Abstract
Background
Maintenance of lean muscle mass and related strength is associated with lower risk for numerous chronic diseases of aging in women.
Objective
To evaluate whether the association between dietary protein and lean mass differs by physical activity level, amino acid composition, and body mass index categories.
Design
Cross-sectional analysis of a prospective cohort.
Participants/setting
Postmenopausal women from the Women’s Health Initiative with body composition measurements by dual-energy X-ray absorptiometry (n=8,298).
Main outcome measures
Percent lean mass, percent fat mass and lean body mass index.
Statistical analyses performed
Linear regression models adjusted for scanner serial number, age, calibrated energy intake, race/ethnicity, neighborhood socioeconomic status, and recreational physical activity were used to determine the relationship between protein intake and body composition measures. Likelihood ratio tests and stratified analysis were used to investigate physical activity and body mass index as potential effect modifiers.
Results
Biomarker-calibrated protein intake was positively associated with percent lean mass; women in the highest protein quintile had 6.3 percentage points higher lean mass than the lowest quintile (P < 0.001). This difference rose to 8.5 percentage points for physically active women in the highest protein quintile (Pinteraction = 0.023). Percent fat mass and lean body mass index were both inversely related to protein intake (both P < 0.001). Physical activity further reduced percent fat mass (Pinteraction = 0.022) and lean body mass index (Pinteraction = 0.011). Leucine intake was associated with lean mass, as were branched chain amino acids combined (both P < 0.001), but not independent of total protein. All associations were observed for normal-weight, overweight, and obese women.
Conclusions
Protein consumption up to 2.02 g/kg body weight daily is positively associated with lean mass in postmenopausal women. Importantly, those that also engage in physical activity have the highest lean mass across body mass index categories.
Keywords: Dietary protein, leucine, physical activity, lean mass, fat mass
INTRODUCTION
Obesity and loss of lean mass, or sarcopenia, are thought to act jointly to increase risk for all-cause mortality.1 The loss of lean mass is hypothesized to contribute to the loss of insulin sensitivity and disturbances in metabolism that are common in overweight and obese individuals.2 Several studies suggest that protein intake is an independent dietary factor important for maintenance of lean mass and its function in glucose homeostasis, energy expenditure, and fat oxidation.3,4 High protein intake during weight loss has been shown to preserve lean mass and its function.5,6 In a recent overfeeding trial, individuals randomized to high protein diets containing 15 or 25% of total calories from protein experienced weight gain due to both increases in lean and fat mass, whereas those randomized to low protein (5%) diets gained fat mass but lost lean mass with attenuated total weight gain.7 More recent work on the effect of individual dietary and supplemental amino acids suggests an important role for specific amino acids on body composition. The branched-chain amino acid (BCAA) leucine has been shown to stimulate muscle protein synthesis, improve insulin sensitivity,8 and may prevent dietand age-related adiposity.9,10 Collectively, these findings support a beneficial role for total protein and specific amino acids on lean mass across a spectrum of behaviors including overeating and inactivity. Given the potential benefits of dietary protein, current recommendations for daily protein intake in older adults (0.8 g protein/kg body weight) have recently been questioned as insufficient for optimal lean mass health,11 and the European Society for Clinical Nutrition and Metabolism has recommended at least 1.0–1.2 g protein/kg body weight daily for healthy, older individuals.12
Despite evidence favoring the metabolic benefits of maintaining a high lean mass, and potential benefits of high-protein diets, the relationship between lean mass and metabolic health is less clear for overweight/obese women. The Monet Study of postmenopausal women supports an adverse relationship between lean mass and metabolic parameters in obese, sedentary older women. These investigators showed that the presence of a high lean body mass index (LBMI) [lean body mass (kg)/height (m2)] in sedentary postmenopausal women was independently associated with reduced insulin sensitivity, poor glucose disposal, and higher levels of circulating pro-inflammatory C-reactive protein.13 These observations suggest caution in recommending higher protein intakes in the absence of other behavioral modifications.
Physical activity is a well-established, independent determinant of both muscle mass and insulin sensitivity, though the intensity, frequency, and duration required for individual benefit remain unclear.14 Further, there is a lack of epidemiological evidence on the impact of physical activity as a modifier of body composition in response to different levels of dietary protein intake, despite evidence that protein demands increase with physical activity.6
To the knowledge of the authors, no studies have evaluated the relationship between dietary protein on muscle and fat mass by physical activity levels or considered the potential impact of body mass index (BMI) on these associations. Associations between total dietary protein (or amino acids, specifically leucine) and lean mass, fat mass, or LBMI were tested, and potential interactions with physical activity were investigated in 8,298 postmenopausal women who participated in the Women’s Health Initiative (WHI) and for whom body composition was assessed by dual-energy X-ray absorptiometry (DXA). Secondarily, these associations were then assessed for differences by BMI category.
PARTICIPANTS AND METHODS
Participants
The WHI included three clinical trials (CT) and an Observational Study (OS) comprising 161,808 postmenopausal women, ages 50–79 years, that launched in 1991 and completed enrollment in 1998.15 Human subjects review committees at 40 participating institutions across the U.S. reviewed and approved each study, and individual participants provided written informed consent. Of 11,020 women who participated in the DXA cohort (conducted at the Arizona, Pittsburgh, and Alabama sites), 10,635 had baseline body composition measures available. Of these, 403 women were excluded from the analysis due to implausible reported energy intake (< 600 or > 5,000 kcal/d), an additional 816 women were excluded for missing data needed to calculate calibrated protein,16 and an additional 1,118 women were excluded for missing data on physical activity. The final study population comprised 8,298 postmenopausal women.
Body size measurements
Height and weight were measured using standardized protocols by trained study personnel in study clinics, BMI was calculated as mass (kg)/height (m2), and LBMI was calculated as lean body mass (kg)/height (m2). Body composition was assessed using whole-body DXA scans, which included measurements of lean (soft tissue) mass and fat mass. All DXA scans were operated by a trained technician certified by the manufacturer. Percent lean mass and percent fat mass were calculated and served as the primary outcome measures throughout the analysis. Detailed methods and quality control are described elsewhere.17
Dietary assessment
Total energy and protein intake at baseline (total as well as animal and plant-based protein) were calculated from the self-administered, 122-item WHI Food Frequency Questionnaire (FFQ).18 Intakes of individual amino acids were computed using estimates derived from the Nutrition Data System for Research nutrient database developed by the Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN.19 Protein intake was evaluated using g protein/kg body weight to be consistent with the current recommended daily allowance (RDA).20 Calibrated energy and calibrated protein intake were calculated according to the WHI Nutritional Biomarkers Study.16 Briefly, the Dietary Modification trial (n = 544) used a doubly labeled water protocol to estimate total energy expenditure and a urinary nitrogen protocol to estimate protein consumption. These results showed that FFQ estimates for total energy were considerably underestimated, and protein was modestly underestimated. The calibrated protein and energy equations correct for these estimations and are therefore considered more accurate. The regression model for calibrated energy equation includes age, BMI, race/ethnicity, income, and physical activity. The calibration equation for protein includes age, BMI, race/ethnicity, income, and education.
