ABSTRACT
An increase in Streptococcus pneumoniae nasopharynx (NP) colonization density during a viral coinfection initiates pathogenesis. To mimic natural S. pneumoniae pathogenesis, we commensally colonized the NPs of adult C57BL/6 mice with S. pneumoniae serotype (ST) 6A or 8 and then coinfected them with mouse-adapted H1N1 influenza A virus (PR/8/34). S. pneumoniae established effective commensal colonization, and influenza virus coinfection caused S. pneumoniae NP density to increase, resulting in bacteremia and mortality. We then studied histidine triad protein D (PhtD), an S. pneumoniae adhesin vaccine candidate, for its ability to prevent invasive S. pneumoniae disease in adult and infant mice. In adult mice, the efficacy of PhtD vaccination was compared with that of PCV13. Vaccination with PCV13 led to a greater reduction of S. pneumoniae NP density (>2.5 log units) than PhtD vaccination (∼1-log-unit reduction). However, no significant difference was observed with regard to the prevention of S. pneumoniae bacteremia, and there was no difference in mortality. Depletion of CD4+ T cells in PhtD-vaccinated adult mice, but not PCV13-vaccinated mice, caused a loss of vaccine-induced protection. In infant mice, passive transfer of antisera or CD4+ T cells from PhtD-vaccinated adult mice led to a nonsignificant reduction in NP colonization density, whereas passive transfer of antisera and CD4+ T cells was needed to cause a significant reduction in NP colonization density. For the first time, these data show an outcome with regard to prevention of invasive S. pneumoniae pathogenesis with a protein vaccine similar to that which occurs with a glycoconjugate vaccine despite a less robust reduction in NP bacterial density.
KEYWORDS: pneumococcal
INTRODUCTION
Streptococcus pneumoniae is part of the human commensal flora in the nasopharynx (NP) (1). Colonization of the NP is asymptomatic and beneficial for the host because it results in the generation of a natural immune response and eventual clearance of the organism (2). However, an increase in the density of S. pneumoniae during a viral upper respiratory coinfection (URI) is associated with pathogenesis (3, 4).
Currently available S. pneumoniae conjugate vaccines (PCVs) lead to nearly complete elimination of S. pneumoniae from the NPs that express vaccine serotype (ST) capsules (5). However, the elimination of these STs has consistently led to the emergence of new, replacement STs (6–8). One current strategy is to develop new PCVs that add additional STs to the vaccine to broaden efficacy against emergent replacement STs. Another strategy is the development of multicomponent S. pneumoniae protein-based vaccines (PPVs) that include as an ingredient surface-exposed, highly conserved proteins expressed by S. pneumoniae (9). However, if PPVs eliminated all S. pneumoniae from the NP in a manner similar to the effect of PCVs, a concern immediately arises regarding the consequences. What organisms will fill the vacated ecological niche? Therefore, the goal of a PPV strategy is to reduce the number of S. pneumoniae bacteria adherent to NP cells to below a pathogenic inoculum during a viral upper respiratory infection.
Using a murine model, we sought to determine the quantitative increase in density of S. pneumoniae in the NP associated with the transition of the organism from commensal to pathogen that occurs during an influenza viral coinfection. We then sought to determine if prior vaccination could effectively prevent the necessary increase in bacterial density permissive to invasive infection. As a vaccine, we used S. pneumoniae histidine triad protein D (PhtD), a highly conserved, surface-exposed adhesin protein that facilitates attachment to the NP and lung epithelium cells (10–12). PhtD as a vaccine component has been shown to be protective in a number of mouse S. pneumoniae infection models (11, 13, 14) and is included in vaccines currently in human trials (15–17). In our current mouse study, the outcome of PhtD vaccine-mediated prevention of invasive S. pneumoniae pathogenesis proved comparable to that achieved with PCV13 vaccination.
RESULTS
S. pneumoniae NP bacterial densities correlate with invasive S. pneumoniae infections.
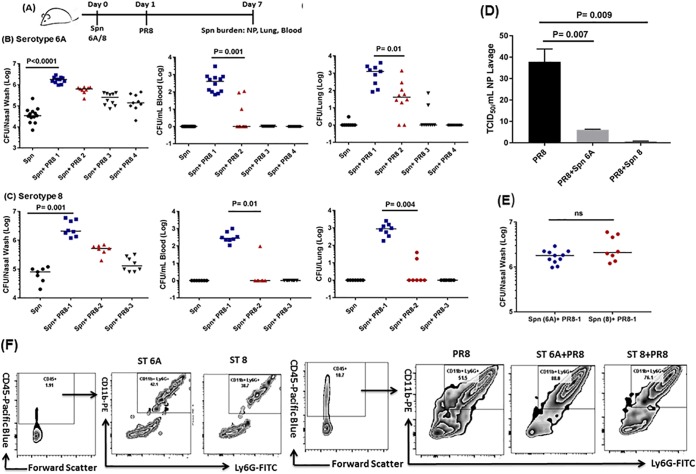
A range of intranasal mouse-adapted H1N1 influenza virus strain PR/8/34 (PR8) inocula (50 times the 50% tissue culture infective dose [TCID50] to 5 times the TCID50) were administered to ST 6A- or ST 8-colonized adult mice as outlined in Fig. 1A. The commensal NP colonization densities for STs 6A and 8 were 1 × 104.5 to 1 × 105.0 CFU. Intranasal administration of 50 times the TCID50 of PR8 caused the NP density of S. pneumoniae to significantly increase by over 1.5 log units to >1 × 106 CFU (P < 0.0001) (Fig. 1B and C), which resulted in bacterial dissemination to the lungs and blood. A reduction in the PR8 infection inoculum (to 25 times the TCID50) resulted in lower S. pneumoniae NP density, which correlated with reduced invasiveness. PR8 infection with an inoculum of 10 times the TCID50 reduced S. pneumoniae NP density to 1.1 × 105 to 1.3 × 105 CFU, a point where invasiveness was almost lost (Fig. 1B and C). Interestingly, compared to mice coinfected with either ST (6A or 8), higher influenza virus titers were observed in the NP lavage fluid of mice infected with PR8 alone (Fig. 1D).
FIG 1.
(A) Six-week-old naive mice were i.n. inoculated (10 μl) with ST 6A (1 × 106 CFU) or ST 8 (1 × 105 CFU). Twenty-four hours later, the mice were inoculated i.n. (10 μl) with different infection doses of H1N1 PR8 influenza virus (PR8-1 at 50 times the TCID50, PR8-2 at 25 times the TCID50, PR8-3 at 10 times the TCID50, or PR8-4 at 5 times the TCID50). Six days later, the mice were euthanized, and the S. pneumoniae (Spn) bacterial burdens in the NP, lungs, and blood were ascertained. (B and C) S. pneumoniae ST 6A and 8 bacterial burdens in the NP, lungs, and blood. The data are representative of the results of 2 or 3 independent experiments (n = 3 or 4 mice per group) and are presented as medians. The data were analyzed by analysis of variance followed by the Dunn post hoc test for differences between two groups. (D) Influenza (PR8) viral titers in the NP lavage fluid of infected mice. The error bars indicate standard deviations (SD). (E) S. pneumoniae NP invasive bacterial densities for STs 6A and 8. ns, not significant. (F) Phenotyping of nasal lavage fluid for neutrophils from mice colonized asymptomatically (S. pneumoniae) and PR8 infected or coinfected (S. pneumoniae plus PR8). The data are expressed as representative flow panels. The numbers in the upper right boxes are the percent neutrophils. FITC, fluorescein isothiocyanate.
