Significance
We report the discovery of complete human interleukin-1 receptor (IL-1R)-associated kinase 1 (IRAK-1) deficiency resulting from a de novo Xq28 microdeletion encompassing MECP2 and IRAK1 in a boy. Like many boys with MECP2 defects, this patient died very early. IRAK-1 is a component of the Toll-like receptor (TLR)/IL-1R (TIR) signaling pathway. Unlike patients with autosomal-recessive complete deficiency of MyD88 or IRAK-4, two other components of the TIR pathway, this patient presented no invasive bacterial infections. We analyzed the impact of human IRAK-1 deficiency in fibroblasts and leukocytes. The role of IRAK-1 in signaling downstream from IL-1R and TLRs differed according to cell type. These findings reveal similarities and differences in the role of IRAK-1 in the TLR and IL-1R pathways between mice and humans.
Keywords: IRAK-1, IRAK-4, Toll-like receptor, interleukin-1 receptor, primary immunodeficiency
Abstract
Most members of the Toll-like receptor (TLR) and interleukin-1 receptor (IL-1R) families transduce signals via a canonical pathway involving the MyD88 adapter and the interleukin-1 receptor-associated kinase (IRAK) complex. This complex contains four molecules, including at least two (IRAK-1 and IRAK-4) active kinases. In mice and humans, deficiencies of IRAK-4 or MyD88 abolish most TLR (except for TLR3 and some TLR4) and IL-1R signaling in both leukocytes and fibroblasts. TLR and IL-1R responses are weak but not abolished in mice lacking IRAK-1, whereas the role of IRAK-1 in humans remains unclear. We describe here a boy with X-linked MECP2 deficiency-related syndrome due to a large de novo Xq28 chromosomal deletion encompassing both MECP2 and IRAK1. Like many boys with MECP2 null mutations, this child died very early, at the age of 7 mo. Unlike most IRAK-4– or MyD88-deficient patients, he did not suffer from invasive bacterial diseases during his short life. The IRAK-1 protein was completely absent from the patient’s fibroblasts, which responded very poorly to all TLR2/6 (PAM2CSK4, LTA, FSL-1), TLR1/2 (PAM3CSK4), and TLR4 (LPS, MPLA) agonists tested but had almost unimpaired responses to IL-1β. By contrast, the patient’s peripheral blood mononuclear cells responded normally to all TLR1/2, TLR2/6, TLR4, TLR7, and TLR8 (R848) agonists tested, and to IL-1β. The death of this child precluded long-term evaluations of the clinical consequences of inherited IRAK-1 deficiency. However, these findings suggest that human IRAK-1 is essential downstream from TLRs but not IL-1Rs in fibroblasts, whereas it plays a redundant role downstream from both TLRs and IL-1Rs in leukocytes.
The interleukin-1 receptor-associated kinase (IRAK) protein complex plays a critical role in the canonical pathway downstream from most Toll-like receptors (TLRs) and IL-1 receptors (IL-1Rs) (1–3). In humans and mice, the IRAK complex has four members: IRAK-1, IRAK-2, IRAK-3/IRAK-M, and IRAK-4 (4–11). All contain an amino-terminal death domain (DD) (12) required for homo- or heterodimerization and a serine/threonine kinase domain (13–15). IRAK-1, IRAK-4, and possibly IRAK-2 have serine/threonine kinase activity (10, 16, 17). Upon stimulation, in both mice and humans, the myeloid differentiation primary response gene 88 (MyD88) adaptor is recruited to TLRs and IL-1Rs via TLR–IL-1R (TIR) interaction; it then recruits IRAK-4 by DD interaction (4, 18–20). Other TIR adapters, such as TIRAP for TLR2 and TLR4 (via MyD88), contribute to TLR-responsive pathways (21, 22). IRAK-4 then associates with IRAK-1 and/or IRAK-2 to form the “Myddosome” (23–26). The phosphorylation of IRAK-1 by IRAK-4 results in the activation of IRAK-1 kinase activity, leading to IRAK-1 hyperphosphorylation (by autophosphorylation). Hyperphosphorylated IRAK-1 dissociates from the Myddosome to associate with TRAF-6 (27, 28) and is then ubiquitinated and degraded or sumoylated (29–32). This ultimately activates both the mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) pathways, resulting in the transcription of target genes, including those encoding proinflammatory cytokines (20, 33). By contrast, the interaction of IRAK-3 with the Myddosome has been shown to have an inhibitory effect, in both mice and humans (28, 34).
In vitro studies in mouse embryonic fibroblasts (MEFs) have shown that the knockout (KO) of Irak1, Irak4, or, to a lesser extent, Irak2 results in impaired responses to TLR2/6 (MALP-2, PGN), TLR4 (LPS), and IL-1R (IL-1β) stimulation (5, 11, 23, 35, 36). Irak3 KO MEFs have not been tested. Irak2−/− (37), Irak4−/− (11), and, to a lesser extent, Irak1−/Y (23, 35) macrophages display impaired responses to TLR4 agonists, whereas Irak3−/− macrophages display enhanced responses to such agonists (28). Irak1−/− splenocytes produce normal amounts of IL1B and TNF mRNA, but their production of IL10 mRNA and protein in response to TLR4 (LPS) stimulation is impaired (31). Irak4−/− splenocytes fail to proliferate in response to the stimulation of TLR2/6 (MALP-2), TLR4 (LPS), TLR7, TLR8 (R848), or TLR9 (CpG), whereas they proliferate normally when stimulated with the nonspecific TLR3 agonist poly(I:C) (37). IL-1R/TLR signaling was not assessed in Irak2−/− and Irak3−/− splenocytes. In vivo studies have shown survival to be higher for Irak1−/−, Irak2−/−, and Irak4−/− mice following LPS injection (Irak3−/− mice were not tested) (11, 38, 39) and lower for Irak1−/− and Irak4−/− mice (the only two strains tested) following Staphylococcus aureus infection (11, 40). Myd88−/− mice and, by inference, Irak4−/− mice are susceptible to many other pathogens not used for the inoculation of the other mutants (41). Overall, these studies suggest that the mouse IRAK-1, IRAK-2, and IRAK-4 proteins play activating roles in TLR and IL-1R responses and protective immunity to bacteria, albeit to different degrees, whereas IRAK-3 seems to have an inhibitory effect (34, 42).
Human patients with autosomal-recessive complete IRAK-4 or MyD88 deficiency have a common clinical phenotype, characterized by extreme susceptibility to a small range of pyogenic bacterial infections, with normal resistance to other bacteria and most viruses, fungi, and parasites (41, 43). IRAK-4– and MyD88-deficient patients present with meningitis, sepsis, arthritis, osteomyelitis, and deep inner-organ/tissue abscesses, mostly caused by gram-positive Streptococcus pneumoniae and S. aureus and, more rarely, by gram-negative Pseudomonas aeruginosa and Shigella sonnei (44–64). They also display superficial skin infections, mostly caused by S. aureus. The first invasive infection occurs before the age of 2 y in 85% of patients and 92% of probands, and before the age of 6 mo in 42% of patients and probands (41, 43, 52, 55, 57, 59–64). The frequency and severity of these infections decrease considerably from adolescence onward, even in the absence of preventive measures, suggesting that the MyD88/IRAK-4–dependent TIR pathway becomes redundant once acquired immunity is fully functional and can ensure protection (43). These disorders have a modest impact on IgM-dependent B-cell immunity, delaying its maturation (65, 66). IRAK-4– and MyD88-deficient patients also display impaired inflammatory responses, such as weak or delayed fever and plasma C-reactive protein (CRP) induction (43).
Fibroblasts from MyD88- and IRAK-4–deficient patients do not respond to IL-1β in terms of IRAK-1 and IκB-α degradation, MAPK activation (p38 phosphorylation), NF-κB DNA-binding activity, transcriptional activity, and proinflammatory cytokine production (44, 52, 55). TLR2/6 and TLR4 responses have not been assessed in MyD88- and IRAK-4–deficient fibroblasts, in which TLR3 responses to poly(I:C) are intact and mediated by the TRIF-dependent pathway (67–69). In addition, CD62L shedding and proinflammatory cytokine production in response to IL-1R agonists (IL-1β) or any of the TLR agonists tested, other than poly(I:C), that signal via receptors other than TLR3 in most human leukocyte subsets (69) (TLR1/2, TLR2/6, TLR5, TLR7, TLR8, TLR9, and partially TLR4) were abolished in all IRAK-4– and MyD88-deficient leukocyte subsets tested ex vivo (granulocytes, monocytes, plasmacytoid and myeloid dendritic cells, NK, T, and B cells) or generated in vitro (monocyte-derived dendritic cells) (52, 55). The weak induction of the IFN-stimulated gene products MIP-1β and MCP-1 in response to LPS was probably mediated by TRIF (52). No patients with IRAK-1, IRAK-2, or IRAK-3 deficiency have yet been described, precluding assessment of the cellular, immunological, and clinical impact of these defects in humans. We describe here a child with X-linked recessive complete IRAK-1 deficiency and the impact of this inborn error of immunity on cellular responses to TLR and IL-1R agonists (70, 71).
Results
Case Report.