Physical activity and other participant characteristics
Recreational physical activity was measured using a validated questionnaire developed for the WHI that determines frequency and duration of several types of activities. In this study, total activity measured is reported as the ratio of work metabolic rate to resting metabolic rate according to the 2011 Ainsworth compendium (MET-hr/wk).21 The minimum American Heart Association recommendation is 7.5 MET-hr/wk, which equates to 150 minutes of moderate117 intensity aerobic physical activity weekly, or 75 minutes of vigorous-intensity aerobic physical activity, or a combination of the two.22,23 Women were stratified by those that achieved the minimum recommendation, and those that did not. Study participants provided general information on demographics and health using study-specific questionnaires completed during the baseline clinic visit. Questionnaires were reviewed by clinic staff for completeness prior to entry into the database. Neighborhood socioeconomic status (NSES) was derived through linkage of individual participant addresses to Federal Information Processing Standards codes from the 2000 U.S. census and tract-level socioeconomic data, with summary measures calculated according to previously described algorithms.24
Statistical analysis
Baseline characteristics of study participants were compared across quintiles of calibrated protein intake [quintiles presented as mean (range), quintile 1: 0.79 (0.54–0.89); quintile 2: 0.94 (0.89–0.99); quintile 3: 1.04 (0.99–1.09); quintile 4; 1.15 (1.09–1.22); quintile 5: 1.35 (1.22–2.02)]. Continuous variables are summarized as mean ± standard deviation. Mean differences are expressed as mean (95% confidence interval (CI)). Protein intake was divided into quintiles in order to avoid assumptions about linearity and to allow visualization of any threshold effects such as U or J-shaped curves. Significant differences across quintiles of protein intake for baseline characteristics were calculated using ANOVA for continuous variables and chi-squared tests for categorical variables. Correlations were measured using Pearson’s correlation coefficients (rho). Associations between protein intake and body composition measures were calculated using linear regression models, adjusted for scanner serial number and variables recognized in the literature to be associated with body composition: age (continuous) and calibrated energy intake (continuous; log-transformed) in the partially adjusted model, and additionally for race/ethnicity [non-Hispanic white, black, Hispanic, other], NSES (continuous), and recreational physical activity (quintiles; MET-hr/wk) in the fully adjusted model. Potential confounders for adjustment in the models were chosen from previously published literature,25,26 and no assessment was made for confounding. Beta-coefficients are interpreted in the same units as the variable described. For these models, beta-coefficients are percentage points for percent lean mass and percent fat mass and are kg/m2 for LBMI. Additional adjustment for alcohol intake, smoking, multivitamin use, oral contraception use, unopposed estrogen use, estrogen + progestin use, history of cancer, history of cardiovascular disease, and history of diabetes did not substantially change the estimates (data not shown). Trends were tested by modeling protein quintiles as an ordinal variable and are reported in each table. In the manuscript text, reported P-values supporting the difference between the fifth and first quintiles of protein intake are calculated from tests assessing the difference in quintile means, not from the tests for trend in each table. Normality was assessed visually using histograms. Physical activity and BMI were investigated as potential effect modifiers of the relationship between protein and body composition using likelihood ratio tests for multiplicative interactions and stratified analysis. BMI was categorized according to standard World Health Organization cut points: normal (18.5–24.9), overweight (25–29.9), and obese (≥ 30). Underweight women (BMI < 18.5; n = 74) were excluded in BMI-stratified analyses due to small sample size. All statistical analyses were conducted using Stata 14.0 (StataCorp, College Station, TX).27 All statistical tests were two-sided with alpha set to 0.05.
RESULTS
Descriptive analysis of participant characteristics
Participant characteristics were compared across quintiles of total calibrated protein intake (g/kg body weight) (Table 1). Black and Native American women were more likely to report lower protein intake than non-Hispanic white and Hispanic women. In general, women that reported higher protein intake also reported higher NSES, physical activity, and alcohol intake, and had a lower BMI, percent fat mass, and slightly lower LBMI, than those that reported lower protein intake. There was a slight inverse relationship between total calibrated energy intake and protein intake (rho = −0.21). Smoking status was not related to protein intake.
Table 1.
Baseline participant characteristics by quintiles of calibrated protein intake (g/kg body weight) in the Women’s Health Initiative Bone Mineral Density cohort (n = 8,298)
| Characteristics | All | Quintile of calibrated protein intake (g/kg body weight) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| n | 8298 | 1660 | 1660 | 1659 | 1660 | 1659 |
| Calibrated protein intake (g/kg body weight), mean (range) |
1.05 (0.54–2.02) |
0.79 (0.54–0.89) |
0.94 (0.89–0.99) |
1.04 (0.99–1.09) |
1.15 (1.09–1.22) |
1.35 (1.22–2.02) |
| Demographics | ||||||
| Age (y), mean ± SD*** | 63.2 ± 7.4 | 65.3 ± 7.2 | 64.4 ± 7.4 | 63.9 ± 7.3 | 62.6 ± 7.1 | 60.2 ± 7.0 |
| Race/ethnicity, n (%) | ||||||
| Non-Hispanic white*** | 6487 (78.2) | 814 (49.0) | 1273 (76.7) | 1416 (85.4) | 1476 (88.9) | 1508 (90.9) |
| Hispanic*** | 482 (5.8) | 49 (3.0) | 110 (6.6) | 102 (6.2) | 113 (6.8) | 108 (6.5) |
| Black*** | 1175 (14.2) | 761 (45.8) | 235 (14.2) | 113 (6.8) | 43 (2.6) | 23 (1.4) |
| Asian*** | 26 (0.31) | 0 (0.00) | 8 (0.48) | 2 (0.12) | 6 (0.36) | 10 (0.60) |
| Native American*** | 96 (1.2) | 28 (1.7) | 27 (1.6) | 21 (1.3) | 13 (0.78) | 7 (0.72) |
| Other*** | 32 (0.39) | 8 (0.48) | 7 (0.42) | 5 (0.30) | 9 (0.54) | 3 (0.18) |
| Neighborhood socioeconomic statusa,b, mean ± SD*** |
71.8 ± 9.1 | 66.1 ± 10.7 | 71.1 ± 8.9 | 72.9 ± 8.2 | 74.0 ± 7.6 | 75.1 ± 7.1 |
| Body size and mass, mean ± SD | ||||||
| Body mass index (kg/m2)*** | 28.2 ± 5.9 | 33.9 ± 6.6 | 29.9 ± 4.8 | 27.7 ± 4.3 | 25.9 ± 3.7 | 23.5 ± 3.4 |
| Lean body mass index (kg/m2)*** | 14.5 ± 1.8 | 15.9 ± 2.0 | 14.8 ± 1.7 | 14.3 ± 1.6 | 13.9 ± 1.4 | 13.5 ± 1.3 |
