. Proc. Natl Acad. Sci. USA. 2009;106:7577–7582. doi: 10.1073/pnas.0902693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berger KL, Kelly SM, Jordan TX, Tartell MA, Randall G. Hepatitis C virus stimulates the phosphatidylinositol 4-kinase IIIα-dependent phosphatidylinositol 4-phosphate production that is essential for its replication. J. Virol. 2011;85:8870–8883. doi: 10.1128/JVI.00059-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reiss S, et al. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe. 2011;9:32–45. doi: 10.1016/j.chom.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tai AW, Salloum S. The role of the phosphatidylinositol 4-kinase PI4KA in hepatitis C virus-induced host membrane rearrangement. PLoS ONE. 2011;6:e26300. doi: 10.1371/journal.pone.0026300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan J, Brill JA. Cinderella story: PI4P goes from precursor to key signaling molecule. Crit. Rev. Biochem. Mol. Biol. 2014;49:33–58. doi: 10.3109/10409238.2013.853024. [DOI] [PubMed] [Google Scholar]
- 79.Altan-Bonnet N, Balla T. Phosphatidylinositol 4-kinases: hostages harnessed to build panviral replication platforms. Trends Biochem. Sci. 2012;37:293–302. doi: 10.1016/j.tibs.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.May ER, Narang A, Kopelevich DI. Role of molecular tilt in thermal fluctuations of lipid membranes. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2007;76:021913. doi: 10.1103/PhysRevE.76.021913. [DOI] [PubMed] [Google Scholar]
- 81.Amako Y, et al. Role of oxysterol binding protein in hepatitis C virus infection. J. Virol. 2009;83:9237–9246. doi: 10.1128/JVI.00958-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H, et al. Oxysterol-binding protein is a phosphatidylinositol 4-kinase effector required for HCV replication membrane integrity and cholesterol trafficking. Gastroenterology. 2014;146:1373–1385. doi: 10.1053/j.gastro.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chao TC, et al. Proline-serine-threonine phosphatase-interacting protein 2 (PSTPIP2), a host membrane-deforming protein, is critical for membranous web formation in hepatitis C virus replication. J. Virol. 2012;86:1739–1749. doi: 10.1128/JVI.06001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frost A, et al. Structural basis of membrane invagination by F-BAR domains. Cell. 2008;132:807–817. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jackson WT. Poliovirus-induced changes in cellular membranes throughout infection. Curr. Opin. Virol. 2014;9:67–73. doi: 10.1016/j.coviro.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arita M. Phosphatidylinositol-4 kinase IIIβ and oxysterol-binding protein accumulate unesterified cholesterol on poliovirus-induced membrane structure. Microbiol. Immunol. 2014;58:239–256. doi: 10.1111/1348-0421.12144. [DOI] [PubMed] [Google Scholar]
- 87.Hsu NY, et al. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 2010;141:799–811. doi: 10.1016/j.cell.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reggiori F, et al. Coronaviruses hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication. Cell Host Microbe. 2010;7:500–508. doi: 10.1016/j.chom.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma M, et al. Japanese encephalitis virus replication is negatively regulated by autophagy and occurs on LC3-I- and EDEM1-containing membranes. Autophagy. 2014;10:1637–1651. doi: 10.4161/auto.29455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Monastyrska I, et al. An autophagy-independent role for LC3 in equine arteritis virus replication. Autophagy. 2013;9:164–174. doi: 10.4161/auto.22743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mackenzie JM, Jones MK, Young PR. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology. 1996;220:232–240. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- 92.Westaway EG, Mackenzie JM, Kenney MT, Jones MK, Khromykh AA. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 1997;71:6650–6661. doi: 10.1128/jvi.71.9.6650-6661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Welsch S, et al. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe. 2009;5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miller S, Kastner S, Krijnse-Locker J, Buhler S, Bartenschlager R. The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. J. Biol. Chem. 2007;282:8873–8882. doi: 10.1074/jbc.M609919200. [DOI] [PubMed] [Google Scholar]
