Abstract
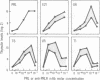
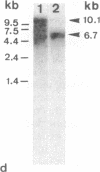
Thymic epithelial cells (TECs), the major component of the thymic microenvironment, can be modulated by pituitary hormones. We have shown previously that prolactin (PRL) can influence the endocrine activity of TECs and stimulate TEC proliferation as well as cytokeratin expression, suggesting the existence of PRL receptors on TECs. Using a series of monoclonal antibodies (mAbs) to the extracellular domain of the rat liver PRL receptor, we have demonstrate that rat TECs bear specific receptors for PRL, as assessed by immunoblotting as well as by immunocytochemistry experiments. Using a probe specific for the long form of PRL receptor, mRNAs of 6.7 and 10.1 kilobases were detected, although by immunoblot the major protein in TECs had a molecular mass of 43 kDa. Functionally, these mAbs were able to modulate thymulin secretion, as well as TEC proliferation. Moreover, the mAbs cross-reacted with human TECs and were able to mimic the action of PRL on these cells. These data bring further support for the general concept of the neuroendocrine immune circuit and extend the notion for a pleiotropic role of PRL as an immunomodulatory hormone.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach J., Bardenne M., Pleau J., Rosa J. Biochemical characterisation of a serum thymic factor. Nature. 1977 Mar 3;266(5597):55–57. doi: 10.1038/266055a0. [DOI] [PubMed] [Google Scholar]
- Ban E., Gagnerault M. C., Jammes H., Postel-Vinay M. C., Haour F., Dardenne M. Specific binding sites for growth hormone in cultured mouse thymic epithelial cells. Life Sci. 1991;48(22):2141–2148. doi: 10.1016/0024-3205(91)90147-4. [DOI] [PubMed] [Google Scholar]
- Bellussi G., Muccioli G., Ghè C., Di Carlo R. Prolactin binding sites in human erythrocytes and lymphocytes. Life Sci. 1987 Aug 24;41(8):951–959. doi: 10.1016/0024-3205(87)90682-5. [DOI] [PubMed] [Google Scholar]
- Berczi I., Nagy E., de Toledo S. M., Matusik R. J., Friesen H. G. Pituitary hormones regulate c-myc and DNA synthesis in lymphoid tissue. J Immunol. 1991 Apr 1;146(7):2201–2206. [PubMed] [Google Scholar]
- Bernton E. W., Meltzer M. S., Holaday J. W. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science. 1988 Jan 22;239(4838):401–404. doi: 10.1126/science.3122324. [DOI] [PubMed] [Google Scholar]
- Boutin J. M., Jolicoeur C., Okamura H., Gagnon J., Edery M., Shirota M., Banville D., Dusanter-Fourt I., Djiane J., Kelly P. A. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988 Apr 8;53(1):69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clevenger C. V., Altmann S. W., Prystowsky M. B. Requirement of nuclear prolactin for interleukin-2--stimulated proliferation of T lymphocytes. Science. 1991 Jul 5;253(5015):77–79. doi: 10.1126/science.2063207. [DOI] [PubMed] [Google Scholar]
- Clevenger C. V., Russell D. H., Appasamy P. M., Prystowsky M. B. Regulation of interleukin 2-driven T-lymphocyte proliferation by prolactin. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6460–6464. doi: 10.1073/pnas.87.16.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardenne M., Pléau J. M., Nabarra B., Lefrancier P., Derrien M., Choay J., Bach J. F. Contribution of zinc and other metals to the biological activity of the serum thymic factor. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5370–5373. doi: 10.1073/pnas.79.17.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardenne M., Savino W., Berrih S., Bach J. F. A zinc-dependent epitope on the molecule of thymulin, a thymic hormone. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7035–7038. doi: 10.1073/pnas.82.20.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMattia G. E., Gellersen B., Bohnet H. G., Friesen H. G. A human B-lymphoblastoid cell line produces prolactin. Endocrinology. 1988 Jun;122(6):2508–2517. doi: 10.1210/endo-122-6-2508. [DOI] [PubMed] [Google Scholar]
- Djiane J., Dusanter-Fourt I., Katoh M., Kelly P. A. Biological activities of binding site specific monoclonal antibodies to prolactin receptors of rabbit mammary gland. J Biol Chem. 1985 Sep 25;260(21):11430–11435. [PubMed] [Google Scholar]
- Djiane J., Houdebine L. M., Kelly P. A. Prolactin-like activity of anti-prolactin receptor antibodies on casein and DNA synthesis in the mammary gland. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7445–7448. doi: 10.1073/pnas.78.12.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edery M., Jolicoeur C., Levi-Meyrueis C., Dusanter-Fourt I., Pétridou B., Boutin J. M., Lesueur L., Kelly P. A., Djiane J. Identification and sequence analysis of a second form of prolactin receptor by molecular cloning of complementary DNA from rabbit mammary gland. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2112–2116. doi: 10.1073/pnas.86.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elberg G., Kelly P. A., Djiane J., Binder L., Gertler A. Mitogenic and binding properties of monoclonal antibodies to the prolactin receptor in Nb2 rat lymphoma cells. Selective enhancement by anti-mouse IgG. J Biol Chem. 1990 Sep 5;265(25):14770–14776. [PubMed] [Google Scholar]
