Abstract
Selenium is an essential micronutrient that is incorporated into at least 25 selenoproteins encoded by the human genome, many of which serve antioxidant functions. Because patients with inflammatory bowel disease (IBD) demonstrate nutritional deficiencies and are at increased risk for colon cancer due to heightened inflammation and oxidative stress, selenoprotein dysfunction may contribute to disease progression. Over the years, numerous studies have analyzed the effects of selenoprotein loss and shown that they are important mediators of intestinal inflammation and carcinogenesis. In particular, recent work has focused on the role of selenoprotein P (SEPP1), a major selenium transport protein which also has endogenous antioxidant function. These experiments determined SEPP1 loss altered immune and epithelial cellular function in a murine model of colitis-associated carcinoma. Here, we discuss the current knowledge of SEPP1 and selenoprotein function in the setting of IBD, colitis, and inflammatory tumorigenesis.
Keywords: Enteroids, Stem cells, Glutathione peroxidase, Selenoprotein P, Inflammation, Interferon-γ, Antioxidant
Introduction
Inflammatory bowel disease (IBD) is estimated to affect over 1 million Americans and 2.5 million Europeans [1]. IBD is primarily comprised of two types of chronic inflammatory disorders of the intestine, Crohn’s disease (CD) and ulcerative colitis (UC) [2]. IBD etiology is incompletely understood, but evidence to-date suggests a complex interplay between microbes, other undefined environmental exposures, genetic susceptibility, and inappropriately sustained and severe autoimmune inflammatory responses, which ultimately results in repetitive injury to the GI tract [3, 4]. Longstanding colonic IBD also predisposes patients to colorectal cancer (CRC). In this situation, sustained inflammation results in a pro-tumorigenic microenvironment in which reactive oxygen species (ROS) induce protein and DNA damage, stimulate immune cell recruitment and polarization, and accelerate epithelial cell proliferation [5].
As greater disease activity is associated with increased cancer risk, understanding the molecular pathogenesis of IBD and identifying modifiable factors affecting disease severity are of paramount importance. Toward that goal, recent studies have implicated the essential micronutrient selenium (Se) as well as specific selenium containing proteins (selenoproteins, SePs), such as selenoprotein P (SEPP1) and members of the glutathione peroxidase (GPx) family, in modifying inflammation and tumorigenesis. The aim of this article is to review the literature on Se and SePs in colitis and colitis-associated carcinoma and pose the argument that Se and SePs are valid targets for therapeutic intervention in IBD.
Selenium and selenoprotein function
Se was discovered by J.J. Berzelius in 1817 and initially recognized to be a toxin when ingested in large amounts [6]. However, Se was later determined to be an essential micronutrient and indispensable for the production of SePs, where it is incorporated as the 21st amino acid selenocysteine (Sec). Functionally, SePs are known to be potent antioxidants, and the majority of characterized SePs catalyze oxidation–reduction reactions using the Sec as an active site [7]. SePs are particularly effective antioxidants owing to the selenol group in Sec, which is more fully ionized than the thiol of cysteine (Cys) at physiological pH. In addition, Sec has a lower pKa (~5.2) and reduction potential than Cys. This makes Sec more reactive than Cys, which is presented within the active site of many non-selenoprotein enzymes [8]. Aside from the normal antioxidant activity contributed by the Sec, selenoprotein S and selenoprotein 15 can process and remove misfolded proteins [9, 10], and MsrB1 is capable of regulating antioxidant protein repair through protein disulfide shuffling [11].
Expression of SePs is tightly controlled by the Sec translational process which is highly dependent on the presence of Se (for reviews on translational regulation of selenoprotein synthesis, see [12–14]). Se deficiency reduces the intracellular amounts of mature tRNA Sec, a special transfer RNA charged with Sec, which in turn results in decreased SeP production. In the setting of limiting Sec tRNA levels, SePs may still be translated; however, there is a “hierarchy” of SeP expression. This hierarchy reflects the relative importance of the selenoproteins in cellular homeostasis. For example, depending on the particular tissue, GPx4 > SEPP1 > thioredoxin reductase 1 > type I deiodinase > GPx1 [15, 16]. Selenoprotein synthesis is also considered to be modulated by differential expression of two Sec tRNA isoforms which are distinguished by the presence of 2′-O-methylribose at position 34 (Um34). The Um34 modification is also dependent on Se availability, with mice maintained on a high Se diet having increased percentages of mcm5Um-containing Sec tRNA [17]. These two Sec tRNA isoforms are differentially associated with production of distinct classes of SePs. The SePs most responsive to the modified mcm5Um-containing Sec tRNA are stress-related SePs, such as GPx1 and GPx3, whereas housekeeping SePs, essential for survival, are not dependent on the Um34 modification allowing higher expression in the context of decreased Se availability [18]. While originally described in mouse models, the mutation of the Sec tRNA gene (TRSP) which interferes with the Um34 modification has recently been described in a human subject, where authors note decreased expression of stress-related SePs, while the expression of housekeeping SePs was largely preserved [19].
Selenium in human disease
Se and SePs contribute greatly to human health, and their functions are most often linked to antioxidant ability. Indeed, Se deficiency, due to Se-poor soil, is correlated with the congestive cardiomyopathy known as Keshan disease [20] and the deforming osteochondropathy, Kashin–Beck disease [21]. Keshan disease incidence has been reduced by the administration of Se-fortified table salt [22], implicating Se deficiency as the etiologic precipitant in this disorder. Fulvic acid supplementation and selenium deficiency in mice recapitulated many of the symptoms of Kashin–Beck disease [23], suggesting that Se supplementation may prevent this disorder. However, a double-blind, randomized control trial of Se supplementation did not affect the clinical course of patients with Kashin–Beck disease [24]. In addition, patients with genetically impaired selenoprotein biosynthesis present with multisystem disorders. For example, the mutation of SBP2 (SECISBP2) is characterized by the failure of spermatogenesis, impaired T lymphocyte proliferation, abnormal mononuclear cytokine secretion, telomere shortening, increased cutaneous ROS, and susceptibility to ultraviolet radiation-induced oxidative damage [25]. Impaired oxidative defenses, muscle defects, and thyroid dysfunction were also observed in the setting of Sec tRNA (TRSP) mutation [19]. Together, these studies underscore the importance of Se and SePs in the maintenance of human health.
Epidemiological studies of patients with below average Se levels have further suggested important biological functions for SePs. Observational studies associate lower serum Se levels with epilepsy [26], age-associated neurological disorders [27], and decreased survival following HIV infection [28]. It should be noted that, as these are observational studies, the causative roles of Se in these diseases have not been proven, but suggests that Se might be protective. Further research on the benefit of Se supplementation in these diseases is essential.