We also observed that fewer ST 8 bacteria (1 × 105 CFU) were needed to cause bacteremia in the coinfection setting with PR8 than with ST 6A (1 × 106 CFU) (Fig. 1E). Therefore, it is the density of S. pneumoniae in the NP that correlates with bacterial dissemination and the burden in the blood and lung. Remarkably, a 1-log-unit decline in the NP bacterial density for STs 6A and 8 was sufficient to observe absence of S. pneumoniae in the blood and lungs (Fig. 1B and C). Mice were observed for 2 weeks, and no morbidity or mortality was seen in mice colonized by ST 6A or 8 alone or infected with the different doses of PR8 alone (data not shown). However, about 30% mortality was observed only in the invasive-coinfection model with 50 times the TCID50 of PR8 (S. pneumoniae plus PR8-1) (data not shown).
Compared to asymptomatically S. pneumoniae-colonized mice, we observed a robust recruitment of neutrophils to the NP (Fig. 1F) of PR8-infected or coinfected mice. The level of neutrophils (CD45+ CD11b+ Ly6G+) in the NP lavage fluid of coinfected mice was significantly higher than in mice infected with PR8 alone (Fig. 1F); 88% of the cells (out of total leukocytes; CD45+) in NP lavage fluid during ST 6 and PR8 coinfection were neutrophils; for ST 8 and PR8 coinfection, 76% of the cells (out of total leukocytes; CD45+) were neutrophils. In the lungs, NP coinfection with S. pneumoniae and PR8 led to a slightly higher recruitment of neutrophils than S. pneumoniae alone, but the differences were not significant (data not shown).
PhtD vaccination reduces the NP densities of diverse S. pneumoniae serotypes.
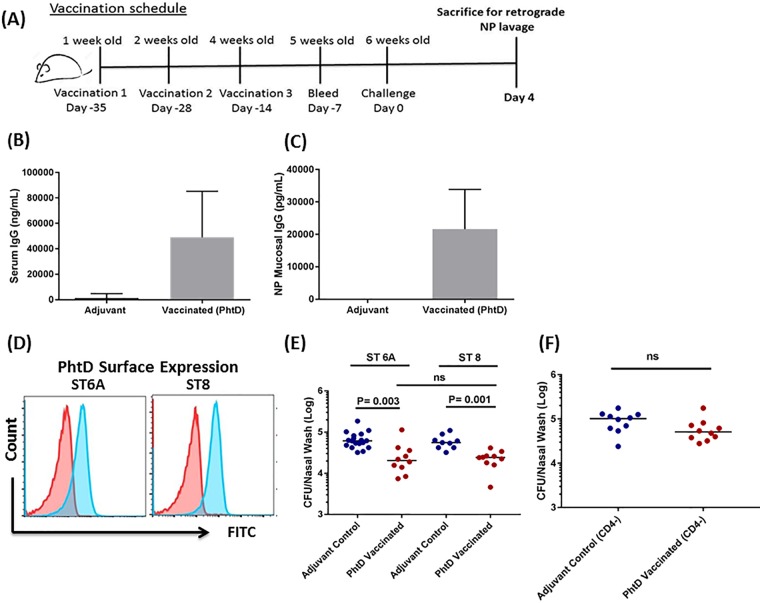
We have previously reported the efficacy of PhtD immunization against NP colonization caused by the S. pneumoniae strain TIGR4 in an adult mouse vaccination model (13). Here, we tested the efficacy of PhtD vaccination against NP colonization using two diverse S. pneumoniae STs (6A and 8) (Fig. 2A). PhtD vaccination resulted in robust serum and mucosal antibody responses (Fig. 2B and C). Sera from PhtD-vaccinated mice reacted to ST 6A and 8 S. pneumoniae, as demonstrated by flow cytometry (Fig. 2D). Compared to adjuvant control, a significantly lower density of S. pneumoniae was observed against both STs in the NP lavage fluid of PhtD-vaccinated mice (ST 6A, P = 0.003; ST 8, P = 0.001) (Fig. 2E). There was no difference between STs 6A and 8 in PhtD-mediated reduction of S. pneumoniae colonization levels (Fig. 2E). After we demonstrated the similarity in efficacy of PhtD vaccination against the two diverse STs, the remaining experiments used ST 6A exclusively. We next sought to understand the role of CD4+ T cells in PhtD-mediated protection against colonization. Depletion of CD4+ T cells from PhtD-vaccinated mice was associated with loss of the reduction in asymptomatic S. pneumoniae NP density that occurred following PhtD vaccination (Fig. 2F).
FIG 2.
(A) Infant (1-week-old) mice were immunized with PhtD with 2 subsequent boosters at weeks 2 and 4. One week later, the mice were bled, and 2 weeks later, they were colonized with S. pneumoniae (STs 6A and 8). Four days after colonization, the mice were euthanized and retrograde NP lavage fluid was collected, serially diluted, and plated on blood agar plates. (B and C) Serum and mucosal anti-PhtD IgG levels in PhtD-vaccinated and adjuvant-vaccinated mice were determined by ELISA. The data are representative of the results of at least 3 independent experiments (n = 5 mice per group) and are expressed as means and SD. (D) Flow cytometry histogram of reactivities of PhtD and adjuvant control antisera against S. pneumoniae STs 6A and 8. (E) S. pneumoniae bacterial densities in NP lavage fluids of PhtD-vaccinated and adjuvant control mice. The data are representative of the results of 2 separate experiments (n = 5 mice per group) expressed as medians and were analyzed by the nonparametric Mann-Whitney test. (F) Effect of CD4+ T cell depletion on S. pneumoniae NP colonization densities in PhtD-vaccinated and control mice (asymptomatic colonization) showing PhtD vaccination and the role of CD4+ T cell depletion in colonization. PhtD-vaccinated or adjuvant control mice were intraperitoneally injected with 200 μg of anti-CD4 antibody with a subsequent booster of 50 μg anti-CD4 antibody 3 days later. CD4+ T cell-depleted mice were asymptomatically colonized with ST 6A. The data are representative of the results of 2 separate experiments (n = 5 or 6 mice per group) expressed as medians. The data were analyzed by the nonparametric Mann-Whitney test.
PhtD vaccination prevents invasive S. pneumoniae pathogenesis in a coinfection model.
