Short abstract
Iran's experience shows that genetic screening can be successful in lower resource countries and also provides some lessons for high resource nations
Progress in controlling communicable diseases increases the relative importance of non-communicable diseases, including genetic disorders.1 In Iran, the development of primary health care over the past 20 years has greatly reduced infant mortality and crude birth rate. Accordingly, in 1991 prevention of non-communicable diseases was added to the primary healthcare programme, and a department for the control of non-communicable disease, including a genetics office, was established within the Ministry of Health and Medical Education. β Thalassaemia, which is an important health problem in Iran,2 was chosen to test the feasibility of preventing non-communicable disease in primary care. We describe how the programme has been implemented.
Primary healthcare infrastructure in Iran
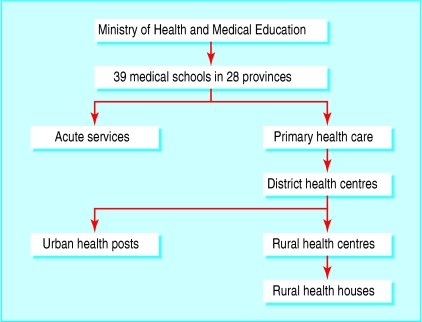
Iran has a five level primary healthcare network covering the entire population of 60 million, in 28 provinces (figure).3 Responsibility for health and medical education are merged throughout the system. Each medical university has a vice chancellor responsible for primary health care. There were 14 826 rural health houses at the start of the thalassaemia project. These are staffed by trained health workers (behvarz) supported by a system of continuing education. As well as premarital health education and blood tests, the responsibilities of primary care staff include an annual census of the population covered, health education, family health (prenatal and postnatal care, children, family planning, immunisation), disease control (tuberculosis, malaria, leprosy, etc), simple treatments, environmental health, and collection, recording, and storage of health information.
Figure 1.
Organisation of the Iranian primary healthcare system
Continuing education for primary care workers is particularly important in developing countries, where the rapid evolution of health priorities requires a flexible response. In Iran, when a new programme is developed, provincial health workers attend an initial meeting at the ministry about programme goals, strategies, and activities, followed by regular updating workshops. Each level of the primary health care system then educates the next level down. Ongoing evaluation is considered equally important. Standardised surveillance data are passed up level by level; each level evaluates its own performance and that of the next level down, and the disease management centre provides feedback to the entire network.
Summary points
Iran's thalassaemia programme was implemented in 1997
Linkage to premarital testing within an effective primary care infrastructure has ensured good take up
Feedback from the community through systematic evaluation led to national acceptance of prenatal diagnosis
Design of thalassaemia prevention programme
The acceptability and effectiveness of preventing thalassaemia by carrier screening and genetic counselling in high risk populations is well established.4-8 The thalassaemia programme was designed to create a general infrastructure for prevention of genetic disorders. Screening was included as part of existing mandatory premarital blood tests. Initially, couples at risk were offered only information and genetic counselling because abortion after prenatal diagnosis was not allowed in Iran. Integration into primary care required development of instruments and methods for educating health workers, the public, and target groups and establishment of professional networks to provide genetic diagnostic services, genetic counselling, and evaluation (surveillance).
Educational component
Primary care genetic counselling network—Genetic counselling teams consisting of a doctor and a professional with a BSc degree in health studies were established in designated accessible urban health posts in every city. Training, organised by specialists attached to the National Genetics Committee, follows the ethical principles recommended by the World Health Organisation.9,10 It includes a distance learning self taught course and interactive face-to-face courses at national, provincial, and district levels. Counsellors sit distance examinations for self taught courses, and written examinations after interactive courses.
Network for informing the public and target groups— Many groups in the community need to be prepared for premarital screening. Relevant experts have been recruited into a national multidisciplinary educational committee, linked to corresponding provincial committees, and so on down the system. Classes about thalassaemia are held for high school students and for young men doing military service (because men are the first to be offered screening). The judiciary is linked to the programme through annual meetings for marriage registrars (many of whom are clergy).
Laboratory diagnostic services
Governmental and private laboratories equipped to screen for thalassaemia have been recruited into an accredited national professional laboratory network, supervised by a national reference laboratory and directorate for laboratory affairs. There are corresponding structures at the provincial level. Laboratory staff follow national screening protocols based on international guidelines,11 participate in quality control, and attend regular educational courses.