- 95.Kaufusi PH, Kelley JF, Yanagihara R, Nerurkar VR. Induction of endoplasmic reticulum-derived replication-competent membrane structures by West Nile virus non-structural protein 4B. PLoS ONE. 2014;9:e84040. doi: 10.1371/journal.pone.0084040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin-Acebes MA, Blazquez AB, Jimenez de Oya N, Escribano-Romero E, Saiz JC. West Nile virus replication requires fatty acid synthesis but is independent on phosphatidylinositol-4-phosphate lipids. PLoS ONE. 2011;6:e24970. doi: 10.1371/journal.pone.0024970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heaton NS, et al. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc. Natl Acad. Sci. USA. 2010;107:17345–17350. doi: 10.1073/pnas.1010811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ke PY, Chen SS. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J. Clin. Invest. 2011;121:37–56. doi: 10.1172/JCI41474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhattacharyya S, Sen U, Vrati S. Regulated IRE1-dependent decay pathway is activated during Japanese encephalitis virus-induced unfolded protein response and benefits viral replication. J. Gen. Virol. 2014;95:71–79. doi: 10.1099/vir.0.057265-0. [DOI] [PubMed] [Google Scholar]
- 100.Hassan IH, et al. Influenza A viral replication is blocked by inhibition of the inositol-requiring enzyme 1 (IRE1) stress pathway. J. Biol. Chem. 2012;287:4679–4689. doi: 10.1074/jbc.M111.284695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang L, Wang A. Virus-induced ER stress and the unfolded protein response. Front. Plant Sci. 2012;3:293. doi: 10.3389/fpls.2012.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li S, Kong L, Yu X. The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Crit. Rev. Microbiol. 2015;41:150–164. doi: 10.3109/1040841X.2013.813899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fung TS, Liu DX. Coronavirus infection, ER stress, apoptosis and innate immunity. Front. Microbiol. 2014;5:296. doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lazar C, Uta M, Branza-Nichita N. Modulation of the unfolded protein response by the human hepatitis B virus. Front. Microbiol. 2014;5:433. doi: 10.3389/fmicb.2014.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jheng JR, Ho JY, Horng JT. ER stress, autophagy, and RNA viruses. Front. Microbiol. 2014;5:388. doi: 10.3389/fmicb.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo Y, Cordes KR, Farese RV, Walther TC. Lipid droplets at a glance. J. Cell Sci. 2009;122:749–752. doi: 10.1242/jcs.037630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salloum S, Wang H, Ferguson C, Parton RG, Tai AW. Rab18 binds to hepatitis C virus NS5A and promotes interaction between sites of viral replication and lipid droplets. PLoS Pathog. 2013;9:e1003513. doi: 10.1371/journal.ppat.1003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tanaka T, et al. Hepatitis C virus NS4B targets lipid droplets through hydrophobic residues in the amphipathic helices. J. Lipid Res. 2013;54:881–892. doi: 10.1194/jlr.M026443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Phan T, Beran RK, Peters C, Lorenz IC, Lindenbach BD. Hepatitis C virus NS2 protein contributes to virus particle assembly via opposing epistatic interactions with the E1–E2 glycoprotein and NS3–NS4A enzyme complexes. J. Virol. 2009;83:8379–8395. doi: 10.1128/JVI.00891-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jirasko V, et al. Structural and functional studies of nonstructural protein 2 of the hepatitis C virus reveal its key role as organizer of virion assembly. PLoS Pathog. 2010;6:e1001233. doi: 10.1371/journal.ppat.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boson B, Granio O, Bartenschlager R, Cosset FL. A concerted action of hepatitis C virus p7 and nonstructural protein 2 regulates core localization at the endoplasmic reticulum and virus assembly. PLoS Pathog. 2011;7:e1002144. doi: 10.1371/journal.ppat.1002144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Counihan NA, Rawlinson SM, Lindenbach BD. Trafficking of hepatitis C virus core protein during virus particle assembly. PLoS Pathog. 