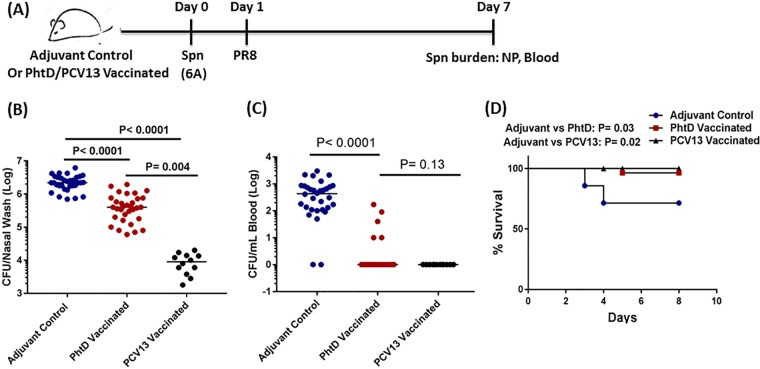
We and others have demonstrated that prior or concurrent upper respiratory influenza virus infection is a major predisposing factor to S. pneumoniae diseases (18–20). Therefore, the invasive-coinfection (S. pneumoniae plus PR8) model provides an ideal tool to evaluate the efficacy of vaccination in circumventing the progression of S. pneumoniae pathogenesis during influenza virus coinfection. PhtD-vaccinated and adjuvant control mice (6 weeks old) were coinfected, and on day 7, the NP and blood bacterial burdens were ascertained (Fig. 3A). Compared to adjuvant-vaccinated mice, the NP bacterial density was nearly 1 log unit lower in PhtD-vaccinated mice (P < 0.001) (Fig. 3B). A 1-log-unit reduction in density to around 1 × 105.5 CFU corresponded to the density observed in earlier experiments where invasiveness was almost lost (Fig. 1B) (S. pneumoniae plus PR8-3). In contrast to PhtD-vaccinated mice with nearly complete protection from bacteremia, more than 80% of adjuvant-vaccinated mice became bacteremic (P < 0.001) (Fig. 3C). PhtD-vaccinated mice also had higher survival during coinfection (P = 0.03) (Fig. 3D). To compare PhtD vaccination-mediated protection with PCV13 that contains ST 6A, we vaccinated mice with PCV13. PCV13 vaccination elicited robust serum 6A-specific IgG titers (see Fig. S3 in the supplemental material) that led to a greater reduction of S. pneumoniae NP density (>2.5 log units) than PhtD vaccination (∼1-log-unit reduction) (Fig. 3B). However, despite the greater reduction of NP bacterial density in PCV13-vaccinated mice, no significant difference was observed with regard to the prevention of S. pneumoniae bacteremia, as >90% of PhtD-vaccinated mice were nonbacteremic (Fig. 3C) (P = 0.13). Also, there was no difference in mortality between PhtD- and PCV13-vaccinated mice (Fig. 3D) (P = 0.28). The protection from invasiveness in PhtD-vaccinated mice occurred as a consequence of preventing an increase in S. pneumoniae NP density that exceeded a pathogenic threshold during coinfection with PR8 influenza virus.
FIG 3.
(A) PhtD- or PCV13-vaccinated or adjuvant control mice (6 weeks old) were i.n. inoculated (10 μl) with 1 × 106 CFU S. pneumoniae (6A). The next day (24 h later), the mice were inoculated i.n. with PR8 influenza virus (50 times the TCID50). Six days later, the mice were euthanized, and the S. pneumoniae bacterial burden was ascertained in NP and blood. (B and C) S. pneumoniae bacterial burden in NP and blood of PhtD-vaccinated and adjuvant control mice coinfected with S. pneumoniae and PR8. The data are represented as medians. The data were analyzed by analysis of variance followed by the Dunn post hoc test for differences between two groups. (D) PhtD- and PCV13-vaccinated and adjuvant control mice (n = 10 to 12/group) were coinfected as described for panel A, and survival was monitored for 10 days. The data were analyzed by the Mantel-Cox test.
PhtD and PCV13 vaccination have divergent correlates of protection in an adult mouse coinfection model.
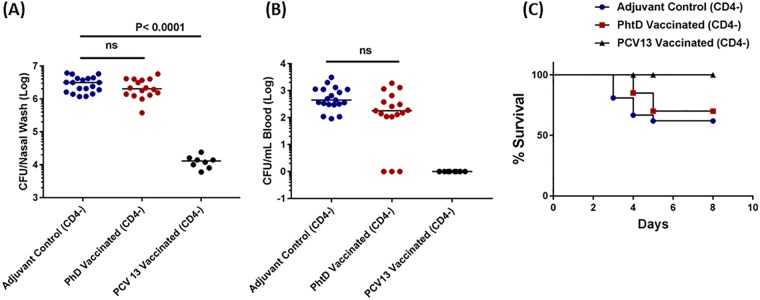
We sought to understand the role of CD4+ T cells and antibody in protection against S. pneumoniae invasiveness and survival during a coinfection. Depletion of CD4+ T cells from PhtD-vaccinated adult mice was associated with a complete loss of the reduction of S. pneumoniae NP density during PR8 coinfection (Fig. 4A). Depletion of CD4+ T cells also abrogated protection from bacteremia and mortality in PhtD-vaccinated mice (Fig. 4B and C). While CD4+ T cells represented an absolute protective correlate against S. pneumoniae invasive pathogenesis in PhtD-vaccinated adult mice, depletion of CD4+ T cells did not impact the protection in PCV13-vaccinated mice. The level of NP bacterial density did not change with CD4+ T cell depletion in PCV13-vaccinated mice, and none of the mice had detectable bacteria in the blood (Fig. 4A and B). Unlike PhtD-vaccinated mice that were CD4+ T cell depleted, all CD4+ T cell-depleted PCV13-vaccinated mice survived (Fig. 4C). These data show that while both protein (PhtD) and glycoconjugate (PCV13) vaccines could protect against S. pneumoniae invasive pathogenesis during an influenza virus coinfection, the induced mechanisms of immune protection against disease pathogenesis differed.
FIG 4.
PhtD and PCV 13 vaccination and the role of CD4+ T cell depletion in survival in a coinfection model. PhtD-vaccinated or adjuvant control mice were intraperitoneally injected with 200 μg of anti-CD4 antibody with a subsequent booster of 50 μg anti-CD4 antibody 3 days later. CD4+ T cell-depleted mice were coinfected with S. pneumoniae (6A) and PR8 influenza virus as explained above. (A and B) Effect of CD4+ depletion on S. pneumoniae burdens in NP and blood in PhtD- and PCV13-vaccinated and control mice (coinfection). The data were analyzed by analysis of variance followed by the Dunn post hoc test for differences between two groups. (C) PhtD- and PCV13-vaccinated and adjuvant control mice (n = 10 to 18/group) were coinfected as described in the legend for Fig. 1, and survival was monitored for 10 days. The data were analyzed by the Mantel-Cox test.
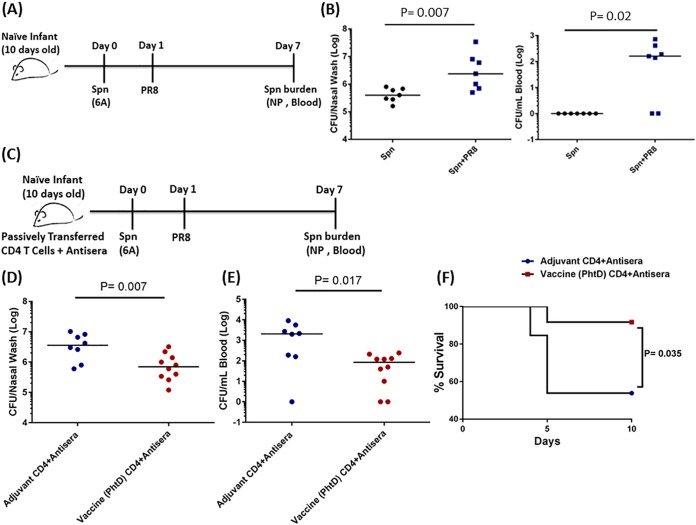
Combined effects of PhtD-specific CD4+ T cells and antibody are required to protect against S. pneumoniae colonization and coinfection in infant mice.