- Goff B. L., Roth J. A., Arp L. H., Incefy G. S. Growth hormone treatment stimulates thymulin production in aged dogs. Clin Exp Immunol. 1987 Jun;68(3):580–587. [PMC free article] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Hartmann D. P., Holaday J. W., Bernton E. W. Inhibition of lymphocyte proliferation by antibodies to prolactin. FASEB J. 1989 Aug;3(10):2194–2202. doi: 10.1096/fasebj.3.10.2787766. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kasahara S., Mori T. A thymic epithelial cell line, IT-45R1, induces the differentiation of prethymic progenitor cells into postthymic cells through direct contact. Thymus. 1982 Feb;4(2):69–75. [PubMed] [Google Scholar]
- Katoh M., Raguet S., Zachwieja J., Djiane J., Kelly P. A. Hepatic prolactin receptors in the rat: characterization using monoclonal antireceptor antibodies. Endocrinology. 1987 Feb;120(2):739–749. doi: 10.1210/endo-120-2-739. [DOI] [PubMed] [Google Scholar]
- Kelley K. W., Brief S., Westly H. J., Novakofski J., Bechtel P. J., Simon J., Walker E. B. GH3 pituitary adenoma cells can reverse thymic aging in rats. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5663–5667. doi: 10.1073/pnas.83.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laussac J. P., Cung M. T., Pasdeloup M., Haran R., Marraud M., Lefrancier P., Dardenne M., Bach J. F. NMR study of thymulin, a lymphocyte differentiating thymic nonapeptide. Conformational states of free peptide in solution. J Biol Chem. 1986 Jun 15;261(17):7784–7790. [PubMed] [Google Scholar]
- Mocchegiani E., Paolucci P., Balsamo A., Cacciari E., Fabris N. Influence of growth hormone on thymic endocrine activity in humans. Horm Res. 1990;33(6):248–255. doi: 10.1159/000181528. [DOI] [PubMed] [Google Scholar]
- Montgomery D. W., Zukoski C. F., Shah G. N., Buckley A. R., Pacholczyk T., Russell D. H. Concanavalin A-stimulated murine splenocytes produce a factor with prolactin-like bioactivity and immunoreactivity. Biochem Biophys Res Commun. 1987 Jun 15;145(2):692–698. doi: 10.1016/0006-291x(87)91020-5. [DOI] [PubMed] [Google Scholar]
- Noble R. L., Beer C. T., Gout P. W. Evidence in vivo and in vitro of a role for the pituitary in the growth of malignant lymphomas in Nb rats. Cancer Res. 1980 Jul;40(7):2437–2440. [PubMed] [Google Scholar]
- Okamura H., Raguet S., Bell A., Gagnon J., Kelly P. A. Purification and protein sequence analysis of rat liver prolactin receptor. J Biol Chem. 1989 Apr 5;264(10):5904–5911. [PubMed] [Google Scholar]
- Okamura H., Zachwieja J., Raguet S., Kelly P. A. Characterization and applications of monoclonal antibodies to the prolactin receptor. Endocrinology. 1989 May;124(5):2499–2508. doi: 10.1210/endo-124-5-2499. [DOI] [PubMed] [Google Scholar]
- Pelletier M., Montplaisir S., Dardenne M., Bach J. F. Thymic hormone activity and spontaneous autoimmunity in dwarf mice and their littermates. Immunology. 1976 Jun;30(6):783–788. [PMC free article] [PubMed] [Google Scholar]
- Russell D. H., Kibler R., Matrisian L., Larson D. F., Poulos B., Magun B. E. Prolactin receptors on human T and B lymphocytes: antagonism of prolactin binding by cyclosporine. J Immunol. 1985 May;134(5):3027–3031. [PubMed] [Google Scholar]
- Savino W., Dardenne M. Analysis of thymic epithelial cell proliferation in vitro by combining bromodeoxyuridine and keratin labeling in an immunofluorescence assay. J Immunol Methods. 1985 Dec 17;85(1):221–226. doi: 10.1016/0022-1759(85)90290-x. [DOI] [PubMed] [Google Scholar]
- Savino W., Dardenne M. Immunohistochemical studies on a human thymic epithelial cell subset defined by the anti-cytokeratin 18 monoclonal antibody. Cell Tissue Res. 1988 Oct;254(1):225–231. doi: 10.1007/BF00220038. [DOI] [PubMed] [Google Scholar]
- Savino W., Dardenne M., Papiernik M., Bach J. F. Thymic hormone-containing cells. Characterization and localization of serum thymic factor in young mouse thymus studied by monoclonal antibodies. J Exp Med. 1982 Aug 1;156(2):628–633. doi: 10.1084/jem.156.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino W., Dardenne M. Thymic hormone-containing cells. VIII. Effects of colchicine, cytochalasin B, and monensin on secretion of thymulin by cultured human thymic epithelial cells. J Histochem Cytochem. 1986 Dec;34(12):1719–1723. doi: 10.1177/34.12.3782779. [DOI] [PubMed] [Google Scholar]
- Shirota M., Banville D., Ali S., Jolicoeur C., Boutin J. M., Edery M., Djiane J., Kelly P. A. Expression of two forms of prolactin receptor in rat ovary and liver. Mol Endocrinol. 1990 Aug;4(8):1136–1143. doi: 10.1210/mend-4-8-1136. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Elsholtz H. P., Tanaka T., Friesen H. G., Gout P. W., Beer C. T., Noble R. L. Receptor-mediated mitogenic action of prolactin in a rat lymphoma cell line. Endocrinology. 1983 Jul;113(1):159–165. doi: 10.1210/endo-113-1-159. [DOI] [PubMed] [Google Scholar]
- Villa-Verde D. M., Defresne M. P., Greimers R., Dardenne M., Savino W., Boniver J. Induction of thymocyte proliferation by supernatants from a mouse thymic epithelial cell line. Cell Immunol. 1991 Aug;136(1):113–121. doi: 10.1016/0008-8749(91)90386-p. [DOI] [PubMed] [Google Scholar]