The proband was a boy born to nonconsanguineous Italian parents at 40 wk of gestation, after an unremarkable pregnancy (Fig. 1A). The family history contained no relevant antecedents. At birth, the baby presented a weak cry, apnea, and hypotonia, and he required neonatal resuscitation and noninvasive respiratory support because of poor respiratory effort. Antibiotics (ampicillin and gentamicin) were administered for the first 3 d of life for a suspected early-onset infection that was not confirmed by microbiological analyses. The child was hospitalized during the first 4 mo of life, due to recurrent episodes of apnea with cyanosis, sometimes accompanied by bradycardia, and poor respiratory effort. He presented severe axial and limb hypotonia, hypokinesia, hyporeactivity, and intermittent proximal lower-limb rigidity, abnormal eye movements (chaotic ocular movements, poor eye contact, and tonic ocular deviation), automatic movements of the tongue, and seizure-like episodes. He also needed oxygen and/or advanced respiratory support through nasal continuous positive airway pressure or mechanical ventilation. He was fed via a nasogastric or orogastric tube due to poor sucking ability and difficulties swallowing. During hospitalization, he had mild conjunctivitis due to S. aureus, a urinary tract infection confirmed by the isolation of Klebsiella pneumoniae from a urine culture and treated with amikacin for 13 d, and bronchospasms in the course of Rhinovirus infection. An electroencephalogram performed due to seizure-like episodes showed a pattern of irritative activity, with high-voltage waves and spikes. The infant was vaccinated with a hexavalent vaccine (against diphtheria/tetanus/acellular pertussis/inactivated poliovirus/hepatitis B/Haemophilus influenzae type b), with no adverse event. At 6 mo of age, percutaneous endoscopic gastrostomy (PEG) was performed. Two days later, the child had a brief temperature peak of 38 °C, with no associated clinical problems. Four days after PEG, at the age of 6 mo, the baby had an episode of acute, severe respiratory failure, during which his temperature briefly peaked at 38 °C and his serum CRP concentration was slightly high, at 10.4 mg/L. Chest X-rays revealed diffuse, heterogeneous, bilateral opacities of the lung parenchyma requiring mechanical ventilation for 4 d. The infant was given ceftriaxone and discharged from hospital 8 d after the onset of respiratory failure (i.e., 4 d after extubation). One month after hospitalization for PEG, the child required mechanical ventilation again, due to progressive respiratory failure. Both axillary temperature and CRP were within the normal range. Chest X-rays were performed and confirmed aspiration pneumonia (SI Appendix, Fig. S1). The child’s neurological and respiratory condition progressively deteriorated. Death occurred at 7 mo of age, due to progressive respiratory failure.
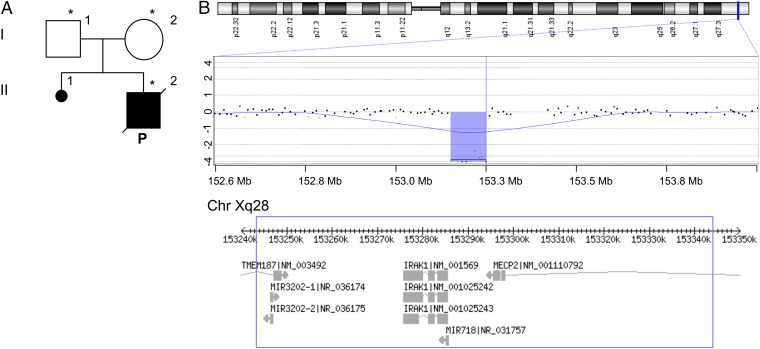
Fig. 1.
De novo large deletion on the X chromosome encompasses the IRAK1 and MECP2 genes. (A) Pedigree of the patient’s family; generations are designated by roman numerals (I, II); the patient is represented by a black square and indicated by “P.” Asterisks indicate the individuals tested for Xq28 deletion. (B) Schematic representation of the X chromosome and the array-CGH profile of the patient’s chromosome Xq28, showing the hemizygous deletion. The experiment was performed with a 180K platform (180K SurePrint G3 Human Kit; Agilent Technologies). A schematic representation of the gene content of the 101-kb deleted region is shown (Bottom).
A Large Xq28 Deletion.
We investigated whether a genetic defect, such as those responsible for Prader–Willi syndrome or 22q11.2 deletion syndrome, could account for the neurological symptoms of the patient. We determined the patient’s karyotype and SNRPN gene methylation profile, and performed FISH for the 22q11.2 region. The patient was found to have a normal 46, XY karyotype, and we excluded both Prader–Willi syndrome and 22q11.2 deletion syndrome. Array comparative genomic hybridization (array-CGH) analysis was also performed to exclude a microduplication/microdeletion syndrome. This assay revealed an intrachromosomal deletion of about 112 kb on the long arm of the X chromosome (Xq28). The proximal breakpoint mapped to between 153,238,518 and 153,246,671 bp, whereas the distal breakpoint mapped to between 153,340,688 and 153,350,516 bp (Fig. 1B). We determined the size of the rearrangement, to a resolution of 1 bp, with specific primers (sequences are available upon request) binding to sequences on either side of the predetermined breakpoints. The breakpoints of the deletion were found at 153,243,653 and 153,344,830 bp. The deleted region was 101,177 bp long, and the presence of the same 12-nt sequence (ACAGAGCAAGAG) at both breakpoints suggests that this rearrangement resulted from nonallelic homologous recombination (72). The deletion encompassed parts of the TMEM187 and MECP2 protein-coding genes and the IRAK1 protein-coding gene, as well as the MIR3202-1, MIR3202-2, and MIR718 RNA-coding genes (Fig. 1B).
The Deletion Encompasses MECP2 and IRAK1.
TMEM187 encodes a putative ubiquitous multipass membrane protein (73). The MIR3202-1, MIR3202-2, and MIR718 genes all encode microRNAs with unknown target genes. MECP2 encodes a widely expressed chromatin-associated protein that specifically binds 5-methyl cytosine residues in CpG dinucleotides and can either activate or repress the transcription of its target genes (74–76). Loss-of-function (LOF) mutations of MECP2 cause Rett syndrome, a progressive neurodevelopmental disorder that predominantly affects girls. Rett syndrome is characterized principally by arrested development at about 12 mo of age, the regression of acquired skills, a reduction or total loss of communication, and stereotypic hand movements (77). Boys carrying LOF mutations of MECP2 present with various neurological phenotypes, from Rett-like syndrome (very rare) to severe neonatal encephalopathy commonly accompanied by respiratory insufficiency, hypotonia, and early childhood death (78–91). MECP2 duplication syndrome is a severe neurodevelopmental disorder (fully penetrant in boys) characterized by hypotonia, severe intellectual disability, speech abnormalities, seizures, and recurrent life-threatening infections (92). About 70 to 75% of patients with MECP2 duplication display susceptibility to recurrent infections. IgA/IgG2 deficiency, low antibody titers against pneumococci, and abnormally strong acute-phase responses have recently been reported in patients with MECP2 duplication syndrome (93). Based on these data, a diagnosis of MECP2-related congenital encephalopathy was established in our patient. Male patients with MECP2-related congenital encephalopathy have been reported to display either MECP2 mutations or, more rarely, large deletions encompassing MECP2. To our knowledge, a deletion encompassing both MECP2 and IRAK1 has never before been reported in a male patient. Such deletions have been reported only in heterozygous female patients (94–102).
Impaired Responses to TLR Stimulation in the Patient’s Fibroblasts.
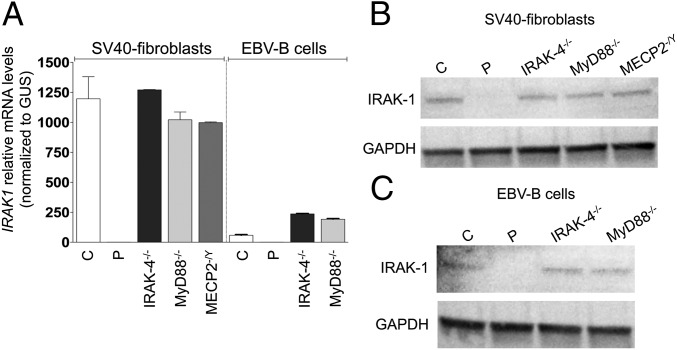
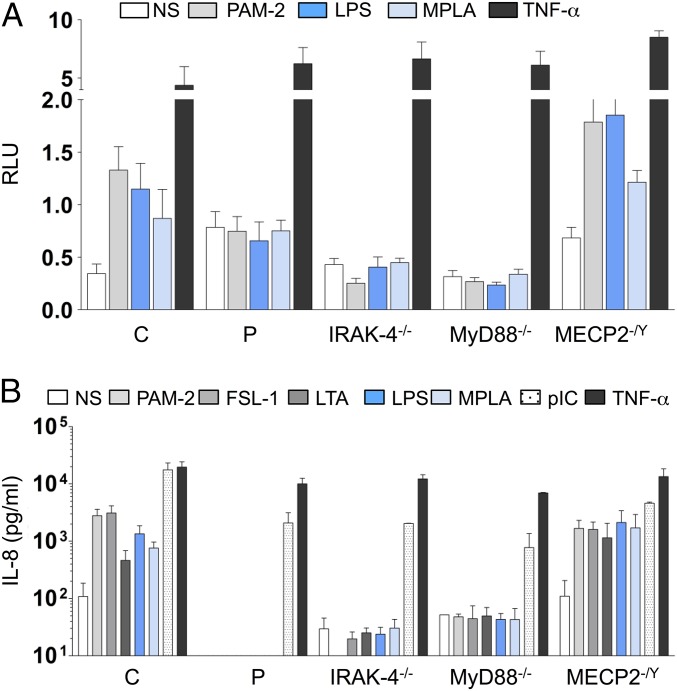
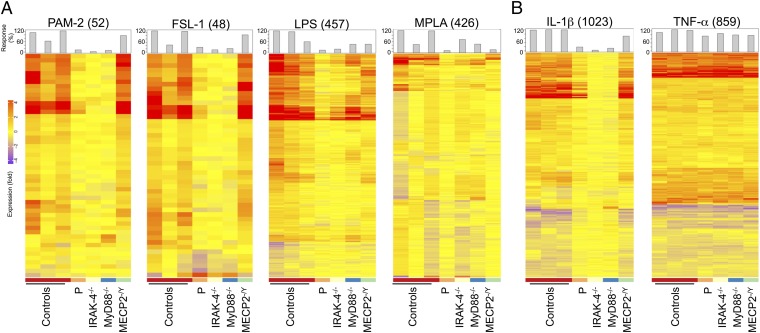
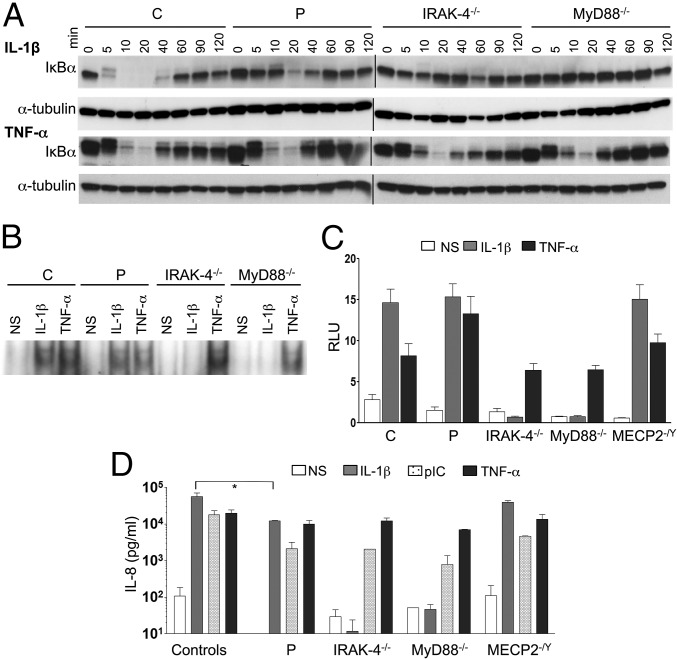