Adults have a more mature immune system than infants and may display different immune protective mechanisms during disease pathogenesis. Although infant mice are initially vaccinated at 1 week old, successive boosters extend into adulthood, as they are 6 weeks older by the time of colonization/coinfection. Therefore, to evaluate the protective PhtD immunity in infant mice, we sought to transfer immunity from 6-week-old PhtD-vaccinated/adjuvant control adult mice to 10-day-old naive infant mice in order to mimic S. pneumoniae pathogenesis in human children, the major target population for protein-based S. pneumoniae vaccination strategies.
In infant mice, passive transfer of antisera or CD4+ T cells from PhtD-vaccinated adult mice led to a nonsignificant reduction (P = 0.17 [Fig. 5B]; P = 0.061 [Fig. 5C]) in NP colonization density, whereas passive transfer of antisera and CD4+ T cells was needed to cause a significant reduction in NP colonization density (P = 0.04) (Fig. 5D). In infant mice, a lower intranasal (i.n.) inoculum (1 × 104 CFU) established colonization, but unlike adult mice, an increase of <1 log unit in S. pneumoniae NP density with PR8 coinfection was associated with invasiveness (Fig. 6B). Under the circumstances of a PR8 coinfection, we found that the PhtD vaccine-induced protection was amplified, resulting in a more robust reduction of S. pneumoniae NP density (P = 0.007) (Fig. 6D) and consequent bacteremia (P = 0.017) (Fig. 6E). Infant mice that received PhtD vaccine immunity also had significantly increased survival compared to mice that received immunity from adjuvant control mice (Fig. 6F).
FIG 5.
(A) Antisera and CD4+ T cells (magnetic sorting) were isolated from PhtD-vaccinated or adjuvant control mice. Naive (10-day-old) infant mice received antisera/CD4+ T cells, separately or combined, from PhtD-vaccinated or adjuvant-vaccinated mice. The mice were colonized, and the S. pneumoniae bacterial burden in NP lavage fluid was determined. (B) Effect of PhtD vaccine or adjuvant control antiserum on S. pneumoniae NP colonization density. (C) Effect of PhtD or control CD4+ T cells on S. pneumoniae NP colonization density. (D) Combined effects of antisera and CD4+ T cells on S. pneumoniae NP colonization density. The data are representative of the results of 2 separate experiments (n = 5 to 7 mice per group) expressed as medians. The data were analyzed by the nonparametric Mann-Whitney test.
FIG 6.
(A and B) Naive infant mice (10 days old) were i.n. inoculated (4 μl) with 1 × 104 S. pneumoniae (ST 6A). The next day (24 h later), the mice were inoculated i.n. (4 μl) with different infection doses of PR8 influenza virus (1.0 times the TCID50). Six days later, the mice were euthanized, and the S. pneumoniae bacterial burden was ascertained in NP and blood. The data are representative of the results of 3 independent experiments (n = 3 per group) and are represented as medians. The data were analyzed by a nonparametric test. (C) Antisera and CD4+ T cells (magnetic sorting) were isolated from PhtD-vaccinated or adjuvant control mice. Naive (10-day-old) infant mice received antisera plus CD4+ T cells from PhtD-vaccinated or adjuvant-vaccinated mice. The mice were coinfected, and the S. pneumoniae bacterial burden was determined in NP lavage fluid and blood. (D) Combined effects of antisera and CD4+ T cells on S. pneumoniae NP bacterial density during coinfection. (E) Combined effects of antisera and CD4+ T cells on the S. pneumoniae blood bacterial burden during coinfection. The data are representative of the results of 1 experiment (n = 13 mice per group) expressed as medians. The data were analyzed by the Mann-Whitney test. (F) Infant mice that received antisera plus CD4+ T cells from PhtD-vaccinated or adjuvant control mice were coinfected as described above, and survival was monitored for 10 days. The data are representative of the results of 1 experiment (n = 13 per group). The data were analyzed by the Mantel-Cox test.
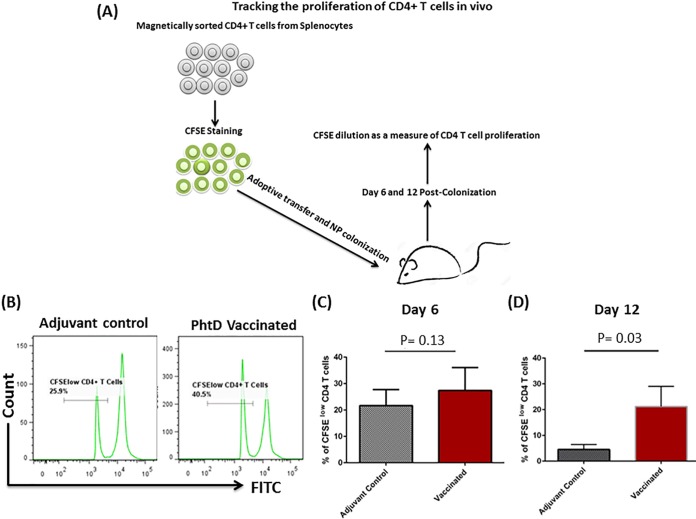
PhtD vaccination-induced CD4+ T cells displayed increased proliferative response in an infant colonization model.
Since vaccine-induced CD4+ T cells were not sufficient for protection in an infant NP colonization model, we sought to determine the proliferative function of adoptively transferred CD4+ T cells in our infant NP colonization model (Fig. 7A). Naive infant mice that received carboxyfluorescein succinimidyl ester (CFSE)-labeled CD4+ T cells from PhtD-vaccinated mice were found to have higher proliferation of CD4+ T (CFSElow) cells than mice that received cells from adjuvant control mice (day 12) (P = 0.03) (Fig. 7B and D). A marginal but nonsignificant difference was observed on day 6 (P = 0.13) (Fig. 7B and C).
FIG 7.
(A) CD4+ T cells from PhtD-vaccinated and adjuvant control mice were magnetically purified, CFSE labeled, and adoptively transferred to naive 10-day-old infant mice. The mice were i.n. inoculated with 1 × 104 CFU of S. pneumoniae (6A). Six and 12 days postcolonization, the spleens were taken out, and CD4+ T cell proliferation was assessed by flow cytometry. (B) Representative histograms of CD4+ T cells from PhtD-vaccinated and adjuvant control mice representing CFSE dilution as a measure of CD4+ T cell proliferation. (C and D) Proliferation of CD4+ T cells on days 6 and 12 postcolonization. The data were normalized and are representative of the results of two separate experiments expressed as means and SD. The data were analyzed by a nonparametric Mann-Whitney test.