As Se may confer protection against disease by reducing chronic oxidative stress and inflammation, it was hypothesized that Se supplementation would protect against cancer development. Indeed, animal models have demonstrated that Se supplementation can reduce the incidence and severity of liver [29], esophageal [30], pancreatic [31], prostatic [32], colon [33], and mammary carcinogenesis [34]. Unfortunately, large clinical trials have yielded mixed results, some suggesting that Se supplementation and/or higher Se status may reduce cancer risk [35–37] and others failing to correlate serum Se levels with cancer risk [38–40]. Thus, the impact of Se supplementation on cancer is a more complex issue than has been heretofore recognized.
Selenium and IBD
Interestingly, the benefit of Se supplementation might be best realized in populations with low baseline Se and high inflammatory burden, such as patients with IBD. IBD patients can have defects in intestinal absorption, leading to nutritional deficiencies which are important to recognize and treat in disease management. Se deficiency and decreased SeP activity have been described in both CD and UC patients, often correlating with disease severity [41–47]. Similar findings have been observed in the dextran sulfate sodium (DSS) mouse model of colitis, where decreased plasma Se levels and GPx activity were observed [48]. However, these studies do not indicate a causal role for Se in IBD development. Nevertheless, experiments analyzing Se deficiency in the context of colitis observed exacerbated disease severity, with higher mortality, decreased body weight, increased diarrhea, more pronounced inflammatory injury, and increased activation of pro-tumorigenic pathways, such as EGF and TGFβ [48]. Increased tumorigenesis and disease progression have also been observed in Se-deficient mice placed on a colitis-associated carcinoma (CAC) protocol, using azoxymethane (AOM) to initiate genetic mutations followed by repeated cycles of DSS-based epithelial injury [48]. Together, these studies suggest a direct role for Se in mediating IBD severity and its associated cancer risk.
Investigating selenoproteins through SEC tRNA mutations
While Se is primarily incorporated into SePs, it was still unclear whether the effects observed with Se were due to the loss of Se-containing proteins or low-molecular weight Se compounds. To broadly investigate the role of SePs, mouse models were developed with modified expression of Sec tRNA which interferes with selenoprotein biosynthesis [49]. Collectively, these models have indicated that SePs exert the bulk of Se’s influence in regulating oxidative stress and tumorigenesis in the gut.
The first developed Sec tRNA mouse model [(i6A−) tRNA[Ser]Sec] relied on transgenic expression of mutated Sec tRNA specifically interfering with synthesis of mcm5Um-containing Sec tRNA. Global transgene expression decreased levels of stress-related SePs [49]. In the gut, Sec tRNA transgenic mice were observed to have increased numbers of aberrant crypt foci (ACF), a type of preneoplastic colonic lesion, after exposure to AOM [50]. Interestingly, these were the first data to show that SeP expression could directly modify the development of colorectal tumorigenesis. To date, this mouse model has also been used to show that decreased SeP expression augments development of prostatic intraepithelial neoplasia, hepatocarcinoma, and inflammatory pyogranulomas, indicating a broad role for stress-related SePs in tumorigenesis across organ systems [51, 52].
As global loss of the mammalian Sec tRNA gene (Trsp) is embryonic lethal, studies to analyze the effect of complete Sec tRNA and selenoprotein loss have relied on a conditional knockout (KO) model to determine tissue-specific effects [53, 54]. However, the effects of tissue-specific Sec tRNA loss were often severe, with SeP expression in many tissues, such as the endothelium, cardiac muscle, liver, and skin, required for survival [55–57]. Nevertheless, Se has long been known to contribute to immune cell function (reviewed in [58]), and this model has provided useful insight into the function of SePs in different immune cell populations, and particularly how they may contribute to inflammatory tumorigenesis in the gut. Trsp knockout in myeloid lineages through a LysM-Cre driver led to increased oxidative stress, upregulated transcription of antioxidant enzymes, accumulation of reactive oxygen species, altered expression of extracellular matrix-related genes, and diminished migration through matrix [56, 59]. Furthermore, placing these mice on an acute DSS-induced colitis protocol resulted in worse colitis characterized by pronounced inflammation, neutrophil infiltration, edema, weight loss, shorter colon length, and expression of pro-inflammatory cytokines relative to WT mice treated with DSS [60]. While intestine epithelial-specific Trsp KO has not yet been described, these data suggest that selenoprotein expression in myeloid-derived immune cells is potent suppressors of inflammation in the gut and likely contributes to inflammatory tumorigenesis.
Glutathione peroxidases
In addition to global SeP loss through the modulation of Sec tRNA expression, other studies have analyzed contributions of individual selenoproteins. While several SePs have been examined in the context of colitis and CAC, some of the best studied are those of the glutathione peroxidase family. As these proteins are characterized by their ability to metabolize hydrogen peroxide (H2O2) and other peroxides, they are considered to be some of the most potent mediators of Se’s effects in oxidative stress and inflammation. In colitis, extracellular GPx levels increase dramatically following DSS treatment, suggesting that these enzymes are upregulated in response to oxidative injury [61]. Specifically, Gpx2, a recently described target of STAT family transcription factors, was determined by gene expression profiling to be one of only seven genes upregulated in three separate models of colitis: DSS, transfer of CD4+ CD45RBhigh T cell populations, and 2,4,6-trinitrobenzene sulphonic acid (TNBS) treatment [62, 63]. GPX2 upregulation was further observed in tissues from both CD and UC patients, as well as colorectal adenomas [63]. Interestingly, GPx1 and GPx2 were also the SePs most affected by expression of the (i6A−) tRNA[Ser]Sec Sec tRNA transgene in the colon, suggesting that reduced GPx expression is a contributing factor to the augmented DSS-induced colitis observed in this model.
Determining the precise role of the GPx family in colitis and inflammatory tumorigenesis has been further aided by the development of individual knockout mouse models. While there are eight GPx family members, GPx’s 5-8 are not SePs in the rodent, as the Sec is substituted for cysteine. Of the remaining GPx family members, global loss of Gpx4 is embryonic lethal, perhaps not surprising given its place at the top of the selenium hierarchy noted above [64]. On the other hand, mice lacking Gpx1, Gpx2, and Gpx3 all develop normally, with no overt baseline phenotypes. However, mice deficient for both Gpx1 and Gpx2 developed spontaneous ileocolitis [65] linked to excess NADPH oxidase-generated ROS [66]. Individual knockout of Gpx1 or Gpx2 also rendered mice more susceptible to salmonella-induced colitis [67], while Gpx2 and Gpx3 knockout mice each have increased inflammation and CAC in AOM/DSS models [68, 69]. Together, these studies suggest a broad role for GPx family selenoproteins in mediating oxidative stress in the context of intestinal inflammation and downstream tumorigenesis.