Neither IRAK1 mRNA (evaluated by quantitative PCR) nor IRAK-1 protein (evaluated by Western blotting) was detected in the patient’s SV40-immortalized fibroblasts (SV40-fibroblasts). By contrast, IRAK-1 mRNA and protein were detected in SV40-fibroblasts from IRAK-4–, MyD88-, and MECP2-deficient patients and healthy individuals, all of whom served as controls in subsequent experiments (Fig. 2 A and B). We first assessed TLR-dependent signaling in the patient’s SV40-fibroblasts, after stimulation with TLR2/6 agonists (PAM2CSK4, known as PAM-2, FSL-1, and LTA-SA), TLR4 agonists (LPS, monophosphoryl lipid A, known as MPLA), and poly(I:C). We had previously checked that human SV40-fibroblasts responded (weakly) to LPS and MPLA stimulation in a TLR4-dependent manner, by knocking down TLR4 mRNA in control SV40-fibroblasts with small interfering RNAs (siRNAs) (SI Appendix, Fig. S2A) and evaluating the resulting levels of IL-8 mRNA and protein. IL-8 mRNA and protein levels in response to stimulation with LPS or MPLA were lower after transfection with an siRNA against TLR4 but not after transfection with a scrambled siRNA; by contrast, no such decrease was observed after PAM-2 stimulation (SI Appendix, Fig. S2 B and C). As in IRAK-4– and MyD88-deficient SV40-fibroblasts, NF-κB DNA-binding activity was abolished in the patient’s SV40-fibroblasts (evaluated by EMSA; SI Appendix, Fig. S3 A and B), together with NF-κB transcriptional activity (luciferase assay; Fig. 3A) and cytokine production (ELISA; Fig. 3B and SI Appendix, Fig. S4A) in response to TLR2/6 and TLR4 stimulation, whereas cytokine production in response to stimulation with poly(I:C) was similar to that in control SV40-fibroblasts. Following TNF-α stimulation, NF-κB DNA-binding and transcriptional activities and cytokine production were similar between SV40-fibroblasts from the patient and from IRAK-4– and MyD88-deficient patients and controls (Fig. 3 and SI Appendix, Figs. S3 A and B and S4A). The responses of MECP2-deficient fibroblasts were similar to those of control SV40-fibroblasts in all conditions and for all readouts tested (Fig. 3 and SI Appendix, Figs. S3 A and B and S4A). Similar results were obtained with primary fibroblasts (SI Appendix, Fig. S5A). Finally, we used microarrays to study the transcriptome of primary fibroblasts, with or without stimulation with TLR2/6 and TLR4 agonists. Primary fibroblasts from the IRAK-1– and MECP2-deficient patient studied displayed much lower levels of target gene induction than cells from healthy controls and an MECP2-deficient patient, as found for fibroblasts from IRAK-4– and MyD88-deficient patients (Fig. 4A). The induction of some target genes was apparently IRAK-4– and MyD88-dependent but IRAK-1–independent. Overall, fibroblasts from the IRAK-1– and MECP2-deficient patient responded poorly to all of the relevant TLR agonists tested.
Fig. 2.
Complete IRAK-1 deficiency. (A) Relative IRAK1 mRNA levels in SV40-fibroblasts and EBV-B cells from controls (labeled C; n = 2 for both SV40-fibroblasts and EBV-B cells) and from the IRAK-1–deficient patient (labeled P) and IRAK-4–, MyD88-, and MECP2-deficient patients (labeled IRAK-4−/−, MyD88−/−, and MECP2−/Y, respectively). The values shown (means ± SEM) were obtained in three independent experiments. (B and C) Western blot analysis of IRAK-1 protein levels in total cell extracts from SV40-fibroblasts (B) and EBV-B cells (C) from controls and the IRAK-1–deficient patient and IRAK-4–, MyD88-, and MECP2-deficient patients. Similar results were obtained in three independent experiments.
Fig. 3.
TLR-dependent signaling in the patient’s fibroblasts. (A) NF-κB–dependent transcription was assessed with the NF-κB reporter luciferase assay in SV40-fibroblasts derived from a healthy control, the IRAK-1–deficient patient, and IRAK-4–, MyD88-, and MECP2-deficient patients. Twenty-four hours after transfection, the cells were left untreated (NS) or stimulated with PAM-2 (10 μg/mL), LPS (10 μg/mL), MPLA (1 μg/mL), or TNF-α (20 ng/mL) for 42 h and then harvested. Reporter gene activities were measured and the values obtained were normalized for transfection efficiency on the basis of Renilla luciferase expression. RLU, relative light units. (B) IL-8 secretion by SV40-fibroblasts from healthy controls (n = 3), the IRAK-1–deficient patient, and IRAK-4–, MyD88-, and MECP2-deficient patients, left unstimulated or stimulated with PAM-2 (10 μg/mL), FSL-1 (1 μg/mL), LTA (10 μg/mL), LPS (10 μg/mL), MPLA (1 μg/mL), poly(I:C) (pIC; 25 μg/mL), and TNF-α (20 ng/mL) as a positive control. The values shown (means ± SEM) were obtained in three independent experiments.
Fig. 4.
Transcriptome analysis of primary fibroblasts. Transcriptional profiles of primary fibroblasts from healthy controls (n = 3), the IRAK-1–deficient patient, and IRAK-4–, MyD88-, and MECP2-deficient patients, stimulated for 6 h with PAM-2 (10 μg/mL), FSL-1 (1 μg/mL), LPS (10 μg/mL), and MPLA (1 μg/mL) (A) and IL-1β (10 ng/mL) or TNF-α (20 ng/mL), as a positive control (B). Shown, as a heat map, are log2-transformed fold-change values for gene expression relative to nonstimulated conditions; columns correspond to subjects, and rows correspond to genes. Genes with induced expression are depicted in red, genes with suppressed expression are shown in blue, and genes displaying no change in expression are shown in yellow. The numbers in parentheses (Top) indicate the total number of responsive transcripts for each set of conditions. Bars represent overall individual responsiveness relative to the mean for control subjects [calculated as (responsive probes in a subject/mean number of responsive probes in healthy control subjects) ×100].
Almost Unimpaired Responses to IL-1R Stimulation in the Patient’s Fibroblasts.
An evaluation of IL-1R signaling revealed much lower levels of phosphorylation of IKK subunits alpha and beta (IKK-α/β) and, to a lesser extent, of p65 in response to IL-1β stimulation in SV40-fibroblasts from the patient than in control cells, whereas the phosphorylation of IKK-α/β and p65 was abolished in IRAK-4–deficient cells (SI Appendix, Fig. S6A). Furthermore, IκB-α degradation upon IL-1β stimulation was both delayed and impaired, but not entirely abolished as in IRAK-4– and MyD88-deficient SV40-fibroblasts (Fig. 5A, Top and SI Appendix, Fig. S6). Moreover, IκB-α phosphorylation levels following IL-1β stimulation were similar in cells from the patient and control cells, whereas this phosphorylation was abolished in IRAK-4–deficient cells (SI Appendix, Fig. S6A). Upon TNF-α stimulation, SV40-fibroblasts from the patient and IRAK-4– and MyD88-deficient patients displayed levels of IκB-α degradation similar to that in control cells (Fig. 5A, Bottom and SI Appendix, Fig. S6B). By contrast to the abolition of responses to IL-1β observed in IRAK-4– and MyD88-deficient SV40-fibroblasts, the patient’s cells displayed NF-κB DNA-binding activity (evaluated by EMSA; Fig. 5B and SI Appendix, Fig. S3C), NF-κB–dependent transcriptional activity (evaluated by a reporter luciferase assay; Fig. 5C), and cytokine production (evaluated by ELISA; Fig. 5D and SI Appendix, Fig. S4B) at levels similar to or only slightly lower than those in control and MECP2-deficient SV40-fibroblasts. Upon TNF-α stimulation [and poly(I:C) stimulation for cytokine production], NF-κB DNA-binding and transcriptional activities and cytokine production were similar in all of the SV40-fibroblast cell lines tested (Fig. 5 B–D and SI Appendix, Figs. S3 and S4B). Similar results were obtained with primary fibroblasts (SI Appendix, Fig. S5B). Finally, the induction of target genes by IL-1β was only modestly affected in primary fibroblasts from the patient, unlike in cells from IRAK-4– and MyD88-deficient patients, as analyzed by genome-wide microarray (Fig. 4B). The response to TNF-α was normal. Overall, our data show that the IRAK-1– and MECP2-deficient fibroblasts from the patient displayed little or no impairment of IL-1R responses, by contrast to the results obtained for IRAK-4– or MyD88-deficient fibroblasts.
Fig. 5.
IL-1R–dependent signaling in the patient’s fibroblasts. (A) IκB-α protein degradation in SV40-fibroblast lines from a healthy control, the IRAK-1–deficient patient, and IRAK-4– and MyD88-deficient patients, left unstimulated or stimulated with IL-1β (10 ng/mL) or TNF-α (20 ng/mL) for various periods of time (min), analyzed by Western blotting. Similar results were obtained in three independent experiments. (B) NF-κB translocation, assessed by EMSA, in SV40-fibroblasts from a healthy control, the IRAK-1–deficient patient, and IRAK-4– and MyD88-deficient patients, following stimulation with IL-1β (10 ng/mL) and TNF-α (20 ng/mL) for 20 min. Similar results were obtained in three independent experiments. (C) NF-κB–dependent transcription was assessed with the NF-κB reporter luciferase assay in SV40-fibroblasts derived from a healthy control, the IRAK-1–deficient patient, and IRAK-4–, MyD88-, and MECP2-deficient patients. Cells were transiently transfected with Renilla luciferase- and NF-κB luciferase-encoding vectors; 24 h after transfection, the cells were left untreated or were stimulated with IL-1β (10 ng/mL) or TNF-α (20 ng/mL) for 42 h and then harvested. Reporter gene activities were measured and the values obtained were normalized for transfection efficiency on the basis of Renilla luciferase expression. The values shown (means ± SEM) were obtained in three independent experiments. (D) IL-8 secretion by SV40-fibroblasts from healthy controls (n = 3), the IRAK-1–deficient patient, and IRAK-4–, MyD88-, and MECP2-deficient patients, left unstimulated or stimulated with IL-1β (10 ng/mL), polyIC (25 μg/mL), and TNF-α (20 ng/mL) as a positive control. The values shown (means ± SEM) were obtained in three independent experiments. *P < 0.05.
