Abstract
Controlling the spread of carcinoma cells to distant organs is the foremost challenge in cancer treatment, as metastatic disease is generally resistant to therapy and is ultimately incurable for the majority of patients. The plasticity of tumor cell phenotype, in which the behaviors and functions of individual tumor cells differ markedly depending upon intrinsic and extrinsic factors, is now known to be a central mechanism in cancer progression. Our expanding knowledge of epithelial and mesenchymal phenotypic states in tumor cells, and the dynamic nature of the transitions between these phenotypes has created new opportunities to intervene to better control the behavior of tumor cells. There are now a variety of innovative pharmacological approaches to preferentially target tumor cells that have acquired mesenchymal features, including cytotoxic agents that directly kill these cells, and inhibitors that block or revert the process of mesenchymalization. Furthermore, novel immunological strategies have been developed to engage the immune system in seeking out and destroying mesenchymalized tumor cells. This review highlights the relevance of phenotypic plasticity in tumor biology, and discusses recently developed pharmacological and immunological means of targeting this phenomenon.
Keywords: epithelial-mesenchymal transition, brachyury, cancer vaccine, stemness
Introduction
The spread of cancer to distant organs represents the foremost challenge in cancer treatment. Whereas localized disease can often be treated with surgery and chemotherapy or radiation, metastatic disease is generally not amenable to surgery, is resistant to chemotherapy and radiation, and is ultimately incurable for the majority of patients. The use of small-molecule inhibitors that target specific cancer molecular alterations (such as EGFR and BRAF inhibitors) or impede tumor angiogenesis (such as VEGFR inhibitors) can succeed in enhancing quality of life and delaying progression, but even these sophisticated treatments eventually become ineffective due to the development of acquired resistance. The current wave of new immunologically targeted therapies (such as anti-PD-1/PD-L1 antibodies) has raised hope for the successful treatment of metastatic disease (Borghaei et al., 2015; Garon et al., 2015; Powles et al., 2014; Ribas et al., 2016), yet even these approaches have so far proven effective for only a proportion of patients. While significantly more research and development in tumor immunotherapy will undoubtedly lead to further breakthroughs in cancer treatment, another novel approach is quietly emerging that endeavors to complement these treatment strategies by targeting the mechanisms underlying tumor progression to metastatic disease.
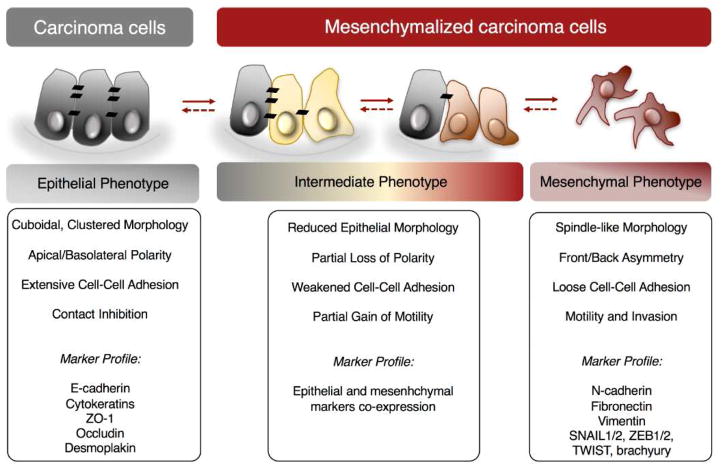
One mechanism that is increasingly being recognized as central to the progression of carcinomas is the phenomenon of tumor cell phenotypic plasticity, exemplified by the observation that the bulk of a tumor mass can be phenotypically and functionally distinct from its invasive front (Brabletz et al., 2001). This phenotypic plasticity of tumor cells often occurs in response to local conditions in the tumor microenvironment, such as hypoxia or inflammation, that lead to the activation of a molecular program designated as the epithelial-mesenchymal transition (EMT), or the reverse phenomenon known as the mesenchymal-epithelial transition (MET) (Kalluri & Weinberg, 2009; Nieto & Cano, 2012; Thiery et al., 2009). During EMT, epithelial cells lose polarity and the expression of molecules distinctive of epithelial status, including cytokeratins, E-cadherin, and several other molecules involved in cell-to-cell adhesion (Figure 1). The cells in turn gain motility, the ability to invade the basement membrane and surrounding tissues, and the expression of mesenchymal molecules, including vimentin, N-cadherin, and a set of transcriptional regulators that include the zinc finger proteins SNAI1/2, the zinc-finger homeodomain proteins ZEB1/2, the helix-loop-helix transcription factors TWIST1/2, the T-box protein brachyury, and others (Bolos et al., 2003; Cano et al., 2000; Eger et al., 2005; Fernando et al., 2010; Yang et al., 2004). These transcriptional regulators, called EMT-TFs, are ultimately responsible for orchestrating the phenotypic changes that take place during an EMT via downregulation and upregulation of epithelial- and mesenchymal-associated genes, respectively (Figure 1). The EMT and MET differentiation programs occur extensively during normal embryonic development, and are also recapitulated in adult tissues during wound healing and fibrosis (Lim & Thiery, 2012). Evidence has now been found for the occurrence of these phenotypic transitions in tumors during the metastatic process, whereby EMT may facilitate the invasion of tumor cells through the basement membrane, their migration to blood and lymphatic vessels, and their extravasation into metastatic sites (Rhim et al., 2012; Tsai et al., 2012), while MET might allow the proliferation of tumor cells at the distant site (Chaffer et al., 2006; Tsai et al., 2012).
Figure 1. Tumor plasticity generates a range of phenotypes in carcinoma cells.
Phenotype characteristics and markers typically expressed are indicated below each phenotype. Arrows indicate the reversibility of the phenomenon.
Realization of the contributions of EMT to cancer pathophysiology has driven the development of novel therapeutic strategies that specifically target tumor cells undergoing this phenomenon. The aims of such strategies are to eliminate mesenchymalized tumor cells in patients that have more advanced disease, or to prevent the development of metastatic disease in high-risk patients with localized tumors. Most of these approaches can be classified as pharmacological and antibody-based targeting of the EMT process, but a novel immunotherapy targeting EMT has also been developed in recent years. In this review, we present a brief survey of the relevance of EMT in tumor biology, and then discuss recently developed pharmacological and immunological means of targeting this phenomenon.
Tumor Cell EMT versus Mesenchymalization
As noted above, the phenomenon of EMT was initially described as a developmental process characterized by the conversion of epithelial cells into highly migratory, invasive mesenchymal cells. During development, EMT allows for the remodeling of various embryonic tissues, including the emergence of the mesoderm from epithelial layers during gastrulation (Hay, 1995, 2005). Well-characterized in the context of embryonic development is also the reverse process named MET, which contributes to the formation of epithelia from mesenchymal cells (Kim et al., 2016). While the epithelial and mesenchymal cell states are well-defined entities in the context of development, in the particular case of carcinomas, however, cellular phenotype is akin to a spectrum, in which intermediate phenotypes comprising characteristics of both epithelial and mesenchymal cell types are apparent (Figure 1). For example, the existence of an intermediate phenotypic status characterized by mixed epithelial and mesenchymal features has been observed with renal carcinoma cells expressing the transcription factor WT1 (Sampson et al., 2014). Further evidence of intermediate phenotypes was demonstrated in lung adenocarcinomas; several tumor cell lines were shown to co-express both epithelial E-cadherin and mesenchymal vimentin, and this hybrid protein signature was also observed in vivo in a proportion (~20%) of lung adenocarcinoma tissues (Schliekelman et al., 2015). Similarly, an intermediate tumor phenotype gene signature that was established in ovarian carcinoma cell lines was also detectable in ovarian cancer tissues, and it correlated with worse clinical outcome when compared to tumors with a defined epithelial or mesenchymal phenotype (Huang et al., 2013b).
One of the challenges in the field of EMT has been the inability to document the occurrence of tumor phenotypic transitions in human tumor tissues, a fact that has originated controversy around the significance of this phenomenon in vivo (Garber, 2008; Tarin et al., 2005). One reason for this limitation is the lack of definitive markers that can effectively discriminate cancer cells that have acquired mesenchymal markers from the normal surrounding mesenchymal stroma cells. The analysis of circulating tumor cells (CTCs) in the peripheral blood of cancer patients has circumvented this problem by evaluating phenotypic traits in isolated tumor cells (Yu et al., 2013). For example, a study conducted with metastatic breast cancer patients measured the expression of transcripts encoding for several epithelial (E-cadherin, keratins, EpCAM) and mesenchymal (fibronectin, N-cadherin) markers in CTCs via in-situ hybridization (Yu et al., 2013). A range of CTC phenotypes were readily detected in most patients, including CTCs with exclusively epithelial or mesenchymal features. More importantly, subgroups characterized by various degrees of co-expression of epithelial and mesenchymal markers were also readily detectable in circulation. In another study, phenotyping of CTCs isolated via a filter-based method from the blood of patients with various tumor types, including liver, colon, lung, and others, resulted in the identification of three subpopulations of CTCs named ‘epithelial,’ ‘epithelial/mesenchymal,’ and ‘mesenchymal,’ with the latter being predominantly enriched in patients with metastasis, as opposed to non-metastatic disease (Wu et al., 2015).
In light of these recent data, we opted to use the term “mesenchymalization” in this review to refer to the acquisition of mesenchymal features by formerly epithelial-like carcinoma cells. To reflect the range of intermediate phenotypes that are possible, we also stipulate that a “mesenchymalized” tumor cell either may, or may not, have relinquished its epithelial characteristics. It is our assertion that this terminology better captures the reality of phenotypic plasticity in vivo, and we refer to this concept throughout this review.
Tumor Mesenchymalization Underlies Metastasis, Stemness, and Resistance
Many lines of evidence indicate that carcinoma mesenchymalization underlies the most lethal aspects of cancer, chiefly metastatic dissemination, stemness-dependent colonization of foreign tissue sites, and resistance to therapeutic intervention. Clinical evidence suggesting an association between tumor phenotypic transitions and cancer progression includes the results of multiple correlative studies demonstrating a positive association between the expression of mesenchymal markers (such as vimentin or N-cadherin) and/or the loss of epithelial E-cadherin in clinical samples with poor prognosis (Gravdal et al., 2007; Tan et al., 2014). Similarly, aberrant expression of transcriptional drivers of the mesenchymal phenotype, including SNAI1, SNAI2, brachyury, and others have been positively correlated with poor clinical outcome in a range of human tumor types (Blanco et al., 2002; Elloul et al., 2005; Gravdal et al., 2007; Hamilton et al., 2016b; Palena et al., 2014; Pinto et al., 2014). Direct evidence for a role of this phenomenon in tumor metastasis, however, originates from murine models of cancer. For example, Rhim and colleagues performed epithelial lineage tracing in a murine model of pancreatic cancer to distinguish cancer cells that retained an epithelial phenotype from cells that had acquired mesenchymal characteristics (Rhim et al., 2012). The authors found that epithelial-tagged cells that invaded and entered the bloodstream co-expressed the mesenchymal proteins ZEB1 or fibroblast-specific protein-1 (FSP-1) and/or lacked expression of epithelial E-cadherin, thus indicating that in vivo mesenchymalization took place and was associated with enhanced cellular invasion and progression.
Other studies, however, have more recently suggested that tumor mesenchymalization is dispensable for tumor dissemination; tumor cells with a deletion of SNAI1 or TWIST1 in a pancreatic tumor model were nevertheless able to effectively metastasize (Zheng et al., 2015), and blockade of mesenchymalization via overexpression of microRNA-200 in a murine model of breast cancer had no effect on lung metastasis development (Fischer et al., 2015), thereby disputing the idea that mesenchymal features are absolutely required for metastasis. Another point of contention is that, as mentioned previously, mesenchymalization may contribute to the invasion and dissemination of tumor cells, but the formation of actively growing macrometastases may depend upon an MET-like process of re-epithelialization. This would imply that dissemination and proliferation are mutually exclusive properties. In this case, metastatic competence would be governed by the ability of tumor cells to undergo dynamic and transient interconversions between phenotypes, as was suggested by a recent report in which an embryonic gene signature representative of extensive cellular plasticity was found to predict distant metastasis in breast cancer (Soundararajan et al., 2015). Still others have proposed that mesenchymalized tumor cells may successfully form macrometastases without phenotypic transition provided they have accumulated genetic alterations to uncouple invasiveness from growth suppression (Brabletz, 2012). Further research will be needed to gain a fuller understanding of these issues.
Aside from metastasis, mesenchymalization is undoubtedly associated with the acquisition of a stem cell-like phenotype which grants tumor cells the ability to initiate and propagate the disease. These two abilities allow tumor cells that have acquired mesenchymal features to survive therapy and repopulate the tumor mass, resulting in clinical recurrence with treatment-insensitive disease. The link between the acquisition of mesenchymal properties and the concomitant gain of features normally attributed to stem cells (i.e., tumor stemness) was initially demonstrated with human mammary epithelial cells (HMLEs) induced into a mesenchymal-like phenotype via overexpression of TWIST1 or SNAI1, which resulted in greater proportion of cells bearing markers associated with stem-like cells (CD44high/CD24low) (Mani et al., 2008). This association was then demonstrated with breast cancer patient tumors obtained after recurrence following treatment with conventional therapies, in which a higher proportion of CD44high/CD24low cells with mesenchymal features was found (Creighton et al., 2009).