DISCUSSION
In this paper, we describe a murine model that mimics natural S. pneumoniae pathogenesis involving the transition of S. pneumoniae from commensal to pathogen during a viral coinfection caused by PR8 influenza virus. We show the influence of an increasing influenza viral load on increased bacterial densities and define quantitatively the NP thresholds of two diverse S. pneumoniae STs (6A and 8) associated with invasive infections. We show that vaccination with PhtD helps prevent the S. pneumoniae NP density from reaching a pathogenic threshold during an influenza virus coinfection. Although, PCV13 vaccination led to a greater reduction of S. pneumoniae NP density, the outcome vis-à-vis prevention of invasive S. pneumoniae pathogenesis did not differ significantly between PhtD- and PCV13-vaccinated mice. We further show that while CD4+ T cells represent the main protective correlate in PhtD-vaccinated adult mice, protection in PCV13-vaccinated mice is CD4+ T cell independent. Infant mice require both PhtD-specific CD4+ T cells and antisera to confer protection against asymptomatic colonization and S. pneumoniae pathogenesis during influenza virus coinfection.
Wolter et al. recently reported that high nasopharyngeal S. pneumoniae density, increased by viral coinfection, was associated with invasive S. pneumoniae pneumonia in children (3). In a mouse coinfection model of S. pneumoniae-influenza A virus (IAV), Diavatopoulos et al. showed that IAV was associated with increased S. pneumoniae colonization, disease, and transmission (21). S. pneumoniae colonization is usually cleared from the NP without progressing to disease (22, 23). Perturbations in the upper respiratory tract colonization niche, driven by viral coinfection, help S. pneumoniae to grow and are essential for progression from an asymptomatic to a pathogenic inoculum (18, 24).
We found that asymptomatic S. pneumoniae colonization elicited a mild inflammatory response in the NP, characterized by a low level of neutrophil recruitment. Introduction of PR8 in the NP led to more robust neutrophil recruitment that correlated with invasive S. pneumoniae pathogenesis. While two different S. pneumoniae strains, expressing ST 6A or 8, had similar NP bacterial thresholds to cause bacteremia, the outcome of invasive infection was achieved with a lower NP inoculum with the more virulent ST 8 strain than with the less virulent ST 6A strain. Wren et al. showed that coinfection with influenza virus enhanced both S. pneumoniae colonization and inflammatory responses within the nasopharynx (25). Nakamura et al. have reported that coinfection with S. pneumoniae and influenza virus modulated the NP inflammatory environment so that it facilitated the rapid proliferation and growth of S. pneumoniae (24). Short et al. reported that local inflammation in the nasal cavity and an increased bacterial load were associated with S. pneumoniae transmission (26). Our findings agree with previously published studies suggesting that although inflammation is required for the resolution of NP colonization (27, 28), an overly exuberant inflammatory response during coinfection is directly associated with proliferation and infection (20, 29, 30).
In the era of PCVs and rapid evolution of new nonvaccine STs (6, 31), next-generation protein-based vaccines are expected to address the shortcomings of existing vaccines (9). The results of the current study provide a new understanding of differences in vaccine-induced protection following PPVs compared to following PCVs. We showed that vaccination with PhtD led to a reduction in the S. pneumoniae NP density sufficient to prevent pathogenesis. This effect was more pronounced during PR8 coinfection than asymptomatic S. pneumoniae colonization in the absence of PR8 coinfection. PCV13 vaccination led to a more robust reduction of S. pneumoniae NP bacterial density than PhtD vaccination. However, the outcomes of vaccination-mediated prevention of invasive-S. pneumoniae pathogenesis did not differ between PhtD- and PCV13-vaccinated mice. While PCV13 vaccination remained completely protective from bacteremia and mortality, a 1-log-unit reduction of S. pneumoniae NP bacterial density achieved by PhtD vaccination was sufficient to significantly reduce bacteremia (>90% of PhtD-vaccinated mice were nonbacteremic) and mortality. These data indicate that PCV13 vaccination is more efficacious in terms of suppressing S. pneumoniae bacterial density in the NP, but prevention of invasive pathogenesis could also be achieved by a moderate reduction of S. pneumoniae bacterial density in the NP, as observed with PhtD vaccination.
Previously, Briles et al. showed that PcpA immunization of adult mice did not reduce NP colonization by S. pneumoniae but nevertheless prevented lung infection and sepsis (32). In another study, pneumolysin (Ply) immunization of adult mice protected against pneumonia and sepsis without affecting the NP colonization (33). Briles et al. showed that immunization with PspA alone reduced NP carriage but that the effect was more pronounced when combined with PsaA (32). In other mouse studies, PspA alone has been reported to reduce pneumonia and sepsis (33). Our results are in agreement with the findings observed with PspA. Thus, immunization with the above-mentioned S. pneumoniae proteins clearly has different effects on NP colonization in mouse studies, but uniformly, such immunizations can protect against S. pneumoniae invasive infection.
To measure the effect of PhtD vaccination on the S. pneumoniae bacterial burden and pathogenesis, the 7-day-postcoinfection time point was deliberately chosen, since time points beyond day 7 likely would include the host's primary immune response to the nasal inoculum challenge strain and vaccination. We cannot exclude the possibility that at later time points, when a mixture of primary response to NP colonization and vaccine response occurs, a greater reduction in the S. pneumoniae bacterial burden might have been observed. Although, vaccination with PhtD reduced NP bacterial density and prevented invasive-S. pneumoniae pathogenesis, further studies are needed to determine the possible herd effect of PhtD vaccination on the prevention of S. pneumoniae bacterial transmission.
Although PCV13 vaccination led to a robust reduction of the S. pneumoniae NP bacterial density, we did not observe elimination of S. pneumoniae in our mouse coinfection model. To our knowledge, this is the first report of a mouse model that shows PCV13-mediated reduction of S. pneumoniae density (nasal lavage fluid) in a coinfection model that mimics natural S. pneumoniae pathogenesis. We recognize that in humans, PCVs result in near elimination of S. pneumoniae vaccine STs from the NP (5). We attribute this difference to the S. pneumoniae infection inoculum. In humans, the bacterial inoculum into the NP from host to host is likely much smaller than the inoculum used in our mouse model (1 × 106 CFU). Clearly elimination of vaccine STs in humans has led to the prevention of S. pneumoniae dissemination and has conferred a herd effect (5). Although vaccination with PhtD reduced NP bacterial density and prevented invasive-S. pneumoniae pathogenesis, further studies are needed to study the herd effects of PhtD vaccination on the prevention of S. pneumoniae bacterial transmission.
Protection mediated by protein-based vaccines may involve immune mechanisms distinct from those engaged by capsule-based glycoconjugate vaccines. In adult mice, we found that depletion of CD4+ T cells from PhtD-vaccinated mice abrogated protection against asymptomatic colonization and increase in S. pneumoniae NP colonization density during coinfection, indicating that CD4+ T cells correlate with reduction of NP colonization density in an antibody-independent manner. Similarly, the depletion of CD4+ T cells in PhtD-vaccinated mice was associated with the loss of survival. However, unlike PhtD, protection in PCV13-vaccinated mice was maintained regardless of CD4+ T cell depletion. In our models, the depletion of CD4+ T cells was performed after vaccination and before infection challenge. Therefore, while postvaccine CD4+ T cells are dispensable in the PCV13-mediated protection, they might be required for the generation of serotype-specific antibodies as it occurs in human (33). Protection from S. pneumoniae NP colonization and disease by glycoconjugate vaccines has been previously shown to be mediated by induction of serotype-specific functional antibodies (34). Taken together, these data suggest that protein vaccines and PCVs have divergent correlates of protection. This is the first report that links the role of protein vaccine-induced CD4+ T cells in protection against S. pneumoniae transition from asymptomatic colonization to disease progression in a model that mimics natural S. pneumoniae pathogenesis. Future studies are needed to understand if the CD4+ T cell-mediated protection is uniform across other S. pneumoniae protein vaccine antigens.