| Whole body fat mass, corrected (%)*** | 43.7 ± 7.3 | 48.8 ± 6.1 | 46.1 ± 6.0 | 44.0 ± 6.1 | 41.9 ± 6.2 | 37.8 ± 6.9 |
| Whole body lean mass, corrected (%)*** | 53.4 ± 7.0 | 48.6 ± 5.9 | 51.1 ± 5.8 | 53.2 ± 5.9 | 55.1 ± 6.0 | 59.0 ± 6.7 |
| Height (cm)*** | 161.6 ± 6.2 | 163.7 ± 6.0 | 162.4 ± 6.0 | 161.5 ± 6.0 | 160.8 ± 6.2 | 159.7 ± 6.2 |
| Weight (kg)*** | 73.6 ± 15.9 | 90.7 ± 17.3 | 78.6 ± 12.3 | 72.1 ± 10.8 | 66.9 ± 8.8 | 59.9 ± 7.9 |
| Physical activity (MET-hr/wk)c, mean ± SD*** | 11.4 ± 13.6 | 7.52 ± 12.9 | 9.81 ± 12.2 | 11.7 ± 13.4 | 12.8 ± 13.8 | 15.4 ± 16.0 |
| Smoking status, n (%) | ||||||
| Never | 4420 (53.9) | 891 (54.6) | 907 (55.0) | 863 (52.5) | 869 (52.9) | 890 (54.3) |
| Past | 3108 (37.9) | 599 (36.7) | 613 (37.2) | 647 (39.4) | 642 (39.1) | 607 (37.1) |
| Current | 676 (8.2) | 141 (8.7) | 129 (7.8) | 133 (8.1) | 132 (8.0) | 141 (8.6) |
| Alcohol intake (drinks/wk), mean ± SD | 1.79 ± 4.2 | 0.95 ± 3.3 | 1.48 ± 3.7 | 1.82 ± 4.3 | 2.16 ± 4.6 | 2.53 ± 4.9 |
| Daily dietary intaked, mean ± SD | ||||||
| Total calibrated energy (kcal)*** | 2070 ± 204 | 2152 ± 265 | 2087 ± 207 | 2055 ± 190 | 2036 ± 162 | 2021 ± 149 |
| Total calibrated protein (g)*** | 75.5 ± 11.5 | 71.6 ± 12.9 | 73.8 ± 11.3 | 75.0 ± 11.1 | 76.8 ± 10.0 | 80.4 ± 9.9 |
| Animal protein (g)*** | 47.8 ± 24.1 | 38.3 ± 22.7 | 43.2 ± 22.4 | 46.9 ± 22.2 | 50.8 ± 22.0 | 59.9 ± 25.1 |
| Vegetable protein (g)*** | 20.3 ± 8.8 | 16.3 ± 7.9 | 18.7 ± 7.8 | 20.3 ± 8.4 | 21.6 ± 8.4 | 24.5 ± 9.2 |
| Animal-to-vegetable protein ratio*** | 2.5 (± 1.2) | 2.5 (± 1.2) | 2.5 (± 1.2) | 2.5 (± 1.3) | 2.5 (± 1.0) | 2.6 (± 1.2) |
| Total leucine (g)*** | 5.5 ± 2.5 | 4.3 ± 2.3 | 4.9 ± 2.2 | 5.4 ± 2.3 | 5.8 ± 2.2 | 6.8 ± 2.5 |
| Total isoleucine (g)*** | 3.1 ± 1.4 | 2.5 ± 1.3 | 2.8 ± 1.3 | 3.1 ± 1.3 | 3.3 ± 2.2 | 3.9 ± 1.4 |
| Total valine (g)*** | 3.6 ± 1.6 | 2.8 ± 1.5 | 3.2 ± 1.4 | 3.5 ± 1.5 | 3.8 ± 1.4 | 4.4 ± 1.6 |
| Total BCAA (leucine + isoleucine + valine) (g)*** | 12.1 ± 5.4 | 9.7 ± 5.1 | 11.0 ± 5.0 | 12.0 ± 5.0 | 12.9 ± 4.9 | 15.1 ± 5.5 |
P < 0.001; significance was calculated using ANOVA for continuous variables and chi-squared tests for categorical variables
Missing data for neighborhood socioeconomic status (n = 142), smoking status (n = 94), and alcohol intake (n = 4)
Neighborhood socioeconomic status (NSES) was derived through linkage of individual participant addresses to Federal Information Processing Standards codes from the 2000 U.S. census and tract-level socioeconomic data, with summary measures calculated according to previously described algorithms
Physical activity was measured as the ratio of work metabolic rate to resting metabolic rate (MET-hr/wk) according to the 2011 Ainsworth compendium
Total energy intake at baseline was calculated from the self-administered, 122-item WHI Food Frequency Questionnaire (FFQ)
Association between protein intake and lean mass, fat mass, and lean body mass index
Percent fat and percent lean mass were negatively correlated (rho < −0.99). LBMI was only modestly correlated with percent lean (rho = −0.24) and percent fat (rho = 0.26) mass. Weight was positively correlated with percent lean (rho = 0.71) and negatively correlated with percent fat (rho = −0.70) mass. In both partially and fully adjusted models, protein intake was strongly associated with percent lean mass (positive) and percent fat mass (negative) in a dose178 response manner (Table 2). In the fully adjusted model, women in quintile 5 of protein intake had a mean 6.3 [95% CI 5.9 to 6.7] percentage points higher lean mass than women in the lowest quintile (P < 0.001). Even incrementally higher levels in reported protein intake (e.g. quintile 2 versus 1; a mean difference of 0.15 g/kg body weight of protein) were associated with higher mean lean mass [1.1 (95% CI 0.8 to 1.5) percentage points higher; P < 0.001]. Likewise, protein intake was strongly associated with lower percent fat mass (quintiles 2– 5 versus 1, all P < 0.001). Individuals in quintile 5 of protein intake had a mean 6.6 (95% CI −7.1 to −6.2) percentage points lower fat mass than individuals in quintile 1. The same dose-dependent responses were observed for black and non-Hispanic white women separately (data not shown; all P <0.001). Stratification by BMI showed a similar dose-dependent relationship across all groups, with individuals in quintile 5 of protein intake having a mean 4.4 (95% CI 3.3 to 5.5), 2.9 (95% CI 2.2 to 3.6), and 3.6 (95% CI 2.4 to 4.8) percentage points higher lean mass than the reference group in normal-weight, overweight, and obese women, respectively. The likelihood ratio test for interaction between protein intake and BMI was significant for percent lean mass (P = 0.001) and percent fat mass (P = 0.001) but not LBMI (P = 0.081).
Table 2.
Association between quintiles of calibrated protein intake (g/kg body weight) and measures of body composition, overall and by BMIa in the Women's Health Initiative Bone Mineral Density cohort: β-coefficient (95% confidence interval)b
| Outcome measure |
Protein quintile |
All participants | Stratified by BMI category | |||
|---|---|---|---|---|---|---|
| Model 1c | Model 2d | Normal 18.5–24.9 |
Overweight 25.0–29.9 |
Obese ≥ 30.0 |
||
| Lean mass %e | ||||||
| 1 | Ref | Ref | Ref | Ref | Ref | |
| 2 | 0.8 (0.4, 1.1) | 1.1 (0.8, 1.5) | 0.7 (−0.4, 1.9) | 1.0 (0.5, 1.5) | 1.1 (0.6, 1.5) | |
| 3 | 2.1 (1.7, 2.4) | 2.3 (1.9, 2.6) | 1.3 (0.2, 2.4) | 1.4 (0.9, 2.0) | 2.2 (1.7, 2.7) | |
| 4 | 3.4 (3.0, 3.7) | 3.5 (3.1, 3.9) | 2.2 (1.1, 3.3) | 2.2 (1.6, 2.8) | 2.7 (2.1, 3.4) | |
| 5 | 6.5 (6.1, 6.9) | 6.3 (5.9, 6.7) | 4.4 (3.3, 5.5) | 2.9 (2.2, 3.6) | 3.6 (2.4, 4.8) | |
| Ptrendf | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Fat mass %e | ||||||
| 1 | Ref | Ref | Ref | Ref | Ref | |
| 2 | −0.8 (−1.2, −0.5) | −1.2 (−1.5, −0.8) | −0.8 (−2.0, 0.3) | −1.0 (−1.6, −0.5) | −1.1 (−1.6, −0.7) | |
| 3 | −2.2 (−2.5, −1.8) | −2.4 (−2.7, −2.0) | −1.3 (−2.4, −0.2) | −1.5 (−2.0, −0.9) | −2.3 (−2.8, −1.8) | |
| 4 | −3.6 (−3.9, −3.2) | −3.6 (−4.0, −3.3) | −2.3 (−3.4, −1.2) | −2.2 (−2.8, −1.6) | −2.9 (−3.5, −2.2) | |
| 5 | −6.9 (−7.2, −6.5) | −6.6 (−7.1, −6.2) | −4.6 (−5.7, −3.5) | −3.0 (−3.7, −2.3) | −3.9 (−5.1, −2.6) | |
| Ptrendf | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| LBMIe,g | ||||||
| 1 | Ref | Ref | Ref | Ref | Ref | |
| 2 | −0.8 (−0.8, −0.7) | −0.5 (−0.6, −0.4) | −0.2 (−0.4, 0.1) | −0.2 (−0.3, −0.1) | −0.5 (−0.6, −0.3) | |
| 3 | −1.0 (−1.1, −0.9) | −0.7, −0.8, −0.6) | −0.2 (−0.4, 0.0) | −0.3 (−0.5, −0.2) | −0.5 (−0.7, −0.4) | |
| 4 | −1.3 (−1.4, −1.2) | −0.9 (−1.0, −0.8) | −0.4 (−0.6, −0.1) | −0.3 (−0.5, −0.2) | −0.7 (−1.0, −0.5) | |
| 5 | −1.6 (−1.7, −1.5) | −1.1 (−1.2, −1.0) | −0.4 (−0.6, −0.2) | −0.4 (−0.6, −0.3) | −0.6 (−1.0, −0.1) | |