Rescue of TLR-Dependent Responsiveness by Wild-Type IRAK1 in the Patient’s Fibroblasts.
Our findings raised questions as to whether the poor TLR response in the fibroblasts of this patient was due to the deletion of IRAK1 or another nearby gene located within the region corresponding to the large X-chromosome deletion (TMEM187, MIR3202-1, MIR3202-2, and MIR718), or a combination of deletions. We addressed this question by transiently transfecting SV40-fibroblasts from the patient, a healthy control, and IRAK-4–, MyD88-, and MECP2-deficient patients with expression vectors encoding wild-type (WT) IRAK-1 or IRAK-4 or an empty vector and leaving them unstimulated or stimulating them with a TLR2, TLR4, or IL-1R agonist. The transfection of the patient’s fibroblasts with WT IRAK1 restored both the production of normal IRAK-1 protein (SI Appendix, Fig. S7A) and TLR2-dependent and TLR4-dependent NF-κB transcriptional activity at levels similar to those in healthy control cells (Fig. 6A). IRAK-4– and MyD88-deficient cells transfected with WT IRAK1 displayed higher levels of constitutive NF-κB activation, whereas control and MECP2-deficient cells transfected with WT IRAK1 had levels of activity higher than those recorded for cells transfected with the empty vector (SI Appendix, Fig. S7B). Moreover, IL-8 production in response to PAM-2, LPS, and MPLA stimulation was partially restored in fibroblasts from the patient transfected with WT IRAK1 but not in patient fibroblasts transfected with an empty vector (SI Appendix, Fig. S7D). The transfection of control and patient fibroblasts with WT IRAK1 further increased NF-κB transcriptional activity in response to IL-1β (Fig. 6B and SI Appendix, Fig. S7C); IL-1β–induced cytokine production levels, which were already very high, were similar to those in nontransfected cells (SI Appendix, Fig. S7E).
Fig. 6.
Causal relationship between complete IRAK-1 deficiency and the phenotype of the patient’s fibroblasts. (A and B) SV40-fibroblasts derived from a healthy control and from the IRAK-1–deficient patient were transiently transfected with vectors encoding Renilla luciferase or NF-κB luciferase, pcDNA3.1-empty (empty vector; EV), or pcDNA3.1-IRAK1 WT (IRAK1). Twenty-four hours after transfection, SV40-fibroblasts were left untreated or were stimulated with PAM-2 (10 μg/mL), LPS (10 μg/mL), and MPLA (1 μg/mL) (A) or with IL-1β (10 ng/mL) (B), and TNF-α (20 ng/mL) as a positive control, for 42 h and then harvested. Reporter gene activities were measured and the values were normalized for transfection efficiency on the basis of Renilla luciferase expression. (C and D) IL-8 secretion by SV40-fibroblasts from a healthy control transfected with siRNAs targeting IRAK1 or with a nonsense siRNA (scrambled) for 48 h, and then left unstimulated or stimulated with PAM-2 (10 μg/mL), LPS (10 μg/mL), and MPLA (1 μg/mL) (C) or with IL-1β (10 ng/mL) (D), and TNF-α (20 ng/mL) as a positive control, for 18 h. The values shown (means ± SEM) were obtained in three independent experiments. ns, not significant; ***P < 0.001, **P < 0.01, and *P < 0.05.
Knocking Down IRAK-1 Expression in Control Fibroblasts Reproduces the Patient’s Phenotype.
We then investigated the causality of the relationship between the lack of IRAK-1 in the patient and his fibroblasts’ impaired responses to TLR2 and TLR4 stimulation, by knocking down IRAK-1 expression in SV40-fibroblasts from a healthy control by transfection with siRNAs. Transfection with the IRAK1 siRNA abolished IRAK-1 expression, with no mRNA or protein detected (SI Appendix, Fig. S8 A and B). Levels of IL-8 mRNA (SI Appendix, Fig. S8C) and protein (Fig. 6C) after stimulation with PAM-2, LPS, or MPLA were significantly lower in the presence of IRAK1 siRNA, whereas no such effect was observed after transfection with scrambled siRNAs. Slightly lower levels of IL-8 mRNA (P = 0.02) and protein (P = 0.01) in response to stimulation with IL-1β were also observed after transfection with si-IRAK1, whereas this siRNA had no effect on the response to TNF-α stimulation (Fig. 6D and SI Appendix, Fig. S8D). Thus, knocking down IRAK-1 expression in control SV40-fibroblasts is sufficient to decrease the responses to TLR2 and TLR4 agonists significantly but has only a very slight effect on the IL-1β–dependent response, reproducing the phenotype of the patient’s fibroblasts.
Knocking Down IRAK-2 Expression Blocks IL-1R Responses in the Patient’s Fibroblasts.
We tested the hypothesis that IRAK-2 might compensate for IRAK-1 deficiency in the patient’s SV40-fibroblasts by mediating some IL-1β–dependent signaling, by using siRNA to knock down IRAK-2 expression in SV40-fibroblasts from a healthy control and the patient (SI Appendix, Fig. S9 A and B). IRAK2 silencing almost totally abolished IL-8 mRNA and protein production in response to IL-1β in the patient’s cells (P = 0.0005 and P < 0.0001, respectively) but only slightly decreased the levels of IL-8 mRNA and protein in the control SV40-fibroblasts (P = 0.07 and P = 0.12, respectively) (SI Appendix, Fig. S9 C and D). IRAK2 silencing had no effect on the production of IL-8 mRNA and protein in response to TNF-α stimulation in SV40-fibroblasts from a healthy control or the patient (SI Appendix, Fig. S9 C and D). Transfection with the scrambled siRNA had no effect on IL-8 mRNA and protein levels in any of the conditions tested (SI Appendix, Fig. S9 C and D). We then knocked down IRAK1 and IRAK2 mRNA levels simultaneously, by transfecting SV40-fibroblasts from a healthy control and an MECP2-deficient patient with siRNAs (SI Appendix, Fig. S10 A and B). We evaluated IL8 mRNA induction in response to IL-1β, TLR2, and TLR4 ligands. The silencing of both IRAK1 and IRAK2 in control and MECP2-deficient fibroblasts resulted in low levels of IL8 mRNA (with a stronger effect than a single-gene knockdown) in response to IL-1β stimulation, as previously observed for the patient’s cells transfected with si-IRAK2 (SI Appendix, Fig. S10C). However, the levels of IL8 mRNA produced in response to stimulation with PAM-2, LPS, or MPLA after the silencing of both IRAK1 and IRAK2 were not significantly lower than those observed after the silencing of IRAK1 alone, in control and MECP2-deficient SV40-fibroblasts (SI Appendix, Fig. S10D). These results strongly suggest that, in the absence of IRAK-1, its IRAK-2 partner can compensate, at least partially, for some of the signaling downstream from IL-1R but not for signaling downstream from TLR in human fibroblasts.
Abolished Response to TLR7 and TLR8 in the Patient’s EBV-B Cells but Normal Responses to All TLR Agonists Tested and to IL-1β in the Patient’s Peripheral Blood Mononuclear Cells.