Evaluation
Evaluation of a programme aiming to provide informed choice requires data on numbers of at risk couples identified and their choices concerning marriage and reproduction; numbers of patients with thalassaemia and their age distribution; and numbers and outcomes of prenatal diagnoses, when this service is available.12-14
Genetic counselling teams report numbers of carrier couples counselled, their choices, and referrals to DNA laboratories. Health houses and health posts report follow up data on carrier couples and register infants born with thalassaemia. Registers of patients and prenatal diagnoses are being developed. The genetics office computerises and evaluates the data, reports back to provincial health centres, and arranges regular field visits by experts. The surveillance system provides guidance on how to adapt the programme to meet the needs of the community.12
Process of screening
Marriage registrars refer prospective couples to a designated local laboratory for premarital screening. The man's red cell indices are checked first. If he has microcytosis (mean cell haemoglobin < 27 pg or mean red cell volume < 80 fl), the woman is tested. When both are microcytic their haemoglobin A2 concentrations are measured. If both have a concentration above 3.5% (diagnostic of β thalassaemia trait11) they are referred to the local designated health post for genetic counselling. Microcytic individuals with a haemoglobin A2 concentration in the normal range are treated with iron and their indices rechecked. All results are sent to the local genetic counselling team. At risk couples attend as many counselling sessions as they need to reach an informed decision (an average of 2.5 sessions, range 1-5). Those who marry after counselling are referred to their local health post or health house for follow up until they have completed their family.
The most important problem encountered is the interpretation of persistent microcytosis with no other abnormality. This is usually due to mild α+ thalassaemia. However, the fact that it may also be due to “normal haemoglobin A2” β thalassaemia11 leads to uncertainty about how to counsel couples when, for example, one partner carries β thalassaemia and the other has persistent microcytosis.
Results of screening programme
Table 1 summarises national data for the first five years. Figures by province are on bmj.com. By the end of 2001, over 2.7 million prospective couples had been screened and 10 298 at risk couples had been identified. The rise over the first three years reflects increasing coverage, plus an annual 7.4% increase in numbers reaching marriageable age. As the programme has become established the average prevalence of carrier couples detected has increased from 3.0/1000 to 4.5/1000. All prospective couples reported at risk have attended genetic counselling, usually with relatives. After counselling, about half proceed to marriage. Many of the rest decide to separate, although the proportion remaining undecided when the data were collected each year (usually 6-12 months after counselling) has increased steadily.
Table 1.
Outcomes in first five years of Iranian thalassaemia prevention programme
|
No of couples
|
Decision of couples (No (%))
|
Rate/1000
|
|||||
|---|---|---|---|---|---|---|---|
| Year | Tested (1000s) | At risk | Married | Separated | Uncertain | At risk couples | Affected births if no prevention* |
| 1997 | 353 | 1 057 | 486 (46) | 473 (45) | 98 (9) | 3.0 | 0.75 |
| 1998 | 520 | 1 534 | 802 (52) | 532 (35) | 200 (13) | 3.0 | 0.74 |
| 1999 | 564 | 2 201 | 1073 (49) | 598 (27) | 530 (24) | 3.9 | 0.98 |
| 2000 | 672 | 2 716 | 1455 (54) | 745 (27) | 516 (19) | 4.0 | 1.01 |
| 2001 | 620 | 2 790 | 1615 (58) | 671 (24) | 504 (18) | 4.5 | 1.13 |
| Total | 2729 | 10 298 | 5431 (53) | 3019 (29) | 1848 (18) | 3.8 | 0.94 |
Based on a simplified assumption of a 1 in 4 risk of an affected child in affected couples and that at risk couples have the same birth rate as the rest of the population.
Preliminary data from the developing national thalassaemia register (table 2) suggests that the affected birth rate had fallen to 30% of expectation by the year 2000. However, it will take time to validate the figures and evaluate the many factors involved. In 1998 the annual number of new cases was already far below expectation as a result of long standing systematic counselling of couples with affected children; if informed couples refrain from having further children, in a population with a large average final family size this can reduce affected births by up to 50%.9 Figures for affected births in the years 2001 and 2002 will rise in the future because many patients present between 1 and 2 years of age.
Table 2.
Number of new patients with thalassaemia major registered at Iranian treatment centres during 1998-2002
| Year of birth | No of new patients reported | % of expected No without intervention* |
|---|---|---|
| 1998 | 480 | 40 |
| 1999 | 416 | 35 |
| 2000 | 341 | 28 |
| 2001 | 206 | 17 |
| 2002 | 78 | 7 |
Without any intervention about 1200 affected children would be born each year.
Effect of evaluation on development of programme
At the outset the options for at risk couples were limited to marrying as planned, separating and finding a non-carrier partner, or postponing marriage or childbearing in the hope of a better solution in the future. However surveillance data, supported by the reported experience of the counsellors, soon showed that the population wanted the option of prenatal diagnosis. This led to intensive and widespread ethical discussions, which concluded in 1998 with a governmental decision to permit abortion before 16 weeks from the last menstrual period if the fetus is known to be affected.