Perhaps the most relevant consequence of a tumor cell’s transition from an epithelial to a mesenchymal-like phenotype is the resulting gain of mechanisms of resistance to a variety of cell death-inducing signals. For instance, although metastatic dissemination was not reduced in the aforementioned studies, suppression of tumor mesenchymalization in pancreatic cancer and breast cancer was reported to significantly enhance susceptibility to the cytotoxic activities of gemcitabine and cyclophosphamide, respectively (Fischer et al., 2015; Zheng et al., 2015). Indeed, multiple studies have now documented both in vitro and in vivo with animal models, the acquisition of resistance to a range of chemotherapeutics, including paclitaxel, oxaliplatin, gemcitabine, docetaxel, and others, as well as resistance to radiation by carcinoma cells transitioned into the mesenchymal phenotype (Fischer et al., 2015; Huang et al., 2013a; Kajiyama et al., 2007; Kurrey et al., 2009; Larocca et al., 2013; Shah et al., 2007; Zheng et al., 2015). The mechanisms involved in this resistance have been studied in detail and include 1) a marked inhibition of cell cycle progression in mesenchymalized carcinoma cells due to the modulation of cell cycle-associated proteins, including p21 and Cyclin D1, among others (Huang et al., 2013a; Larocca et al., 2013; Mejlvang et al., 2007; Vega et al., 2004); 2) the blockade of DNA damage responses (Zhang et al., 2015); and 3) the upregulation of drug efflux pumps of the family of ATP-biding cassette (ABC) transporters in mesenchymal-like tumor cells (Saxena et al., 2011).
In addition to inducing resistance to conventional therapeutics, tumor cells undergoing a transition towards the mesenchymal phenotype have also been shown to exhibit resistance to a variety of targeted therapies, including resistance to epidermal growth factor receptor (EGFR) kinase inhibitors (Byers et al., 2013; Thomson et al., 2005; Thomson et al., 2008), HER2-directed therapy (Kim et al., 2014), hormonal therapy (Hiscox et al., 2006), and others (Richard et al., 2016). Although the molecular mechanisms of resistance induced via the acquisition of mesenchymal features in response to each of these targeted therapies may vary, overall, resistance has always been associated with the activation of compensatory signaling pathways which might be able to sustain the growth and survival of the resistant cells even in the presence of the targeted drug. Additional evidence to link therapy-resistance with tumor plasticity was documented in studies conducted with the blood of patients pre- vs. post-therapy, in which increased numbers of mesenchymal CTCs were associated with progressive disease, while patients who responded to therapy exhibited a decrease in the number of CTCs and/or a proportional decrease in mesenchymal versus epithelial CTCs (Yu et al., 2013).
Direct Targeting of Mesenchymalized Tumor Cells
Given the crucial roles that phenotype transitions play in many aspects of tumor progression, an attractive therapeutic approach is to directly target the subpopulation of mesenchymalized tumor cells with agents that exhibit preferential toxicity toward these cells as opposed to the more epithelial cells that comprise the bulk of the tumor. The drugs referenced below were specifically developed for targeting CSCs, but may be applicable to tumor cells with a mesenchymal phenotype more generally (Table 1). Perhaps the first agent to be identified with such properties is the polyether ionophore salinomycin, which was identified by Gupta and colleagues in a high-throughput screen of 16,000 compounds using isogenic HMLE breast cells that had been engineered to undergo a transition towards the mesenchymal-like phenotype via shRNA-mediated knockdown of the E-cadherin gene (Gupta et al., 2009). In this study, salinomycin was reported to reduce the proportion of CSCs by greater than 100-fold relative to the chemotherapy drug paclitaxel, and such preferential killing of drug-resistant and stem-like tumor cells has subsequently been shown in several malignancies (reviewed in (Naujokat & Steinhart, 2012)). Other ionophores have also been reported to impact cells with mesenchymal attributes; the polyether ionophores nigericin (Gupta et al., 2009) and monensin (Ketola et al., 2012; Shaik et al., 2004) have been reported to selectively kill stem-like tumor cells or to reduce drug-resistance and stemness markers, respectively, and the channel-forming ionophore gramicidin A was shown to inhibit hypoxia-inducible factor (HIF) (David et al., 2014), a well-known driver of mesenchymalization in solid tumors (Haase, 2009).
Table 1.
Pharmacological and immunological agents with the potential to target mesenchymalization in solid tumors.
| Agent | Target | Stage | Current Clinical Trials in Solid Tumors | Clinical Trial Indication |
|---|---|---|---|---|
| Ionophores | ||||
| 1. Salinomycin | Broad | Preclinical | ||
| 2. Nigericin | Broad | Preclinical | ||
| 3. Monensin | Broad | Preclinical | ||
| 4. Gramicidin A | HIF, Broad | Preclinical | ||
|
| ||||
| Small Molecules | ||||
| 1. ML239 | Nf-κB, undefined | Preclinical | ||
| 2. Cinnamides | Undefined | Preclinical | ||
|
| ||||
| Inhibitors | ||||
| 1. Midostaurin (PKC412) | SYK, AURKA, broad | Phase I | NCT01282502 | Locally advanced rectal cancer |
| 2. VS-5584 | PI3K, mTOR | Phase I | NCT01991938 | Non-hematologic cancers, metastatic cancer, lymphoma |
| 3. TEW-7197 | TGFβRI/ALK5 | Phase I | NCT02160106 | Advanced stage solid tumors |
| 4. Galunisertib (LY2157299) | TGFβRI/ALK5 | Phase I/II/III | NCT02906397, NCT02672475, NCT02734160, NCT02423343, NCT02688712, NCT02452008 | Hepatocellular carcinoma, triple-negative breast cancer, solid tumors, non-small cell lung cancer, glioblastoma, rectal cancer, castration-resistant prostate cancer |
| 5. LGK974 | Porcupine | Phase I | NCT01351103 | Pancreatic adenocarcinoma, BRAF-mutant colorectal cancer, other tumor types |
| 6. PRI-724 | β-catenin-CBP binding | Phase I/II | NCT01606579, NCT02413853 | Advanced myeloid malignancies, metastatic colorectal cancer |
| 7. Reparixin | CXCR1, 2 | Phase II | NCT02370238 | Metastatic breast cancer |
| 8. Fulvestrant | ER | FDA-approved | Multiple | Hormone-receptor positive breast cancer |
| 9. Vorinostat | Class I HDAC | FDA-approved | Multiple | Multiple |
|
| ||||
| Antibodies | ||||
| 1. Fresolimumab (GC1008) | TGF-β1, 2, 3 | Phase I/II | NCT02581787, NCT01401062 | Early-stage non-small cell lung cancer, metastatic breast cancer |
| 2. Vantictumab (OMP-18R5) | Fzd1, 2, 5, 7, 8 | Phase I | NCT01973309, NCT02005315, NCT01957007 | Metastatic breast cancer, pancreatic cancer, non-small cell lung cancer |
| 3. Siltuximab | IL-6 | FDA-approved | ||
| 4. Tocilizumab | IL-6R | FDA-approved | NCT02767557 | Pancreatic carcinoma |
| 6. HuMax-IL8 | IL-8 | Phase I | NCT02536469 | Solid Tumors |
|
| ||||
| Fusion Proteins | ||||
| 1. OMP-54F28 | Wnt ligands | Phase I | NCT02050178, NCT02069145, NCT02092363 | Pancreatic cancer, hepatocellular cancer, ovarian cancer |
|
| ||||
| Antisense Oligonucleotides | ||||
| 1. Trabedersen (AP- 12009) | TGF-β2 | Phase I/II | ||
|
| ||||
| Immunotherapies | ||||
| 1. MVA-Brachyury-TRICOM | Brachyury vaccine | Phase I | NCT02179515 | Solid tumors |
| 2. Yeast-Brachyury (GI-6301) | Brachyury vaccine | Phase I/II | NCT01519817, NCT02383498 | Solid tumors, chordoma |
| 3. Ad5-Brachyury | Brachyury vaccine | Preclinical | ||
| 4. Pembrolizumab | PD-1 | FDA-approved | Multiple | Multiple |
| 5. Nivolumab | PD-1 | FDA-approved | Multiple | Multiple |
| 6. Atezolizumab | PD-L1 | FDA-approved | Multiple | Multiple |
What could account for the differential sensitivity of mesenchymal versus epithelial tumor cells to ionophore drugs? One possibility is that mesenchymalized tumor cells in general may be more susceptible to disruptions in cellular cation homeostasis due to intrinsic deficits in ion transport function. Indeed, EMT has been shown to increase the concentration of intracellular sodium and decrease the expression of a gene that regulates sodium transport (Espineda et al., 2004; Rajasekaran et al., 2010), while alterations in potassium and pH regulation are known to play a role in cancer cell resistance to apoptosis (Pedersen et al., 2013). Other potential mechanisms that could contribute to the selectivity of these drugs for mesenchymalized tumor cells include inhibition of the TGF-β pathway (Zhang et al., 2016), inhibition of the Wnt/β-catenin pathway (Lu et al., 2011; Tumova et al., 2014), inhibition of drug efflux transporters (Riccioni et al., 2010), induction of ER stress (Yoon et al., 2013), and disruption of mitochondrial function (Mitani et al., 1976). However, it must be noted that these agents can also induce significant toxic side-effects in vivo, including neurotoxicity (Boehmerle & Endres, 2011). The clinical feasibility of ionophore drugs will therefore likely require the development of novel preventative measures (Boehmerle et al., 2014) or drug-delivery approaches (Zhao et al., 2016) to eliminate toxicity to normal tissues.
Other research groups have also used similar high-throughput screening (HTS) strategies to identify CSC-selective cytotoxic agents. Using the same HMLE cell line system employed by Gupta and colleagues (Gupta et al., 2009), a much larger screen of over 300,000 compounds was conducted in which a compound derived from an acyl hydrazone scaffold termed ML239 was found to be 23-fold more cytotoxic to CSC-like cells (Carmody et al., 2012). Mechanistic analyses indicated that ML239 may regulate NF-κB signaling, although the specific molecular target was not elucidated (Carmody et al., 2012). Selective cytotoxicity was also reported for cinnamide derivatives in this screen (Germain et al., 2013).
HTS was also used to identify drugs to target triple-negative breast cancer (TNBC), a subtype of breast cancer that lacks expression of the estrogen receptor, the progesterone receptor, and the HER2/NEU/ERBB2 oncogene, and that often exhibits mesenchymal features (Prat et al., 2010). Using an isogenic MCF10A cell line model with ectopic expression of the transcription factor TWIST, a driver of mesenchymalization, a library of 20,000 chemicals was screened and identified midostaurin (PKC412) as a selectively toxic compound in mesenchymalized tumor cells (Muellner et al., 2015). Midostaurin is a derivative of the natural alkaloid staurosporine, a broad-spectrum kinase inhibitor, and it is being investigated in several clinical trials for leukemia and a phase I trial in combination with radiation and chemotherapy for advanced rectal cancer (NCT01282502). Additional studies with midostaurin demonstrated selective cytotoxicity in other tumor models of mesenchymalization, including in cells treated with TGF-β, in mesenchymal-like tumor xenograft explants, in TNBC cell lines, and in a TNBC patient-derived xenograft (Muellner et al., 2015). Target analysis revealed that midostaurin inhibits both Aurora kinase A (AURKA), a target previously implicated in basal-like breast cancer (Staff et al., 2010), and spleen tyrosine kinase (SYK), a critical mediator of immune receptor signaling (Turner et al., 2000) that was previously unrecognized in TNBC pathology. SYK inhibition was found to abrogate signaling by STAT3, a pathway known to be associated with CSC features in breast cancer (Marotta et al., 2011), and also synergized with inhibition of AURKA, showing that the multiple targets of midostaurin can be targeted in combination to kill mesenchymal, basal-like breast tumor cells (Muellner et al., 2015).
Other investigations also suggest that mesenchymalized tumors share molecular vulnerabilities that can be targeted with multi-kinase inhibitors. The phosphatidylinositol-3-kinase/Akt/mechanistic target of rapamycin (PI3K/Akt/mTOR) signaling pathway is a vital pathway in tumor development that governs proliferation, survival, metabolism, angiogenesis (Engelman, 2009; Porta et al., 2014), and even EMT and stemness (Xia & Xu, 2015). VS-5584 is a potent dual kinase inhibitor that blocks all four class I isoforms of PI3K along with mTOR complexes 1 and 2 (mTORC1/2). This drug was found to be up to 30-fold more effective at inhibiting CSC proliferation and survival in vitro compared to the non-CSC population in breast tumor cells (Kolev et al., 2015). VS-5584 also preferentially abolished CSCs in vivo in breast tumor cell line xenografts, and diminished CSCs in ex vivo human tumor explants (Kolev et al., 2015). Importantly, VS-5584 was found to potentiate the effects of chemotherapy in vivo by significantly delaying tumor regrowth following treatment with cisplatin (Kolev et al., 2015), thereby validating the notion that selective targeting of CSCs combined with conventional tumor debulking treatments can more effectively delay or prevent tumor relapse. VS-5584 is currently being evaluated in phase I clinical trials (NCT01991938).
