Abstract
Purpose
Few studies have evaluated the degree to which prescription drug initiators are correctly identified using claims data. We examine the prevalence and predictors of recent statin possession in statin initiators identified using claims data.
Methods
Among Medicare Current Beneficiary Survey (MCBS) respondents, we used Medicare Part D claims from 2006–2011 to identify statin initiators using a 12-month baseline period of no prior statin claims. Using MCBS interview data, we identified those with self-reported statins obtained during the baseline period. We used log-binomial regression to estimate adjusted prevalence ratios (adjPR) and 95% confidence intervals (CI) for predictors of recent statin possession.
Results
Among 766 statin initiators identified in prescription claims, 155 (20%) reported recent statin possession during baseline. Beneficiaries with no Part D claims in the past 30 days (adjPR=1.49, 95% CI: 1.13, 1.96), those with no inpatient, outpatient or physician visits in the past 30 days (adjPR=1.50, 95% CI: 1.11, 2.03), those with a brand name statin index claim (adjPR=1.55, 95% CI: 1.19, 2.02), and those with an index claim in January or February (adjPR=1.55, 95% CI: 1.00, 2.26) had an increased probability of recent statin possession.
Conclusions
In a cohort of statin initiators identified using prescription claims, 20% had evidence of statin possession during the baseline period. Pharmacoepidemiologic new user studies may benefit from including sensitivity analyses within subgroups less likely to include prevalent users to assess the robustness of key findings to misidentification of the time of treatment initiation.
Keywords: new user design, statins, administrative claims, Medicare Current Beneficiary Survey
Introduction
Pharmacoepidemiologic research based on insurance claims data leverages data on both dispensed prescriptions and healthcare encounters for large populations, generally necessary to study the real world dynamics of pharmacological therapy and rare events.1 However, researchers must consider the implications of using administratively collected insurance data. Of primary interest for the current study is the completeness of prescription claims data which are routinely used to characterize treatment initiation and adherence.
These data are continuously and prospectively collected for all insured with a drug benefit plan and are believed to provide a more accurate and complete picture of prescription use compared to survey data and self-reported drug use, which may be subject to recall and social desirability bias and hardly ever capture drug exposures without interruption.2 However, prescription claims data may not contain records for off-formulary prescriptions, those paid for completely out-of-pocket, those processed in out-of-network pharmacies, or samples given to patients by physicians.3, 4 Furthermore, the Medicare Part D benefit includes a coverage gap, where Medicare discontinues reimbursement for drugs once beneficiaries reach a certain limit in drug spending in a calendar year.5 The coverage gap has been found to impact patient decisions on prescription fulfillment leading to alternative methods to receive drugs (e.g. Canadian online pharmacies, physician samples), which could result in prescription use not captured in Medicare Part D claims.6 Incomplete prescription data may lead researchers to misidentify the date of treatment initiation and adherence patterns, or completely overlook treatment episodes.
We focus on potential errors in the identification of treatment initiation, as the new user study design is widely used in pharmacoepidemiologic research. This study design avoids biases stemming from time-varying hazards and selection of persistent users, while facilitating the correct temporal relation between ascertainment of baseline covariates, exposure, and outcome.7–11 We examined the prevalence and predictors of potential prevalent use in individuals identified as new users based on claims data. Specifically, among a cohort of statin initiators identified using prescription claims data, we examined the prevalence of self-reported statins obtained prior to the claims-based treatment initiation date. We further identified factors associated with having obtained prior statins to characterize populations in which the timing of treatment initiation based on prescription claims alone may be subject to greater misclassification.
Methods
Data Source
We used Medicare Current Beneficiary Survey (MCBS) Cost and Use data from 2006 to 2011 for this study. The MCBS is a longitudinal rotating panel survey, with participants sampled from Medicare enrollment files to be nationally representative of Medicare beneficiaries enrolled in Part A or Part B. The disabled (<65) and elderly (≥80) are oversampled to ensure large enough numbers for statistically reliable data in these groups.12 CMS provides cross-sectional weights for each respondent to estimate levels of services for the entire enrolled Medicare population. The Cost and Use file aims to estimate total annual healthcare utilization and expenditures for both Medicare-covered and non-covered services.13 Participants are interviewed three times a year for four consecutive years on demographic information, health status and functioning, and prescription medications.12, 14 Of primary interest for our study is the Prescribed Medicine Events (PME) questionnaire. Respondents are asked to report all prescription medications obtained and all prescriptions filled since the prior interview (approximately 3 months ago). To aid in accurate and complete reporting, respondents are instructed to retain prescription bottles and receipts and are prompted about refills for prescriptions reported in prior interviews.15 Questionnaires are made publically available by the Centers for Medicare & Medicaid Services (CMS), and can be downloaded from their website.16 In-person interview data were used to obtain self-reported prescriptions, age, race, gender, and region of residence. Medicare claims and administrative data were used to obtain enrollment history, low-income subsidy (LIS) status, Part D prescription claims, copay, and past healthcare utilization.