Humans and mice have a less mature immune response during infancy than in adulthood (35–38). Human infants will be a primary vulnerable population targeted for next-generation PPVs. To understand if PhtD vaccine immunity could reduce S. pneumoniae colonization density in infant mice, we transferred antisera and/or CD4+ T cells from PhtD-vaccinated or adjuvant control adult mice to 10-day-old infant mice and colonized/coinfected the mice. PhtD vaccine antisera or CD4+ T cells alone led to marginal but nonsignificant reductions in S. pneumoniae colonization. However, a combined transfer of vaccine antisera and CD4+ T cells led to a significant reduction of S. pneumoniae NP colonization density, protected mice from S. pneumoniae pathogenesis during coinfection, and increased their survival. Protein vaccine antigen-specific CD4+ T cells of adult mice are mature and can participate in clearance of colonization more effectively even in the absence of antibodies (39–41). However, the function of CD4+ T cells in infancy vis-à-vis clearance of colonization could be impaired significantly in the light of their immature and less well developed immune systems (35, 42–44). Therefore, the role of protein antigen-specific antibodies and CD4+ T cells appears to be important in the context of the infant immune system and its ability to clear colonization in contrast to adult mice, where CD4+ T cells are sufficient.
In an infant colonization/coinfection model, sufficient levels of NP mucosal antibodies to adhesin proteins, such as PhtD, can maintain NP colonization density to below a pathogenic inoculum for protection from invasive S. pneumoniae disease. PhtD-vaccine-induced CD4+ T cells may further supplement the clearance. The immune response most likely would be orchestrated in sequence. In that regard, we found that adoptively transferred CD4+ T cells from PhtD-vaccinated mice significantly increased in proliferation by day 12, suggesting that a CD4+ T cell response would not be sufficient in mediating protection against colonization on day 4. The protective efficacy of PhtD with the other two S. pneumoniae vaccines, PcpA and Ply, in trivalent formulation is currently being investigated in our infant mouse model. The ability of protein-based vaccines to maintain S. pneumoniae NP colonization density below a pathogenic threshold during a viral coinfection should be actively explored as an advanced vaccination strategy to contain S. pneumoniae infections.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were purchased from Jackson Laboratory, bred in house, and used with the approval and in accordance with the guidelines of the Rochester General Hospital Animal Care and Use Committee.
Strains and reagents.
S. pneumoniae ST 6A strain BG7322 was obtained from Sanofi Pasteur, and ST 8 strain 6308 was obtained from the ATCC. Both strains have been used in animal infection experiments (45, 46). Recombinant PhtD (good manufacturing practice [GMP] grade) was provided by Sanofi Pasteur. PCV13 (Pfizer) was provided by Michael Pichichero from Legacy Pediatrics (Rochester, NY). Mouse-adapted H1N1 influenza virus strain PR/8/34 (PR8) was obtained from the ATCC and expanded in embryonated chicken eggs (47). A TCID50 assay was performed to determine the influenza virus infection inoculum and titers in the NP lavage fluid of infected mice (18).
Colonization and invasive-coinfection models.
To develop asymptomatic colonization, naive (adult [6-week-old]) C57BL/6 mice were lightly anesthetized with isoflurane and inoculated i.n. with S. pneumoniae infection doses (10 μl phosphate-buffered saline [PBS] containing 1 × 106 CFU for STs 6A and 8). The inoculum of each ST that did not disseminate to the lungs or blood and was sustained in the NP for at least 2 weeks was established in preliminary experiments (data not shown). The mice were euthanized, and upper respiratory tract lavage fluid was collected from the nostrils using 200 μl of PBS as previously described (13, 45). The nasal lavage fluids were plated on blood agar plates, and colonies were enumerated the next day. Total CFU in the nasal lavage fluid were then calculated based on the volume of lavage fluid recovered. For the development of the S. pneumoniae-PR8 coinfection models, naive C57BL/6 mice (6 weeks old) were i.n. inoculated with S. pneumoniae ST 6A or 8 (1 × 105 to 1 × 106 CFU). Twenty-four hours later, the colonized mice were i.n. inoculated with a range of PR8 inocula (PR8-1 at 50 times the TCID50, PR8-2 at 25 times the TCID50, PR8-3 at 10 times the TCID50, or PR8-4 at 5 times the TCID50). Six days post-PR8 inoculation, the mice were bled and euthanized as described above, and the S. pneumoniae burdens in the NP, lungs, and blood were determined.
Vaccination of mice.
One-week-old infant mice were vaccinated intramuscularly with 50 μl of PhtD vaccine formulation (2 μg PhtD emulsified with 25 μg of alum adjuvant), PCV13 (diluted 1:20 in saline), or adjuvant control (25 μg of alum adjuvant), followed by two boosters at weeks 2 and 4. The mice were bled at week 5 and colonized/coinfected at week 6.
Quantitation of serum and mucosal antibodies and surface expression analysis of PhtD.
Anti-PhtD serum and mucosal quantitative antibody titers (IgG) were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (48). S. pneumoniae 6A-specific serum IgG antibodies were determined by ELISA as described previously (49). Surface expression of PhtD was determined using an LSR II flow cytometer (BD) as previously described (13).
Immune phenotyping of NP lavage fluid.
Nasal washes from mice colonized with S. pneumoniae alone or coinfected with PR8 were pooled and washed twice with PBS. Samples were stained to detect neutrophils (CD45+ CD11b+ Ly6G+) as previously described (45). An LSR II flow cytometer was used to acquire 10,000 (colonization) or 50,000 to 100,000 (coinfection) events, and the data were analyzed using FlowJo (Tree Star).
Depletion of CD4+ T cells.
One week after the third vaccination (5-week-old mice) and 1 week before colonization/coinfection, mice were depleted of CD4+ T cells by intraperitoneal (i.p.) injection of anti-CD4+ T cell monoclonal antibody (clone GK1.5; eBioscience) as previously reported (50). The efficacy of depletion was established by the analysis of blood by flow cytometry (see Fig. S1 in the supplemental material).
Adoptive transfer of PhtD vaccine immunity (CD4+ T cells and antisera) and infant mouse colonization and coinfection.
Ten-day-old infant mice were intranasally inoculated with S. pneumoniae (4 μl containing 1 × 104 CFU ST 6A). The next day (24 h later), S. pneumoniae-colonized mice were intranasally inoculated with PR8 (4 μl containing 1.0 times the TCID50). Six days post-PR8 inoculation, the mice were bled and euthanized as described above, and the S. pneumoniae burdens in the NP and blood were determined. To test the effect of PhtD vaccination on the prevention of invasive S. pneumoniae pathogenesis in infant mice, PhtD-vaccinated and adjuvant control adult mice were bled 2 weeks after the third vaccination (6-week-old mice), and sera were prepared. Single-cell suspensions were prepared from spleens, and CD4+ T cells were magnetically sorted using a CD4+ T cell enrichment kit (Miltenyi Biotec). The purity of enrichment was assessed by flow cytometry (see Fig. S2 in the supplemental material). Each naive mouse (10 days old) received 2.5 million cells retro-orbitally or 100 μl of antisera i.p. from either PhtD-vaccinated or adjuvant-vaccinated mice. The mice were i.n. inoculated with 1 × 104 CFU of S. pneumoniae (6A) after either 24 h of CD4+ T cell transfer or 3 h of antiserum transfer. Four days later, the mice were euthanized, and the bacterial burden in the NP lavage fluid was determined as described above. In order to determine the combined effects of CD4+ T cells and antisera, naive infant mice received CD4+ T cells and antisera (CD4+ T cells on day 0 and antisera 24 h later) before being colonized/coinfected as described above. Four (for colonization) or 6 (for coinfection) days later, the mice were euthanized, and the bacterial burdens in the NP lavage fluid and blood were determined as described above.