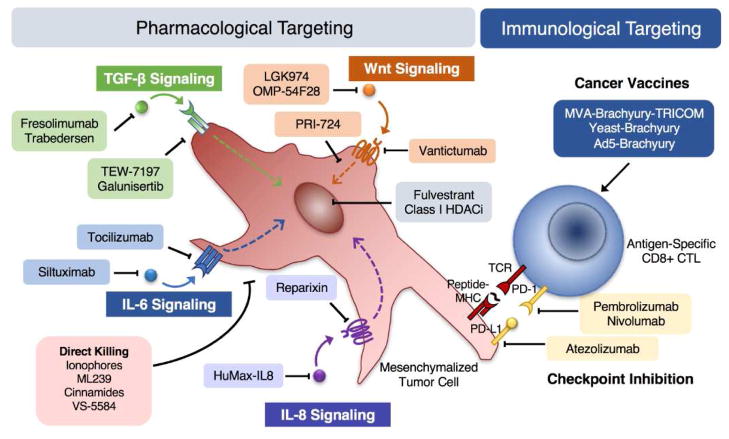
Altogether, these studies support the notion that targeting a cell phenotypic state, as opposed to specific oncogenic mutations, is a therapeutically feasible strategy (Figure 2). Furthermore, the studies of multi-kinase inhibition indicate that selective killing of mesenchymalized tumor cells may require the simultaneous targeting of multiple targets in order to shut down signaling pathway redundancies that can compensate for single target inhibition.
Figure 2. Pharmacological and immunological agents with the potential to target mesenchymalization in solid tumors.
Selected agents used for signaling pathway inhibition or for targeting mesenchymalized carcinoma cells via direct or immune-mediated killing.
Blocking and Reverting Tumor Mesenchymalization
The acquisition of mesenchymal features involves the activation of specific signaling events that ultimately control the expression of EMT-TFs. Mesenchymalization-inducing signaling can be the result of cell-intrinsic and irreversible mechanisms, such as oncogenic activation, or it may be induced by extrinsic factors that arise from the many complex interactions between tumor cells, host cells, and the tumor microenvironment (Savagner, 2015). Furthermore, epigenetic mechanisms are known to play a key role in controlling cancer cell phenotypic plasticity (Wang & Shang, 2013). Because this plasticity is reversible in principle, targeting the various signaling pathways that promote it can be used to block phenotypic transition, or to restore epithelial-like differentiation in tumor cells that have already undergone mesenchymalization.
Although many signaling pathways have a demonstrated role in the mesenchymalization of carcinomas (Pattabiraman & Weinberg, 2014), this review aims to cover only selected TGF-β and Wnt pathway-targeting agents that have progressed into clinical evaluation (Table 1). Additionally, the previously underappreciated role for interleukins in promoting mesenchymalization of cancer, along with associated therapeutic strategies, is discussed. Finally, recent applications of HTS technology to identify novel modulators of tumor cell phenotype is highlighted.
Targeting the TGF-β Signaling Pathway
One of the best-described signaling pathways that induces mesenchymalization is the TGF-β pathway, which mediates developmental EMT during induction of the mesendoderm lineage, neural crest migration, heart valve formation, and palate fusion (Derynck & Akhurst, 2007). TGF-β exerts similar phenotypic transitions in carcinomas; TGF-β signaling is well-known to upregulate the expression of several EMT-TFs (Larocca et al., 2013; Shirakihara et al., 2007; Thuault et al., 2006), and it plays a vital role in cancer stemness and therapy resistance (Bhola et al., 2013; Larocca et al., 2013; Mani et al., 2008). In human tumors, the cells with mesenchymal-like features are often located at the invasive front of tumors, and this region is known to be rich in stromal TGF-β (Massague, 2008). Tumors benefit greatly from the TGF-β-induced mesenchymalization process that promotes invasion and migration, angiogenesis, apoptosis-resistance, and immunoevasion (Massague, 2008; Padua & Massague, 2009). Indeed, increased TGF-β expression is correlated with tumor progression in lung, colorectal, prostate, and gastric cancers (Padua et al., 2009). However, TGF-β signaling paradoxically suppresses tumor growth during the early stages of malignancy, and this suppressive effect must be disabled for tumors to unleash the tumor-promoting potential of TGF-β signaling.
TGF-β is the prototypic member of a gene superfamily that consists of over 40 factors divided into various subfamilies, including bone morphogenetic proteins, activins, and inhibins (Lebrun, 2012). The TGF-β subfamily consists of three distinct ligands (TGF-β1, -β2, and -β3) that mediate canonical and non-canonical signaling based upon the dependency for Smad activation. Canonical TGF-β signaling occurs when TGF-β ligands that have been cleaved into their active form bind and activate a heterotetrameric receptor complex composed of two TGF-β type 1 (TGFβRI/ALK-5) and two TGF-β type 2 (TGFβRII) receptors, which in turn phosphorylates Smads 2 and 3. These phosphorylated Smads then form a complex with Smad4, translocate into the nucleus, and mediate the activation of transcriptional targets that suppress epithelial traits while promoting mesenchymal properties. Non-canonical TGF-β signaling occurs when activation of other signaling cascades (PI3K/Akt, Erk, JNK, p38, RhoA, etc.) takes place independent of Smad activation (Zhang, 2009).
Approaches to inhibiting the TGF-β pathway include small molecule inhibitors, antibodies, and antisense oligonucleotides (Figure 2). Several novel TGFβRI/ALK-5 inhibitors have been developed and investigated preclinically, but only two have progressed to clinical trials in carcinoma (Kothari et al., 2014). Galunisertib (LY2157299) is a TGFβRI/ALK-5 inhibitor that has been undergoing clinical development since 2006 (Herbertz et al., 2015). In preclinical studies, galunisertib was shown to delay tumor growth and confer a survival advantage in xenograft tumor models (Herbertz et al., 2015). Furthermore, it demonstrated the ability to prevent CSC-mediated tumor recurrence after treatment with paclitaxel in triple-negative breast cancer xenografts (Bhola et al., 2013), and it was shown to potentiate the antiproliferative and proapoptotic effects of sorafenib in human hepatocellular whole tumor tissue samples treated ex vivo (Serova et al., 2015). In order to reduce the severe cardiac toxicity seen with other TGFβRI/ALK-5 inhibitors, an optimized intermittent dosing regimen was developed in first-in-human studies in cancer patients (Rodon et al., 2015). Galunisertib is now undergoing clinical evaluation in numerous trials in combination with other agents; 1) phase I combination with radiotherapy for hepatocellular carcinoma (NCT02906397); 2) phase I combination with paclitaxel for triple-negative breast cancer (NCT02672475); 3) phase I combination with durvalumab (a novel anti-PD-L1 monoclonal antibody) for metastatic pancreatic cancer (NCT02734160); 4) phase I/II combination with nivolumab for solid tumors, non-small lung cancer, and glioblastoma (NCT02423343); 5) phase II combination with chemotherapy and radiation for rectal cancer (NCT02688712); 6) phase II combination with enzalutamide for castration-resistant prostate cancer (NCT02452008).
TEW-7197 is another TGFβRI/ALK-5 inhibitor that was shown to inhibit cell migration and invasion in vitro, and to block breast cancer metastasis to the lung in vivo (Son et al., 2014). This agent also enhanced the intratumoral infiltration and cytolytic potential of cytotoxic T lymphocytes, thereby indicating that TGF-β pathway blockade by TEW-7197 can augment antitumor immunity. TEW-7197 is now undergoing evaluation in a phase I dose-escalation study in advanced, refractory solid tumors (NCT02160106).
Antibody-based targeting of TGF-β ligands has been studied extensively in preclinical mouse models, and has been shown to diminish tumor growth, attenuate metastasis, augment antitumor immunity, and to synergize with chemotherapy and radiation (Lonning et al., 2011). Fresolimumab (GC1008) is a fully human IgG4 monoclonal antibody that neutralizes the active forms of human TGF-β1, -β2, and -β3. The safety of this agent was established in phase I trials for melanoma and renal cell carcinoma, in which multiple doses were generally well tolerated and no dose limiting toxicities reported (Morris et al., 2014). Preliminary evidence of efficacy in patients with melanoma was also reported, as one patient achieved a partial response and six patients experienced stable disease. Fresolimumab is now undergoing clinical trials in combination with radiotherapy for early stage non-small cell lung cancer (NCT02581787) and metastatic breast cancer (NCT01401062).
Trabedersen (AP-12009) is an antisense oligonucleotide targeting TGF-β2. It was tested in clinical trials in patients with glioma, and demonstrated an acceptable safety profile (Jaschinski et al., 2011). In preclinical studies, trabedersen was reported to reduce tumor growth, lymph node metastasis, and angiogenesis in orthotopic pancreatic tumor xenografts (Schlingensiepen et al., 2011).
Targeting the Wnt Signaling Pathway
The Wnt signaling pathway is a central regulatory pathway that is integral to numerous biological processes in both normal and malignant cells. This pathway has been associated with cancer since its initial discovery in which proviral insertion into the WNT1 gene locus induced tumor formation in a murine model of breast cancer (Nusse et al., 1984). Wnt pathway components are known to be mutated in many cancer types, most notably in colorectal tumors (Tai et al., 2015), and target genes for this pathway include regulators of proliferation (Myc, cyclin D1), survival (p-glycoprotein, survivin), migration (MMP7, MMP14), and angiogenesis (VEGF) (Herbst et al., 2014a). Similar to the TGF-β pathway, Wnt signaling is a known inducer of mesenchymalization during embryonic development (gastrulation, cardiac valve formation, mammary bud formation) and in cancer (Micalizzi et al., 2010; Zhan et al., 2016). Examples include the induction of SNAI1 and vimentin in breast cancer cells (Yook et al., 2006), activation of stem-like characteristics and metastasis in colon cancer (Brabletz et al., 2005; Zhan et al., 2016). These insights indicate that targeting Wnt signaling may be another means of interfering with tumor cell mesenchymalization (Figure 2).
Similar to the TGF-β pathway, the Wnt signaling cascade is divided into canonical and non-canonical pathways that reflect their involvement with the transcriptional coactivator β-catenin (outlined in (Zhan et al., 2016)). Briefly, in both types of pathways, signal initiation occurs when Wnt ligands glycoproteins bind to a family of G-protein coupled receptors known as Frizzled receptors (Fzd). There are 19 Wnts and 10 Fzds, thus highlighting to complexity of this pathway. During canonical signaling, the usual destruction of β-catenin is inhibited, and β-catenin translocates into the nucleus where it acts as a transcriptional coactivator with the TCF/LEF family of transcription factors to regulate the expression of target genes. Non-canonical Wnt signaling leads to the activation of Rho family GTPases that regulate the cytoskeleton, and activation of the stress kinase JNK, phospholipase C (PLC), intracellular calcium fluxes, activation of protein kinase C (PKC), and downstream modulation of transcriptional responses.
Numerous Wnt pathway inhibitors are undergoing development and preclinical testing, and some have progressed into clinical trials (Figure 2). LGK974 is an inhibitor of a membrane-bound O-acyltransferase known as Porcupine (gene name PORCN), which catalyzes the palmitoylation of Wnt ligand (Kadowaki et al., 1996; Willert et al., 2003). This posttranslational modification is crucial for Wnt secretion and interaction with Fzd receptors. In preclinical models, LGK974 was shown to inhibit Wnt signaling and reduce target gene expression in vitro and in vivo (Liu et al., 2013). Additionally, this drug induced strong anti-tumor effects in murine MMTV-Wnt1 tumors and in human HN30 and NSU1076 head and neck squamous cell carcinoma (HNSCC) xenografts (Liu et al., 2013). This agent is currently in phase I clinical trials for patients with various tumor types (NCT01351103).
OMP-54F28 is another inhibitor of Wnt ligand signaling. This agent is a recombinant fusion protein that combines the ligand-binding domain of Fzd7 with the human IgG1 Fc region. It acts as a soluble decoy receptor that competes with native Fzd8 to bind and sequester Wnt ligands. Similar to LGK974, OMP-54F28 was shown to block Wnt signaling and inhibit the growth of murine MMTV-Wnt1 tumors (Le et al., 2015). Furthermore, this agent reduced the growth of patient-derived xenografts (PDXs) of pancreatic tumor cancer, and showed additive anti-tumor effects with chemotherapy (Le et al., 2015). Importantly, OMP-54F28 exhibited the ability to reduce the frequency of CSCs and re-sensitize resistant tumors to chemotherapy, thereby demonstrating an ability to revert features associated with tumor mesenchymalization (Le et al., 2015). A phase 1 dose-escalation study in solid tumors was recently completed (NCT01608867), and no dose-limiting toxicities have been reported to date (Tai et al., 2015). Three phase 1b trials are now ongoing to test OMP-54F28 in combination with nab-paclitaxel and gemcitabine in pancreatic cancer (NCT02050178), with sorafenib in hepatocellular carcinoma (NCT02069145), and with paclitaxel and carboplatin in ovarian cancer (NCT02092363).