| Ptrendf | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
BMI: Body Mass Index
Beta-coefficients are presented as a mean difference from the reference and are interpreted in the same units as the variable described (percentage points for lean mass % and fat mass % and kg/m2 for LBMI)
Linear regression model adjusted for age and calibrated energy intake (continuous; log-transformed kcal)
Further adjusted for race/ethnicity (non-Hispanic white, black, Hispanic, other), neighborhood socioeconomic status (continuous), physical activity (MET-hr/wk; quintiles), and scanner serial number
Likelihood ratio test for BMI-by-protein interaction on percent lean mass (P = 0.001), percent fat mass (P = 0.001), and LBMI (P = 0.081)
Ptrend calculated by modeling protein quintiles as an ordinal variable
LBMI: Lean Body Mass Index. LBMI was calculated as: lean body mass (kg)/height (m2)
Physical activity modifies the association between protein and lean/fat mass overall and across BMI categories
Tests for two-way interactions revealed that physical activity modifies the association between protein and percent lean mass (P = 0.023), percent fat mass (P = 0.022), and LBMI (P = 0.011). For example, women in quintile 5 for protein intake and ≥ 7.5 MET-hr/wk had a mean of 8.5 (95% CI 8.0 to 9.0) percentage points higher lean mass than women in quintile 1 for protein intake with < 7.5 MET-hr/wk (Table 3). Among women that did not reach the physical activity recommendation, however, women in quintile 5 for protein intake had only a mean of 5.9 (95% CI 5.4 to 6.5) percentage points higher lean mass than the reference. Further stratification by non-Hispanic white and black women showed the same patterns across all comparisons (data not shown). When the full cohort was stratified by BMI, physically active women in quintile 5 of protein intake had higher lean mass across all BMI categories compared to both quintile 1 of protein of active women and to less-active women across all protein quintiles. In models stratified by physical activity group (data not shown), significant trends were found across protein quintiles for all women and within each BMI category (all Ptrend < 0.05). The highest mean percent lean mass was observed among normal-weight, physically active women with high protein intake (60.1%) compared to 54.7% and 53.7% for active overweight and obese women, respectively (Supplemental Table 1). Notably, active obese women that consumed at or above quintile 3 of protein had a mean lean mass (51.6%) comparable to that of active overweight women in quintile 1 of protein intake (51.5%). The reverse pattern was observed for percent fat mass. For example, active, normal-weight women in protein quintile 1 had the lowest mean fat mass (36.6%) compared to 42.3% and 43.4% for active overweight and obese women, respectively, whereas inactive normal-weight, overweight, and obese women in protein quintile 1 had means of 42.3%, 46.8%, and 49.4% fat mass respectively. The same pattern was not observed with LBMI. The likelihood ratio test for the 3-way interaction between BMI, physical activity, and protein was not significant for percent lean mass (P = 0.87), percent fat mass (P = 0.86) or LBMI (P = 0.45). However, there were statistically significant two-way interactions between protein and BMI for percent lean mass (P = 0.001) and percent fat mass (P = 0.001), though not for LBMI (P = 0.13).
Table 3.
Effect of physical activity on the association between quintiles of calibrated protein intake (g/kg body weight) and measures of body composition, overall and stratified by BMIa in the Women's Health Initiative Bone Mineral Density cohort: β-coefficient (95% confidence interval)b,c
| Outcome measure |
Physical activity level (MET-hr/wk)d |
Protein quintile |
Stratified by physical activity |
Additionally stratified by BMI category | ||
|---|---|---|---|---|---|---|
| Normal 18.5–24.9 |
Overweight 25.0–29.9 |
Obese ≥ 30.0 |
||||
| Lean mass %e | ||||||
| < 7.5 | 1 | Reference | Reference | Reference | Reference | |
| < 7.5 | 2 | 1.9 (0.6, 1.5) | 0.3 (−1.3, 1.9) | 1.0 (0.3, 1.7) | 1.0 (0.5, 1.5) | |
| < 7.5 | 3 | 2.0 (1.5, 2.5) | 0.6 (−0.9, 2.2) | 1.4 (0.6, 2.1) | 1.9 (1.3, 2.5) | |
| < 7.5 | 4 | 3.4 (2.9, 3.9) | 1.7 (0.2, 3.2) | 2.1 (1.3, 2.8) | 3.0 (2.3, 3.8) | |
| < 7.5 | 5 | 5.9 (5.4, 6.5) | 3.5 (2.0, 5.1) | 2.6 (1.7, 3.5) | 3.1 (1.4, 4.7) | |
| ≥ 7.5 | 1 | 1.2 (0.7, 1.7) | 0.8 (−1.1, 2.7) | 1.2 (0.4, 2.0) | 1.0 (0.5, 1.5) | |
| ≥ 7.5 | 2 | 2.7 (2.2, 3.1) | 2.0 (0.4, 3.7) | 2.2 (1.5, 2.9) | 2.3 (1.7, 2.9) | |
| ≥ 7.5 | 3 | 4.2 (3.7, 4.7) | 2.8 (1.3, 4.3) | 2.6 (1.9, 3.4) | 3.6 (3.0, 4.3) | |
| ≥ 7.5 | 4 | 5.3 (4.9, 5.8) | 3.4 (1.9, 4.9) | 3.4 (2.6, 4.2) | 3.6 (2.7, 4.5) | |
| ≥ 7.5 | 5 | 8.5 (8.0, 9.0) | 5.8 (4.3, 7.3) | 4.3 (3.5, 5.2) | 5.5 (3.8, 7.3) | |
| Fat mass %e | ||||||
| < 7.5 | 1 | Reference | Reference | Reference | Reference | |
| < 7.5 | 2 | −1.2 (−1.6, −0.7) | −0.4 (−2.0, 1.3) | −1.0 (−1.7, −0.3) | −1.1 (−1.6, −0.6) | |
| < 7.5 | 3 | −2.1 (−2.6, −1.6) | −0.7 (−2.2, 0.9) | −1.4 (−2.2, −0.7 | −2.0 (−2.6, −1.4) | |
| < 7.5 | 4 | −3.6 (−4.1, −3.1 | −1.8 (−3.4, −0.2 | −2.1 (−2.9, −1.3 | −3.2 (−4.0, −2.4) | |
| < 7.5 | 5 | −6.2 (−6.8, −5.7 | −3.7 (−5.3, −2.2 | −2.7 (−3.6, −1.8 | −3.2 (−4.9, −1.6) | |
| ≥ 7.5 | 1 | −1.3 (−1.8, −0.8 | −0.9 (−2.9, 1.0 | −1.2 (−2.1, −0.4 | −1.1 (−1.6, −0.5) | |
| ≥ 7.5 | 2 | −2.8 (−3.3, −2.4 | −2.2 (−3.9, −0.5 | −2.3 (−3.1, −1.6 | −2.4 (−3.0, −1.8) | |
| ≥ 7.5 | 3 | −4.4 (−4.9, −3.9 | −2.9 (−4.4, −1.4 | −2.7 (−3.5, −2.0 | −3.8 (−4.5, −3.1) | |
| ≥ 7.5 | 4 | −5.6 (−6.1, −5.1 | −3.6 (−5.2, −2.1 | −3.5 (−4.3, −2.8 | −3.8 (−4.7, −2.8) | |
| ≥ 7.5 | 5 | −8.9 (−9.4, −8.4 | −6.1 (−7.6, −4.6 | −4.5 (−5.4, −3.6 | −5.9 (−7.6, −4.2) | |
| LBMIe,f | ||||||
| < 7.5 | 1 | Reference | Reference | Reference | Reference | |
| < 7.5 | 2 | −0.6 (−0.7, −0.5 | −0.3 (−0.6, 0.0 | −0.2 (−0.4, −0.0 | −0.6 (−0.7, −0.4) | |
| < 7.5 | 3 | −0.8 (−0.9, −0.7 | −0.3 (−0.6, 0.0 | −0.4 (−0.6, −0.2 | −0.7 (−0.9, −0.5) | |
| < 7.5 | 4 | −1.0 (−1.1, −0.9 | −0.5 (−0.8, −0.2 | −0.4 (−0.6, −0.2 | −0.7 (−1.0, −0.4) | |
| < 7.5 | 5 | −1.2 (−1.4, −1.1 | −0.5 (−0.8, −0.2 | −0.6 (−0.8, −0.4 | −0.9 (−1.5, 0.3) | |
| ≥ 7.5 | 1 | −0.2 (−0.4, −0.1 | −0.1 (−0.5, 0.3 | −0.0 (−0.3, 0.2 | −0.2 (−0.3, −0.0) | |
| ≥ 7.5 | 2 | −0.6 (−0.7, −0.5 | −0.1 (−0.5, 0.2 | −0.2 (−0.4, 0.0 | −0.5 (−0.7, −0.3) | |
| ≥ 7.5 | 3 | −0.8 (−0.9, −0.7 | −0.2 (−0.5, 0.1 | −0.3 (−0.5, −0.1 | −0.5 (−0.7, −0.2) | |
| ≥ 7.5 | 4 | −1.0 (−1.2, −0.9 | −0.4 (−0.7, −0.1 | −0.3 (−0.5, −0.1 | −0.9 (−1.2, −0.6) | |
| ≥ 7.5 | 5 | −1.2 (−1.3, −1.1 | −0.4 (−0.7, −0.1 | −0.4 (−0.6, −0.1 | −0.4 (−1.0, −0.2) | |
BMI: Body Mass Index.