2011;7:e1002302. doi: 10.1371/journal.ppat.1002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Popescu CI, et al. NS2 protein of hepatitis C virus interacts with structural and non-structural proteins towards virus assembly. PLoS Pathog. 2011;7:e1001278. doi: 10.1371/journal.ppat.1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Samsa MM, et al. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 2009;5:e1000632. doi: 10.1371/journal.ppat.1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moss B. Poxvirus membrane biogenesis. Virology. 2015;479:619–626. doi: 10.1016/j.virol.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krijnse Locker J, Chlanda P, Sachsenheimer T, Brugger B. Poxvirus membrane biogenesis: rupture not disruption. Cell. Microbiol. 2013;15:190–199. doi: 10.1111/cmi.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kolli S, et al. Structure–function analysis of vaccinia virus H7 protein reveals a novel phosphoinositide binding fold essential for poxvirus replication. J. Virol. 2015;89:2209–2219. doi: 10.1128/JVI.03073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee JY, et al. Apolipoprotein E likely contributes to a maturation step of infectious hepatitis C virus particles and interacts with viral envelope glycoproteins. J. Virol. 2014;88:12422–12437. doi: 10.1128/JVI.01660-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Catanese MT, et al. Ultrastructural analysis of hepatitis C virus particles. Proc. Natl Acad. Sci. USA. 2013;110:9505–9510. doi: 10.1073/pnas.1307527110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schmeiser S, Mast J, Thiel HJ, Konig M. Morphogenesis of pestiviruses: new insights from ultrastructural studies of strain Giraffe-1. J. Virol. 2014;88:2717–2724. doi: 10.1128/JVI.03237-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patient R, Hourioux C, Roingeard P. Morphogenesis of hepatitis B virus and its subviral envelope particles. Cell. Microbiol. 2009;11:1561–1570. doi: 10.1111/j.1462-5822.2009.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Crawford SE, Hyser JM, Utama B, Estes MK. Autophagy hijacked through viroporin-activated calcium/calmodulin-dependent kinase kinase-β signaling is required for rotavirus replication. Proc. Natl Acad. Sci. USA. 2012;109:E3405–E3413. doi: 10.1073/pnas.1216539109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Charpilienne A, et al. Solubilized and cleaved VP7, the outer glycoprotein of rotavirus, induces permeabilization of cell membrane vesicles. J. Gen. Virol. 1997;78:1367–1371. doi: 10.1099/0022-1317-78-6-1367. [DOI] [PubMed] [Google Scholar]
- 124.Maruri-Avidal L, Lopez S, Arias CF. Endoplasmic reticulum chaperones are involved in the morphogenesis of rotavirus infectious particles. J. Virol. 2008;82:5368–5380. doi: 10.1128/JVI.02751-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aldabe R, Irurzun A, Carrasco L. Poliovirus protein 2BC increases cytosolic free calcium concentrations. J. Virol. 1997;71:6214–6217. doi: 10.1128/jvi.71.8.6214-6217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Jong AS, et al. Functional analysis of picornavirus 2B proteins: effects on calcium homeostasis and intracellular protein trafficking. J. Virol. 2008;82:3782–3790. doi: 10.1128/JVI.02076-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sharon-Friling R, Goodhouse J, Colberg-Poley AM, Shenk T. Human cytomegalovirus pUL37×1 induces the release of endoplasmic reticulum calcium stores. Proc. Natl Acad. Sci. USA. 2006;103:19117–19122. doi: 10.1073/pnas.0609353103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Norman JP, et al. HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS ONE. 2008;3:e3731. doi: 10.1371/journal.pone.0003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mayne M, Holden CP, Nath A, Geiger JD. Release of calcium from inositol 1,4,5-trisphosphate receptor-regulated stores by HIV-1 Tat regulates TNF-α production in human macrophages. J. Immunol. 2000;164:6538–6542. doi: 10.4049/jimmunol.164.12.6538. [DOI] [PubMed] [Google Scholar]