A basis already existed for the rapid development of prenatal diagnosis because many Iranian thalassaemia mutations were known, and some private laboratories were already providing prenatal diagnosis in response to demand.15-17 The country also already had a few experts in chorionic villus sampling. A national DNA laboratory network, including two national genetic reference laboratories and six other laboratories experienced with thalassaemia, was initiated in 1999 and began to function in 2001. The laboratories follow national guidelines, accept referrals from primary care, and return surveillance data.
Since the creation of the DNA laboratory network, the number of couples seeking prenatal diagnosis has risen sharply (H Najmabadi, personal communication). In addition, all known at risk couples who married before prenatal diagnosis was available have been informed of its availability and offered genetic work-up in case they become pregnant, and prenatal diagnosis is offered systematically to parents of affected children.
Financial aspects
The government health budget covers planning, education, counselling, and surveillance. Couples pay for their own screening tests, which cost about $5 (£2.70, €3.90). The (governmental) health insurance companies cover DNA tests and prenatal diagnosis. Over 90% of the population are insured, and help is available for couples who are not uninsured.
Problems and further development
The Iranian thalassaemia programme is far from complete or perfect. At this stage it is not possible to accurately assess the completeness of screening or follow up. However, incomplete coverage will inevitably come to light because of unexpected affected births. Overdiagnosis of β thalassaemia in people who have microcytosis but no other abnormality could have contributed to the rise in prevalence of carrier couples. However, surveillance data indicate that the rise is more likely to be due to increased coverage of high risk areas and improved screening performance. The programme also fails to detect structural haemoglobin variants such as haemoglobin S, which is common in some provinces. Pilot studies of screening for risk of sickle cell disorders are now in progress. The second phase of the programme, the systematic offer of screening to couples who married before the programme began, has now been initiated.
Lessons from the Iranian experience
The programme is economically viable because it works through the established primary healthcare and educational systems, focuses existing (though scattered) genetic expertise on a common objective, and added thalassaemia screening to existing pre-marital blood tests. Couples willingly pay for screening because they want a healthy family and are prepared for expenses associated with marriage. The (governmental) insurance companies pay for prenatal diagnosis because it helps to limit the escalating cost of patient care.1,9,18 This enables the laboratory network to expand to meet demand, which could exceed 3000 requests for prenatal diagnosis a year. Mass referrals provide laboratories and universities with resources and scientific data, so promoting further development of genetic knowledge and technology in the country, and expanding capacity for other genetic services.
When planning a programme, the initial tendency is to focus on technical aspects. However the most difficult, expensive, and time consuming component is establishing sustainable education for health workers and the community. Ongoing evaluation is equally important because it provides objective feedback that permits the programme to adapt to the needs of the community. The role of evaluation in the rapid evolution of social attitudes to abortion for serious fetal abnormality in Iran is highly relevant for other Islamic countries and for Muslim minorities in high resource countries.
Figure 2.
Prenatal diagnosis has developed in response to public demand in Iran
Credit: SHEHZAD NOORANI/STILL PICTURES
Primary care based genetic screening must be inclusive rather than focused on a single disorder. The recognition that thalassaemia screening is simply a first step in the application of genetic knowledge in primary care has been crucial for its acceptance.
Advantages of genetic screening in primary care
The most important advantage of premarital screening is that it gives carriers and carrier couples the widest possible range of informed choice. However, no single screening strategy can meet the needs of a whole population. Primary care based screening has the advantage of allowing the flexible use of multiple complementary strategies—for example, offering testing systematically to newly-weds, or as part of family planning, or as soon as a pregnancy is recognised. Primary care workers can also offer carrier testing to relatives of patients and carriers, a particularly important strategy in countries like Iran where consanguineous marriage is common.19 We believe that these advantages make primary care based genetic screening the approach of choice for the future.
Supplementary Material
 Details of the programme's performance are on bmj.com
Details of the programme's performance are on bmj.com
The thalassaemia programme was initiated under the direction of M S Akbari, under-secretary for health affairs. We also thank A A Sayari, Melek Afsali, M M Gooya and R Labafghasemi for guidance and support. We acknowledge the contribution of the following members of the National Genetic Advisory Committee: L Hosseini Gohari, D Daneshvar-Farhud, M H Karimi-Nejad, M T Arzanian, P Vosogh, T Akbari, S Zeinali, N Najmabadi, R Amini, H Abolghasemi, M Izadyar, S Seif, P Passalar and SR Ghafarii; the professionals and health workers in the genetics office; K Shadpoor and all those working in the primary health care system who have made this programme a reality.