Inhibition of Wnt signaling via receptor inhibition is another strategy that is being explored. Vantictumab (OMP-18R5) is a fully human IgG2 monoclonal antibody that was initially found to bind the Fzd7 receptor to block Wnt ligand interaction. Subsequent analysis determined that it also binds Fzd1, 2, 5, and 8 (Gurney et al., 2012). Vantictumab demonstrated significant growth inhibition and synergy with several chemotherapeutic agents in preclinical studies of human PDXs from breast, colon, pancreas, lung tumors (Gurney et al., 2012). Furthermore, treatment was shown to reduce the expression of stemness-related genes in breast tumors (CD44, ALDH1A1, SOX1, SOX2), to restore mucinous differentiation in pancreatic tumors, and to reduce tumor-initiating cell frequency, thereby indicating the ability to revert mesenchymal features (Gurney et al., 2012). A phase 1 dose-escalation study in solid tumors was recently completed (NCT01345201) in which Wnt pathway inhibition was confirmed in pharmacodynamics studies of hair follicles, and prolonged stable disease was achieved in three patients with radiologically progressing neuroendocrine tumors (Tai et al., 2015). Three combination trials are now ongoing to test vantictumab with paclitaxel in breast cancer (NCT01973309), with nab-paclitaxel and gemcitabine in pancreatic cancer (NCT02005315), and with docetaxel in lung cancer (NCT01957007).
An alternative strategy to target the Wnt pathway is to interfere with β-catenin-dependent transcriptional regulation. PRI-724 is a small molecule inhibitor that prevents the binding of β-catenin with the transcriptional coactivator CBP (cAMP response-element binding [CREB] binding protein). Targeting this association reduces stemness and enhances differentiation (Lenz & Kahn, 2014; Tai et al., 2015). In a phase 1 dose-escalation testing in solid tumors (NCT01302405), PRI-724 was reported to have a very acceptable safety profile, and no maximum tolerated dose was identified (Tai et al., 2015). Although objective responses were not observed in the 18 patients that were evaluated, reduced β-catenin target gene expression circulating tumor cells was reported (Tai et al., 2015). PRI-724 is now undergoing clinical evaluation in myeloid malignancies (NCT01606579) and in combination with chemotherapy and the anti-VEGF antibody bevacizumab in colon cancer (NCT02413853).
Targeting the IL-6 and IL-8 Pathway
Cytokine expression in the tumor microenvironment can often have beneficial effects on tumor growth and progression. These factors are often secreted by infiltrating immune cells and other cell types found within the tumor microenvironment. The tumor cells themselves can often acquire the expression of certain cytokine receptors to gain the ability to respond to signaling by these molecules. Furthermore, tumor cells may also be capable of secreting numerous cytokines to exploit their chemoattractant abilities to engage and remodel the surrounding stroma. Several studies have now implicated key cytokines and chemokines as playing a major role in the acquisition of mesenchymal characteristics, particularly interleukin-6 (IL-6) and IL-8 (Palena et al., 2012).
IL-6 is a pleiotropic cytokine that performs many functions critical to innate and adaptive immunity (Hirano et al., 1986). The first report of IL-6 in cancer was seen in myeloma, in which IL-6-targeting antibodies reduced proliferation (Kawano et al., 1988). IL-6 is now recognized as one of the most ubiquitously expressed and dysregulated cytokines in cancer, with increased expression found in multiple cancers (Becker et al., 2004; Fulciniti et al., 2009; Okamoto et al., 1997; Qu et al., 2015; Salgado et al., 2003). This cytokine has also been shown to induce mesenchymal features in cancers of the cervix (Miao et al., 2014), prostate (Shiota et al., 2013), lung (Shintani et al., 2016), and head and neck (Yadav et al., 2011), among others. Sullivan and colleagues (Sullivan et al., 2009) showed that exposure to exogenous IL-6 repressed E-cadherin expression in breast cancer cells, and ectopic expression resulted in a mesenchymal-like phenotype and gene expression pattern both in vitro and in vivo. Furthermore, constitutive overexpression of the EMT-TF TWIST was found to induce the production of IL-6 and activate STAT3 signaling (Sullivan et al., 2009). In line with these findings, treatment of colorectal cancer cells with IL-6 markedly increased mesenchymal marker expression, reduced E-cadherin expression, and enhanced invasion. These effects were abrogated via knockdown of either the IL-6 receptor or STAT3 (Rokavec et al., 2014).
Inhibitors and antibodies that target IL-6 or its receptor are currently in clinical use for treating rheumatoid arthritis, Castleman disease, and inflammatory bowel disease (Kang et al., 2015). In particular, the IL-6-targeting antibody siltuximab and the IL-6 receptor-targeting antibody tocilizumab are FDA-approved agents that have advanced into clinical trials for cancer treatment (Figure 2). Siltuximab was recently evaluated as a monotherapy in a dose-escalation phase I/II study and was observed to be well tolerated in patients with advanced solid tumors, although no clinical benefit was observed (Angevin et al., 2014). It was also studied in combination with docetaxel in a phase I trial for patients with castration-resistant prostate cancer (Hudes et al., 2013). The combination therapy was well tolerated in patients, and preliminary evidence of efficacy was observed as 62% of patients treated with the combination achieved decreased PSA levels of 50% or greater. Tocilizumab was recently evaluated in combination with chemotherapy in a phase I trial for patients with epithelial ovarian cancer (Dijkgraaf et al., 2015). This trial demonstrated safety as well as potential immunological benefit, as changes indicative of enhanced anti-tumor immunity were reported. A phase II trial of tocilizumab in combination with nab-paclitaxel and gemcitabine for pancreatic cancer is currently underway (NCT02767557).
IL-8 is a chemotactic cytokine that stimulates the activation and migration of leukocytes in response to tissue injury and inflammation. The biological effects of IL-8 are mediated via binding with two G protein-coupled receptors, CXCR1(IL-8Rα) and CXCR2 (IL-8Rβ) (Holmes et al., 1991; Rollins, 1997). IL-8 signaling can potentially promote tumor growth by activating multiple signaling pathways, including PI3K/Akt/mTOR (MacManus et al., 2007), MAPK (Knall et al., 1996), FAK (Feniger-Barish et al., 2003), JAK/STAT (Fu et al., 2015), and elevated IL-8 expression has been implicated as a marker of poor prognosis, particularly in lung cancer (Pine et al., 2011) and breast cancer (Benoy et al., 2004). IL-8 has also been linked to mesenchymalization of carcinomas; enhanced expression of IL-8 and CXCR1, for example, was observed to occur after TGF-β treatment of colon carcinoma cells (Bates et al., 2004). Similarly, upregulation of the EMT-TF brachyury was shown to induce EMT along with IL-8 secretion, and neutralization of IL-8 reversed this phenotype (Fernando et al., 2011). Finally, resistance to EGFR inhibition in EGFR-mutant lung cancer cells was shown to enhance IL-8 secretion along with features of tumor mesenchymalization and stemness, and blockade of IL-8 was sufficient to revert these features and re-sensitize these cells to treatment with EGFR inhibitors (Fernando et al., 2016; Liu et al., 2015). Further evidence linking IL-8 and tumor mesenchymalization can be found in a recent review (David et al., 2016).
Therapeutic targeting of IL-8 signaling can be accomplished by IL-8-neutralizing antibodies or via small molecule inhibitors targeting CXCR1/2 (Figure 2). Monoclonal antibodies that target IL-8 include ABX-IL8 and HuMax-IL8. ABX-IL8 was evaluated in preclinical studies and was shown to decrease invasion through downregulation of MMP2 and MMP9 and to reduce the growth of urothelial tumors in vivo (Mian et al., 2003). Similar results were also seen in melanoma (Huang et al., 2002). HuMax-IL8 has been studied clinically in the inflammatory disease palmoplantar pustulosis, and it demonstrated safety along with effective sequestration of IL-8 and associated decreased activation and migration of neutrophils (Skov et al., 2008). It is currently undergoing evaluation in a phase I clinical trial in solid tumors (NCT02536469). The majority of IL-8 receptor-targeting studies in cancer have utilized the small molecule antagonist reparixin (formerly known as repertaxin), a noncompetitive inhibitor of CXCR1 and CXCR2 that prevents IL-8 signaling by locking the receptors in an inactive form (Casilli et al., 2005). In preclinical analyses, reparixin was shown to effectively reduce tumor growth, to deplete CSC frequency in vitro and in vivo (Ginestier et al., 2010), and to synergize with chemotherapy to reduce metastases (Brandolini et al., 2015). Reparixin was recently evaluated in a phase 1b trial of patients with HER2 negative breast cancer in which it demonstrated acceptable safety and tolerability in combination with paclitaxel (NCT02001974). It is currently in a phase 2 clinical trial in combination with paclitaxel for metastatic triple-negative breast cancer (NCT02370238).
High-Throughput Screens Identify Novel Modulators of Tumor Phenotype
As noted above, HTS technology has been used to identify cytotoxic agents capable of directly killing mesenchymalized tumor cells. However, in recent years a relatively new application of HTS technology has emerged in which the goal is to identify compounds that are not necessarily cytotoxic, but are instead able to prevent or revert features of tumor mesenchymalization. One early example was a relatively small screen of 267 compounds designed to identify compounds capable of inhibiting the spot migration of tumor cells that had been treated with EMT-inducing growth factors (Chua et al., 2012). Another example used a cell-based reporter assay system to identify compounds capable of upregulating the expression of a cell adhesion molecule that is frequently downregulated during mesenchymalization (Huynh et al., 2015). Validation studies determined that the identified compounds could attenuate tumor cell motility and invasiveness in vitro, while also reducing tumor xenograft growth and local invasion in vivo. These examples confirm the feasibility and potential utility of this novel approach.
Screening with a collection of over 2,000 FDA-approved compounds was recently undertaken to identify novel modulators of tumor phenotype that could be repurposed from their original indications (Hamilton et al., 2016a). Unlike similar assays, which screened mesenchymal cell line models that had been stably transfected with exogenous DNA, this study utilized single-cell cloning to derive an isogenic pair of highly homogeneous epithelial-like or mesenchymal-like clones of the H460 lung cancer cell line that had not been artificially manipulated. Furthermore, this screen was also unique in that it was designed to identify non-toxic compounds capable of enhancing the susceptibility of mesenchymalized tumor cell clone to killing by TNF-related apoptosis-inducing ligand (TRAIL), which was used as a surrogate for immune-mediated killing. The estrogen receptor antagonist fulvestrant was identified as the lead compound from this screen. Treatment with fulvestrant was found to not only improve TRAIL-mediated killing of the mesenchymalized clone, but to also markedly enhance the susceptibility of these cells to chemotherapy and to lysis by innate and antigen-specific immune cells. Mechanistic studies identified a robust association between mesenchymal features and expression of estrogen receptor 1 (ER-α) in lung cancer, and blockade of estrogen signaling with fulvestrant was able to revert these features. These results suggest that fulvestrant (Figure 2) can be used to alter the phenotype of lung tumors to become more amenable to treatment with chemotherapy and/or immunotherapy.
In addition to cell signaling, phenotype modulation in tumor cells is also regulated by epigenetic mechanisms. Functional loss of E-cadherin expression, a pivotal occurrence during tumor plasticity, can be accomplished through silencing of the CDH1 gene (encoding E-cadherin) via promoter hypermethylation (Lombaerts et al., 2006). CDH1 silencing can also be accomplished by EMT-TF-mediated recruitment of histone deacetylase (HDAC) enzymes at the CDH1 promoter, which deacetylate histone proteins thereby causing chromosomal condensation and gene repression (Peinado et al., 2004). Indeed, genome-wide reprogramming of histone modifications in specific chromatin domains was reported to occur in cells undergoing TGF-β-mediated mesenchymalization (McDonald et al., 2011). Accordingly, a recent screening employing restoration of E-cadherin expression as the readout identified inhibitors of HDAC enzymes (HDACi) as candidate drugs to restore epithelial differentiation in tumor cells (Tang et al., 2016). Among 41 HDACi screened, 28 showed the ability to upregulate E-cadherin promoter activity, and the top ranking compounds all had selectivity for class I HDACs. Subsequent studies using class I HDACi (vorinostat, mocetinostat, entinostat, and/or CI994) determined that only mesenchymal-like tumor cells were susceptible to phenotype reversal, and that restoration of epithelial differentiation by HDACi reduced both anchorage-independent growth and spheroid forming efficiency. Notably, class I HDACi (vorinostat, romidepsin) are already FDA-approved agents for treating patients with T-cell lymphoma, and several other HDACi are currently undergoing evaluation in numerous clinical trials for cancer. The results of this screen suggest that tumor phenotype modulation may be another promising indication for HDACi (Figure 2).