Selenoprotein P and cancer
In addition to GPx family SePs, selenoprotein P (SEPP1) has also been implicated in mediating Se’s effect on inflammatory tumorigenesis. Unlike the majority of SePs which are best characterized by their enzymatic activity, SEPP1 is better known as the predominant Se transport protein. SEPP1 is primarily expressed in the liver, where the majority of Se metabolism takes place, incorporating Se in ten Sec residues within its primary structure (in comparison, most SePs only have 1 Sec). The majority of these Secs exist within SEPP1’s Se-rich C-terminal domain, which is necessary for the delivery of Se to distant tissues via the plasma, where it can be taken up and degraded to free Se for the synthesis of other SePs. SEPP1 currently has two known receptors which are differentially expressed based on tissue type. In tissues such as the brain and testes, SEPP1 is taken up by apoER2-mediated endocytosis, although in other tissues, such as the kidney, the primary SEPP1 receptor is megalin, a lipoprotein receptor localized to the proximal tubule epithelium within the kidney [70–72]. To illustrate the effect of SEPP1 in Se transport, hepatocyte-specific Sepp1 knock out resulted in a 90 % reduction in plasma Se levels, greatly reducing the whole body and tissue Se [73]. However, it is important to note that SEPP1 can also function as an antioxidant through a single N-terminal Sec, which exists within a UXXC motif that catalyzes the oxidation of glutathione (GSH) by a hydrogen peroxide or phosphatidylcholine hydroperoxide [74, 75]. Thus, both N- and C-terminal domains contribute to the overall function of SEPP1, making it vital for the production of other selenoproteins within target organs and giving it the ability to serve in an antioxidant function.
SEPP1 levels and activity are significantly decreased in colon tumors, human prostate tumors, C3(1)/Tag transgenic mouse tumors, and in prostate cancer cell lines [76, 77]. Furthermore, several SNPs have been identified in SEPP1 that may contribute to decreased expression in colorectal adenomas and have been associated with cancer risk [78–80]. Indeed, SEPP1 transcript levels are decreased as early as the adenoma stage in CRC [81, 82]. Collectively, these data suggest that SEPP1 regulates intestinal homeostasis and protects from colitis and CAC.
SEPP1 modifies CAC
Recently, a global Sepp1 knockout mouse model was used to investigate the contribution of SEPP1 to intestinal injury and development of CAC. In this study, Sepp1 wild type (WT, Sepp1 +/+), heterozygous (Sepp1 +/−), and null (Sepp1 −/−) mice were subjected to the AOM/DSS initiation–promotion protocol to model inflammatory tumorigenesis. These studies suggest that SEPP1 functions as a haploinsufficient tumor suppressor, with Sepp1 +/− mice displaying increased tumor multiplicity, a higher degree of dysplasia, increased intratumoral proliferation, and a greater extent of oxidative DNA lesions relative to both Sepp1 +/+ and Sepp1 −/− mice. Thus, reducing, but not eliminating, SEPP1 results in significantly increased tumor burden. Contrary to expectations, complete Sepp1 deficiency (Sepp1 −/−) resulted in decreased tumorigenesis concomitant with increased apoptosis, decreased proliferation, and high genomic instability [82]. Thus, it is postulated that this observation is due to the “double-edged-sword” of oxidative stress where instead of promotion of malignancy with increased ROS production, critically high levels of oxidative injury lead to the clearance of initiated Sepp1 −/− cells. This is supported by the observation that when Sepp1 −/− mice are treated with either AOM or DSS as single modalities tumor multiplicity is increased [82].
Specific assessment of the role of SEPP1 in tumorigenesis is confounded by the fact that SEPP1 participates in Se transport and contributes to the production of other SePs which may influence colitis. To test whether SEPP1’s Se transport capacity contributes to CAC development, mice with truncated Se-rich C-terminal domain [70] were subjected to AOM/DSS treatment. These mice show increased tumor number and dysplasia, although not to the extent of Sepp1 heterozygous mice [82], indicating that at least some of the phenotype observed in SEPP1 deficiency was due to loss of this domain. However, a contribution of the Sec redox active was also observed in mice containing an enzymatically dead serine in place of Sec [75] and also had increased tumor number and size with associated increased proliferation and DNA damage. Thus, both the Se transport and enzymatic functions of SEPP1 contribute to protect against intestinal injury and CAC. As complete knockout of SEPP1 resulted in a phenotype that differed significantly from that seen with loss of either component alone, other impacts of SEPP1 loss cannot be ruled out.
Cell-type specific roles for SEPP1
SEPP1 is expressed in the intestinal epithelium and immune cells, and in the context of global SEPP1 loss it remained unclear which cell type was mediating the observed phenotypes. Macrophages contribute to the pathogenesis of colitis and tumor development, and loss of SeP synthesis within macrophages increases inflammatory injury in DSS-based colitis models [60]. Interestingly, SEPP1 is also the most upregulated gene in pro-inflammatory tumor-conditioned macrophages [83] suggesting an important role for SEPP1 in macrophage function. It is likely that multiple selenoproteins contribute to the inflammatory microenvironment and a loss of balance within the macrophage selenoproteome alters immune cell activity. Moreover, SEPP1 expression and/or secretion is decreased by the cytokines TGF-β1, interleukin 1β, tumor necrosis factor α, and interferon γ (IFN-γ) [84, 85], further complicating the role of SEPP1 in immune cell activity. Experimentally, an increase in total and M2 macrophages was observed in tumors from AOM/DSS-treated Sepp1 +/− mice. The increase in M2 macrophages was determined to be due to skewed polarization as opposed to recruitment, as direct stimulation with either IFN-γ and LPS or IL-13 led to decreased M1 polarization and increased M2 polarization, respectively, in SEPP1 heterozygous naïve macrophages. This only occurred in heterozygous macrophages and was not seen in full knockout macrophages, indicating that tight regulation of SEPP1 levels is required for proper macrophage function. Thus, SEPP1 may protect against inflammatory tumorigenesis through its attenuation of pro-inflammatory immune cell polarization, though the roles of SEPP1 in the immune environment are complex.