CD4+ T cell proliferation in an infant mouse colonization model.
Single-cell suspensions were prepared from the spleens of PhtD-vaccinated or adjuvant-vaccinated mice, and CD4+ T cells were magnetically sorted as described above. CD4+ T cells were labeled with CFSE dye (eBioscience) according to the manufacturer's instructions. Naive infant mice (10 days old) received 2.5 million CFSE-labeled CD4+ T cells retro-orbitally from PhtD-vaccinated or adjuvant control mice. After 24 h, the mice were i.n. inoculated with 1 × 104 CFU (4 μl) of S. pneumoniae (6A). CD4+ T cell proliferation was measured in the spleens of colonized mice (6 and 12 days postcolonization).
Statistical analysis.
The data were analyzed using GraphPad Prism software. Statistical significance among multiple groups was determined by means of analysis of variance, followed by Dunn post hoc test for comparisons between the groups. A Mann-Whitney test was used to compare the data representing just two groups. Survival data were analyzed by the Mantel-Cox log rank test. A P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Akhila Sunkara and Karin Pryharski for technical assistance. We thank Robert Zagursky for his critical reading of the manuscript.
We declare no conflict of interest.
This work was supported by Sanofi Pasteur Ltd. through an investigator-initiated grant to Michael E. Pichichero.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00530-16.
REFERENCES
- 1.Bogaert D, De Groot R, Hermans PW. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 2.Richards L, Ferreira DM, Miyaji EN, Andrew PW, Kadioglu A. 2010. The immunising effect of pneumococcal nasopharyngeal colonisation; protection against future colonisation and fatal invasive disease. Immunobiology 215:251–263. doi: 10.1016/j.imbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Wolter N, Tempia S, Cohen C, Madhi SA, Venter M, Moyes J, Walaza S, Malope-Kgokong B, Groome M, du Plessis M, Magomani V, Pretorius M, Hellferscee O, Dawood H, Kahn K, Variava E, Klugman KP, von Gottberg A. 2014. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J Infect Dis 210:1649–1657. doi: 10.1093/infdis/jiu326. [DOI] [PubMed] [Google Scholar]
- 4.McCullers JA. 2006. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, O'Brien KL, Pneumococcal Carriage Group . 2012. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines 11:841–855. doi: 10.1586/erv.12.53. [DOI] [PubMed] [Google Scholar]
- 6.Croucher NJ, Harris SR, Fraser C, Quail MA, Burton J, van der Linden M, McGee L, von Gottberg A, Song JH, Ko KS, Pichon B, Baker S, Parry CM, Lambertsen LM, Shahinas D, Pillai DR, Mitchell TJ, Dougan G, Tomasz A, Klugman KP, Parkhill J, Hanage WP, Bentley SD. 2011. Rapid pneumococcal evolution in response to clinical interventions. Science 331:430–434. doi: 10.1126/science.1198545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey JR, Kaur R, Friedel VC, Pichichero ME. 2013. Acute otitis media otopathogens during 2008 to 2010 in Rochester, New York. Pediatr Infect Dis J 32:805–809. doi: 10.1097/INF.0b013e31828d9acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pichichero ME, Casey JR. 2007. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA 298:1772–1778. doi: 10.1001/jama.298.15.1772. [DOI] [PubMed] [Google Scholar]
- 9.Pichichero ME, Khan MN, Xu Q. 2016. Next generation protein based Streptococcus pneumoniae vaccines. Hum Vaccin Immunother 12:194–205. doi: 10.1080/21645515.2015.1052198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rioux S, Neyt C, Di Paolo E, Turpin L, Charland N, Labbe S, Mortier MC, Mitchell TJ, Feron C, Martin D, Poolman JT. 2011. Transcriptional regulation, occurrence and putative role of the Pht family of Streptococcus pneumoniae. Microbiology 157:336–348. doi: 10.1099/mic.0.042184-0. [DOI] [PubMed] [Google Scholar]
- 11.Adamou JE, Heinrichs JH, Erwin AL, Walsh W, Gayle T, Dormitzer M, Dagan R, Brewah YA, Barren P, Lathigra R, Langermann S, Koenig S, Johnson S. 2001. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect Immun 69:949–958. doi: 10.1128/IAI.69.2.949-958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan MN, Pichichero ME. 2012. Vaccine candidates PhtD and PhtE of Streptococcus pneumoniae are adhesins that elicit functional antibodies in humans. Vaccine 30:2900–2907. doi: 10.1016/j.vaccine.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan MN, Pichichero ME. 2013. CD4 T cell memory and antibody responses directed against the pneumococcal histidine triad proteins PhtD and PhtE following nasopharyngeal colonization and immunization and their role in protection against pneumococcal colonization in mice. Infect Immun 81:3781–3792. doi: 10.1128/IAI.00313-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godfroid F, Hermand P, Verlant V, Denoel P, Poolman JT. 2011. Preclinical evaluation of the Pht proteins as potential cross-protective pneumococcal vaccine antigens. Infect Immun 79:238–245. doi: 10.1128/IAI.00378-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leroux-Roels G, Maes C, De Boever F, Traskine M, Ruggeberg JU, Borys D. 2014. Safety, reactogenicity and immunogenicity of a novel pneumococcal protein-based vaccine in adults: a phase I/II randomized clinical study. Vaccine 32:6838–6846. doi: 10.1016/j.vaccine.2014.02.052. [DOI] [PubMed] [Google Scholar]
- 16.Prymula R, Pazdiora P, Traskine M, Ruggeberg JU, Borys D. 2014. Safety and immunogenicity of an investigational vaccine containing two common pneumococcal proteins in toddlers: a phase II randomized clinical trial. Vaccine 32:3025–3034. doi: 10.1016/j.vaccine.2014.03.066. [DOI] [PubMed] [Google Scholar]
- 17.Bologa M, Kamtchoua T, Hopfer R, Sheng X, Hicks B, Bixler G, Hou V, Pehlic V, Yuan T, Gurunathan S. 2012. Safety and immunogenicity of pneumococcal protein vaccine candidates: monovalent choline-binding protein A (PcpA) vaccine and bivalent PcpA-pneumococcal histidine triad protein D vaccine. Vaccine 30:7461–7468. doi: 10.1016/j.vaccine.2012.10.076. [DOI] [PubMed] [Google Scholar]
- 18.Smith AM, Adler FR, Ribeiro RM, Gutenkunst RN, McAuley JL, McCullers JA, Perelson AS. 2013. Kinetics of coinfection with influenza A virus and Streptococcus pneumoniae. PLoS Pathog 9:e1003238. doi: 10.1371/journal.ppat.1003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rynda-Apple A, Robinson KM, Alcorn JF. 2015. Influenza and bacterial superinfection: illuminating the immunologic mechanisms of disease. Infect Immun 83:3764–3770. doi: 10.1128/IAI.00298-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metzger DW, Sun K. 2013. Immune dysfunction and bacterial coinfections following influenza. J Immunol 191:2047–2052. doi: 10.4049/jimmunol.1301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diavatopoulos DA, Short KR, Price JT, Wilksch JJ, Brown LE, Briles DE, Strugnell RA, Wijburg OL. 2010. Influenza A virus facilitates Streptococcus pneumoniae transmission and disease. FASEB J 24:1789–1798. doi: 10.1096/fj.09-146779. [DOI] [PubMed] [Google Scholar]