Immunological Targeting of Mesenchymalized Tumor Cells
The landscape of cancer medicine has been profoundly altered in recent years by the successful implementation of immunotherapy. Such approaches include tumor vaccines, immune checkpoint inhibition, and adoptive T-cell transfer with native or modified T cells. The prospect of using immunotherapy to activate antitumor responses against specifically those malignant cells that exhibit a mesenchymalized phenotype represents a radical new approach to cancer immunotherapy, as it would seek to educate the patient’s own immune cells to preferentially eliminate those cells that contribute the most to metastatic spread and therapy resistance. As with pharmacological targeting of EMT, the generation of robust immune responses against these cells could ideally be combined with conventional therapies that eradicate the epithelial-like component of the tumor, thereby resulting in better long-term tumor control. To our knowledge, there is only one immunotherapy that is specifically designed to target mesenchymal-like tumor cells which has progressed to clinical trials. However, emerging research also suggests that mesenchymalized tumors in general may be more susceptible to certain immune-based treatments. These recent advances are discussed below.
Antitumor Immune Responses
Immune responses can be classified as innate (non-specific) and adaptive (specific) responses. Adaptive immunity depends upon the detection of an antigen that is recognized as foreign to the host, and these responses exhibit persistence upon re-challenge with the antigen, a phenomenon known as immunological memory. To generate adaptive immunity, antigen must first be internalized, processed into short peptides, and displayed upon major histocompatibility complex (MHC) molecules by antigen-presenting cells such as dendritic cells (DCs). Peptide-MHC complexes are in turn bound by T-cell receptors (TCRs) found on T cells that are capable of recognizing the specific antigen. T cells that express the cell surface marker CD4 bind to MHC class II, while T cells that express CD8 bind to MHC class I. Once activated, T cells migrate to the periphery, proliferate rapidly, and enter the effector phase in which they exert their immune functions; CD4+ cells, known as T helper cells (TH), secrete inflammatory cytokines to support other immune cells, while the CD8+ cells, known as cytotoxic T lymphocytes (CTLs), seek out and destroy other cells that express the recognized antigen. Once the provoking immune event has been resolved, a subset of these activated cells become memory T cells that persist for many years in order to rapidly respond to the offending stimulus should it ever be encountered again.
Adaptive responses can also be generated artificially by vaccination, and this approach can be exploited against tumors in the form of a cancer vaccine. Unlike vaccines for infectious diseases that seek to primarily elicit B cell-dependent antibody responses, cancer vaccines are designed to provoke T-cell responses, especially CTL functions that can recognize and destroy tumor cells. Cancer vaccines are designed to target antigens expressed solely by the tumor (known as tumor-specific antigens) or those expressed at higher levels in tumor cells compared to normal cells (termed tumor-associated antigens) thereby preventing or limiting undesirable autoimmunity. A key advantage of anticancer vaccines is the ability to target any protein expressed by tumor cells, as all cells process virtually all expressed proteins and display the resulting peptides with MHC class I molecules on the cell surface. This is in contrast to small molecule inhibitors, which cannot target “undruggable” molecules that lack a specific binding groove, or antibodies, which can only target cell surface proteins.
Brachyury as a Cancer Vaccine Target
To specifically target elements of the mesenchymalization process with a cancer vaccine, the choice of an appropriate antigen is critical; the antigen must exhibit a tissue-restricted pattern of expression in primarily neoplastic tissues, and it must be capable of inducing an immune response. Molecules commonly upregulated during mesenchymalization of carcinomas, such as vimentin or N-cadherin, would not be good candidates as they are also abundantly expressed on various normal cell types. Efforts to find a suitable tumor-associated antigen of relevance to the phenomenon of mesenchymalization identified the T-box transcription factor brachyury encoded by the T gene). This embryonal gene is transiently expressed in mesodermal precursor cells during formation of the posterior mesoderm, but is virtually undetectable in normal adult tissues other than the testes and thyroid (Roselli et al., 2012). However, brachyury has been found to be overexpressed in a wide variety of carcinomas, including lung, breast, colon, and prostate, among others (Palena & Hamilton, 2015). In tumor cells, brachyury has been shown to be a driver of mesenchymalization, as high expression of brachyury resulted in loss of epithelial markers (including repression of E-cadherin), acquisition of mesenchymal markers, gain of motility and invasiveness, increased metastatic dissemination (Fernando et al., 2010), increased stemness characteristics (Sarkar et al., 2012), and enhanced resistance to radiation and chemotherapy (Huang et al., 2013a; Larocca et al., 2013). In recent years, high levels of brachyury expression were reported to associate with poor clinical outcome in patients with hormone-receptor positive breast cancer (Palena et al., 2014), triple-negative breast cancer (Hamilton et al., 2016b), colorectal cancer (Kilic et al., 2011; Sarkar et al., 2012), lung cancer (Haro et al., 2013), prostate cancer (Pinto et al., 2014), gastrointestinal stromal tumors (Pinto et al., 2016), hepatocellular carcinoma (Du et al., 2014), and oral squamous cell carcinoma (Imajyo et al., 2012).
In addition to satisfying the first requirement of an anticancer vaccine target by exhibiting a restricted pattern of expression in primarily neoplastic tissues, brachyury has also been shown to satisfy the second requirement by demonstrating immunogenicity both in vitro and in vivo. Using blood from cancer patients, brachyury-specific CD8+ T cells could be expanded by in vitro stimulation with an HLA-A0201-restricted epitope of brachyury identified using an HLA-binding prediction algorithm (Palena et al., 2007), and these patient-derived CD8+ T cells proved capable of lysing brachyury-expressing carcinoma cells (Palena et al., 2007; Palena et al., 2014; Roselli et al., 2012). In addition, the development of brachyury-reactive CD8+ T cells was observed in prostate cancer patients who received vaccinations against CEA or PSA (Madan et al., 2012), likely due to cross-presentation of antigen following tumor destruction in response to vaccine, a phenomenon known as antigen cascade. These data indicate that anti-brachyury immunity can be developed both directly and indirectly in cancer patients, thereby paving the way for the development of brachyury-targeted vaccines.
Brachyury-Targeting Cancer Vaccines
Several approaches can be used to vaccinate a patient against a given tumor antigen, including 1) the direct delivery of the antigenic peptide(s) or protein(s); 2) the use of DCs loaded with peptides, proteins, or mRNA encoding for a given antigen; 3) the use of whole-tumor cell vaccines; and 4) the use of vector-based vaccines in which a recombinant viral, yeast, or bacterial vector is engineered to overexpress an antigen of choice (Schlom et al., 2014). In the particular case of brachyury, three vector-based cancer vaccine platforms have been developed, and two of these are currently undergoing clinical testing as delineated below.
The first brachyury cancer vaccine that reached the clinical stage is a recombinant yeast-brachyury vector (designated as GI-6301) that consists of heat-killed Saccharomyces cerevisiae engineered to express the full-length human brachyury protein. In vitro studies conducted with human DCs incubated with recombinant yeast-brachyury initially showed the ability of this vector to activate previously established human brachyury-specific T-cell lines, as well as to expand brachyury-specific CD4+ and CD8+ T cells from the blood of cancer patients (Hamilton et al., 2013). Experiments conducted in vivo also showed the ability of this vaccine to activate and expand murine brachyury-specific CD4+ and CD8+ T-cell responses, while inducing tumor control in a murine model of experimental metastasis (Hamilton et al., 2013). A phase I clinical trial of this vaccine (NCT01519817) was recently completed in patients with advanced carcinomas or chordoma (Heery et al., 2015b). The vaccine was well tolerated with no associated toxicities and elicited brachyury-specific CD8+ and/or CD4+ T-cell responses in approximately half of the patients, post-vaccination. Some evidence of clinical activity was also reported, particularly in two of the chordoma patients enrolled in the study in whom a confirmed partial response and a reduction of tumor burden were observed, respectively. Based on these results, a phase II study of the yeast-brachyury vaccine is currently ongoing in patients with chordoma (NCT02383498).
Another platform undergoing extensive clinical investigation in the field of cancer immunotherapy consists of recombinant poxvirus vectors, including vaccinia and fowlpox viruses modified to express tumor antigen(s) and costimulatory molecules (Gulley et al., 2014). Recently, an MVA-brachyury-TRICOM vaccine has been developed. This vaccine consists of a highly attenuated and safe strain of vaccinia, namely Modified Vaccinia Ankara (MVA), engineered to express the full-length brachyury protein and a triad of costimulatory molecules named TRICOM (B7-1, ICAM-1, LFA-3). This vector has been evaluated in a recently completed phase I clinical trial in patients with advanced carcinomas or chordoma (NCT02179515), demonstrating the ability to elicit CD4+ and/or CD8+ T-cell responses post-vaccination in up to 80% of the patients, in the absence of toxicities (Heery et al., 2015a).
A novel adenoviral-based vaccine encoding for brachyury was also recently developed (Gabitzsch et al., 2015). In preclinical studies, this vaccine demonstrated the ability to elicit the activation of antigen-specific human T cells in vitro, and induced antigen-specific CD4+ and CD8+ T cells in vaccinated mice.
Immune Checkpoint Inhibition
As discussed above, activated CD8+ T cells enter into the effector phase in which they are capable of destroying tumor cells that express the recognized antigen. However, the degree and duration of effector responses depends upon the balance between co-stimulatory versus inhibitory signals that are received by the T cell. Such inhibitory signals can be delivered via binding with immune checkpoint molecules expressed by antigen-presenting cells or tumor cells. In normal physiology, these inhibitory signals serve to maintain self-tolerance and regulate peripheral responses to pathogens in order to prevent excessive tissue damage caused by chronic inflammation. However, these same pathways are often co-opted during tumor progression to prevent host anti-tumor immune responses.
Monoclonal antibodies designed to inhibit immune checkpoints or their ligands, including cytotoxic T lymphocyte associated protein-4 (CTLA-4), programmed cell death-1 (PD-1), and programmed cell death ligand-1 (PD-L1), have yielded striking clinical responses in subsets of patients with melanoma and cancers of the lung, kidney, and bladder (Brahmer et al., 2012; Rosenberg et al., 2016; Topalian et al., 2012; Wolchok et al., 2013). These therapies work by relieving T cell inhibitory signals that maintain tumor immune tolerance, thereby unleashing a patient’s own pre-existing anti-tumor immune response. Early investigations into predictive correlates for PD-1/PD-L1 checkpoint inhibitor response have found that tumor and immune cell PD-L1 expression as well as the presence of tumor-infiltrating lymphocytes (TIL) can discriminate responding patients (Herbst et al., 2014b; Tumeh et al., 2014).
Continued investigation into predictive parameters of response has now revealed an intriguing potential connection between checkpoint immunotherapy and tumor cell mesenchymalization. The first line of evidence comes from the independent development of two different universal ‘EMT scoring’ methods that quantitatively assessed the degree of tumor mesenchymalization across an array of cancer types (Mak et al., 2016; Tan et al., 2014). In these analyses, those tumor types known to respond well to checkpoint inhibition (melanoma, renal, and lung) also tended to have a more mesenchymal score. Second, a preclinical study in human and murine cell lines revealed that ZEB1-driven mesenchymalization suppresses host immunity by controlling the expression of tumor cell PD-L1 (Chen et al., 2014). Using murine tumor models, the authors showed that mesenchymal-like tumors, but not epithelial tumors, are susceptible to PD-L1 blockade therapy (Chen et al., 2014). Lastly, an analysis of patient tumor genomic and proteomic profiles across 11 cancer types showed a strong correlation between mesenchymal score and markers of immune activation (Mak et al., 2016). These results were confirmed in a subsequent analysis of lung adenocarcinoma (Lou et al., 2016), indicating that immune checkpoint inhibitors may be well suited to exploit these preexisting inflammatory immune responses. However, the putative connection between checkpoint immunotherapy and tumor phenotypic status may be tumor-type specific, as the mesenchymal phenotype was found to be associated with innate resistance to PD-1 blockade in melanomas (Hugo et al., 2016). There are currently two FDA-approved PD-1- targeting antibodies (pembrolizumab, nivolumab), one FDA-approved PD-L1-targeting antibody (atezolizumab), and several other antibodies undergoing clinical trials. Whether PD-1/PD-L1 checkpoint inhibition can truly be thought of as indirectly targeting tumor mesenchymalization remains a tantalizing possibility that awaits further experimental validation.
Conclusions
As researchers have probed the complexity of cancer, it has become abundantly clear that individual carcinoma cells that comprise a tumor are not a homogeneous group, but instead exist as a heterogeneous assembly of cells that exhibit markedly differing biological behaviors and functions due to intrinsic factors (mutational diversity) and extrinsic factors (oxygenation, proximity to microenvironmental factors, etc.). These factors dictate the phenotypic state in which tumor cells reside, and these differing states signify important functional attributes that influence tumor cell behavior and disease progression. Continuing knowledge of these phenotypic states and the dynamic nature of tumor cell phenotype transitions has created new opportunities to intervene to control the behavior of tumor cells.