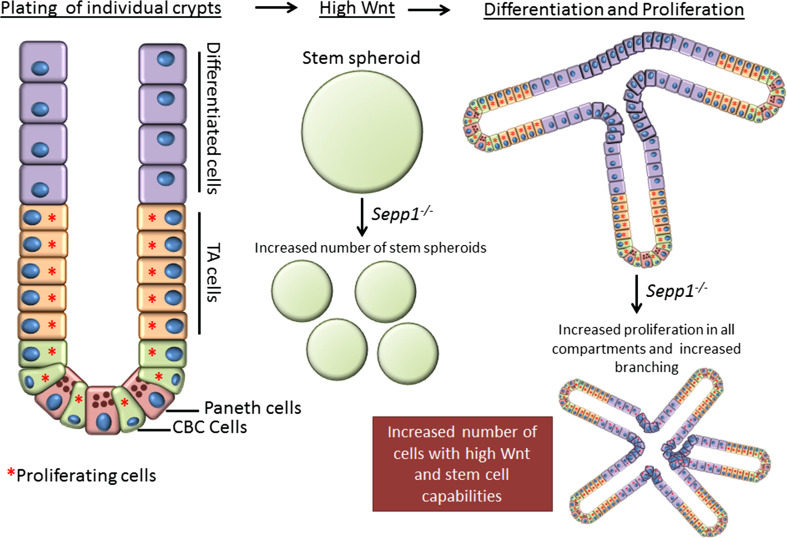
On the other hand, Sepp1 −/− mice demonstrated increased DNA damage and significantly altered apoptosis and proliferation within the epithelial compartment, suggesting that this cell population may be differentially affected by SEPP1 loss. To determine whether SEPP1 mediates epithelial tissue-autonomous effects, small intestinal organoids (enteroids) [86] were generated from WT and Sepp1 −/− mice. These studies demonstrated increased plating efficiency, branching, and stem spheroids (Fig. 1) in enteroids from Sepp1 −/− mice, all indicators of increased stem-cell function [87–89]. These data suggest that loss of SEPP1 in epithelial cells drives them to a more stem-cell-like and potentially pro-tumorigenic phenotype. Moreover, the assessment of tumor tissue isolated from Sepp1 −/− mice revealed increased expression of genes regulated by the WNT signaling pathway, a pathway heavily implicated in maintaining stem-cell populations as well as being a key driver in intestinal tumorigenesis [90]. Together, these functional alterations in Sepp1 −/− enteroids highlight changes that are likely occurring within the intestinal epithelial cells which may independently contribute to cell transformation and tumor promotion.
Fig. 1.
Sepp1 −/− enteroids demonstrate increased stem-cell characteristics. This schematic shows normal growth characteristics upon plating single intestinal crypts. Proliferating cells (red asterisk) include stem cells (CBCs, crypt-based columnar cells) and transient amplifying (TA) cells. Upon differentiation, cells no longer proliferate but complete the crypt structure. When WNT is added to the Matrigel matrix, an increased propensity to form stem spheroids occurs. Once WNT has been expended, enteroids proliferate and component cells differentiate. In the case of Sepp1 knockout, enteroids form more stem spheroids, indicative of increased WNT tone. Sepp1 −/− enteroids also demonstrate increased branching, which suggests a higher percentage of stem cells within the population, and higher proliferation even in regions, where cells should be differentiated and quiescent. All these characteristics indicate that loss of SEPP1 contributes to increased tumorigenic properties in epithelial cells
Conclusions
Pre-clinical studies strongly indicate that antioxidants, such as many of the SePs which are produced depending on local Se concentration, should be chemopreventative agents in malignancy, but human trials have proven a disappointment. The US case–control study testing the efficacy of Se and vitamin E supplementation in cancer (SELECT) did not demonstrate a protective effect of Se supplementation on risk of CRC [91], and an intervention trial meta-analysis determined that oral administration of Se was not effective in preventing colorectal neoplasia [40]. However, research on SePs in inflammatory cancer suggests that patient selection will play a significant role in the success of Se supplementation studies in humans. Thus, targeted supplementation in Se-deficient populations may be an effective prevention strategy. Indeed, some patients with IBD are Se deficient, with SEPP1 expression decreased as much as 50 % in patients compared to healthy controls [92, 93]. As SEPP1 haploinsufficiency leads to increased tumorigenesis in rodent CAC models, the degree of SEPP1 reduction observed in IBD patients is to a level that, in animals, promotes tumorigenesis. In further support of a protective role for Se supplementation, a significant survival benefit was demonstrated in mouse cohorts fed a high Se diet (1.0 PPM) as opposed to an Se sufficient diet (0.25 PPM) when subjected to the AOM/DSS protocol [82]. As selenoprotein expression should be optimized at 0.25 PPM, it may be that the protective effect of the high Se diet occurs due to reduced Se uptake in mice subjected to the inflammatory carcinogenesis protocol.
Se supplementation may additionally benefit populations with decreased SEPP1 expression due to genetic polymorphisms affecting its expression. Case control studies of incident prostate cancer cases and matched controls indicated increased prostate cancer risk in patients harboring SEPP1 SNPs, possibly influenced by decreased plasma SEPP1 [78, 94]. Four SEPP1 variants are significantly associated with advanced colorectal adenoma risk [79], and genetic instability has been observed in the SEPP1 promoter (T)17 repeat motif in CRC in the context of the MSI-CRC mutator phenotype [80]. Though these polymorphisms are incompletely understood, they tend to be linked with increased cancer risk and modulate either expression or isoform proportion of SEPP1. Genotyping of SEPP1 in patients with CAC may predict increased responsiveness to Se supplementation.
In conclusion, this review presents a broad role for SePs in protection against inflammatory carcinogenesis. Studies relying on mutation of selenocysteine tRNA indicate a protective role of SePs in inflammatory tumorigenesis, but do not identify the SePs responsible. It is likely that multiple SePs can contribute to this phenotype. For example, loss of both GPx1 and GPx2 worsens colitis, indicating that these two SePs are important in mitigating intestinal inflammation. Furthermore, decreases in SEPP1 contribute to inflammatory tumorigenesis by reducing redox capacity, enhancing stem-cell characteristics and proliferation of epithelial cells, and modulating immune cell polarization toward a pro-tumorigenic phenotype. Loss of GPx3, in a similar model, results in increased tumorigenesis and dysplasia concomitant with increased proliferation, hyperactive WNT signaling, and increased DNA damage. It is likely that, with more thorough study of SePs in inflammatory tumorigenesis, we will see a common trend amongst SePs which will further promote Se and SePs as bona fide therapeutic targets in the prevention of inflammatory tumorigenesis.