Linear regression model adjusted for age, energy intake (continuous; log-transformed kcal), race/ethnicity (non-Hispanic white, black, Hispanic, other), neighborhood socioeconomic status (continuous), and scanner serial number
Beta-coefficients are presented as a mean difference from the reference and are interpreted in the same units as the variable described (percentage points for lean mass % and fat mass % and kg/m2 for LBMI)
Physical activity was measured as the ratio of work metabolic rate to resting metabolic rate (MET-hr/wk) according to the 2011 Ainsworth compendium
Likelihood ratio test for protein-by-physical activity interaction on percent lean mass (P = 0.023), percent fat mass (P = 0.022), and LBMI (P = 0.011)
LBMI: Lean Body Mass Index. LBMI was calculated as: lean body mass (kg)/height (m2)
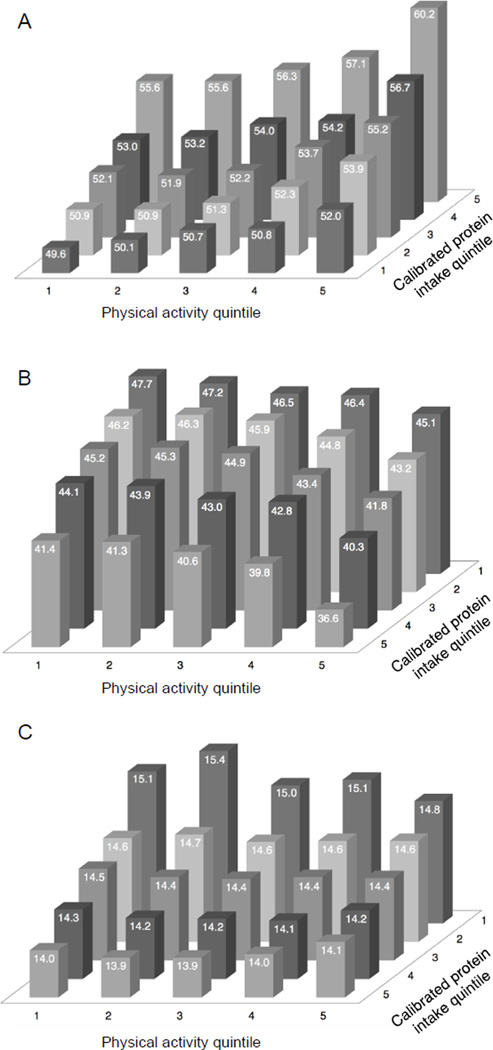
To assess the effect of habitual physical activity and protein intake on percent lean mass and percent fat mass, participants were further categorized by physical activity quintiles. Women in quintile 5 of both protein intake and physical activity had the highest adjusted mean lean mass (60.2%), whereas women in quintile 1 of both protein and physical activity had the lowest mean lean mass (49.6%) (Figure 1A). Overall, the association supports a dose-response relationship of protein intake plus physical activity on percent lean mass. The results do not differ by BMI category (data not shown). Results for percent fat mass (Figure 1B) were inverse, with higher protein intake and higher activity associated with lower percent fat mass. Higher protein intake was also associated with lower LBMI; however, the effect of physical activity on LBMI was modest with higher levels of physical activity resulting in a slightly lower LBMI only among those in quintile 1 of protein intake, and no obvious effect in other quintiles (Figure 1C). In these analyses the likelihood ratio test showed a significant interaction between protein and physical activity on percent lean mass (P < 0.001), percent fat mass (P < 0.001), and LBMI (P = 0.012).
Figure 1. Adjusted mean values of (A) percent lean mass, (B) percent fat mass, and (C) lean body mass index (LBMI) across quintiles of calibrated protein intake and physical activity.
Multivariate linear regression models are adjusted for age, calibrated energy intake (continuous; log-transformed kcal), race/ethnicity (non-Hispanic white, black, Hispanic, other), neighborhood socioeconomic status (continuous), and scanner serial number. Likelihood ratio tests for interaction between protein and physical activity on percent lean mass (P < 0.001), percent fat mass (P < 0.001), and LBMI (P = 0.012). Note that (B) and (C) have a reversed protein axis for improved visualization.
Association between branched chain amino acids and percent lean or fat mass
When compared to the lowest quintile of leucine intake, those in quintile 5 had a mean 12.6 (95% CI 11.9 to 13.2) percentage points greater lean mass (P < 0.001; Table 4). Total BCAA showed a similar relationship. When total amino acid intake minus leucine (or minus BCAA), as well as other amino acid combinations, were explored for evidence of independent effects of specific amino acids, there were no differences in the observed associations (data not shown). Like total protein, high leucine intake was associated with less fat mass and lower LBMI, although the relationship with LBMI was weaker. The high correlation (rho = 0.99) between leucine and (uncalibrated) total protein, however, limits testing of any independent effects of leucine.
Table 4.