- 130.Ehrlich LS, et al. Activation of the inositol (1,4,5)-triphosphate calcium gate receptor is required for HIV-1 Gag release. J. Virol. 2010;84:6438–6451. doi: 10.1128/JVI.01588-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coller KE, et al. Molecular determinants and dynamics of hepatitis C virus secretion. PLoS Pathog. 2012;8:e1002466. doi: 10.1371/journal.ppat.1002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Benedicto I, et al. Clathrin mediates infectious hepatitis C virus particle egress. J. Virol. 2015;89:4180–4190. doi: 10.1128/JVI.03620-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mirazimi A, von Bonsdorff CH, Svensson L. Effect of brefeldin A on rotavirus assembly and oligosaccharide processing. Virology. 1996;217:554–563. doi: 10.1006/viro.1996.0150. [DOI] [PubMed] [Google Scholar]
- 134.Jourdan N, et al. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J. Virol. 1997;71:8268–8278. doi: 10.1128/jvi.71.11.8268-8278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Clayson ET, Brando LV, Compans RW. Release of simian virus 40 virions from epithelial cells is polarized and occurs without cell lysis. J. Virol. 1989;63:2278–2288. doi: 10.1128/jvi.63.5.2278-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Inal JM, Jorfi S. Coxsackievirus B transmission and possible new roles for extracellular vesicles. Biochem. Soc. Trans. 2013;41:299–302. doi: 10.1042/BST20120272. [DOI] [PubMed] [Google Scholar]
- 137.Bar S, Rommelaere J, Nuesch JP. Vesicular transport of progeny parvovirus particles through ER and Golgi regulates maturation and cytolysis. PLoS Pathog. 2013;9:e1003605. doi: 10.1371/journal.ppat.1003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rust RC, et al. Cellular COPII proteins are involved in production of the vesicles that form the poliovirus replication complex. J. Virol. 2001;75:9808–9818. doi: 10.1128/JVI.75.20.9808-9818.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trahey M, Oh HS, Cameron CE, Hay JC. Poliovirus infection transiently increases COPII vesicle budding. J. Virol. 2012;86:9675–9682. doi: 10.1128/JVI.01159-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li MY, et al. KDEL receptors assist dengue virus exit from the endoplasmic reticulum. Cell Rep. 2015;10:1496–1507. doi: 10.1016/j.celrep.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 141.Chen YH, et al. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell. 2015;160:619–630. doi: 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Robinson SM, et al. Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS Pathog. 2014;10:e1004045. doi: 10.1371/journal.ppat.1004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chang J, Block TM, Guo JT. Antiviral therapies targeting host ER α-glucosidases: current status and future directions. Antiviral Res. 2013;99:251–260. doi: 10.1016/j.antiviral.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tardif KD, Mori K, Kaufman RJ, Siddiqui A. Hepatitis C virus suppresses the IRE1–XBP1 pathway of the unfolded protein response. J. Biol. Chem. 2004;279:17158–17164. doi: 10.1074/jbc.M312144200. [DOI] [PubMed] [Google Scholar]
- 145.Chan SW. Unfolded protein response in hepatitis C virus infection. Front. Microbiol. 2014;5:233. doi: 10.3389/fmicb.2014.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Diwaker D, Mishra KP, Ganju L. Effect of modulation of unfolded protein response pathway on dengue virus infection. Acta Biochim. Biophys. Sin. (Shanghai) 2015;47:960–968. doi: 10.1093/abbs/gmv108. [DOI] [PubMed] [Google Scholar]
- 147.Anderson DJ, et al. Targeting the AAA ATPase p97 as an approach to treat cancer through disruption of protein homeostasis. Cancer Cell. 2015;28:653–665. doi: 10.1016/j.ccell.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou HJ, et al. Discovery of a first-in-class, potent, selective and orally bioavailable inhibitor of the p97 AAA ATPase (CB-5083) J. Med. Chem. 2015;58:9480–9497. doi: 10.1021/acs.jmedchem.5b01346. [DOI] [PubMed] [Google Scholar]
- 149.Zhang Y, et al. Hsp70 molecular chaperone facilitates endoplasmic reticulum-associated protein degradation of cystic fibrosis transmembrane conductance regulator in yeast. Mol. Biol. Cell. 2001;12:1303–1314. doi: 10.1091/mbc.12.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Houck SA, et al. Quality control autophagy degrades soluble ERAD-resistant conformers of the misfolded membrane protein GnRHR. Mol. Cell. 2014;54:166–179. doi: 10.1016/j.molcel.