Abbreviations
- SEPP1
Selenoprotein P
- CAC
Colitis-associated cancer
- IBD
Inflammatory bowel disease
- CD
Crohn’s disease
- UC
Ulcerative colitis
- SeP
Selenoprotein
- GPx
Glutathione Peroxidase
- ROS
Reactive oxygen species
- Se
Selenium
- CRC
Colorectal cancer
- AOM
Azoxymethane
- DSS
Dextran sulfate sodium
- Sec
Selenocysteine
- Cys
Cysteine
- GSH
Glutathione
- ACF
Aberrant crypt foci
- TNBS
2,4,6-Trinitrobenzene sulphonic acid
- IFN-γ
Interferon-γ
References
- 1.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky DK. Inflammatory bowel disease (1) N Engl J Med. 1991;325:928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 3.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roessner A, Kuester D, Malfertheiner P, Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract. 2008;204:511–524. doi: 10.1016/j.prp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 6.Emsley J. Nature’s building blocks: an A–Z guide to the elements. Oxford: Oxford University Press; 2011. [Google Scholar]
- 7.Stadtman TC. Selenium biochemistry. Mammalian selenoenzymes. Ann N Y Acad Sci. 2000;899:399–402. doi: 10.1111/j.1749-6632.2000.tb06203.x. [DOI] [PubMed] [Google Scholar]
- 8.Stadtman TC. Selenocysteine. Annu Rev Biochem. 1996;65:83–100. doi: 10.1146/annurev.bi.65.070196.000503. [DOI] [PubMed] [Google Scholar]
- 9.Labunskyy VM, Yoo MH, Hatfield DL, Gladyshev VN. Sep15, a thioredoxin-like selenoprotein, is involved in the unfolded protein response and differentially regulated by adaptive and acute ER stresses. Biochemistry. 2009;48:8458–8465. doi: 10.1021/bi900717p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature. 2004;429:841–847. doi: 10.1038/nature02656. [DOI] [PubMed] [Google Scholar]
- 11.Kaya A, Lee BC, Gladyshev VN. Regulation of protein function by reversible methionine oxidation and the role of selenoprotein MsrB1. Antioxid Redox Signal. 2015;23:814–822. doi: 10.1089/ars.2015.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burk RF, Hill KE. Regulation of selenoproteins. Annu Rev Nutr. 1993;13:65–81. doi: 10.1146/annurev.nu.13.070193.000433. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann PR, Berry MJ. Selenoprotein synthesis: a unique translational mechanism used by a diverse family of proteins. Thyroid. 2005;15:769–775. doi: 10.1089/thy.2005.15.769. [DOI] [PubMed] [Google Scholar]
- 14.Turanov AA, Xu XM, Carlson BA, Yoo MH, Gladyshev VN, Hatfield DL. Biosynthesis of selenocysteine, the 21st amino acid in the genetic code, and a novel pathway for cysteine biosynthesis. Adv Nutr. 2011;2:122–128. doi: 10.3945/an.110.000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schriever SC, Barnes KM, Evenson JK, Raines AM, Sunde RA. Selenium requirements are higher for glutathione peroxidase-1 mRNA than gpx1 activity in rat testis. Exp Biol Med (Maywood) 2009;234:513–521. doi: 10.3181/0812-RM-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes KM, Evenson JK, Raines AM, Sunde RA. Transcript analysis of the selenoproteome indicates that dietary selenium requirements of rats based on selenium-regulated selenoprotein mRNA levels are uniformly less than those based on glutathione peroxidase activity. J Nutr. 2009;139:199–206. doi: 10.3945/jn.108.098624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatfield D, Lee BJ, Hampton L, Diamond AM. Selenium induces changes in the selenocysteine tRNA[Ser]Sec population in mammalian cells. Nucleic Acids Res. 1991;19:939–943. doi: 10.1093/nar/19.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatfield DL, Carlson BA, Xu XM, Mix H, Gladyshev VN. Selenocysteine incorporation machinery and the role of selenoproteins in development and health. Prog Nucleic Acid Res Mol Biol. 2006;81:97–142. doi: 10.1016/S0079-6603(06)81003-2. [DOI] [PubMed] [Google Scholar]
- 19.Schoenmakers E, Carlson B, Agostini M, Moran C, Rajanayagam O, Bochukova E, Tobe R, Peat R, Gevers E, Muntoni F, Guicheney P, Schoenmakers N, Farooqi S, Lyons G, Hatfield D, Chatterjee K. Mutation in human selenocysteine transfer RNA selectively disrupts selenoprotein synthesis. J Clin Invest. 2016;126:992–996. doi: 10.1172/JCI84747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J. An original discovery: selenium deficiency and Keshan disease (an endemic heart disease) Asia Pacific J Clin Nutr. 2012;21:320–326. [PubMed] [Google Scholar]
- 21.Yao Y, Pei F, Kang P. Selenium, iodine, and the relation with Kashin–Beck disease. Nutrition. 2011;27:1095–1100. doi: 10.1016/j.nut.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Cheng YY, Qian PC. The effect of selenium-fortified table salt in the prevention of Keshan disease on a population of 1.05 million. Biomed Environ Sci BES. 1990;3:422–428. [PubMed] [Google Scholar]
- 23.Yang C, Niu C, Bodo M, Gabriel E, Notbohm H, Wolf E, Muller PK. Fulvic acid supplementation and selenium deficiency disturb the structural integrity of mouse skeletal tissue. An animal model to study the molecular defects of Kashin–Beck disease. Biochem J. 1993;289(Pt 3):829–835. doi: 10.1042/bj2890829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreno-Reyes R, Mathieu F, Boelaert M, Begaux F, Suetens C, Rivera MT, Neve J, Perlmutter N, Vanderpas J. Selenium and iodine supplementation of rural Tibetan children affected by Kashin–Beck osteoarthropathy. Am J Clin Nutr. 2003;78:137–144. doi: 10.1093/ajcn/78.1.137. [DOI] [PubMed] [Google Scholar]
- 25.Schoenmakers E, Agostini M, Mitchell C, Schoenmakers N, Papp L, Rajanayagam O, Padidela R, Ceron-Gutierrez L, Doffinger R, Prevosto C, Luan J, Montano S, Lu J, Castanet M, Clemons N, Groeneveld M, Castets P, Karbaschi M, Aitken S, Dixon A, Williams J, Campi I, Blount M, Burton H, Muntoni F, O’Donovan D, Dean A, Warren A, Brierley C, Baguley D, Guicheney P, Fitzgerald R, Coles A, Gaston H, Todd P, Holmgren A, Khanna KK, Cooke M, Semple R, Halsall D, Wareham N, Schwabe J, Grasso L, Beck-Peccoz P, Ogunko A, Dattani M, Gurnell M, Chatterjee K. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest. 2010;120:4220–4235. doi: 10.1172/JCI43653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashrafi MR, Shams S, Nouri M, Mohseni M, Shabanian R, Yekaninejad MS, Chegini N, Khodadad A, Safaralizadeh R. A probable causative factor for an old problem: selenium and glutathione peroxidase appear to play important roles in epilepsy pathogenesis. Epilepsia. 2007;48:1750–1755. doi: 10.1111/j.1528-1167.2007.01143.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Rocourt C, Cheng WH. Selenoproteins and the aging brain. Mech Ageing Dev. 2010;131:253–260. doi: 10.1016/j.mad.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Baum MK, Shor-Posner G, Lai S, Zhang G, Lai H, Fletcher MA, Sauberlich H, Page JB. High risk of HIV-related mortality is associated with selenium deficiency. J Acquir Immune Defic Syndr Hum Retrovirol Off Publ Int Retrovirol Assoc. 1997;15:370–374. doi: 10.1097/00042560-199708150-00007. [DOI] [PubMed] [Google Scholar]