Association between intake quintiles (g/kg body weight) of leucine, and BCAAa (as well as all amino acids minus leucine and BCAA) and measures of body composition in the Women's Health Initiative Bone Mineral Density cohort: β-coefficient (95% confidence interval)b,c
| Outcome measure |
Quintile | Leucine intake | Branched-chain amino acid intake | ||
|---|---|---|---|---|---|
| Leucine | Non-leucine | BCAA | Non-BCAA | ||
| Lean mass % | |||||
| 1 | Ref | Ref | Ref | Ref | |
| 2 | 3.7 (3.3, 4.2) | 4.1 (3.6, 4.5) | 3.7 (3.3, 4.2) | 4.1 (3.7, 4.6) | |
| 3 | 6.2 (5.7, 6.7) | 6.7 (6.2, 7.2) | 6.3 (5.8, 6.8) | 6.8 (6.3, 7.3) | |
| 4 | 8.5 (8.0, 9.0) | 9.3 (8.8, 9.9) | 8.6 (8.1, 9.2) | 9.4 (8.8, 9.9) | |
| 5 | 12.6 (11.9, 13.2) | 13.5 (12.9, 14.2) | 12.8 (12.1, 13.4) | 13.6 (13.0, 14.2) | |
| Ptrendd | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Fat mass % | |||||
| 1 | Ref | Ref | Ref | Ref | |
| 2 | −3.9 (−4.4, −3.4) | −4.3 (−4.8, −3.8) | −3.9 (−4.4, −3.5) | −4.3 (−4.8, −3.9) | |
| 3 | −6.6 (−7.1, −6.1) | −7.1 (−7.6, −6.6) | −6.7 (−7.2, −6.2) | −7.2 (−7.7, −6.7) | |
| 4 | −8.9 (−9.5, −8.4) | −9.8 (−10.4, −9.3) | −9.1 (−9.7, −8.6) | −9.9 (−10.4, −9.3) | |
| 5 | −13.3 (−13.9, −12.6) | −14.2 (−14.9, −13.6) | −13.5 (−14.1, −12.8) | −14.3 (−15.0, −13.7) | |
| Ptrendd | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| LBMIe | |||||
| 1 | Ref | Ref | Ref | Ref | |
| 2 | −0.8 (−0.9, −0.7) | −0.9 (−1.0, −0.8) | −0.8 (−1.0, −0.7) | −0.9 (−1.0, −0.8) | |
| 3 | −1.4 (−1.5, −1.2) | −1.4 (−1.6, −1.3) | −1.4 (−1.5, −1.2) | −1.4 (−1.6, −1.3) | |
| 4 | −1.8 (−2.0, −1.7) | −2.0 (−2.1, −1.8) | −1.9 (−2.0, −1.7) | −2.0 (−2.1, −1.8) | |
| 5 | −2.6 (−2.8, −2.5) | −2.8 (−3.0, −2.6) | −2.7 (−2.8, −2.5) | −2.8 (−3.0, −2.7) | |
| Ptrendd | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
BCAA: branched chain amino acids
Linear regression model adjusted for age, energy intake (continuous; log-transformed kcal), race/ethnicity (non-Hispanic white, black, Hispanic, other), neighborhood socioeconomic status (continuous), physical activity (MET-hr/wk; quintiles), and scanner serial number
Beta-coefficients are presented as a mean difference from the reference and are interpreted in the same units as the variable described (percentage points for lean mass % and fat mass %, and kg/m2 for LBMI)
Ptrend calculated by modeling amino acid quintiles as an ordinal variable
LBMI: lean body mass index. LBMI was calculated as: lean body mass (kg)/height (m2)
DISCUSSION
These results support a strong positive relationship between protein consumption and percent lean mass in postmenopausal women, and that physical activity modifies this relationship across BMI categories. For women with the highest intake of protein, percent lean mass was higher than all other quintiles; women in the fifth quintile of protein intake had a mean 6.5 (95% CI 6.1 to 6.9) percentage points higher lean mass than the lowest quintile (Table 2). For women with the highest intake of protein and who also reported the highest level of physical activity, percent lean mass was higher than all other quintiles; this top group had a mean 8.5 (95% CI 8.0 to 9.0) percentage points higher lean mass, and −8.9 (95% CI −9.4 to −8.4) percentage points lower fat mass, than inactive women in the lowest protein quintile (Table 3). According to a table developed by classic body composition scientists Jackson and Pollock,28 for women over age 56 years, a body fat percentage of 26–31% is considered to be within an ideal range, 32–37% is considered overweight, and 38% or above is considered obese. The difference between each of these categories is about 6 percentage points; this is less than the mean 8.9 percentage-point difference in percent fat observed in the current study between the highest and lowest categories of combined protein and physical activity (Table 3), as well as less than the mean 6.9 percentage-point difference observed with the highest and lowest categories of protein intake alone (Table 2). The combined benefit of high dietary protein and physical activity on higher percent lean mass and lower fat mass was present for women across all BMI groups. These observed differences could have important clinical implications, particularly for patients that have trouble reducing total caloric intake. In this study, however, less than 5% of women were in the 26–31% body fat range, regardless of protein intake or physical activity level.
While supplementation with leucine and BCAA has been shown to improve muscle mass, prevent loss, and enhance function, particularly in older individuals,9,29 the highly correlated nature of individual amino acids with total protein in this study limits the interpretation of the association between BCAA or leucine and percent lean mass.
In multiple cohorts, high lean mass has protective health outcomes. Allison et al. showed that in two large epidemiological studies, fat loss, but not total weight loss (indicating a relative increase in lean mass) was associated with reduced overall mortality.30 Others showed that proportionally higher lean mass had lower overall mortality among patients with diastolic heart failure, independent of fat mass.31 A large body of epidemiological evidence suggests that a higher BMI later in life is protective against overall mortality; however, these studies have not examined the effect of percent lean or fat mass.32 Within the DXA cohort of the WHI, Bea et al. showed that quintile 5 of percent lean mass was associated with higher overall mortality than quintile 1 among older women (ages 70–79 years); however, in younger women (ages 50–59 years), this association was reversed. Younger women in quintile 5 of percent lean body mass had 59% reduced mortality compared to quintile 1.33 It is possible that “ideal” percent lean mass ranges could be age- and outcome-specific; therefore, it is difficult to interpret the clinical significance of the differences in lean mass observed in the current study.
Findings from the Monet study suggest that the benefit of lean mass may differ by fat mass distribution. Specifically, sedentary postmenopausal women with high LBMI and high visceral fat had worse insulin sensitivity compared to individuals with low LBMI.13 However, sedentary women with high LBMI and low visceral fat had comparatively greater insulin sensitivity. Consistent with an overall benefit for metabolism, an inverse relationship between both percent fat mass and LBMI with higher protein intake was observed. BMI and LBMI are strongly positively correlated (rho = 0.76); therefore it is possible that a lower LBMI may simply reflect the lower absolute levels of lean mass that occur with lower body weight. Importantly, the relative lean mass (percent lean mass) was still greater with higher protein. As cited above, higher relative lean mass may be more clinically important than absolute lean mass gains or losses. Also notable is that the effect of protein on LBMI was consistent across BMI categories, indicating that the effect of protein on LBMI is not dependent on BMI despite their correlation. For sedentary women (<7.5 MET-hr/wk), LBMI was lower for all women in quintile 5 of protein intake, regardless of BMI category. Interestingly, Figure 1 suggests a non-linear relationship between physical activity and protein intake for LBMI in obese women. This relationship may reflect potential beneficial effects of protein and physical activity on LBMI that, in the absence of information on fat distribution (i.e., visceral fat), limits any interpretation supporting a positive metabolic benefit with higher LBMI in these women.
While largely beneficial, the impact of high dietary protein on health outcomes has been inconsistent. Within WHI and other large cohorts, higher calibrated protein intake (>1.2 g/kg daily compared to <1.2g/kg daily) has been associated with improved physical function,25 fewer health problems,34 and lower risk of frailty,35 sarcopenia,26 and stroke.36 Conversely, higher dietary protein has been related to greater risk for ischemic heart disease in several European cohorts,37–39 although not in the Nurses’ Health Study.40 In the WHI41 and the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort42 higher dietary protein was related to an increased risk of developing diabetes. None of these analyses, however, evaluated the modifying effects of physical activity on the outcome assessed.
Physical activity has been shown to increase lean body mass in older individuals43 and is consistently associated with decreased risk of multiple chronic diseases,44 including diabetes and postmenopausal breast cancer,45,46 independent of weight loss.47,48 In the current study, physical activity strongly modified the relationship between protein intake and percent lean mass (Pinteraction < 0.001). In this study, physical activity levels ranged from 0–134 MET-hr/wk. Notably, physically active obese women had percent lean mass levels comparable to inactive overweight women at each quintile of protein intake.
The RDA for protein is 0.8 g/kg body weight.20 In the current study, protein intakes ranged from 0.54–2.02 g/kg body weight. Women in quintile 5 of protein intake consumed 1.22–2.02 g/kg body weight (i.e., greater than the RDA) and had an average 59.0 ± 6.7% lean mass [37.8 ± 6.9% fat mass]. Percent lean mass was higher than the other quintiles (Ptrend < 0.001). Strategies to preserve lean mass in postmenopausal women are important, since they annually lose 0.6% lean mass on average.49 This estimated loss equates to a mean 0.24 kg lean mass for the cohort in this study. In this analysis, even a small increase in reported protein intake (e.g. 0.15 g/kg body weight for quintile 2 versus 1) was associated with a mean lean mass difference equivalent to 0.89 kg, which more than makes up for projected yearly losses. While higher percent lean mass is reported to have health benefits, the optimal level of percent lean mass for postmenopausal women is not known. Similarly, there are no updated accepted recommendations for percent fat mass for postmenopausal women since Jackson and Pollock,28 other than what has been calculated from BMI.50
Protein and animal sources may also differentially affect lean mass as well as other components of muscle. In this study, the mean ratio of animal to plant protein (uncalibrated) was 2.5 across quintiles 1–4, and slightly higher in quintile 5 [2.6 (± 1.2)]. Because the relative proportions of plant and animal protein remained fairly consistent across quintiles, it is unlikely that the observed associations are strongly influenced by protein source. Recently, two large clinical trials published results describing the effect of protein source on lean mass. In a cross-sectional analysis of the Framingham cohort, lean mass in older women was positively associated with intake of total and animal protein, but not plant-based protein. Conversely, quadriceps strength was associated with plant protein, but not total or animal protein, suggesting that plant-based protein plays a role in muscle quality beyond lean mass.51 In a prospective study in Finland, both total protein and animal protein, but not plant protein, were positively related to total lean mass at baseline and to lean mass changes after 3 years’ follow up. Plant protein alone, however, was positively associated with appendicular lean mass preservation.52 While beyond the scope of this manuscript, future studies should investigate whether physical activity modifies the association between animal or plant-based protein on lean mass.