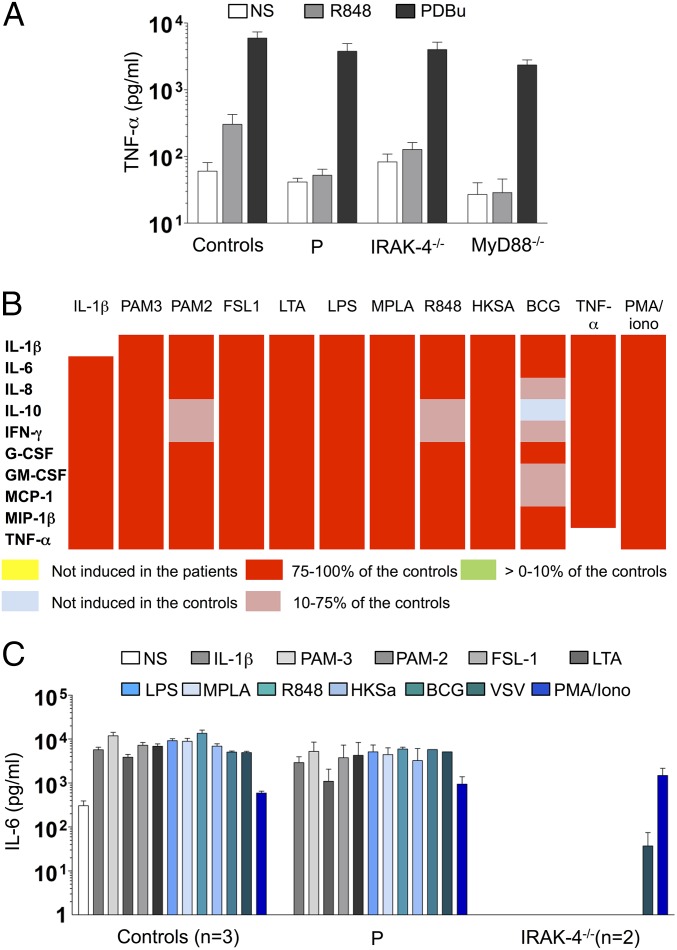
We then evaluated the response of EBV-transformed B lymphocytes (EBV-B cells) from the patient, which were also found to have no IRAK-1 mRNA or protein (Fig. 2 A and C), to a TLR7 and TLR8 agonist (R848). Like IRAK-4– and MyD88-deficient cells, these cells produced no TNF-α, whereas all three types of mutant cells responded normally to PDBu (phorbol 12,13-dibutyrate) (Fig. 7A). We then compared the cytokine production of the patient’s peripheral blood mononuclear cells (PBMCs) (which did not produce IRAK1 mRNA, and in which it was not possible to evaluate IRAK-1 protein levels) with that of PBMCs from three healthy controls and two IRAK-4–deficient patients, after 36 h of stimulation with TLR1/2 (PAM3CSK4), TLR2/6, TLR4, TLR7, and TLR8 agonists, heat-killed S. aureus (HK-SA), live Mycobacterium bovis Bacillus Calmette–Guérin (bacillus Calmette–Guérin), and rhabdoviruses (VSV; vesicular stomatitis virus infection), and IL-1β. Levels of IL-1β, IL-6, IL-8, IL-10, IFN-γ, G-CSF, GM-CSF, MCP-1, MIP-1β, and TNF-α production by the patient’s PBMCs were similar to those in control PBMCs in response to IL-1β and all TLR agonists tested (Fig. 7 B and C). By contrast, cytokine production was either strongly impaired or totally abolished in IRAK-4–deficient PBMCs, with the exception of MCP-1 and MIP-1β production in response to stimulation with LPS and MPLA, which was within the control range, probably reflecting use of the IRAK-4–independent TLR4 signaling pathway. Cytokine production by PBMCs from the patient and IRAK-4–deficient patients was similar to that in healthy controls in response to stimulation with TNF-α or phorbol 12-myristate 13-acetate (PMA)/ionomycin (Fig. 7 B and C). We tested the hypothesis that IRAK-2 might compensate for IRAK-1 deficiency in the patient’s PBMCs, by knocking down IRAK1, IRAK2, or both with siRNA in PBMCs from healthy controls (SI Appendix, Fig. S11A). The single-gene knockdowns (IRAK1 or IRAK2) did not significantly affect IL8 mRNA production in response to any of the TLR/IL-1R agonists tested. By contrast, the silencing of both IRAK1 and IRAK2 resulted in significantly lower levels of IL8 mRNA in response to all TLR/IL-1R agonists (SI Appendix, Fig. S11B). Transfection with scrambled siRNA had no effect on IL8 mRNA levels in any of the conditions tested (SI Appendix, Fig. S11B). The limited numbers of PBMCs available from the patient precluded further functional analyses of the leukocyte subsets. These results nevertheless strongly suggest that IRAK-1 plays a redundant role in the myeloid and lymphoid subsets of human blood mononuclear cells, contrary to the findings for EBV-B cells and fibroblasts. These results also suggest that, in the absence of IRAK-1, its IRAK-2 partner can compensate for some of the signaling downstream from TLR/IL-1R in human leukocytes.
Fig. 7.
TIR-dependent signaling in the patient’s leukocytes. (A) TNF-α secretion by EBV-transformed B cells from controls (n = 4), the IRAK-1–deficient patient, and IRAK-4– and MyD88-deficient patients, left unstimulated or stimulated with R848 (3 μg/mL) and PDBu (10−7 M) as a positive control. The values shown (means ± SEM) were obtained in three independent experiments. (B and C) PBMCs from healthy controls (n = 3), the IRAK-1–deficient patient, and two IRAK-4–deficient patients were left unstimulated or stimulated with IL-1β (20 ng/mL), PAM-3 (1 μg/mL), PAM-2 (1 μg/mL), FSL-1 (100 ng/mL), LTA (1 μg/mL), LPS (10 ng/mL), MPLA (1 μg/mL), R848 (1 μg/mL), heat-killed S. aureus (107 HKSA per mL), bacillus de Calmette et Guérin, and, as positive controls, TNF-α (20 ng/mL) and PMA/ionomycin (10−7 M/10−5 M). The production of multiple cytokines in IRAK-1–deficient PBMCs was evaluated by multiplex ELISA. Cytokine levels are represented as ratios of the response observed in the patient to mean levels in controls. For none of the stimulations tested, the patient showed either no induction or at 0-10% of the controls. (B). IL-6 production, as determined by ELISA (C). The values shown (means ± SEM) were obtained in two independent experiments.
Discussion
We describe here a male infant with a large X-chromosome deletion encompassing the gene encoding IRAK-1. This 100-kb de novo Xq28 deletion encompassed all or part of the TMEM187, MIR3202-1, MIR3202-2, IRAK1, MIR718, and MECP2 genes. The absence of the MECP2 gene was consistent with the patient’s clinical phenotype, as congenital encephalopathy, hypotonia, and respiratory insufficiency are commonly reported in boys with neonatal encephalopathy due to MECP2 deficiency (78–91). The lack of TMEM187, MIR3202-1, MIR3202-2, and MIR718 was not associated with any overt phenotype at the time of the patient’s death at the age of 7 mo. It was not possible to attribute any particular clinical phenotype to the lack of IRAK-1, as severe respiratory failure and pulmonary infections are commonly seen in male patients with isolated MECP2 deficiency (83, 88, 90). However, this child presented only a slight, transient increase in temperature (37.8 °C) during a bout of bilateral pneumonia, and his serum CRP concentration remained below 2 mg/L. None of the previously described MECP2-deficient male patients were reported to have impaired inflammatory responses during infections (83, 88, 90). Laboratory/biochemical tests reportedly gave normal results for these patients. Body temperature was provided for only one child presenting with severe neonatal encephalopathy, recurrent respiratory infections, and dysfunctions who died of respiratory infection in a context of high fever (88). CRP levels were not reported for any of these patients. By contrast, poor inflammatory responses during infectious episodes are characteristic of IRAK-4– and MyD88-deficient patients (43). Unlike the patient described here, who suffered from only mild infections such as conjunctivitis, urinary infection, and rhinovirus infections, responding well to standard treatments, IRAK-4– and MyD88-deficient patients typically present with recurrent, invasive pyogenic infections, which are reported in 46% of these patients (probands 49%; relatives 42%) before the age of 7 mo (43, 44, 52, 55). Our findings thus suggest that the IRAK-1–dependent TLR and IL-1R pathway is more redundant in host defense than that controlled by IRAK-4 and MyD88. However, as only one patient with complete IRAK-1 deficiency has been identified, it would be premature to draw firm conclusions about the role of human IRAK-1 in protective immunity.
Our study is more informative concerning the cellular consequences of IRAK-1 deficiency. The criteria for genetic studies in a single patient are fulfilled (103), with a causal relationship between the IRAK1 genotype and the TLR/IL-1R cellular phenotype. The patient’s SV40-fibroblasts responded very poorly to TLR2/6 and TLR4 agonists but, surprisingly, responded normally to IL-1β. Neither IRAK-4– nor MyD88-deficient fibroblasts responded to any of the TLR agonists tested (except for TLR3) or IL-1β. Like IRAK-4– and MyD88-deficient cells (52), the patient’s EBV-B cells did not respond to TLR7 and TLR8 agonists. By contrast, the patient’s PBMCs responded normally to all TLR (TLR1/2, TLR2/6, TLR4, TLR7, and TLR8) agonists tested and to IL-1β (in terms of the production of many cytokines), whereas neither IRAK-4– nor MyD88-deficient PBMCs responded to any of these TLR (except for some residual TLR4 signaling, presumably via TRIF) and IL-1R (SI Appendix, Table S1) agonists. By contrast, Irak1-deficient MEFs displayed strong but incomplete impairment of the response to both TLRs (TLR2 and TLR4) (23) and IL-1Rs (5, 23, 36). This result is at odds with the almost complete abolition of responses to TLR2/6 and TLR4 agonists, and the preserved response to IL-1β observed in human IRAK-1–deficient fibroblasts. Upon TLR4 stimulation, the production of IL-10 mRNA and protein was strongly impaired in splenocytes from Irak1-deficient mice, but these cells were able to produce IL-1β and TNF-α at levels similar to those in WT splenocytes (31). After the stimulation of TLR4, TLR1/2, TLR2/6, TLR7, TLR8, or IL-1R, the levels of IL-10, IL-1β, and TNF-α production by the patient’s PBMCs were similar to those of control PBMCs. We were unable to test leukocyte subsets or responses to agonists of other members of the TLR (e.g., TLR5 and TLR9) and IL-1R (e.g., IL-18R and IL-33R) families. Overall, our results, somewhat at odds with the data obtained for mutant mice (5, 23, 36) (SI Appendix, Table S2), suggest that human IRAK-1 is largely redundant downstream from both TLRs and IL-1R in mononuclear blood cells and downstream from IL-1R in fibroblasts, whereas it plays an essential role, at least downstream from TLR2/6, TLR4, TLR7, and TLR8, in fibroblasts and EBV-B cells.
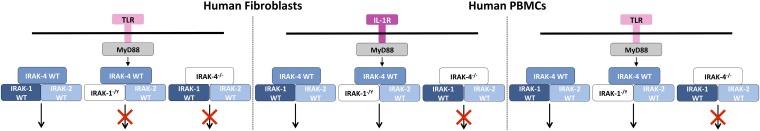
Our study also provides new insight into the contributions of IRAK-1 and IRAK-2 in the signaling pathways downstream from TLRs and IL-1R (Fig. 8). In vitro studies have shown that IRAK-1 plays an important role downstream from IL-1R, as an adaptor protein crucial for Myddosome formation and the activation of downstream signaling (16, 104–106). We show that the patient’s fibroblasts were able to respond to IL-1β stimulation almost normally, like human fibroblasts from healthy controls silenced for IRAK1 (or IRAK2). However, the IL-1 response of fibroblasts from a healthy donor was strongly impaired when both IRAK1 and IRAK2 were silenced. These data suggest that IRAK-1 and IRAK-2 play mutually redundant roles, at least downstream from IL-1R, in human fibroblasts. Mouse studies have shown that IRAK-2 is crucial for cytokine production by bone marrow-derived macrophages in response to TLR4 stimulation (17, 39). Similarly, IRAK-2 has been shown to be essential for the LPS-induced response of human HEK-293 cells and human PBMCs (107). Our findings suggest that IRAK-2 is dispensable for TLR-mediated signaling in human fibroblasts, because IRAK2 silencing does not impair the production of IL-8 mRNA or protein in response to PAM-2 or LPS, whereas IRAK1 silencing greatly decreases this response. We further suggest that IRAK-2 drives the preserved TLR/IL-1R–dependent signaling displayed by IRAK-1–deficient PBMCs, as the silencing of both IRAK1 and IRAK2 in control PBMCs impaired TLR/IL-1R responses, at least in terms of IL8 mRNA induction. Overall, our findings suggest that signaling downstream from TLRs and IL-1R in human fibroblasts diverges at the IRAK proteins: IRAK-1 is essential downstream from TLRs, whereas signaling downstream from IL-1R can be mediated by IRAK-1, IRAK-2, or both. In leukocytes, the TLR/IL-1R pathway also seems to be mediated by IRAK-1, IRAK-2, or both. The identification of humans with mutations affecting other components of the TIR pathway should clarify the relative contributions of each human IRAK protein to host defense. These findings are important, given current efforts to develop inhibitors of IRAK-1 and IRAK-4 for the treatment of various human diseases (108, 109).