- 29.Daoud AH, Griffin AC. Effect of retinoic acid, butylated hydroxytoluene, selenium and sorbic acid on azo-dye hepatocarcinogenesis. Cancer Lett. 1980;9:299–304. doi: 10.1016/0304-3835(80)90021-X. [DOI] [PubMed] [Google Scholar]
- 30.van Rensburg SJ, Hall JM, Gathercole PS. Inhibition of esophageal carcinogenesis in corn-fed rats by riboflavin, nicotinic acid, selenium, molybdenum, zinc, and magnesium. Nutr Cancer. 1986;8:163–170. doi: 10.1080/01635588609513890. [DOI] [PubMed] [Google Scholar]
- 31.Woutersen RA, Appel MJ, Van Garderen-Hoetmer A. Modulation of pancreatic carcinogenesis by antioxidants. Food Chem Toxicol. 1999;37:981–984. doi: 10.1016/S0278-6915(99)00093-9. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Bonorden MJ, Li GX, Lee HJ, Hu H, Zhang Y, Liao JD, Cleary MP, Lu J. Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prev Res (Phila) 2009;2:484–495. doi: 10.1158/1940-6207.CAPR-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baines AT, Holubec H, Basye JL, Thorne P, Bhattacharyya AK, Spallholz J, Shriver B, Cui H, Roe D, Clark LC, Earnest DL, Nelson MA. The effects of dietary selenomethionine on polyamines and azoxymethane-induced aberrant crypts. Cancer Lett. 2000;160:193–198. doi: 10.1016/S0304-3835(00)00585-1. [DOI] [PubMed] [Google Scholar]
- 34.Ip C, Thompson HJ, Ganther HE. Selenium modulation of cell proliferation and cell cycle biomarkers in normal and premalignant cells of the rat mammary gland. Cancer Epidemiol Biomark Prev. 2000;9:49–54. [PubMed] [Google Scholar]
- 35.Clark LC, Dalkin B, Krongrad A, Combs GF, Jr, Turnbull BW, Slate EH, Witherington R, Herlong JH, Janosko E, Carpenter D, Borosso C, Falk S, Rounder J. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81:730–734. doi: 10.1046/j.1464-410x.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 36.Duffield-Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH, Jacobs ET, Marshall JR, Clark LC. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–612. doi: 10.1046/j.1464-410X.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs ET, Jiang R, Alberts DS, Greenberg ER, Gunter EW, Karagas MR, Lanza E, Ratnasinghe L, Reid ME, Schatzkin A, Smith-Warner SA, Wallace K, Martinez ME. Selenium and colorectal adenoma: results of a pooled analysis. J Natl Cancer Inst. 2004;96:1669–1675. doi: 10.1093/jnci/djh310. [DOI] [PubMed] [Google Scholar]
- 38.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Jr, Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallace K, Byers T, Morris JS, Cole BF, Greenberg ER, Baron JA, Gudino A, Spate V, Karagas MR. Prediagnostic serum selenium concentration and the risk of recurrent colorectal adenoma: a nested case-control study. Cancer Epidemiol Biomark Prev. 2003;12:464–467. [PubMed] [Google Scholar]
- 40.Papaioannou D, Cooper KL, Carroll C, Hind D, Squires H, Tappenden P, Logan RF. Antioxidants in the chemoprevention of colorectal cancer and colorectal adenomas in the general population: a systematic review and meta-analysis. Colorectal Dis. 2011;13:1085–1099. doi: 10.1111/j.1463-1318.2010.02289.x. [DOI] [PubMed] [Google Scholar]
- 41.Geerling BJ, Badart-Smook A, Stockbrugger RW, Brummer RJ. Comprehensive nutritional status in patients with long-standing Crohn disease currently in remission. Am J Clin Nutr. 1998;67:919–926. doi: 10.1093/ajcn/67.5.919. [DOI] [PubMed] [Google Scholar]
- 42.Hinks LJ, Inwards KD, Lloyd B, Clayton B. Reduced concentrations of selenium in mild Crohn’s disease. J Clin Pathol. 1988;41:198–201. doi: 10.1136/jcp.41.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuroki F, Matsumoto T, Iida M. Selenium is depleted in Crohn’s disease on enteral nutrition. Dig Dis. 2003;21:266–270. doi: 10.1159/000073346. [DOI] [PubMed] [Google Scholar]
- 44.Loeschke K, Konig A, Trebert Haeberlin S, Lux F. Low blood selenium concentration in Crohn disease. Ann Intern Med. 1987;106:908. doi: 10.7326/0003-4819-106-6-908_1. [DOI] [PubMed] [Google Scholar]
- 45.Penny WJ, Mayberry JF, Aggett PJ, Gilbert JO, Newcombe RG, Rhodes J. Relationship between trace elements, sugar consumption, and taste in Crohn’s disease. Gut. 1983;24:288–292. doi: 10.1136/gut.24.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rannem T, Ladefoged K, Hylander E, Hegnhoj J, Jarnum S. Selenium status in patients with Crohn’s disease. Am J Clin Nutr. 1992;56:933–937. doi: 10.1093/ajcn/56.5.933. [DOI] [PubMed] [Google Scholar]
- 47.Ringstad J, Kildebo S, Thomassen Y. Serum selenium, copper, and zinc concentrations in Crohn’s disease and ulcerative colitis. Scand J Gastroenterol. 1993;28:605–608. doi: 10.3109/00365529309096096. [DOI] [PubMed] [Google Scholar]
- 48.Barrett CW, Singh K, Motley AK, Lintel MK, Matafonova E, Bradley AM, Ning W, Poindexter SV, Parang B, Reddy VK, Chaturvedi R, Fingleton BM, Washington MK, Wilson KT, Davies SS, Hill KE, Burk RF, Williams CS. Dietary selenium deficiency exacerbates DSS-induced epithelial injury and AOM/DSS-induced tumorigenesis. PLoS One. 2013;8:e67845. doi: 10.1371/journal.pone.0067845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moustafa ME, Carlson BA, El-Saadani MA, Kryukov GV, Sun QA, Harney JW, Hill KE, Combs GF, Feigenbaum L, Mansur DB, Burk RF, Berry MJ, Diamond AM, Lee BJ, Gladyshev VN, Hatfield DL. Selective inhibition of selenocysteine tRNA maturation and selenoprotein synthesis in transgenic mice expressing isopentenyladenosine-deficient selenocysteine tRNA. Mol Cell Biol. 2001;21:3840–3852. doi: 10.1128/MCB.21.11.3840-3852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Irons R, Carlson BA, Hatfield DL, Davis CD. Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J Nutr. 2006;136:1311–1317. doi: 10.1093/jn/136.5.1311. [DOI] [PubMed] [Google Scholar]