To the best of the knowledge of the authors, this is the first report to directly evaluate whether physical activity modified the association between protein and lean mass. Strengths of this study are the robust dataset, large sample size, calibrated protein and energy intake measures, and DXA-measured body composition. The use of calibrated protein intake corrects for error in self-reporting of protein intake. DXA has been used in multiple clinical trials with postmenopausal women, although it may underestimate fat mass in leaner individuals.53,54 A limitation for this study is that the uppermost intake level did not afford an opportunity to determine if there is a “ceiling” effect in relation to protein intake and lean mass gains in postmenopausal women. This is interesting given that women in quintile 5 of protein intake in this study were consuming more than twice the current RDA of 0.8 g protein/kg body weight. Other limitations include self-report of diet data and computed amino acid data. Lifetime physical activity behavior may also influence these associations; in this study, the exposure was limited to self-reported activity at the time of study entry. While stratification by race/ethnicity suggests that the effect of protein and physical activity on lean mass and fat mass among black women follows a similar pattern to that in non-Hispanic white women, the presented results are generalizable only to postmenopausal non-Hispanic white women. Additionally, while there is a range of leucine content in dietary protein sources, particularly when comparing vegetable- and animal-based proteins (in terms of both total leucine/total protein and leucine per serving),55 it was not possible to test independent relationships between amino acids on the outcomes assessed in this study. Controlled feeding studies with a high proportion of dietary leucine and/or BCAA, or direct measurement of blood levels of dietary amino acids, are warranted to test these relationships.
CONCLUSIONS
While it is known that protein supplementation increases muscle mass in the presence of exercise (particularly resistance training) or physical activity, this is the first study to demonstrate a cross-sectional association between habitual activity levels on lean mass in postmenopausal women within a range of usual dietary protein intakes. Protein's positive association with lean mass, as well as the additive effect of physical activity, was observed regardless of BMI category. These results suggest that dietary protein's association with lean body mass is maximized when combined with physical activity at or above the minimum American Heart Association recommendations. Future research should consider physical activity level as a modifier when relating dietary protein intake to body composition and health outcomes.
Supplementary Material
Acknowledgments
Please see the Short List of WHI Investigators.
Sources of Funding: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. Support was also provided by Susan G. Komen CCR14299136.
SHORT LIST OF WHI INVESTIGATORS
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
For a list of all the investigators who have contributed to WHI science, please visit: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: There are no conflicts of interest to disclose.
Contributor Information
Jessica A. Martinez, Department of Nutritional Sciences, University of Arizona Cancer Center, University of Arizona, Tucson, AZ, phone: (520) 626-6326, jam1@email.arizona.edu.
Betsy C. Wertheim, University of Arizona Cancer Center, Tucson, AZ, phone: (520) 777-1666, BWertheim@uacc.arizona.edu.
Cynthia A. Thomson, Mel & Enid Zuckerman College of Public Health, University of Arizona Cancer Center, University of Arizona, Tucson, AZ, phone: (520) 940-1759 cthomson@email.arizona.edu.
Jennifer W. Bea, Department of Nutritional Sciences, University of Arizona Cancer Center Tucson, AZ, phone: (520) 626-0912, JBea@uacc.arizona.edu.
Robert Wallace, Department of Epidemiology, MD, MSc, University of Iowa, Iowa City, IA, phone: (319) 384-1551, robertwallace@uiowa.edu.
Matthew Allison, Department of Family Medicine and Public Health, University of California, San Diego, CA, phone: (858) 822-7671, mallison@ucsd.edu.
Linda Snetselaa, Department of Epidemiology, College of Public Health, University of Iowa, Iowa City, IA, phone: (319) 384-1553, linda-snetselaar@uiowa.edu.
Zhao Chen, Department of Epidemiology and Biostatistics, Mel and Enid Zuckerman College of Public Health, phone: (520) 626-901, zchen@email.arizona.edu.
Rami Nassir, Department of Biochemistry and Molecular Medicine, University of California, Davis, CA phone: (530) 754-6016, rmnassir@ucdavis.edu.
Patricia A. Thompson, Department of Pathology, Stony Brook University, Stony Brook, NY, phone: (631) 444-6818, patricia.thompsoncarino@stonybrookmedicine.edu.
REFERENCES
- 1.Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: A new category of obesity in the elderly. Nutrition, Metabolism and Cardiovascular Diseases. 2008;18(5):388–395. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Hurley BF, Hanson ED, Sheaff AK. Strength training as a countermeasure to aging muscle and chronic disease. Sports medicine. 2011;41(4):289–306. doi: 10.2165/11585920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression 1. Am J Clin Nutr. 2006;83(2):260–274. doi: 10.1093/ajcn/83.2.260. [DOI] [PubMed] [Google Scholar]
- 4.Layman DK, Evans EM, Erickson D, et al. A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. The Journal of Nutrition. 2009;139(3):514–521. doi: 10.3945/jn.108.099440. [DOI] [PubMed] [Google Scholar]
- 5.Mojtahedi MC, Thorpe MP, Karampinos DC, et al. The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2011;66(11):1218–1225. doi: 10.1093/gerona/glr120. [DOI] [PubMed] [Google Scholar]
- 6.Phillips SM, Van Loon LJ. Dietary protein for athletes: from requirements to optimum adaptation. J Sports. Sci. 2011;29(Suppl 1):S29–S38. doi: 10.1080/02640414.2011.619204. [DOI] [PubMed] [Google Scholar]
- 7.Bray GA, Smith SR, de Jonge L, et al. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA. 2012;307(1):47–55. doi: 10.1001/jama.2011.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laplante M, Sabatini DM. mTOR Signaling in Growth Control and Disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casperson SL, Sheffield-Moore M, Hewlings SJ, Paddon-Jones D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clinical Nutrition. 2012 doi: 10.1016/j.clnu.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin LQ, Xun P, Bujnowski D, et al. Higher branched-chain amino acid intake is associated with a lower prevalence of being overweight or obese in middle-aged East Asian and Western adults. The Journal of Nutrition. 2011;141(2):249–254. doi: 10.3945/jn.110.128520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volpi E, Campbell WW, Dwyer JT, et al. Is the Optimal Level of Protein Intake for Older Adults Greater Than the Recommended Dietary Allowance? The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013;68(6):677–681. doi: 10.1093/gerona/gls229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clinical Nutrition. 2014;33(6):929–936. doi: 10.1016/j.clnu.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brochu M, Mathieu ME, Karelis AD, et al. Contribution of the lean body mass to insulin resistance in postmenopausal women with visceral obesity: a Monet study. Obesity (Silver Spring) 2008;16(5):1085–1093. doi: 10.1038/oby.2008.23. [DOI] [PubMed] [Google Scholar]
- 14.O'Gorman DJ, Krook A. Exercise and the treatment of diabetes and obesity. Med Clin North Am. 2011;95(5):953–969. doi: 10.1016/j.mcna.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Controlled Clinical Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 16.Neuhouser ML, Tinker L, Shaw PA, et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women's Health Initiative. Am J Epidemiol. 2008;167(10):1247–1259. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Bassford T, Green SB, et al. Postmenopausal hormone therapy and body composition--a substudy of the estrogen plus progestin trial of the Women's Health Initiative. Am J Clin Nutr. 2005;82(3):651–656. doi: 10.1093/ajcn.82.3.651. [DOI] [PubMed] [Google Scholar]
- 18.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Annals of Epidemiology. 1999;9(3):178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 19.Schakel SF. Maintaining a nutrient database in a changing marketplace: Keeping pace with changing food products - A research perspective. J Food Comp and Anal. 2001;14:315–322. [Google Scholar]
- 20.US Department of Agriculture. Dietary Reference Intakes: Estimated Average Requirements and Recommended Intakes. [Accessed 06/30/2016]; http://fnic.nal.usda.gov/dietary-guidance/dietary-reference-intakes/dri-tables-and-application-reports.