- 22.Neill DR, Coward WR, Gritzfeld JF, Richards L, Garcia-Garcia FJ, Dotor J, Gordon SB, Kadioglu A. 2014. Density and duration of pneumococcal carriage is maintained by transforming growth factor beta1 and T regulatory cells. Am J Respir Crit Care Med 189:1250–1259. doi: 10.1164/rccm.201401-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sleeman KL, Griffiths D, Shackley F, Diggle L, Gupta S, Maiden MC, Moxon ER, Crook DW, Peto TE. 2006. Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J Infect Dis 194:682–688. doi: 10.1086/505710. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura S, Davis KM, Weiser JN. 2011. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest 121:3657–3665. doi: 10.1172/JCI57762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wren JT, Blevins LK, Pang B, King LB, Perez AC, Murrah KA, Reimche JL, Alexander-Miller MA, Swords WE. 2014. Influenza A virus alters pneumococcal nasal colonization and middle ear infection independently of phase variation. Infect Immun 82:4802–4812. doi: 10.1128/IAI.01856-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Short KR, Reading PC, Wang N, Diavatopoulos DA, Wijburg OL. 2012. Increased nasopharyngeal bacterial titers and local inflammation facilitate transmission of Streptococcus pneumoniae. mBio 3:e00255-12. doi: 10.1128/mBio.00255-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puchta A, Verschoor CP, Thurn T, Bowdish DM. 2014. Characterization of inflammatory responses during intranasal colonization with Streptococcus pneumoniae. J Vis Exp 83:e50490. doi: 10.3791/50490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira DM, Neill DR, Bangert M, Gritzfeld JF, Green N, Wright AK, Pennington SH, Bricio-Moreno L, Moreno AT, Miyaji EN, Wright AD, Collins AM, Goldblatt D, Kadioglu A, Gordon SB. 2013. Controlled human infection and rechallenge with Streptococcus pneumoniae reveals the protective efficacy of carriage in healthy adults. Am J Respir Crit Care Med 187:855–864. doi: 10.1164/rccm.201212-2277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cauley LS, Vella AT. 2015. Why is coinfection with influenza virus and bacteria so difficult to control? Discov Med 19:33–40. [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis GT, Davidson S, Crotta S, Branzk N, Papayannopoulos V, Wack A. 2015. TRAIL+ monocytes and monocyte-related cells cause lung damage and thereby increase susceptibility to influenza-Streptococcus pneumoniae coinfection. EMBO Rep 16:1203–1218. doi: 10.15252/embr.201540473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Croucher NJ, Finkelstein JA, Pelton SI, Mitchell PK, Lee GM, Parkhill J, Bentley SD, Hanage WP, Lipsitch M. 2013. Population genomics of post-vaccine changes in pneumococcal epidemiology. Nat Genet 45:656–663. doi: 10.1038/ng.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briles DE, Ades E, Paton JC, Sampson JS, Carlone GM, Huebner RC, Virolainen A, Swiatlo E, Hollingshead SK. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect Immun 68:796–800. doi: 10.1128/IAI.68.2.796-800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pichichero ME. 2013. Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum Vaccin Immunother 9:2505–2523. doi: 10.4161/hv.26109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 4:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bogaert D, Weinberger D, Thompson C, Lipsitch M, Malley R. 2009. Impaired innate and adaptive immunity to Streptococcus pneumoniae and its effect on colonization in an infant mouse model. Infect Immun 77:1613–1622. doi: 10.1128/IAI.00871-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES III, Hajjar AM, Hawkins NR, Self SG, Wilson CB. 2009. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kollmann TR, Levy O, Montgomery RR, Goriely S. 2012. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 37:771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pichichero ME. 2014. Challenges in vaccination of neonates, infants and young children. Vaccine 32:3886–3894. doi: 10.1016/j.vaccine.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. 2005. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A 102:4848–4853. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moffitt KL, Gierahn TM, Lu YJ, Gouveia P, Alderson M, Flechtner JB, Higgins DE, Malley R. 2011. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe 9:158–165. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trzcinski K, Thompson CM, Srivastava A, Basset A, Malley R, Lipsitch M. 2008. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infect Immun 76:2678–2684. doi: 10.1128/IAI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchant A, Kollmann TR. 2015. Understanding the ontogeny of the immune system to promote immune-mediated health for life. Front Immunol 6:77. doi: 10.3389/fimmu.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan MN, Pichichero ME. 2014. The host immune dynamics of pneumococcal colonization: implications for novel vaccine development. Hum Vaccin Immunother 10:3688–3699. doi: 10.4161/21645515.2014.979631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Debock I, Flamand V. 2014. Unbalanced neonatal CD4(+) T-cell immunity. Front Immunol 5:393. doi: 10.3389/fimmu.2014.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan MN, Coleman JR, Vernatter J, Varshney AK, Dufaud C, Pirofski LA. 2014. An ahemolytic pneumolysin of Streptococcus pneumoniae manipulates human innate and CD4+ T-cell responses and reduces resistance to colonization in mice in a serotype-independent manner. J Infect Dis 210:1658–1669. doi: 10.1093/infdis/jiu321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brooks-Walter A, Briles DE, Hollingshead SK. 1999. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect Immun 67:6533–6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woolcock PR. 2008. Avian influenza virus isolation and propagation in chicken eggs. Methods Mol Biol 436:35–46. [DOI] [PubMed] [Google Scholar]
- 48.Verhoeven D, Xu Q, Pichichero ME. 2014. Vaccination with a Streptococcus pneumoniae trivalent recombinant PcpA, PhtD and PlyD1 protein vaccine candidate protects against lethal pneumonia in an infant murine model. Vaccine 32:3205–3210. doi: 10.1016/j.vaccine.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Wernette CM, Frasch CE, Madore D, Carlone G, Goldblatt D, Plikaytis B, Benjamin W, Quataert SA, Hildreth S, Sikkema DJ, Kayhty H, Jonsdottir I, Nahm MH. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol 10:514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mochimaru H, Usui T, Yaguchi T, Nagahama Y, Hasegawa G, Usui Y, Shimmura S, Tsubota K, Amano S, Kawakami Y, Ishida S. 2008. Suppression of alkali burn-induced corneal neovascularization by dendritic cell vaccination targeting VEGF receptor 2. Invest Ophthalmol Vis Sci 49:2172–2177. doi: 10.1167/iovs.07-1396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.