- 51.Diwadkar-Navsariwala V, Prins GS, Swanson SM, Birch LA, Ray VH, Hedayat S, Lantvit DL, Diamond AM. Selenoprotein deficiency accelerates prostate carcinogenesis in a transgenic model. Proc Natl Acad Sci USA. 2006;103:8179–8184. doi: 10.1073/pnas.0508218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moustafa ME, Carlson BA, Anver MR, Bobe G, Zhong N, Ward JM, Perella CM, Hoffmann VJ, Rogers K, Combs GF, Jr, Schweizer U, Merlino G, Gladyshev VN, Hatfield DL. Selenium and selenoprotein deficiencies induce widespread pyogranuloma formation in mice, while high levels of dietary selenium decrease liver tumor size driven by TGFalpha. PLoS One. 2013;8:e57389. doi: 10.1371/journal.pone.0057389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bosl MR, Takaku K, Oshima M, Nishimura S, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp) Proc Natl Acad Sci USA. 1997;94:5531–5534. doi: 10.1073/pnas.94.11.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumaraswamy E, Carlson BA, Morgan F, Miyoshi K, Robinson GW, Su D, Wang S, Southon E, Tessarollo L, Lee BJ, Gladyshev VN, Hennighausen L, Hatfield DL. Selective removal of the selenocysteine tRNA [Ser]Sec gene (Trsp) in mouse mammary epithelium. Mol Cell Biol. 2003;23:1477–1488. doi: 10.1128/MCB.23.5.1477-1488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shrimali RK, Weaver JA, Miller GF, Starost MF, Carlson BA, Novoselov SV, Kumaraswamy E, Gladyshev VN, Hatfield DL. Selenoprotein expression is essential in endothelial cell development and cardiac muscle function. Neuromuscul Disord. 2007;17:135–142. doi: 10.1016/j.nmd.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlson BA, Xu XM, Gladyshev VN, Hatfield DL. Selective rescue of selenoprotein expression in mice lacking a highly specialized methyl group in selenocysteine tRNA. J Biol Chem. 2005;280:5542–5548. doi: 10.1074/jbc.M411725200. [DOI] [PubMed] [Google Scholar]
- 57.Sengupta A, Lichti UF, Carlson BA, Ryscavage AO, Gladyshev VN, Yuspa SH, Hatfield DL. Selenoproteins are essential for proper keratinocyte function and skin development. PLoS One. 2010;5:e12249. doi: 10.1371/journal.pone.0012249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16:705–743. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki T, Kelly VP, Motohashi H, Nakajima O, Takahashi S, Nishimura S, Yamamoto M. Deletion of the selenocysteine tRNA gene in macrophages and liver results in compensatory gene induction of cytoprotective enzymes by Nrf2. J Biol Chem. 2008;283:2021–2030. doi: 10.1074/jbc.M708352200. [DOI] [PubMed] [Google Scholar]
- 60.Kaushal N, Kudva AK, Patterson AD, Chiaro C, Kennett MJ, Desai D, Amin S, Carlson BA, Cantorna MT, Prabhu KS. Crucial role of macrophage selenoproteins in experimental colitis. J Immunol. 2014;193:3683–3692. doi: 10.4049/jimmunol.1400347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tham DM, Whitin JC, Cohen HJ. Increased expression of extracellular glutathione peroxidase in mice with dextran sodium sulfate-induced experimental colitis. Pediatr Res. 2002;51:641–646. doi: 10.1203/00006450-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 62.Hiller F, Besselt K, Deubel S, Brigelius-Flohe R, Kipp AP. GPx2 induction is mediated through STAT transcription factors during acute colitis. Inflamm Bowel Dis. 2015;21:2078–2089. doi: 10.1097/MIB.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 63.Te Velde AA, Pronk I, de Kort F, Stokkers PC. Glutathione peroxidase 2 and aquaporin 8 as new markers for colonic inflammation in experimental colitis and inflammatory bowel diseases: an important role for H2O2? Eur J Gastroenterol Hepatol. 2008;20:555–560. doi: 10.1097/MEG.0b013e3282f45751. [DOI] [PubMed] [Google Scholar]
- 64.Yant LJ, Ran Q, Rao L, Van Remmen H, Shibatani T, Belter JG, Motta L, Richardson A, Prolla TA. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496–502. doi: 10.1016/S0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 65.Esworthy RS, Aranda R, Martin MG, Doroshow JH, Binder SW, Chu FF. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G848–G855. doi: 10.1152/ajpgi.2001.281.3.G848. [DOI] [PubMed] [Google Scholar]
- 66.Esworthy RS, Kim BW, Chow J, Shen B, Doroshow JH, Chu FF. Nox1 causes ileocolitis in mice deficient in glutathione peroxidase-1 and -2. Free Radic Biol Med. 2014;68:315–325. doi: 10.1016/j.freeradbiomed.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esworthy RS, Kim BW, Wang Y, Gao Q, Doroshow JH, Leto TL, Chu FF. The Gdac1 locus modifies spontaneous and Salmonella-induced colitis in mice deficient in either Gpx2 or Gpx1 gene. Free Radic Biol Med. 2013;65:1273–1283. doi: 10.1016/j.freeradbiomed.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barrett CW, Ning W, Chen X, Smith JJ, Washington MK, Hill KE, Coburn LA, Peek RM, Chaturvedi R, Wilson KT, Burk RF, Williams CS. Tumor suppressor function of the plasma glutathione peroxidase gpx3 in colitis-associated carcinoma. Cancer Res. 2013;73:1245–1255. doi: 10.1158/0008-5472.CAN-12-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krehl S, Loewinger M, Florian S, Kipp AP, Banning A, Wessjohann LA, Brauer MN, Iori R, Esworthy RS, Chu FF, Brigelius-Flohe R. Glutathione peroxidase-2 and selenium decreased inflammation and tumors in a mouse model of inflammation-associated carcinogenesis whereas sulforaphane effects differed with selenium supply. Carcinogenesis. 2012;33:620–628. doi: 10.1093/carcin/bgr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill KE, Zhou J, Austin LM, Motley AK, Ham AJ, Olson GE, Atkins JF, Gesteland RF, Burk RF. The selenium-rich C-terminal domain of mouse selenoprotein P is necessary for the supply of selenium to brain and testis but not for the maintenance of whole body selenium. J Biol Chem. 2007;282:10972–10980. doi: 10.1074/jbc.M700436200. [DOI] [PubMed] [Google Scholar]