There are now a variety of pharmacological approaches to targeting tumor mesenchymalization, and novel immunological means have emerged in recent years. The time for evaluating the various strategies of targeting mesenchymalized tumor cells has arrived, and it is conceivable that combinations with conventional treatments could be used as to eliminate those susceptible tumor cells that exhibit more epithelial-like features, thus granting better long-term tumor control. Furthermore, use of immunological targeting of tumor mesenchymalization could be administered concurrently to engage the immune system in seeking and destroying those cells that may have avoided pharmacological targeting.
In summary, advances in our understanding of tumor cell phenotypic plasticity in the laboratory combined with the advent of personalized medicine approaches in the clinic has presented a rich opportunity to develop a new arsenal of both pharmacological and immunological therapies to target tumor mesenchymalization. Such an opportunity must be seized in order to produce the next big leap in cancer therapy.
Acknowledgments
Financial Support: This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Abbreviations
- CSC
cancer stem cell
- CTC
circulating tumor cell
- EMT
epithelial-mesenchymal transition
- HTS
high-throughput screening
- MET
mesenchymal-epithelial transition
Footnotes
Conflicts of Interest Statement
The authors declare that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angevin E, Tabernero J, Elez E, Cohen SJ, Bahleda R, van Laethem JL, Ottensmeier C, Lopez-Martin JA, Clive S, Joly F, Ray-Coquard I, Dirix L, Machiels JP, Steven N, Reddy M, Hall B, Puchalski TA, Bandekar R, van de Velde H, Tromp B, Vermeulen J, Kurzrock R. A phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2014;20:2192–2204. doi: 10.1158/1078-0432.CCR-13-2200. [DOI] [PubMed] [Google Scholar]
- Bates RC, DeLeo MJ, 3rd, Mercurio AM. The epithelial-mesenchymal transition of colon carcinoma involves expression of IL-8 and CXCR-1-mediated chemotaxis. Exp Cell Res. 2004;299:315–324. doi: 10.1016/j.yexcr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Galle PR, Blessing M, Rose-John S, Neurath MF. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpé S, Vermeulen PB, Dirix LY. Increased Serum Interleukin-8 in Patients with Early and Metastatic Breast Cancer Correlates with Early Dissemination and Survival. Clinical Cancer Research. 2004;10:7157–7162. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- Bhola NE, Balko JM, Dugger TC, Kuba MG, Sanchez V, Sanders M, Stanford J, Cook RS, Arteaga CL. TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest. 2013;123:1348–1358. doi: 10.1172/JCI65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–3246. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- Boehmerle W, Endres M. Salinomycin induces calpain and cytochrome c-mediated neuronal cell death. Cell Death Dis. 2011;2:e168. doi: 10.1038/cddis.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmerle W, Muenzfeld H, Springer A, Huehnchen P, Endres M. Specific targeting of neurotoxic side effects and pharmacological profile of the novel cancer stem cell drug salinomycin in mice. J Mol Med (Berl) 2014;92:889–900. doi: 10.1007/s00109-014-1155-0. [DOI] [PubMed] [Google Scholar]
- Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005;179:56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- Brabletz T. To differentiate or not--routes towards metastasis. Nat Rev Cancer. 2012;12:425–436. doi: 10.1038/nrc3265. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandolini L, Cristiano L, Fidoamore A, De Pizzol M, Di Giacomo E, Florio TM, Confalone G, Galante A, Cinque B, Benedetti E, Ruffini PA, Cifone MG, Giordano A, Alecci M, Allegretti M, Cimini A. Targeting CXCR1 on breast cancer stem cells: signaling pathways and clinical application modelling. Oncotarget. 2015;6:43375–43394. doi: 10.18632/oncotarget.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, Shen L, Fan Y, Giri U, Tumula PK, Nilsson MB, Gudikote J, Tran H, Cardnell RJ, Bearss DJ, Warner SL, Foulks JM, Kanner SB, Gandhi V, Krett N, Rosen ST, Kim ES, Herbst RS, Blumenschein GR, Lee JJ, Lippman SM, Ang KK, Mills GB, Hong WK, Weinstein JN, Wistuba II, Coombes KR, Minna JD, Heymach JV. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2013;19:279–290. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Carmody LC, Germain AR, VerPlank L, Nag PP, Munoz B, Perez JR, Palmer MA. Phenotypic high-throughput screening elucidates target pathway in breast cancer stem cell-like cells. J Biomol Screen. 2012;17:1204–1210. doi: 10.1177/1087057112458317. [DOI] [PubMed] [Google Scholar]
- Casilli F, Bianchini A, Gloaguen I, Biordi L, Alesse E, Festuccia C, Cavalieri B, Strippoli R, Cervellera MN, Di Bitondo R, Ferretti E, Mainiero F, Bizzarri C, Colotta F, Bertini R. Inhibition of interleukin-8 (CXCL8/IL-8) responses by repertaxin, a new inhibitor of the chemokine receptors CXCR1 and CXCR2. Biochem Pharmacol. 2005;69:385–394. doi: 10.1016/j.bcp.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, Diao L, Wang J, Roybal JD, Patel M, Ungewiss C, Peng D, Antonia S, Mediavilla-Varela M, Robertson G, Jones S, Suraokar M, Welsh JW, Erez B, Wistuba II, Chen L, Peng D, Wang S, Ullrich SE, Heymach JV, Kurie JM, Qin FX. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua KN, Sim WJ, Racine V, Lee SY, Goh BC, Thiery JP. A cell-based small molecule screening method for identifying inhibitors of epithelial-mesenchymal transition in carcinoma. PLoS One. 2012;7:e33183. doi: 10.1371/journal.pone.0033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JM, Owens TA, Inge LJ, Bremner RM, Rajasekaran AK. Gramicidin A blocks tumor growth and angiogenesis through inhibition of hypoxia-inducible factor in renal cell carcinoma. Mol Cancer Ther. 2014;13:788–799. doi: 10.1158/1535-7163.MCT-13-0891. [DOI] [PubMed] [Google Scholar]
- David JM, Dominguez C, Hamilton DH, Palena C. The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines (Basel) 2016;4 doi: 10.3390/vaccines4030022. pii: E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat Cell Biol. 2007;9:1000–1004. doi: 10.1038/ncb434. [DOI] [PubMed] [Google Scholar]
- Dijkgraaf EM, Santegoets SJ, Reyners AK, Goedemans R, Wouters MC, Kenter GG, van Erkel AR, van Poelgeest MI, Nijman HW, van der Hoeven JJ, Welters MJ, van der Burg SH, Kroep JR. A phase I trial combining carboplatin/doxorubicin with tocilizumab, an anti-IL-6R monoclonal antibody, and interferon-alpha2b in patients with recurrent epithelial ovarian cancer. Ann Oncol. 2015;26:2141–2149. doi: 10.1093/annonc/mdv309. [DOI] [PubMed] [Google Scholar]
- Du R, Wu S, Lv X, Fang H, Wu S, Kang J. Overexpression of brachyury contributes to tumor metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. J Exp Clin Cancer Res. 2014;33:105. doi: 10.1186/s13046-014-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- Elloul S, Elstrand MB, Nesland JM, Trope CG, Kvalheim G, Goldberg I, Reich R, Davidson B. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103:1631–1643. doi: 10.1002/cncr.20946. [DOI] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Espineda CE, Chang JH, Twiss J, Rajasekaran SA, Rajasekaran AK. Repression of Na,K-ATPase beta1-subunit by the transcription factor snail in carcinoma. Mol Biol Cell. 2004;15:1364–1373. doi: 10.1091/mbc.E03-09-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feniger-Barish R, Yron I, Meshel T, Matityahu E, Ben-Baruch A. IL-8-induced migratory responses through CXCR1 and CXCR2: association with phosphorylation and cellular redistribution of focal adhesion kinase. Biochemistry. 2003;42:2874–2886. doi: 10.1021/bi026783d. [DOI] [PubMed] [Google Scholar]
- Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010;120:533–544. doi: 10.1172/JCI38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71:5296–5306. doi: 10.1158/0008-5472.CAN-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando RI, Hamilton DH, Dominguez C, David JM, McCampbell KK, Palena C. IL-8 signaling is involved in resistance of lung carcinoma cells to erlotinib. Oncotarget. 2016;71:5296–5306. doi: 10.18632/oncotarget.9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ, Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB, Fan J. Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int J Oncol. 2015;46:587–596. doi: 10.3892/ijo.2014.2761. [DOI] [PubMed] [Google Scholar]
- Fulciniti M, Hideshima T, Vermot-Desroches C, Pozzi S, Nanjappa P, Shen Z, Patel N, Smith ES, Wang W, Prabhala R, Tai YT, Tassone P, Anderson KC, Munshi NC. A high-affinity fully human anti-IL-6 mAb, 1339, for the treatment of multiple myeloma. Clin Cancer Res. 2009;15:7144–7152. doi: 10.1158/1078-0432.CCR-09-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabitzsch ES, Tsang KY, Palena C, David JM, Fantini M, Kwilas A, Rice AE, Latchman Y, Hodge JW, Gulley JL, Madan RA, Heery CR, Balint JP, Jr, Jones FR, Schlom J. The generation and analyses of a novel combination of recombinant adenovirus vaccines targeting three tumor antigens as an immunotherapeutic. Oncotarget. 2015;6:31344–31359. doi: 10.18632/oncotarget.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K. Epithelial-to-mesenchymal transition is important to metastasis, but questions remain. J Natl Cancer Inst. 2008;100:232–233. 239. doi: 10.1093/jnci/djn032. [DOI] [PubMed] [Google Scholar]
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- Germain AR, Carmody LC, Nag PP, Morgan B, Verplank L, Fernandez C, Donckele E, Feng Y, Perez JR, Dandapani S, Palmer M, Lander ES, Gupta PB, Schreiber SL, Munoz B. Cinnamides as selective small-molecule inhibitors of a cellular model of breast cancer stem cells. Bioorg Med Chem Lett. 2013;23:1834–1838. doi: 10.1016/j.bmcl.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, Wicha MS. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–7011. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- Gulley JL, Madan RA, Tsang KY, Jochems C, Marte JL, Farsaci B, Tucker JA, Hodge JW, Liewehr DJ, Steinberg SM, Heery CR, Schlom J. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res. 2014;2:133–141. doi: 10.1158/2326-6066.CIR-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, Lam A, Lazetic S, Ma S, Mitra S, Park IK, Pickell K, Sato A, Satyal S, Stroud M, Tran H, Yen WC, Lewicki J, Hoey T. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH. Oxygen regulates epithelial-to-mesenchymal transition: insights into molecular mechanisms and relevance to disease. Kidney Int. 2009;76:492–499. doi: 10.1038/ki.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DH, Litzinger MT, Jales A, Huang B, Fernando RI, Hodge JW, Ardiani A, Apelian D, Schlom J, Palena C. Immunological targeting of tumor cells undergoing an epithelial-mesenchymal transition via a recombinant brachyury-yeast vaccine. Oncotarget. 2013;4:1777–1790. doi: 10.18632/oncotarget.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DH, Matthews Griner L, Keller JM, Hu X, Southall N, Marugan J, David JM, Ferrer M, Palena C. Targeting estrogen receptor signaling with fulvestrant enhances immune and chemotherapy-mediated cytotoxicity of human lung cancer. Clin Cancer Res. 2016a doi: 10.1158/1078-0432.CCR-15-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DH, Roselli M, Ferroni P, Costarelli L, Cavaliere F, Taffuri M, Palena C, Guadagni F. Brachyury, a vaccine target, is overexpressed in triple-negative breast cancer. Endocr Relat Cancer. 2016b;23:783–796. doi: 10.1530/ERC-16-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro A, Yano T, Kohno M, Yoshida T, Koga T, Okamoto T, Takenoyama M, Maehara Y. Expression of Brachyury gene is a significant prognostic factor for primary lung carcinoma. Ann Surg Oncol. 2013;20(Suppl 3):S509–516. doi: 10.1245/s10434-013-2914-9. [DOI] [PubMed] [Google Scholar]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- Heery CR, Donahue RN, Lepone L, Grenga I, Richards J, Metenou S, Fernando RI, Dirmeier U, Singh H, Madan RA, Gulley JL, Schlom J. Phase I, dose-escalation, clinical trial of MVA-Brachyury-TRICOM vaccine demonstrating safety and brachyury-specific T cell responses. Journal for ImmunoTherapy of Cancer. 2015a;3:1–2. [Google Scholar]
- Heery CR, Singh BH, Rauckhorst M, Marte JL, Donahue RN, Grenga I, Rodell TC, Dahut W, Arlen PM, Madan RA, Schlom J, Gulley JL. Phase I Trial of a Yeast-Based Therapeutic Cancer Vaccine (GI-6301) Targeting the Transcription Factor Brachyury. Cancer Immunol Res. 2015b;3:1248–1256. doi: 10.1158/2326-6066.CIR-15-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbertz S, Sawyer JS, Stauber AJ, Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, Desaiah D, Guba SC, Benhadji KA, Slapak CA, Lahn MM. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther. 2015;9:4479–4499. doi: 10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst A, Jurinovic V, Krebs S, Thieme SE, Blum H, Goke B, Kolligs FT. Comprehensive analysis of beta-catenin target genes in colorectal carcinoma cell lines with deregulated Wnt/beta-catenin signaling. BMC Genomics. 2014a;15:74. doi: 10.1186/1471-2164-15-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014b;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Hiscox S, Jiang WG, Obermeier K, Taylor K, Morgan L, Burmi R, Barrow D, Nicholson RI. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer. 2006;118:290–301. doi: 10.1002/ijc.21355. [DOI] [PubMed] [Google Scholar]
- Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- Huang B, Cohen JR, Fernando RI, Hamilton DH, Litzinger MT, Hodge JW, Palena C. The embryonic transcription factor Brachyury blocks cell cycle progression and mediates tumor resistance to conventional antitumor therapies. Cell Death Dis. 2013a;4:e682. doi: 10.1038/cddis.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RY, Wong MK, Tan TZ, Kuay KT, Ng AH, Chung VY, Chu YS, Matsumura N, Lai HC, Lee YF, Sim WJ, Chai C, Pietschmann E, Mori S, Low JJ, Choolani M, Thiery JP. An EMT spectrum defines an anoikis-resistant and spheroidogenic intermediate mesenchymal state that is sensitive to e-cadherin restoration by a src-kinase inhibitor, saracatinib (AZD0530) Cell Death Dis. 2013b;4:e915. doi: 10.1038/cddis.2013.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD, Gudas JM, Bar-Eli M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol. 2002;161:125–134. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudes G, Tagawa ST, Whang YE, Qi M, Qin X, Puchalski TA, Reddy M, Cornfeld M, Eisenberger M. A phase 1 study of a chimeric monoclonal antibody against interleukin-6, siltuximab, combined with docetaxel in patients with metastatic castration-resistant prostate cancer. Invest New Drugs. 2013;31:669–676. doi: 10.1007/s10637-012-9857-z. [DOI] [PubMed] [Google Scholar]
- Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, Seja E, Lomeli S, Kong X, Kelley MC, Sosman JA, Johnson DB, Ribas A, Lo RS. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh TP, Barwe SP, Lee SJ, McSpadden R, Franco OE, Hayward SW, Damoiseaux R, Grubbs SS, Petrelli NJ, Rajasekaran AK. Glucocorticoids suppress renal cell carcinoma progression by enhancing Na,K-ATPase beta-1 subunit expression. PLoS One. 2015;10:e0122442. doi: 10.1371/journal.pone.0122442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajyo I, Sugiura T, Kobayashi Y, Shimoda M, Ishii K, Akimoto N, Yoshihama N, Kobayashi I, Mori Y. T-box transcription factor Brachyury expression is correlated with epithelial-mesenchymal transition and lymph node metastasis in oral squamous cell carcinoma. Int J Oncol. 2012;41:1985–1995. doi: 10.3892/ijo.2012.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaschinski F, Rothhammer T, Jachimczak P, Seitz C, Schneider A, Schlingensiepen KH. The antisense oligonucleotide trabedersen (AP 12009) for the targeted inhibition of TGF-beta2. Curr Pharm Biotechnol. 2011;12:2203–2213. doi: 10.2174/138920111798808266. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–3128. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, Kikkawa F. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31:277–283. [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Tanaka T, Kishimoto T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int Immunol. 2015;27:21–29. doi: 10.1093/intimm/dxu081. [DOI] [PubMed] [Google Scholar]
- Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, Asaoku H, Tang B, Tanabe O, Tanaka H, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332:83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Ketola K, Vuoristo A, Orešič M, Kallioniemi O, Iljin K. Monensin Induced Oxidative Stress Reduces Prostate Cancer Cell Migration and Cancer Stem Cell Population. In: Lushchak V, Gospodaryov DV, editors. Oxidative Stress and Diseases. InTech; 2012. [Google Scholar]
- Kilic N, Feldhaus S, Kilic E, Tennstedt P, Wicklein D, Wasielewski R, Viebahn C, Kreipe H, Schumacher U. Brachyury expression predicts poor prognosis at early stages of colorectal cancer. Eur J Cancer. 2011;47:1080–1085. doi: 10.1016/j.ejca.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Kim HP, Han SW, Song SH, Jeong EG, Lee MY, Hwang D, Im SA, Bang YJ, Kim TY. Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene. 2014;33:3334–3341. doi: 10.1038/onc.2013.285. [DOI] [PubMed] [Google Scholar]
- Kim HY, Jackson TR, Davidson LA. On the role of mechanics in driving mesenchymal-to-epithelial transitions. Semin Cell Dev Biol. 2016 doi: 10.1016/j.semcdb.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knall C, Young S, Nick JA, Buhl AM, Worthen GS, Johnson GL. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J Biol Chem. 1996;271:2832–2838. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- Kolev VN, Wright QG, Vidal CM, Ring JE, Shapiro IM, Ricono J, Weaver DT, Padval MV, Pachter JA, Xu Q. PI3K/mTOR dual inhibitor VS-5584 preferentially targets cancer stem cells. Cancer Res. 2015;75:446–455. doi: 10.1158/0008-5472.CAN-14-1223. [DOI] [PubMed] [Google Scholar]
- Kothari AN, Mi Z, Zapf M, Kuo PC. Novel clinical therapeutics targeting the epithelial to mesenchymal transition. Clin Transl Med. 2014;3:35. doi: 10.1186/s40169-014-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–2068. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- Larocca C, Cohen JR, Fernando RI, Huang B, Hamilton DH, Palena C. An autocrine loop between TGF-beta1 and the transcription factor brachyury controls the transition of human carcinoma cells into a mesenchymal phenotype. Mol Cancer Ther. 2013;12:1805–1815. doi: 10.1158/1535-7163.MCT-12-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. 2015;146:1–11. doi: 10.1016/j.pharmthera.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun JJ. The Dual Role of TGFbeta in Human Cancer: From Tumor Suppression to Cancer Metastasis. ISRN Mol Biol. 2012;2012:381428. doi: 10.5402/2012/381428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz HJ, Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014;105:1087–1092. doi: 10.1111/cas.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139:3471–3486. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, Li J, Tompkins C, Pferdekamper A, Steffy A, Cheng J, Kowal C, Phung V, Guo G, Wang Y, Graham MP, Flynn S, Brenner JC, Li C, Villarroel MC, Schultz PG, Wu X, McNamara P, Sellers WR, Petruzzelli L, Boral AL, Seidel HM, McLaughlin ME, Che J, Carey TE, Vanasse G, Harris JL. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci U S A. 2013;110:20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YN, Chang TH, Tsai MF, Wu SG, Tsai TH, Chen HY, Yu SL, Yang JC, Shih JY. IL-8 confers resistance to EGFR inhibitors by inducing stem cell properties in lung cancer. Oncotarget. 2015;6:10415–10431. doi: 10.18632/oncotarget.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaerts M, van Wezel T, Philippo K, Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de Water B, Cornelisse CJ, Cleton-Jansen AM. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer. 2006;94:661–671. doi: 10.1038/sj.bjc.6602996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonning S, Mannick J, McPherson JM. Antibody targeting of TGF-beta in cancer patients. Curr Pharm Biotechnol. 2011;12:2176–2189. doi: 10.2174/138920111798808392. [DOI] [PubMed] [Google Scholar]
- Lou Y, Diao L, Cuentas ER, Denning WL, Chen L, Fan YH, Byers LA, Wang J, Papadimitrakopoulou VA, Behrens C, Rodriguez JC, Hwu P, Wistuba II, Heymach JV, Gibbons DL. Epithelial-Mesenchymal Transition Is Associated with a Distinct Tumor Microenvironment Including Elevation of Inflammatory Signals and Multiple Immune Checkpoints in Lung Adenocarcinoma. Clin Cancer Res. 2016;22:3630–3642. doi: 10.1158/1078-0432.CCR-15-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Choi MY, Yu J, Castro JE, Kipps TJ, Carson DA. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci U S A. 2011;108:13253–13257. doi: 10.1073/pnas.1110431108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacManus CF, Pettigrew J, Seaton A, Wilson C, Maxwell PJ, Berlingeri S, Purcell C, McGurk M, Johnston PG, Waugh DJ. Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol Cancer Res. 2007;5:737–748. doi: 10.1158/1541-7786.MCR-07-0032. [DOI] [PubMed] [Google Scholar]
- Madan RA, Bilusic M, Heery C, Schlom J, Gulley JL. Clinical evaluation of TRICOM vector therapeutic cancer vaccines. Semin Oncol. 2012;39:296–304. doi: 10.1053/j.seminoncol.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak MP, Tong P, Diao L, Cardnell RJ, Gibbons DL, William WN, Skoulidis F, Parra ER, Rodriguez-Canales J, Wistuba II, Heymach JV, Weinstein JN, Coombes KR, Wang J, Byers LA. A Patient-Derived, Pan-Cancer EMT Signature Identifies Global Molecular Alterations and Immune Target Enrichment Following Epithelial-to-Mesenchymal Transition. Clin Cancer Res. 2016;22:609–620. doi: 10.1158/1078-0432.CCR-15-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ, Choudhury SA, Maruyama R, Wu Z, Gonen M, Mulvey LA, Bessarabova MO, Huh SJ, Silver SJ, Kim SY, Park SY, Lee HE, Anderson KS, Richardson AL, Nikolskaya T, Nikolsky Y, Liu XS, Root DE, Hahn WC, Frank DA, Polyak K. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121:2723–2735. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald OG, Wu H, Timp W, Doi A, Feinberg AP. Genome-scale epigenetic reprogramming during epithelial-to-mesenchymal transition. Nat Struct Mol Biol. 2011;18:867–874. doi: 10.1038/nsmb.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejlvang J, Kriajevska M, Vandewalle C, Chernova T, Sayan AE, Berx G, Mellon JK, Tulchinsky E. Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol Biol Cell. 2007;18:4615–4624. doi: 10.1091/mbc.E07-05-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian BM, Dinney CP, Bermejo CE, Sweeney P, Tellez C, Yang XD, Gudas JM, McConkey DJ, Bar-Eli M. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin Cancer Res. 2003;9:3167–3175. [PubMed] [Google Scholar]
- Miao JW, Liu LJ, Huang J. Interleukin-6-induced epithelial-mesenchymal transition through signal transducer and activator of transcription 3 in human cervical carcinoma. Int J Oncol. 2014;45:165–176. doi: 10.3892/ijo.2014.2422. [DOI] [PubMed] [Google Scholar]
- Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani M, Yamanishi T, Miyazaki Y, Otake N. Salinomycin effects on mitochondrial ion translocation and respiration. Antimicrob Agents Chemother. 1976;9:655–660. doi: 10.1128/aac.9.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Tan AR, Olencki TE, Shapiro GI, Dezube BJ, Reiss M, Hsu FJ, Berzofsky JA, Lawrence DP. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One. 2014;9:e90353. doi: 10.1371/journal.pone.0090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellner MK, Mair B, Ibrahim Y, Kerzendorfer C, Lechtermann H, Trefzer C, Klepsch F, Muller AC, Leitner E, Macho-Maschler S, Superti-Furga G, Bennett KL, Baselga J, Rix U, Kubicek S, Colinge J, Serra V, Nijman SM. Targeting a cell state common to triple-negative breast cancers. Mol Syst Biol. 2015;11:789. doi: 10.15252/msb.20145664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujokat C, Steinhart R. Salinomycin as a drug for targeting human cancer stem cells. J Biomed Biotechnol. 2012;2012:950658. doi: 10.1155/2012/950658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Cano A. The epithelial-mesenchymal transition under control: global programs to regulate epithelial plasticity. Semin Cancer Biol. 2012;22:361–368. doi: 10.1016/j.semcancer.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984;307:131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997;57:141–146. [PubMed] [Google Scholar]
- Padua D, Massague J. Roles of TGFbeta in metastasis. Cell Res. 2009;19:89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- Palena C, Polev DE, Tsang KY, Fernando RI, Litzinger M, Krukovskaya LL, Baranova AV, Kozlov AP, Schlom J. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13:2471–2478. doi: 10.1158/1078-0432.CCR-06-2353. [DOI] [PubMed] [Google Scholar]
- Palena C, Hamilton DH, Fernando RI. Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncol. 2012;8:713–722. doi: 10.2217/fon.12.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palena C, Roselli M, Litzinger MT, Ferroni P, Costarelli L, Spila A, Cavaliere F, Huang B, Fernando RI, Hamilton DH, Jochems C, Tsang KY, Cheng Q, Lyerly HK, Schlom J, Guadagni F. Overexpression of the EMT driver brachyury in breast carcinomas: association with poor prognosis. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palena C, Hamilton DH. Immune Targeting of Tumor Epithelial-Mesenchymal Transition via Brachyury-Based Vaccines. Adv Cancer Res. 2015;128:69–93. doi: 10.1016/bs.acr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, Hoffmann EK, Novak I. Cell volume regulation in epithelial physiology and cancer. Front Physiol. 2013;4:233. doi: 10.3389/fphys.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, Bowman ED, Engels EA, Caporaso NE, Harris CC. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst. 2011;103:1112–1122. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto F, Pertega-Gomes N, Pereira MS, Vizcaino JR, Monteiro P, Henrique RM, Baltazar F, Andrade RP, Reis RM. T-box transcription factor brachyury is associated with prostate cancer progression and aggressiveness. Clin Cancer Res. 2014;20:4949–4961. doi: 10.1158/1078-0432.CCR-14-0421. [DOI] [PubMed] [Google Scholar]