Fig. 8.
Suggested model for signaling downstream from TLR or IL-1R in human fibroblasts and PBMCs, in the presence and absence of IRAK-1 and IRAK-4.
Methods
cDNA Preparation and Real-Time Quantitative PCR.
Total RNA was extracted from EBV-B cells and SV40-fibroblasts with the Qiagen RNA Mini Kit, according to the manufacturer’s instructions. cDNA was synthesized with SuperScript II reverse transcriptase (Life Technologies). Real-time quantitative PCR (qPCR) was performed with TaqMan Gene Expression Assays (Life Technologies; Hs 01018347_m1), according to the manufacturer’s instructions. mRNA levels are expressed relative to GUS mRNA levels, as determined by the 2−ΔΔC(t) method.
Western Blots and EMSA.
Cells were lysed in a buffer containing 50 mM Tris⋅HCl (pH 7.4), 150 mM NaCl, 0.5% Triton X-100, and 2 mM EDTA supplemented with protease inhibitors and phosphatase inhibitor mixture (Roche). Western blotting was performed with polyclonal anti–IRAK-1 (sc-7883) and anti-GAPDH (sc-25778) antibodies (Santa Cruz Biotechnology). The degradation of IκB-α upon stimulation with IL-1β (10 ng/mL; R&D Systems) or TNF-α (20 ng/mL; R&D Systems) was evaluated with an anti–IκB-α polyclonal antibody (sc-371), with an antibody against α-tubulin (sc-5286) as a loading control (Santa Cruz Biotechnology). For EMSA, SV40-fibroblasts were stimulated for 20 min with IL-1β (10 ng/mL; R&D Systems) or TNF-α (20 ng/mL; R&D Systems). Nuclear extracts were then prepared and incubated (10 μg protein) with a 32P-labeled double-stranded NF-κB–specific oligonucleotide κB probe (5′-GATCATGGGGAATCCCCA-3′ and 5′-GATCTGGGGATTCCCCAT-3′).
Cell Stimulation, Cytokine Determinations, and Luciferase Assays.
IL-8 and IL-6 levels were assessed in SV40-fibroblasts incubated for 18 h in the presence of 10 ng/mL IL-1β (R&D Systems), 20 ng/mL TNF-α (R&D Systems), 10 μg/mL PAM-2 (a TLR2/6 agonist), 1 µg/mL FSL-1 (a TLR2/6 agonist), 10 μg/mL LTA-SA (a TLR2 agonist), 1 μg/mL MPLA (a TLR4 agonist) (all from InvivoGen), 10 μg/mL LPS-SE (a TLR4 agonist; Sigma-Aldrich), or 25 μg/mL poly(I:C) (GE Healthcare). TNF-α levels were assessed in EBV-B cells incubated for 24 h in the presence of 3 μg/mL R848 (imidazoquinoline compound, a TLR7/8 agonist; InvivoGen) or PDBu (a protein kinase C agonist; Sigma-Aldrich). IL-6 levels were assessed in frozen and thawed PBMCs incubated for 36 h in the presence of 20 ng/mL IL-1β (R&D Systems), 20 ng/mL TNF-α (R&D Systems), 1 μg/mL PAM3CSK4 (PAM-3; a TLR1/2 agonist), 1 μg/mL PAM2CSK4 (PAM-2; a TLR2/6 agonist), 100 ng/mL FSL-1 (a TLR2/6 agonist), 1 μg/mL pLTA-SA (a TLR2 agonist), imidazoquinoline (R848; a TLR7/8 agonist), 107 heat-killed S. aureus (HKSA) per mL (mainly a TLR2 agonist) (all from InvivoGen), lipopolysaccharides (rough strains) from Salmonella enterica serotype minnesota Re 595 (LPS; Sigma), live bacillus Calmette–Guérin, and VSV (multiplicity of infection 0.5) or PMA/ionomycin (Sigma-Aldrich; positive control; 10−7 M/10−5 M). Supernatants were collected and ELISA (Sanquin) was performed in accordance with kit instructions. For the simultaneous determination of multiple cytokines in PBMC supernatants, we used a fluorescence-based assay capable of detecting 17 cytokines (Bio-Plex Pro Human Cytokine 17-plex Assay M5000031YV; Bio-Rad). NF-κB luciferase activity was assessed by transiently transfecting SV40-fibroblasts with 100 ng of NF-κB–dependent firefly luciferase vector with five NF-κB binding sites in the promoter (Agilent Technologies) and 40 ng of Renilla luciferase vector (pRL-SV40-d238) (110) as an internal control with the Lipofectamine LTX Kit (Life Technologies; 15338100), according to the manufacturer’s instructions. Fibroblasts were then stimulated for 24 h with IL-1β, PAM-2, LPS, MPLA, or TNF-α, as described above. The cells were lysed in passive lysis buffer, and luciferase activities were measured in the Dual-Luciferase Reporter Assay (Promega).
Complementation and Silencing.
For the complementation experiments, IRAK-1−/Y, IRAK-4−/−, MyD88−/−, MeCP2−/Y, and healthy control SV40-fibroblasts were transiently transfected by incubation for 24 h with 200 ng pcDNA3.1 empty vector or pcDNA3.1-IRAK1 WT (Invitrogen). The cells were then stimulated with various ligands for 24 h, and luciferase activity or cytokine production was assessed as described above. For the silencing of IRAK-1, IRAK-2, or both, SV40-fibroblasts were transiently transfected with 50 nM siRNA targeting IRAK1 or IRAK2 or a mixture of siRNAs targeting IRAK1 and IRAK2 (50 nM final concentration) or scrambled siRNA (50 nM) with Lipofectamine RNAiMAX (Life Technologies; 13778030), according to the manufacturer’s instructions. Fibroblasts were then stimulated for 4 h for the determination of IL8, IRAK1, IRAK2, and IRAK4 mRNA levels by RT-qPCR, or for 18 h for the determination of IRAK-1 protein levels by Western blotting and IL-8 protein levels by ELISA. RNA was extracted with the ZR RNA MicroPrep Kit (ZR1061; Zymo Research), according to the manufacturer’s instructions.
Statistics.
For single comparisons of independent groups, t tests were performed; ***P < 0.001, **P < 0.01, and *P < 0.05. Analyses were performed with GraphPad software.
Study Approval.
The study was approved by the ethics committee of IRCCS San Matteo Hospital Foundation, Pavia (ID-PDS 20140000324); informed consent was obtained from the patient’s parents, in accordance with the World Medical Association guidelines, Helsinki Declaration, and EU directives.
Supplementary Material
Acknowledgments
We thank the family for participating in this study. We also thank Lahouari Amar and Yelena Nemirovskaya and all members of the Laboratory of Human Genetics of Infectious Diseases. The “Cell lines and DNA bank of Rett Syndrome, X-linked mental retardation and other genetic diseases,” a member of Telethon Network of Genetic Biobanks Project GTB12001, funded by Telethon Italy, and of the EuroBioBank network and the “Associazione Italiana Rett O.N.L.U.S.” provided us with specimens. This work was funded by the French National Research Agency (ANR) under the “Investments for the Future” program (Grant ANR-10-IAHU-01), Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases (ANR-10-LABX-62-IBEID), Institut National de la Santé et de la Recherche Médicale (INSERM), Paris Descartes University, Jeffrey Modell Centers Network, The Rockefeller University, and St. Giles Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620139114/-/DCSupplemental.