- 21.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services (HHS) Physical activity guidelines for Americans. Washington: HHS; 2008. Office of Disease Prevention and Health Promotion. [Google Scholar]
- 23.Shiroma EJ, Sesso HD, Lee IM. Physical activity and weight gain prevention in older men. International Journal of Obesity. 2012;36(9):1165–1169. doi: 10.1038/ijo.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi L, Nassir R, Kosoy R, et al. Relationship between diabetes risk and admixture in postmenopausal African-American and Hispanic-American women. Diabetologia. 2012;55(5):1329–1337. doi: 10.1007/s00125-012-2486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beasley JM, Wertheim BC, LaCroix AZ, et al. Biomarker-calibrated protein intake and physical function in the Women's Health Initiative. J Am Geriatr. Soc. 2013;61(11):1863–1871. doi: 10.1111/jgs.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beasley JM, Shikany JM, Thomson CA. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr Clin Pract. 2013;28(6):684–690. doi: 10.1177/0884533613507607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. Stata (for Macintosh) [computer program] [Google Scholar]
- 28.Jackson AS, Pollock ML. Factor analysis and multivariate scaling of anthropometric variables for the assessment of body composition. Med Sci Sports. 1976;8(3):196–203. doi: 10.1249/00005768-197600830-00012. [DOI] [PubMed] [Google Scholar]
- 29.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. American Journal of Physiology. Endocrinology and Metabolism. 2006;291(2):E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 30.Allison DB, Zannolli R, Faith MS, et al. Weight loss increases and fat loss decreases all-cause mortality rate: results from two independent cohort studies. International journal of obesity and related metabolic disorders. Journal of the International Association for the Study of Obesity. 1999;23(6):603–611. doi: 10.1038/sj.ijo.0800875. [DOI] [PubMed] [Google Scholar]
- 31.De Schutter A, Lavie CJ, Kachur S, Patel DA, Milani RV. Body composition and mortality in a large cohort with preserved ejection fraction: untangling the obesity paradox. Mayo Clin Proc. 2014;89(8):1072–1079. doi: 10.1016/j.mayocp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Oreopoulos A, Kalantar-Zadeh K, Sharma AM, Fonarow GC. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr. Med. 2009;25(4):643–659. viii. doi: 10.1016/j.cger.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Bea JW, Thomson CA, Wertheim BC, et al. Risk of Mortality According to Body Mass Index and Body Composition Among Postmenopausal Women. Am J Epidemiol. 2015;182(7):585–596. doi: 10.1093/aje/kwv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vellas BJ, Hunt WC, Romero LJ, Koehler KM, Baumgartner RN, Garry PJ. Changes in nutritional status and patterns of morbidity among free-living elderly persons: a 10-year longitudinal study. Nutrition. 1997;13(6):515–519. doi: 10.1016/s0899-9007(97)00029-4. [DOI] [PubMed] [Google Scholar]
- 35.Beasley JM, LaCroix AZ, Neuhouser ML, et al. Protein intake and incident frailty in the Women's Health Initiative observational study. Journal of the American Geriatrics Society. 2010;58(6):1063–1071. doi: 10.1111/j.1532-5415.2010.02866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prentice RL, Huang Y, Kuller LH, et al. Biomarker-calibrated energy and protein consumption and cardiovascular disease risk among postmenopausal women. Epidemiology. 2011;22(2):170–179. doi: 10.1097/EDE.0b013e31820839bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344:e4026. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trichopoulou A, Psaltopoulou T, Orfanos P, Hsieh CC, Trichopoulos D. Low-carbohydrate-high-protein diet and long-term survival in a general population cohort. European Journal of Clinical Nutrition. 2007;61(5):575–581. doi: 10.1038/sj.ejcn.1602557. [DOI] [PubMed] [Google Scholar]
- 39.Sjogren P, Becker W, Warensjo E, et al. Mediterranean and carbohydrate-restricted diets and mortality among elderly men: a cohort study in Sweden. Am J Clin Nutr. 2010;92(4):967–974. doi: 10.3945/ajcn.2010.29345. [DOI] [PubMed] [Google Scholar]
- 40.Hu FB, Stampfer MJ, Manson JE, et al. Dietary protein and risk of ischemic heart disease in women. Am J Clin Nutr. 1999;70(2):221–227. doi: 10.1093/ajcn.70.2.221. [DOI] [PubMed] [Google Scholar]
- 41.Tinker LF, Sarto GE, Howard BV, et al. Biomarker-calibrated dietary energy and protein intake associations with diabetes risk among postmenopausal women from the Women's Health Initiative. Am J Clin Nutr. 2011;94(6):1600–1606. doi: 10.3945/ajcn.111.018648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sluijs I, Beulens JW, van der AD, Spijkerman AM, Grobbee DE, van der Schouw YT. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care. 2010;33(1):43–48. doi: 10.2337/dc09-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Medicine and Science in Sports and Exercise. 2011;43(2):249–258. doi: 10.1249/MSS.0b013e3181eb6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ : Canadian Medical Association Journal. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zisser H, Gong P, Kelley CM, Seidman JS, Riddell MC. Exercise and diabetes. Int J Clin Pract Suppl. (170):71–75. doi: 10.1111/j.1742-1241.2010.02581.x. [DOI] [PubMed] [Google Scholar]
- 46.Magne N, Melis A, Chargari C, et al. Recommendations for a lifestyle which could prevent breast cancer and its relapse: physical activity and dietetic aspects. Crit Rev Oncol Hematol. 80(3):450–459. doi: 10.1016/j.critrevonc.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Gaesser GA, Angadi SS, Sawyer BJ. Exercise and diet, independent of weight loss, improve cardiometabolic risk profile in overweight and obese individuals. The Physician and Sportsmedicine. 2011;39(2):87–97. doi: 10.3810/psm.2011.05.1898. [DOI] [PubMed] [Google Scholar]
- 48.Rajarajeswaran P, Vishnupriya R. Exercise in cancer. Indian Journal of Medical and Paediatric Oncology. 2009;30(2):61–70. doi: 10.4103/0971-5851.60050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rolland YM, Perry HM, 3rd, Patrick P, Banks WA, Morley JE. Loss of appendicular muscle mass and loss of muscle strength in young postmenopausal women. J Gerontol A Biol Sci. Med Sci. 2007;62(3):330–335. doi: 10.1093/gerona/62.3.330. [DOI] [PubMed] [Google Scholar]
- 50.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72(3):694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 51.Sahni S, Mangano KM, Hannan MT, Kiel DP, McLean RR. Higher Protein Intake Is Associated with Higher Lean Mass and Quadriceps Muscle Strength in Adult Men and Women. J Nutr. 2015;145(7):1569–1575. doi: 10.3945/jn.114.204925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isanejad M, Mursu J, Sirola J, et al. Association of protein intake with the change of lean mass among elderly women: The Osteoporosis Risk Factor and Prevention - Fracture Prevention Study (OSTPRE-FPS) J Nutr. Sci. 2015;4:e41. doi: 10.1017/jns.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Der Ploeg GE, Withers RT, Laforgia J. Percent body fat via DEXA: comparison with a four-compartment model. J Appl Physiol. 2003;94(2):499–506. doi: 10.1152/japplphysiol.00436.2002. [DOI] [PubMed] [Google Scholar]
- 54.Williams JE, Wells JC, Wilson CM, Haroun D, Lucas A, Fewtrell MS. Evaluation of Lunar Prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model. Am J Clin Nutr. 2006;83(5):1047–1054. doi: 10.1093/ajcn/83.5.1047. [DOI] [PubMed] [Google Scholar]
- 55.US Department of Agriculture. Food Composition Databases. [Accessed 06/30/2016];2016 May; http://ndb.nal.usda.gov/ndb/foods.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.