Contributors and sources: BM visited Iran on three occasions as WHO consultant to the programme. She is a retired Wellcome principal research fellow. AS visited the United Kingdom on a one year training fellowship. Both authors contributed to writing the article and BM is the guarantor.
Funding: BM's visits to Iran were funded by the Eastern Mediterranean Regional Office of WHO. AS's training in the UK was funded by the Ministry of Health of the Islamic Republic of Iran.
Competing interests: None declared.
References
- 1.Alwan A, Modell B. Recommendations for introducing genetics services into developing countries. Nature Rev Genetics 2003;4: 61-8. [DOI] [PubMed] [Google Scholar]
- 2.Farhud D, Sadighi H. Investigation of prevalence of thalassaemia in Iran. Iran J Public Health 1997;26: 1-2. [Google Scholar]
- 3.Shadpour K. The PHC experience in Iran. Teheran: Unicef, 1994.
- 4.Cao A, Rosatelli. Screening and prenatal diagnosis of the haemoglobinopathies. Balliere's Clin Haematol 1993;6: 263-86. [DOI] [PubMed] [Google Scholar]
- 5.Angastiniotis MA, Hadjiminas MG. Prevention of thalassaemia in Cyprus. Lancet 1981;i: 369-70. [DOI] [PubMed] [Google Scholar]
- 6.Loukopoulos D. Current status of thalassaemia and the sickle cell syndromes in Greece. Semin Hematol 1996;33: 76-86. [PubMed] [Google Scholar]
- 7.Maggio A, Caronia F, Orlandi F. Prenatal diagnosis of haemoglobinopathies in Sicily. Lancet 1992;339: 1361-2. [DOI] [PubMed] [Google Scholar]
- 8.Modell B, Ward RHT, Fairweather DVI. Effect of introducing antenatal diagnosis on the reproductive behaviour of families at risk for thalassaemia major. BMJ 1980;ii: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alwan AA, Modell B. Community control of genetic and congenital disorders. Cairo: World Health Organisation Regional Office for the Eastern Mediterranean, 1997. (EMRO Technical Publications Series 24.)
- 10.Report of a WHO meeting on ethical issues in medical genetics. Proposed international guidelines on ethical issues in medical genetics and genetics services. http://www.who.int/genomics/publications/en/ethicalguidelines1998.pdf (accessed 1 Nov 2004).
- 11.General Haematology Task Force of the British Committee for Standards in Haematology. The laboratory diagnosis of haemoglobinopathies. Br J Haematol 1998;101; 783-92. [DOI] [PubMed] [Google Scholar]
- 12.Modell B, Darlison M, Khan M, Harris R. Role of genetic diagnosis registers in ongoing consultation with the community. Comm Gen 2000;3: 144-7. [Google Scholar]
- 13.Modell B, Khan M, Darlison M, King A, Layton M, Old J, et al. Use of a national diagnosis register for surveillance of an inherited disorder: the example of beta thalassaemia in the United Kingdom. Bull World Health Organ 2001;79: 1006-13. [PMC free article] [PubMed] [Google Scholar]
- 14.Modell B, Harris R, Lane B, Khan M, Darlison M, Petrou M, et al. Informed choice in genetic screening for thalassaemia during pregnancy: audit from a national confidential enquiry. BMJ 2000;320: 325-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gohari LH, Petrou M, Felekis X, Christopoulos G, Kleanthous M. Identification of alpha-thalassemia mutations in Iranian individuals with abnormal hematological indices and normal Hb A2. Hemoglobin 2003;27: 129-32. [DOI] [PubMed] [Google Scholar]
- 16.Akbari M, Izadi P. The incidence of beta thalassaemia gene mutations and prenatal diagnosis in Iran. 7th meeting of the American Society for Human Genetics, Baltimore, USA, 1997. Am J Hum Genet 1997;61(suppl):abstract No 829.
- 17.Najmabadi H, Karimi-Nejad R, Sahebjam S, Pourfarzad F, Teimourian S, Sahebjam F, et al. The beta-thalassemia mutation spectrum in the Iranian population. Hemoglobin. 2001;25: 285-96. [DOI] [PubMed] [Google Scholar]
- 18.Angastiniotis MA, Kyriakidou S, Hadjiminas M. How thalassaemia was controlled in Cyprus. World Health Forum 1986;7; 291-7. [Google Scholar]
- 19.Ahmed S, Saleem M, Modell B, Petrou M. Screening extended families for genetic haemoglobin disorders in Pakistan. N Engl J Med 2001;347: 1162-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.