References
- 1.Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol. 2011;21(13):R488–R493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 2.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: Back to the future. Immunity. 2013;39(6):1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casanova JL, Abel L, Quintana-Murci L. Human TLRs and IL-1Rs in host defense: Natural insights from evolutionary, epidemiological, and clinical genetics. Annu Rev Immunol. 2011;29:447–491. doi: 10.1146/annurev-immunol-030409-101335. [DOI] [PubMed] [Google Scholar]
- 4.Cao Z, Henzel WJ, Gao X. IRAK: A kinase associated with the interleukin-1 receptor. Science. 1996;271(5252):1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 5.Thomas JA, et al. Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J Immunol. 1999;163(2):978–984. [PubMed] [Google Scholar]
- 6.Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278(5343):1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 7.Conner JR, Smirnova II, Poltorak A. A mutation in Irak2c identifies IRAK-2 as a central component of the TLR regulatory network of wild-derived mice. J Exp Med. 2009;206(7):1615–1631. doi: 10.1084/jem.20090490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wesche H, et al. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J Biol Chem. 1999;274(27):19403–19410. doi: 10.1074/jbc.274.27.19403. [DOI] [PubMed] [Google Scholar]
- 9.Deng JC, et al. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116(9):2532–2542. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Strelow A, Fontana EJ, Wesche H. IRAK-4: A novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci USA. 2002;99(8):5567–5572. doi: 10.1073/pnas.082100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki N, et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416(6882):750–756. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- 12.Park HH, et al. The death domain superfamily in intracellular signaling of apoptosis and inflammation. Annu Rev Immunol. 2007;25:561–586. doi: 10.1146/annurev.immunol.25.022106.141656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11(2):293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 14.Neumann D, Kollewe C, Resch K, Martin MU. The death domain of IRAK-1: An oligomerization domain mediating interactions with MyD88, Tollip, IRAK-1, and IRAK-4. Biochem Biophys Res Commun. 2007;354(4):1089–1094. doi: 10.1016/j.bbrc.2007.01.104. [DOI] [PubMed] [Google Scholar]
- 15.Gosu V, Basith S, Durai P, Choi S. Molecular evolution and structural features of IRAK family members. PLoS One. 2012;7(11):e49771. doi: 10.1371/journal.pone.0049771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song KW, et al. The kinase activities of interleukin-1 receptor associated kinase (IRAK)-1 and 4 are redundant in the control of inflammatory cytokine expression in human cells. Mol Immunol. 2009;46(7):1458–1466. doi: 10.1016/j.molimm.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Yin W, et al. The kinase activity of interleukin-1 receptor-associated kinase 2 is essential for lipopolysaccharide-mediated cytokine and chemokine mRNA stability and translation. J Interferon Cytokine Res. 2011;31(5):415–422. doi: 10.1089/jir.2010.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: An adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7(6):837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 19.Medzhitov R, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2(2):253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 20.Watters TM, Kenny EF, O’Neill LA. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol Cell Biol. 2007;85(6):411–419. doi: 10.1038/sj.icb.7100095. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald KA, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413(6851):78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 22.Kenny EF, O’Neill LA. Signalling adaptors used by Toll-like receptors: An update. Cytokine. 2008;43(3):342–349. doi: 10.1016/j.cyto.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Kawagoe T, et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9(6):684–691. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]
- 24.Motshwene PG, et al. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J Biol Chem. 2009;284(37):25404–25411. doi: 10.1074/jbc.M109.022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465(7300):885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gay NJ, Gangloff M, O’Neill LA. What the Myddosome structure tells us about the initiation of innate immunity. Trends Immunol. 2011;32(3):104–109. doi: 10.1016/j.it.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383(6599):443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi K, et al. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110(2):191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 29.Böl G, Kreuzer OJ, Brigelius-Flohé R. Translocation of the interleukin-1 receptor-associated kinase-1 (IRAK-1) into the nucleus. FEBS Lett. 2000;477(1–2):73–78. doi: 10.1016/s0014-5793(00)01759-2. [DOI] [PubMed] [Google Scholar]
- 30.Böl GF, Jurrmann N, Brigelius-Flohé R. Cellular trafficking of the IL-1RI-associated kinase-1 requires intact kinase activity. Biochem Biophys Res Commun. 2005;332(1):279–287. doi: 10.1016/j.bbrc.2005.04.121. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Li T, Sane DC, Li L. IRAK1 serves as a novel regulator essential for lipopolysaccharide-induced interleukin-10 gene expression. J Biol Chem. 2004;279(49):51697–51703. doi: 10.1074/jbc.M410369200. [DOI] [PubMed] [Google Scholar]
- 32.Su J, Richter K, Zhang C, Gu Q, Li L. Differential regulation of interleukin-1 receptor associated kinase 1 (IRAK1) splice variants. Mol Immunol. 2007;44(5):900–905. doi: 10.1016/j.molimm.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Gottipati S, Rao NL, Fung-Leung WP. IRAK1: A critical signaling mediator of innate immunity. Cell Signal. 2008;20(2):269–276. doi: 10.1016/j.cellsig.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Zhou H, et al. IRAK-M mediates Toll-like receptor/IL-1R-induced NFκB activation and cytokine production. EMBO J. 2013;32(4):583–596. doi: 10.1038/emboj.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandra R, et al. IRAK1-dependent signaling mediates mortality in polymicrobial sepsis. Inflammation. 2013;36(6):1503–1512. doi: 10.1007/s10753-013-9692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanakaraj P, et al. Interleukin (IL)-1 receptor-associated kinase (IRAK) requirement for optimal induction of multiple IL-1 signaling pathways and IL-6 production. J Exp Med. 1998;187(12):2073–2079. doi: 10.1084/jem.187.12.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawagoe T, et al. Essential role of IRAK-4 protein and its kinase activity in Toll-like receptor-mediated immune responses but not in TCR signaling. J Exp Med. 2007;204(5):1013–1024. doi: 10.1084/jem.20061523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swantek JL, Tsen MF, Cobb MH, Thomas JA. IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J Immunol. 2000;164(8):4301–4306. doi: 10.4049/jimmunol.164.8.4301. [DOI] [PubMed] [Google Scholar]
- 39.Wan Y, et al. Interleukin-1 receptor-associated kinase 2 is critical for lipopolysaccharide-mediated post-transcriptional control. J Biol Chem. 2009;284(16):10367–10375. doi: 10.1074/jbc.M807822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verdrengh M, Thomas JA, Hultgren OH. IL-1 receptor-associated kinase 1 mediates protection against Staphylococcus aureus infection. Microbes Infect. 2004;6(14):1268–1272. doi: 10.1016/j.micinf.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 41.von Bernuth H, Picard C, Puel A, Casanova JL. Experimental and natural infections in MyD88- and IRAK-4-deficient mice and humans. Eur J Immunol. 2012;42(12):3126–3135. doi: 10.1002/eji.201242683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su J, Xie Q, Wilson I, Li L. Differential regulation and role of interleukin-1 receptor associated kinase-M in innate immunity signaling. Cell Signal. 2007;19(7):1596–1601. doi: 10.1016/j.cellsig.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picard C, et al. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 2010;89(6):403–425. doi: 10.1097/MD.0b013e3181fd8ec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Picard C, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299(5615):2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 45.Enders A, et al. Two siblings with lethal pneumococcal meningitis in a family with a mutation in interleukin-1 receptor-associated kinase 4. J Pediatr. 2004;145(5):698–700. doi: 10.1016/j.jpeds.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 46.Day N, et al. Interleukin receptor-associated kinase (IRAK-4) deficiency associated with bacterial infections and failure to sustain antibody responses. J Pediatr. 2004;144(4):524–526. doi: 10.1016/j.jpeds.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 47.Chapel H, Puel A, von Bernuth H, Picard C, Casanova JL. Shigella sonnei meningitis due to interleukin-1 receptor-associated kinase-4 deficiency: First association with a primary immune deficiency. Clin Infect Dis. 2005;40(9):1227–1231. doi: 10.1086/428733. [DOI] [PubMed] [Google Scholar]
- 48.Takada H, et al. Delayed separation of the umbilical cord in two siblings with interleukin-1 receptor-associated kinase 4 deficiency: Rapid screening by flow cytometer. J Pediatr. 2006;148(4):546–548. doi: 10.1016/j.jpeds.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 49.McDonald DR, Brown D, Bonilla FA, Geha RS. Interleukin receptor-associated kinase-4 deficiency impairs Toll-like receptor-dependent innate antiviral immune responses. J Allergy Clin Immunol. 2006;118(6):1357–1362. doi: 10.1016/j.jaci.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Cardenes M, et al. Autosomal recessive interleukin-1 receptor-associated kinase 4 deficiency in fourth-degree relatives. J Pediatr. 2006;148(4):549–551. doi: 10.1016/j.jpeds.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 51.Lavine E, et al. Cellular and humoral aberrations in a kindred with IL-1 receptor-associated kinase 4 deficiency. J Allergy Clin Immunol. 2007;120(4):948–950. doi: 10.1016/j.jaci.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 52.Ku CL, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204(10):2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoarau C, et al. TLR9 activation induces normal neutrophil responses in a child with IRAK-4 deficiency: Involvement of the direct PI3K pathway. J Immunol. 2007;179(7):4754–4765. doi: 10.4049/jimmunol.179.7.4754. [DOI] [PubMed] [Google Scholar]
- 54.de Beaucoudrey L, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205(7):1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Bernuth H, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321(5889):691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krause JC, et al. Very late-onset group B Streptococcus meningitis, sepsis, and systemic shigellosis due to interleukin-1 receptor-associated kinase-4 deficiency. Clin Infect Dis. 2009;49(9):1393–1396. doi: 10.1086/630206. [DOI] [PubMed] [Google Scholar]
- 57.Picard C, Casanova JL, Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IκBα deficiency. Clin Microbiol Rev. 2011;24(3):490–497. doi: 10.1128/CMR.00001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giardino G, et al. Targeted next-generation sequencing revealed MYD88 deficiency in a child with chronic yersiniosis and granulomatous lymphadenitis. J Allergy Clin Immunol. 2016;137(5):1591–1595.e4. doi: 10.1016/j.jaci.2015.09.050. [DOI] [PubMed] [Google Scholar]