- Pinto F, Campanella NC, Abrahao-Machado LF, Scapulatempo-Neto C, de Oliveira AT, Brito MJ, Andrade RP, Guimaraes DP, Reis RM. The embryonic Brachyury transcription factor is a novel biomarker of GIST aggressiveness and poor survival. Gastric Cancer. 2016;19:651–659. doi: 10.1007/s10120-015-0505-0. [DOI] [PubMed] [Google Scholar]
- Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Sun F, Zhou J, Li L, Shapiro SD, Xiao G. Interleukin-6 Prevents the Initiation but Enhances the Progression of Lung Cancer. Cancer Res. 2015;75:3209–3215. doi: 10.1158/0008-5472.CAN-14-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran SA, Huynh TP, Wolle DG, Espineda CE, Inge LJ, Skay A, Lassman C, Nicholas SB, Harper JF, Reeves AE, Ahmed MM, Leatherman JM, Mullin JM, Rajasekaran AK. Na,K-ATPase subunits as markers for epithelial-mesenchymal transition in cancer and fibrosis. Mol Cancer Ther. 2010;9:1515–1524. doi: 10.1158/1535-7163.MCT-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, Gangadhar TC, Hersey P, Dronca R, Joseph RW, Zarour H, Chmielowski B, Lawrence DP, Algazi A, Rizvi NA, Hoffner B, Mateus C, Gergich K, Lindia JA, Giannotti M, Li XN, Ebbinghaus S, Kang SP, Robert C. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. Jama. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- Riccioni R, Dupuis ML, Bernabei M, Petrucci E, Pasquini L, Mariani G, Cianfriglia M, Testa U. The cancer stem cell selective inhibitor salinomycin is a p-glycoprotein inhibitor. Blood Cells Mol Dis. 2010;45:86–92. doi: 10.1016/j.bcmd.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Richard G, Dalle S, Monet MA, Ligier M, Boespflug A, Pommier RM, de la Fouchardiere A, Perier-Muzet M, Depaepe L, Barnault R, Tondeur G, Ansieau S, Thomas E, Bertolotto C, Ballotti R, Mourah S, Battistella M, Lebbe C, Thomas L, Puisieux A, Caramel J. ZEB1-mediated melanoma cell plasticity enhances resistance to MAPK inhibitors. EMBO Mol Med. 2016 doi: 10.15252/emmm.201505971. pii: e201505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodon J, Carducci MA, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, Brana I, Sicart E, Gueorguieva I, Cleverly AL, Pillay NS, Desaiah D, Estrem ST, Paz-Ares L, Holdhoff M, Blakeley J, Lahn MM, Baselga J. First-inhuman dose study of the novel transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res. 2015;21:553–560. doi: 10.1158/1078-0432.CCR-14-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokavec M, Oner MG, Li H, Jackstadt R, Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, Slotta-Huspenina J, Bader FG, Greten FR, Hermeking H. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124:1853–1867. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Roselli M, Fernando RI, Guadagni F, Spila A, Alessandroni J, Palmirotta R, Costarelli L, Litzinger M, Hamilton D, Huang B, Tucker J, Tsang KY, Schlom J, Palena C. Brachyury, a driver of the epithelial-mesenchymal transition, is overexpressed in human lung tumors: an opportunity for novel interventions against lung cancer. Clin Cancer Res. 2012;18:3868–3879. doi: 10.1158/1078-0432.CCR-11-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P, Dirix LY. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–646. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- Sampson VB, David JM, Puig I, Patil PU, de Herreros AG, Thomas GV, Rajasekaran AK. Wilms’ tumor protein induces an epithelial-mesenchymal hybrid differentiation state in clear cell renal cell carcinoma. PLoS One. 2014;9:e102041. doi: 10.1371/journal.pone.0102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Shields B, Davies ML, Muller J, Wakeman JA. BRACHYURY confers cancer stem cell characteristics on colorectal cancer cells. Int J Cancer. 2012;130:328–337. doi: 10.1002/ijc.26029. [DOI] [PubMed] [Google Scholar]
- Savagner P. Epithelial-mesenchymal transitions: from cell plasticity to concept elasticity. Curr Top Dev Biol. 2015;112:273–300. doi: 10.1016/bs.ctdb.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Saxena M, Stephens MA, Pathak H, Rangarajan A. Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011;2:e179. doi: 10.1038/cddis.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliekelman MJ, Taguchi A, Zhu J, Dai X, Rodriguez J, Celiktas M, Zhang Q, Chin A, Wong CH, Wang H, McFerrin L, Selamat SA, Yang C, Kroh EM, Garg KS, Behrens C, Gazdar AF, Laird-Offringa IA, Tewari M, Wistuba II, Thiery JP, Hanash SM. Molecular portraits of epithelial, mesenchymal, and hybrid States in lung adenocarcinoma and their relevance to survival. Cancer Res. 2015;75:1789–1800. doi: 10.1158/0008-5472.CAN-14-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingensiepen KH, Jaschinski F, Lang SA, Moser C, Geissler EK, Schlitt HJ, Kielmanowicz M, Schneider A. Transforming growth factor-beta 2 gene silencing with trabedersen (AP 12009) in pancreatic cancer. Cancer Sci. 2011;102:1193–1200. doi: 10.1111/j.1349-7006.2011.01917.x. [DOI] [PubMed] [Google Scholar]
- Schlom J, Hodge JW, Palena C, Tsang KY, Jochems C, Greiner JW, Farsaci B, Madan RA, Heery CR, Gulley JL. Therapeutic cancer vaccines. Adv Cancer Res. 2014;121:67–124. doi: 10.1016/B978-0-12-800249-0.00002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serova M, Tijeras-Raballand A, Dos Santos C, Albuquerque M, Paradis V, Neuzillet C, Benhadji KA, Raymond E, Faivre S, de Gramont A. Effects of TGF-beta signalling inhibition with galunisertib (LY2157299) in hepatocellular carcinoma models and in ex vivo whole tumor tissue samples from patients. Oncotarget. 2015;6:21614–21627. doi: 10.18632/oncotarget.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- Shaik MS, Chatterjee A, Singh M. Effects of monensin liposomes on the cytotoxicity, apoptosis and expression of multidrug resistance genes in doxorubicin-resistant human breast tumour (MCF-7/dox) cell-line. J Pharm Pharmacol. 2004;56:899–907. doi: 10.1211/0022357023772. [DOI] [PubMed] [Google Scholar]
- Shintani Y, Fujiwara A, Kimura T, Kawamura T, Funaki S, Minami M, Okumura M. IL-6 Secreted from Cancer-Associated Fibroblasts Mediates Chemoresistance in NSCLC by Increasing Epithelial-Mesenchymal Transition Signaling. J Thorac Oncol. 2016;11:1482–1492. doi: 10.1016/j.jtho.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Shiota M, Bishop JL, Nip KM, Zardan A, Takeuchi A, Cordonnier T, Beraldi E, Bazov J, Fazli L, Chi K, Gleave M, Zoubeidi A. Hsp27 regulates epithelial mesenchymal transition, metastasis, and circulating tumor cells in prostate cancer. Cancer Res. 2013;73:3109–3119. doi: 10.1158/0008-5472.CAN-12-3979. [DOI] [PubMed] [Google Scholar]
- Shirakihara T, Saitoh M, Miyazono K. Differential regulation of epithelial and mesenchymal markers by deltaEF1 proteins in epithelial mesenchymal transition induced by TGF-beta. Mol Biol Cell. 2007;18:3533–3544. doi: 10.1091/mbc.E07-03-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov L, Beurskens FJ, Zachariae CO, Reitamo S, Teeling J, Satijn D, Knudsen KM, Boot EP, Hudson D, Baadsgaard O, Parren PW, van de Winkel JG. IL-8 as antibody therapeutic target in inflammatory diseases: reduction of clinical activity in palmoplantar pustulosis. J Immunol. 2008;181:669–679. doi: 10.4049/jimmunol.181.1.669. [DOI] [PubMed] [Google Scholar]
- Son JY, Park SY, Kim SJ, Lee SJ, Park SA, Kim MJ, Kim SW, Kim DK, Nam JS, Sheen YY. EW-7197, a novel ALK-5 kinase inhibitor, potently inhibits breast to lung metastasis. Mol Cancer Ther. 2014;13:1704–1716. doi: 10.1158/1535-7163.MCT-13-0903. [DOI] [PubMed] [Google Scholar]
- Soundararajan R, Paranjape AN, Barsan V, Chang JT, Mani SA. A novel embryonic plasticity gene signature that predicts metastatic competence and clinical outcome. Sci Rep. 2015;5:11766. doi: 10.1038/srep11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff S, Isola J, Jumppanen M, Tanner M. Aurora-A gene is frequently amplified in basal-like breast cancer. Oncol Rep. 2010;23:307–312. [PubMed] [Google Scholar]
- Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai D, Wells K, Arcaroli J, Vanderbilt C, Aisner DL, Messersmith WA, Lieu CH. Targeting the WNT Signaling Pathway in Cancer Therapeutics. Oncologist. 2015;20:1189–1198. doi: 10.1634/theoncologist.2015-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TZ, Miow QH, Miki Y, Noda T, Mori S, Huang RY, Thiery JP. Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol Med. 2014;6:1279–1293. doi: 10.15252/emmm.201404208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HM, Kuay KT, Koh PF, Asad M, Tan TZ, Chung VY, Lee SC, Thiery JP, Huang RJ. An epithelial marker promoter induction screen identifies histone deacetylase inhibitors to restore epithelial differentiation and abolishes anchorage independence growth in cancers. Cell Death Discov. 2016;2:16041. doi: 10.1038/cddiscovery.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. discussion 6000–5991. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Thomson S, Buck E, Petti F, Griffin G, Brown E, Ramnarine N, Iwata KK, Gibson N, Haley JD. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65:9455–9462. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- Thomson S, Petti F, Sujka-Kwok I, Epstein D, Haley JD. Kinase switching in mesenchymal-like non-small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clin Exp Metastasis. 2008;25:843–854. doi: 10.1007/s10585-008-9200-4. [DOI] [PubMed] [Google Scholar]
- Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174:175–183. doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumova L, Pombinho AR, Vojtechova M, Stancikova J, Gradl D, Krausova M, Sloncova E, Horazna M, Kriz V, Machonova O, Jindrich J, Zdrahal Z, Bartunek P, Korinek V. Monensin inhibits canonical Wnt signaling in human colorectal cancer cells and suppresses tumor growth in multiple intestinal neoplasia mice. Mol Cancer Ther. 2014;13:812–822. doi: 10.1158/1535-7163.MCT-13-0625. [DOI] [PubMed] [Google Scholar]
- Turner M, Schweighoffer E, Colucci F, Di Santo JP, Tybulewicz VL. Tyrosine kinase SYK: essential functions for immunoreceptor signalling. Immunol Today. 2000;21:148–154. doi: 10.1016/s0167-5699(99)01574-1. [DOI] [PubMed] [Google Scholar]
- Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shang Y. Epigenetic control of epithelial-to-mesenchymal transition and cancer metastasis. Exp Cell Res. 2013;319:160–169. doi: 10.1016/j.yexcr.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Liu S, Liu Z, Huang J, Pu X, Li J, Yang D, Deng H, Yang N, Xu J. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One. 2015;10:e0123976. doi: 10.1371/journal.pone.0123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am J Cancer Res. 2015;5:1602–1609. [PMC free article] [PubMed] [Google Scholar]
- Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011;9:1658–1667. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim NH, Cha SY, Ryu JK, Choi YJ, Kim J, Fearon ER, Weiss SJ. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006;8:1398–1406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- Yoon MJ, Kang YJ, Kim IY, Kim EH, Lee JA, Lim JH, Kwon TK, Choi KS. Monensin, a polyether ionophore antibiotic, overcomes TRAIL resistance in glioma cells via endoplasmic reticulum stress, DR5 upregulation and c-FLIP downregulation. Carcinogenesis. 2013;34:1918–1928. doi: 10.1093/carcin/bgt137. [DOI] [PubMed] [Google Scholar]
- Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2016 doi: 10.1038/onc.2016.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Lu Y, Li Q, Mao J, Hou Z, Yu X, Fan S, Li J, Gao T, Yan B, Wang B, Song B, Li L. Salinomycin suppresses TGF-beta1-induced epithelial-to-mesenchymal transition in MCF-7 human breast cancer cells. Chem Biol Interact. 2016;248:74–81. doi: 10.1016/j.cbi.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Zhang P, Sun Y, Ma L. ZEB1: at the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14:481–487. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Xia G, Dong S, Jiang ZX, Chen M. An iTEP-salinomycin nanoparticle that specifically and effectively inhibits metastases of 4T1 orthotopic breast tumors. Biomaterials. 2016;93:1–9. doi: 10.1016/j.biomaterials.2016.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]