- 71.Olson GE, Winfrey VP, Nagdas SK, Hill KE, Burk RF. Apolipoprotein E receptor-2 (ApoER2) mediates selenium uptake from selenoprotein P by the mouse testis. J Biol Chem. 2007;282:12290–12297. doi: 10.1074/jbc.M611403200. [DOI] [PubMed] [Google Scholar]
- 72.Burk RF, Hill KE, Olson GE, Weeber EJ, Motley AK, Winfrey VP, Austin LM. Deletion of apolipoprotein E receptor-2 in mice lowers brain selenium and causes severe neurological dysfunction and death when a low-selenium diet is fed. J Neurosci. 2007;27:6207–6211. doi: 10.1523/JNEUROSCI.1153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hill KE, Wu S, Motley AK, Stevenson TD, Winfrey VP, Capecchi MR, Atkins JF, Burk RF. Production of selenoprotein P (Sepp1) by hepatocytes is central to selenium homeostasis. J Biol Chem. 2012;287:40414–40424. doi: 10.1074/jbc.M112.421404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saito Y, Hayashi T, Tanaka A, Watanabe Y, Suzuki M, Saito E, Takahashi K. Selenoprotein P in human plasma as an extracellular phospholipid hydroperoxide glutathione peroxidase. Isolation and enzymatic characterization of human selenoprotein p. J Biol Chem. 1999;274:2866–2871. doi: 10.1074/jbc.274.5.2866. [DOI] [PubMed] [Google Scholar]
- 75.Kurokawa S, Eriksson S, Rose KL, Wu S, Motley AK, Hill S, Winfrey VP, McDonald WH, Capecchi MR, Atkins JF, Arner ES, Hill KE, Burk RF. Sepp1(UF) forms are N-terminal selenoprotein P truncations that have peroxidase activity when coupled with thioredoxin reductase-1. Free Radic Biol Med. 2014;69:67–76. doi: 10.1016/j.freeradbiomed.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Taie OH, Uceyler N, Eubner U, Jakob F, Mork H, Scheurlen M, Brigelius-Flohe R, Schottker K, Abel J, Thalheimer A, Katzenberger T, Illert B, Melcher R, Kohrle J. Expression profiling and genetic alterations of the selenoproteins GI-GPx and SePP in colorectal carcinogenesis. Nutr Cancer. 2004;48:6–14. doi: 10.1207/s15327914nc4801_2. [DOI] [PubMed] [Google Scholar]
- 77.Calvo A, Xiao N, Kang J, Best CJ, Leiva I, Emmert-Buck MR, Jorcyk C, Green JE. Alterations in gene expression profiles during prostate cancer progression: functional correlations to tumorigenicity and down-regulation of selenoprotein-P in mouse and human tumors. Cancer Res. 2002;62:5325–5335. [PubMed] [Google Scholar]
- 78.Steinbrecher A, Meplan C, Hesketh J, Schomburg L, Endermann T, Jansen E, Akesson B, Rohrmann S, Linseisen J. Effects of selenium status and polymorphisms in selenoprotein genes on prostate cancer risk in a prospective study of European men. Cancer Epidemiol Biomark Prev. 2010;19:2958–2968. doi: 10.1158/1055-9965.EPI-10-0364. [DOI] [PubMed] [Google Scholar]
- 79.Peters U, Chatterjee N, Hayes RB, Schoen RE, Wang Y, Chanock SJ, Foster CB. Variation in the selenoenzyme genes and risk of advanced distal colorectal adenoma. Cancer Epidemiol Biomark Prev. 2008;17:1144–1154. doi: 10.1158/1055-9965.EPI-07-2947. [DOI] [PubMed] [Google Scholar]
- 80.Al-Taie OH, Seufert J, Mork H, Treis H, Mentrup B, Thalheimer A, Starostik P, Abel J, Scheurlen M, Kohrle J, Jakob F. A complex DNA-repeat structure within the Selenoprotein P promoter contains a functionally relevant polymorphism and is genetically unstable under conditions of mismatch repair deficiency. Eur J Hum Genet. 2002;10:499–504. doi: 10.1038/sj.ejhg.5200811. [DOI] [PubMed] [Google Scholar]
- 81.Smith JJ, Deane NG, Wu F, Merchant NB, Zhang B, Jiang A, Lu P, Johnson JC, Schmidt C, Bailey CE, Eschrich S, Kis C, Levy S, Washington MK, Heslin MJ, Coffey RJ, Yeatman TJ, Shyr Y, Beauchamp RD. Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology. 2010;138:958–968. doi: 10.1053/j.gastro.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barrett CW, Reddy VK, Short SP, Motley AK, Lintel MK, Bradley AM, Freeman T, Vallance J, Ning W, Parang B, Poindexter SV, Fingleton B, Chen X, Washington MK, Wilson KT, Shroyer NF, Hill KE, Burk RF, Williams CS. Selenoprotein P influences colitis-induced tumorigenesis by mediating stemness and oxidative damage. J Clin Invest. 2015;125:2646–2660. doi: 10.1172/JCI76099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, Pasqualini F, Nebuloni M, Chiabrando C, Mantovani A, Allavena P. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 2010;185:642–652. doi: 10.4049/jimmunol.1000413. [DOI] [PubMed] [Google Scholar]
- 84.Dreher I, Jakobs TC, Kohrle J. Cloning and characterization of the human selenoprotein P promoter. Response of selenoprotein P expression to cytokines in liver cells. J Biol Chem. 1997;272:29364–29371. doi: 10.1074/jbc.272.46.29364. [DOI] [PubMed] [Google Scholar]
- 85.Mostert V, Dreher I, Kohrle J, Abel J. Transforming growth factor-beta1 inhibits expression of selenoprotein P in cultured human liver cells. FEBS Lett. 1999;460:23–26. doi: 10.1016/S0014-5793(99)01298-3. [DOI] [PubMed] [Google Scholar]
- 86.Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 87.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, Perret C, Shroyer NF, Romagnolo B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc Natl Acad Sci USA. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farin HF, Van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143(1518–1529):e1517. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 90.Clevers H. Wnt breakers in colon cancer. Cancer Cell. 2004;5:5–6. doi: 10.1016/S1535-6108(03)00339-8. [DOI] [PubMed] [Google Scholar]
- 91.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ojuawo A, Keith L. The serum concentrations of zinc, copper and selenium in children with inflammatory bowel disease. Cent Afr J Med. 2002;48:116–119. [PubMed] [Google Scholar]
- 93.Andoh A, Hirashima M, Maeda H, Hata K, Inatomi O, Tsujikawa T, Sasaki M, Takahashi K, Fujiyama Y. Serum selenoprotein-P levels in patients with inflammatory bowel disease. Nutrition. 2005;21:574–579. doi: 10.1016/j.nut.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 94.Cooper ML, Adami HO, Gronberg H, Wiklund F, Green FR, Rayman MP. Interaction between single nucleotide polymorphisms in selenoprotein P and mitochondrial superoxide dismutase determines prostate cancer risk. Cancer Res. 2008;68:10171–10177. doi: 10.1158/0008-5472.CAN-08-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]