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saeed M, et al. Role of the endoplasmic reticulum-associated degradation (ERAD) pathway in degradation of hepatitis C virus envelope proteins and production of virus particles. J. Biol. Chem. 2011;286:37264–37273. doi: 10.1074/jbc.M111.259085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Noack J, Bernasconi R, Molinari M. How viruses hijack the ERAD tuning machinery. J. Virol. 2014;88:10272–10275. doi: 10.1128/JVI.00801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Taguwa S, et al. Defining Hsp70 subnetworks in dengue virus replication reveals key vulnerability in flavivirus infection. Cell. 2015;163:1108–1123. doi: 10.1016/j.cell.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Villareal VA, Rodgers MA, Costello DA, Yang PL. Targeting host lipid synthesis and metabolism to inhibit dengue and hepatitis C viruses. Antiviral Res. 2015;124:110–121. doi: 10.1016/j.antiviral.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van de Weijer ML, Luteijn RD, Wiertz EJ. Viral immune evasion: lessons in MHC class I antigen presentation. Semin. Immunol. 2015;27:125–137. doi: 10.1016/j.smim.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 156.van den Boomen DJ, Lehner PJ. Identifying the ERAD ubiquitin E3 ligases for viral and cellular targeting of MHC class I. Mol. Immunol. 2015;68:106–111. doi: 10.1016/j.molimm.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wiertz EJ, et al. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 158.Machold RP, Wiertz EJ, Jones TR, Ploegh HL. The HCMV gene products US11 and US2 differ in their ability to attack allelic forms of murine major histocompatibility complex (MHC) class I heavy chains. J. Exp. Med. 1997;185:363–366. doi: 10.1084/jem.185.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang X, Ye Y, Lencer W, Hansen TH. The viral E3 ubiquitin ligase mK3 uses the Derlin/p97 endoplasmic reticulum-associated degradation pathway to mediate down-regulation of major histocompatibility complex class I proteins. J. Biol. Chem. 2006;281:8636–8644. doi: 10.1074/jbc.M513920200. [DOI] [PubMed] [Google Scholar]
- 160.Herr RA, Wang X, Loh J, Virgin HW, Hansen TH. Newly discovered viral E3 ligase pK3 induces endoplasmic reticulum-associated degradation of class I major histocompatibility proteins and their membrane-bound chaperones. J. Biol. Chem. 2012;287:14467–14479. doi: 10.1074/jbc.M111.325340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Horst D, Ressing ME, Wiertz EJ. Exploiting human herpesvirus immune evasion for therapeutic gain: potential and pitfalls. Immunol. Cell Biol. 2011;89:359–366. doi: 10.1038/icb.2010.129. [DOI] [PubMed] [Google Scholar]
- 162.Wycisk AI, et al. Epstein–Barr viral BNLF2a protein hijacks the tail-anchored protein insertion machinery to block antigen processing by the transport complex TAP. J. Biol. Chem. 2011;286:41402–41412. doi: 10.1074/jbc.M111.237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Verweij MC, et al. The varicellovirus UL49.5 protein blocks the transporter associated with antigen processing (TAP) by inhibiting essential conformational transitions in the 6 + 6 transmembrane TAP core complex. J. Immunol. 2008;181:4894–4907. doi: 10.4049/jimmunol.181.7.4894. [DOI] [PubMed] [Google Scholar]
- 164.Willey RL, Maldarelli F, Martin MA, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 1992;66:7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Magadan JG, et al. Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Pathog. 2010;6:e1000869. doi: 10.1371/journal.ppat.1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Arganaraz ER, Schindler M, Kirchhoff F, Cortes MJ, Lama J. Enhanced CD4 down-modulation by late stage HIV-1 nef alleles is associated with increased Env incorporation and viral replication. J. Biol. Chem. 2003;278:33912–33919. doi: 10.1074/jbc.M303679200. [DOI] [PubMed] [Google Scholar]
- 167.Nieva JL, Agirre A, Nir S, Carrasco L. Mechanisms of membrane permeabilization by picornavirus 2B viroporin. FEBS Lett. 2003;552:68–73. doi: 10.1016/s0014-5793(03)00852-4. [DOI] [PubMed] [Google Scholar]
- 168.Ambrose RL, Mackenzie JM. West Nile virus differentially modulates the unfolded protein response to facilitate replication and immune evasion. J. Virol. 2011;85:2723–2732. doi: 10.1128/JVI.02050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hartshorn KL, et al. Effects of influenza A virus on human neutrophil calcium metabolism. J. Immunol. 1988;141:1295–1301. [PubMed] [Google Scholar]