- 59.Ku CL, et al. IRAK4 and NEMO mutations in otherwise healthy children with recurrent invasive pneumococcal disease. J Med Genet. 2007;44(1):16–23. doi: 10.1136/jmg.2006.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Comeau JL, et al. Staphylococcal pericarditis, and liver and paratracheal abscesses as presentations in two new cases of interleukin-1 receptor associated kinase 4 deficiency. Pediatr Infect Dis J. 2008;27(2):170–174. doi: 10.1097/INF.0b013e318157ad01. [DOI] [PubMed] [Google Scholar]
- 61.Conway DH, Dara J, Bagashev A, Sullivan KE. Myeloid differentiation primary response gene 88 (MyD88) deficiency in a large kindred. J Allergy Clin Immunol. 2010;126(1):172–175. doi: 10.1016/j.jaci.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 62.Schnappauf C, et al. Invasive pneumococcal diseases in children and adolescents—A single centre experience. BMC Res Notes. 2014;7:145. doi: 10.1186/1756-0500-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKelvie B, et al. Fatal pneumococcal meningitis in a 7-year-old girl with interleukin-1 receptor activated kinase deficiency (IRAK-4) despite prophylactic antibiotic and IgG responses to Streptococcus pneumoniae vaccines. J Clin Immunol. 2014;34(3):267–271. doi: 10.1007/s10875-014-9996-4. [DOI] [PubMed] [Google Scholar]
- 64.Stergiopoulou T, et al. Deficiency of interleukin-1 receptor-associated kinase 4 presenting as fatal Pseudomonas aeruginosa bacteremia in two siblings. Pediatr Infect Dis J. 2015;34(3):299–300. doi: 10.1097/INF.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 65.Weller S, et al. IgM+IgD+CD27+ B cells are markedly reduced in IRAK-4-, MyD88-, and TIRAP- but not UNC-93B-deficient patients. Blood. 2012;120(25):4992–5001. doi: 10.1182/blood-2012-07-440776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maglione PJ, et al. IRAK-4 and MyD88 deficiencies impair IgM responses against T-independent bacterial antigens. Blood. 2014;124(24):3561–3571. doi: 10.1182/blood-2014-07-587824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casrouge A, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314(5797):308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 68.Zhang SY, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317(5844):1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 69.Zhang SY, et al. TLR3 immunity to infection in mice and humans. Curr Opin Immunol. 2013;25(1):19–33. doi: 10.1016/j.coi.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Casanova JL, Abel L. The genetic theory of infectious diseases: A brief history and selected illustrations. Annu Rev Genomics Hum Genet. 2013;14:215–243. doi: 10.1146/annurev-genom-091212-153448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casanova JL, Abel L, Quintana-Murci L. Immunology taught by human genetics. Cold Spring Harb Symp Quant Biol. 2013;78:157–172. doi: 10.1101/sqb.2013.78.019968. [DOI] [PubMed] [Google Scholar]
- 72.Inoue K, Lupski JR. Molecular mechanisms for genomic disorders. Annu Rev Genomics Hum Genet. 2002;3:199–242. doi: 10.1146/annurev.genom.3.032802.120023. [DOI] [PubMed] [Google Scholar]
- 73.Faranda S, et al. Characterization and fine localization of two new genes in Xq28 using the genomic sequence/EST database screening approach. Genomics. 1996;34(3):323–327. doi: 10.1006/geno.1996.0293. [DOI] [PubMed] [Google Scholar]
- 74.Nan X, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393(6683):386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 75.Zoghbi HY. MeCP2 dysfunction in humans and mice. J Child Neurol. 2005;20(9):736–740. doi: 10.1177/08830738050200090701. [DOI] [PubMed] [Google Scholar]
- 76.Chahrour M, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320(5880):1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 78.Claes S, et al. X-linked severe mental retardation and a progressive neurological disorder in a Belgian family: Clinical and genetic studies. Clin Genet. 1997;52(3):155–161. doi: 10.1111/j.1399-0004.1997.tb02536.x. [DOI] [PubMed] [Google Scholar]
- 79.Wan M, et al. Rett syndrome and beyond: Recurrent spontaneous and familial MECP2 mutations at CpG hotspots. Am J Hum Genet. 1999;65(6):1520–1529. doi: 10.1086/302690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Villard L, et al. Two affected boys in a Rett syndrome family: Clinical and molecular findings. Neurology. 2000;55(8):1188–1193. doi: 10.1212/wnl.55.8.1188. [DOI] [PubMed] [Google Scholar]
- 81.Meloni I, et al. A mutation in the Rett syndrome gene, MECP2, causes X-linked mental retardation and progressive spasticity in males. Am J Hum Genet. 2000;67(4):982–985. doi: 10.1086/303078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Couvert P, et al. MECP2 is highly mutated in X-linked mental retardation. Hum Mol Genet. 2001;10(9):941–946. doi: 10.1093/hmg/10.9.941. [DOI] [PubMed] [Google Scholar]
- 83.Zeev BB, et al. Rett syndrome: Clinical manifestations in males with MECP2 mutations. J Child Neurol. 2002;17(1):20–24. doi: 10.1177/088307380201700105. [DOI] [PubMed] [Google Scholar]
- 84.Lynch SA, Whatley SD, Ramesh V, Sinha S, Ravine D. Sporadic case of fatal encephalopathy with neonatal onset associated with a T158M missense mutation in MECP2. Arch Dis Child Fetal Neonatal Ed. 2003;88(3):F250–F252. doi: 10.1136/fn.88.3.F250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Christodoulou J, Grimm A, Maher T, Bennetts B. RettBASE: The IRSA MECP2 variation database—A new mutation database in evolution. Hum Mutat. 2003;21(5):466–472. doi: 10.1002/humu.10194. [DOI] [PubMed] [Google Scholar]
- 86.Leuzzi V, Di Sabato ML, Zollino M, Montanaro ML, Seri S. Early-onset encephalopathy and cortical myoclonus in a boy with MECP2 gene mutation. Neurology. 2004;63(10):1968–1970. doi: 10.1212/01.wnl.0000144350.97844.94. [DOI] [PubMed] [Google Scholar]
- 87.Kankirawatana P, et al. Early progressive encephalopathy in boys and MECP2 mutations. Neurology. 2006;67(1):164–166. doi: 10.1212/01.wnl.0000223318.28938.45. [DOI] [PubMed] [Google Scholar]
- 88.Lundvall M, Samuelsson L, Kyllerman M. Male Rett phenotypes in T158M and R294X MeCP2-mutations. Neuropediatrics. 2006;37(5):296–301. doi: 10.1055/s-2006-924613. [DOI] [PubMed] [Google Scholar]
- 89.Venâncio M, Santos M, Pereira SA, Maciel P, Saraiva JM. An explanation for another familial case of Rett syndrome: Maternal germline mosaicism. Eur J Hum Genet. 2007;15(8):902–904. doi: 10.1038/sj.ejhg.5201835. [DOI] [PubMed] [Google Scholar]
- 90.Jülich K, Horn D, Burfeind P, Erler T, Auber B. A novel MECP2 mutation in a boy with neonatal encephalopathy and facial dysmorphism. J Pediatr. 2009;155(1):140–143. doi: 10.1016/j.jpeds.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 91.Lombardi LM, Baker SA, Zoghbi HY. MECP2 disorders: From the clinic to mice and back. J Clin Invest. 2015;125(8):2914–2923. doi: 10.1172/JCI78167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van Esch H. MECP2 duplication syndrome. Mol Syndromol. 2011;2(3–5):128–136. doi: 10.1159/000329580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bauer M, et al. Infectious and immunologic phenotype of MECP2 duplication syndrome. J Clin Immunol. 2015;35(2):168–181. doi: 10.1007/s10875-015-0129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Erlandson A, Hallberg B, Hagberg B, Wahlström J, Martinsson T. MECP2 mutation screening in Swedish classical Rett syndrome females. Eur Child Adolesc Psychiatry. 2001;10(2):117–121. doi: 10.1007/s007870170034. [DOI] [PubMed] [Google Scholar]
- 95.Yaron Y, et al. MECP2 mutations in Israel: Implications for molecular analysis, genetic counseling, and prenatal diagnosis in Rett syndrome. Hum Mutat. 2002;20(4):323–324. doi: 10.1002/humu.9069. [DOI] [PubMed] [Google Scholar]
- 96.Erlandson A, et al. Multiplex ligation-dependent probe amplification (MLPA) detects large deletions in the MECP2 gene of Swedish Rett syndrome patients. Genet Test. 2003;7(4):329–332. doi: 10.1089/109065703322783707. [DOI] [PubMed] [Google Scholar]
- 97.Schollen E, Smeets E, Deflem E, Fryns JP, Matthijs G. Gross rearrangements in the MECP2 gene in three patients with Rett syndrome: Implications for routine diagnosis of Rett syndrome. Hum Mutat. 2003;22(2):116–120. doi: 10.1002/humu.10242. [DOI] [PubMed] [Google Scholar]
- 98.Ariani F, et al. Real-time quantitative PCR as a routine method for screening large rearrangements in Rett syndrome: Report of one case of MECP2 deletion and one case of MECP2 duplication. Hum Mutat. 2004;24(2):172–177. doi: 10.1002/humu.20065. [DOI] [PubMed] [Google Scholar]
- 99.Laccone F, et al. Large deletions of the MECP2 gene detected by gene dosage analysis in patients with Rett syndrome. Hum Mutat. 2004;23(3):234–244. doi: 10.1002/humu.20004. [DOI] [PubMed] [Google Scholar]
- 100.Ravn K, et al. Large genomic rearrangements in MECP2. Hum Mutat. 2005;25(3):324. doi: 10.1002/humu.9320. [DOI] [PubMed] [Google Scholar]
- 101.Archer HL, et al. Gross rearrangements of the MECP2 gene are found in both classical and atypical Rett syndrome patients. J Med Genet. 2006;43(5):451–456. doi: 10.1136/jmg.2005.033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hardwick SA, et al. Delineation of large deletions of the MECP2 gene in Rett syndrome patients, including a familial case with a male proband. Eur J Hum Genet. 2007;15(12):1218–1229. doi: 10.1038/sj.ejhg.5201911. [DOI] [PubMed] [Google Scholar]
- 103.Casanova JL, Conley ME, Seligman SJ, Abel L, Notarangelo LD. Guidelines for genetic studies in single patients: Lessons from primary immunodeficiencies. J Exp Med. 2014;211(11):2137–2149. doi: 10.1084/jem.20140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Neumann D, et al. Threonine 66 in the death domain of IRAK-1 is critical for interaction with signaling molecules but is not a target site for autophosphorylation. J Leukoc Biol. 2008;84(3):807–813. doi: 10.1189/jlb.0507290. [DOI] [PubMed] [Google Scholar]
- 105.Li X, Commane M, Jiang Z, Stark GR. IL-1-induced NFkappa B and c-Jun N-terminal kinase (JNK) activation diverge at IL-1 receptor-associated kinase (IRAK) Proc Natl Acad Sci USA. 2001;98(8):4461–4465. doi: 10.1073/pnas.071054198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kollewe C, et al. Sequential autophosphorylation steps in the interleukin-1 receptor-associated kinase-1 regulate its availability as an adapter in interleukin-1 signaling. J Biol Chem. 2004;279(7):5227–5236. doi: 10.1074/jbc.M309251200. [DOI] [PubMed] [Google Scholar]
- 107.Keating SE, Maloney GM, Moran EM, Bowie AG. IRAK-2 participates in multiple Toll-like receptor signaling pathways to NFkappaB via activation of TRAF6 ubiquitination. J Biol Chem. 2007;282(46):33435–33443. doi: 10.1074/jbc.M705266200. [DOI] [PubMed] [Google Scholar]
- 108.Bahia MS, Kaur M, Silakari P, Silakari O. Interleukin-1 receptor associated kinase inhibitors: Potential therapeutic agents for inflammatory- and immune-related disorders. Cell Signal. 2015;27(6):1039–1055. doi: 10.1016/j.cellsig.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 109.Li Z, et al. Inhibition of IRAK1/4 sensitizes T cell acute lymphoblastic leukemia to chemotherapies. J Clin Invest. 2015;125(3):1081–1097. doi: 10.1172/JCI75821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ho CK, Strauss JF. Activation of the control reporter plasmids pRL-TK and pRL-SV40 by multiple GATA transcription factors can lead to aberrant normalization of transfection efficiency. BMC Biotechnol. 2004;4:10. doi: 10.1186/1472-6750-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.