Eligible Population
We identified statin initiators using Medicare Part D claims for MCBS participants. Eligible participants were 65 years of age or older with at least one Part D claim for a statin between 2007 and 2011. We required 12 months of prior continuous Medicare Part A (inpatient), B (outpatient/physician care), and D (outpatient drug) enrollment, and beneficiaries must have completed the baseline MCBS questionnaire at least 12 months prior to the statin claim date. We applied a 12-month baseline period during which beneficiaries had no Part D claims for a statin to define incident use, and we excluded beneficiaries if they resided in a long-term care facility (e.g. nursing or group home) during baseline, as survey and claims data are less reliable in these settings. If an individual had multiple instances of statin initiation, the first eligible prescription dispensing date for each beneficiary was chosen as the index statin claim. This method of identifying statin initiators mirrors methods commonly used in claims-based pharmacoepidemiologic studies.9, 17, 18
Recent Statin Possession
At each interview, participants were asked to report all prescription medications obtained or filled since the prior interview. Interview windows were thus defined as beginning one day after the prior interview, and ending on the date of the current interview. Any interview windows falling completely within the 12-month baseline period were considered eligible in the current analysis (Figure 1).
Figure 1.
Timeline for identification of new users of statins. Statin initiators are identified using Part D Claims with a 12-month baseline period. All MCBS interview windows falling completely within the 12-month baseline period are examined for self-reported statin prescriptions obtained within the clean period.
Any statins obtained during eligible interview windows may indicate misclassification of prevalent statin users as statin initiators. To examine the extent of potential misclassification of the timing of statin initiation, we calculated the percentage of claims-based statin initiators who had reported obtaining statins within the 12-month baseline. If a participant reported obtaining statins within an eligible interview window, they were flagged as having recent statin possession. Given the wording of the questionnaire, recent statin possession may include prescriptions obtained in a pharmacy, regardless of whether claimed through the Part D benefit, as well as those obtained from physicians as samples.16
Statistical Analyses
We compared the proportion of beneficiaries with recent statin possession among different patient subgroups. We used a log-binomial regression model to directly estimate prevalence ratios, as odds ratios estimated using logistic regression would overestimate the risk ratio because the prevalence of recent statin possession is relatively high.19 We report multivariate adjusted prevalence ratios and their 95% confidence intervals for predictors of recent statin possession. Potential predictors included age, sex, race, brand name vs generic drug, amount of copay, and region of residence. The following characteristics were also added as potential covariates due to imbalance observed during initial descriptive analyses: calendar month of index claim, number of prescription drug claims in the month prior, and number of inpatient, outpatient, and physician visits in the month prior.
Because the Medicare Part D program began in 2006, we conducted a sensitivity analysis using only data from 2007–2011. We conducted all analyses in SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina). This study was approved by UNC’s IRB (13-2402).
Results
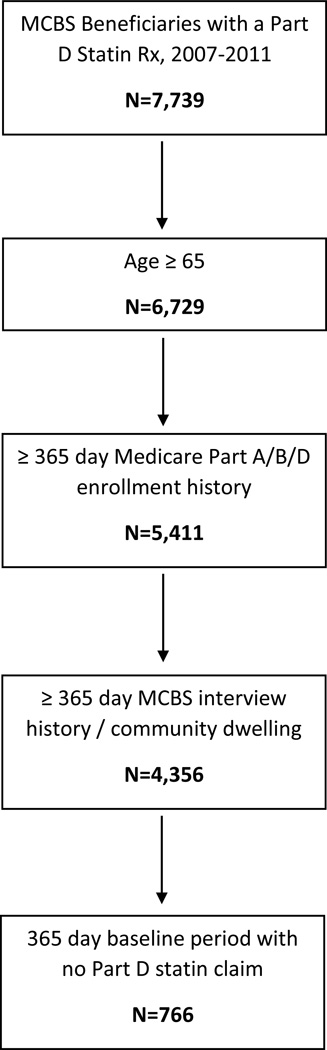
Among 7,739 MCBS participants who had claims for a statins between 2007 and 2011, we identified 766 eligible statin initiators using the Part D claims data (Figure 2). Statin initiators had a mean age of 75.8 years, 63% were female, 81% were white, and 25% qualified for LIS (Table 1). Applying cross-sectional sampling weights had very little effect on patient demographics and prevalence of recent statin prescriptions (data not shown).
Figure 2.
Eligibility criteria for identification of new users of statins. MCBS beneficiaries with 1 or more Part D claims for a statin prescription between 2007 and 2011 are identified. We then impose eligibility criteria for age, enrollment history, and a baseline to define a final cohort of statin initiators according to Part D Claims data.
Table 1.
Characteristics of statin initiators identified in Medicare Part D claims, 2006–2011
| Claims-based Statin Initiators (n=766) |
||
|---|---|---|
| N | % | |
| Age, Mean (Std. Deviation) | 75.8 | (6.5) |
| Female | 483 | 63.1% |
| Race | ||
| White | 617 | 80.5% |
| Black | 80 | 10.4% |
| Other | 69 | 9.0% |
| Low-income Subsidy Recipient (LIS) |
191 | 24.9% |
| Healthcare Past 30 Days | ||
| 1+ Rx | 579 | 75.6% |
| 1+ Inpatient or Outpatient Visit |
404 | 52.7% |
| Comorbidities | ||
| Diabetes | 203 | 26.5% |
| Hypertension | 404 | 52.7% |
| Hyperlipidemia | 400 | 52.2% |
| Ischemic heart disease | 173 | 22.6% |
| Arterial fibrillation | 61 | 8.0% |
| Heart Failure | 93 | 12.1% |
| Acute Events | ||
| Myocardial infarction | 28 | 3.7% |
| Unstable angina | 27 | 3.5% |
| Stroke | 107 | 14.0% |
| Cardiovascular Disease Management |
||
| Angiography | 44 | 5.7% |
| Cardiac stress test | 76 | 9.9% |
| Echocardiograph | 134 | 17.5% |
| Lipid testing | 279 | 36.4% |
| Inpatient Stays | ||
| 0 Stays | 707 | 92.3% |
| 1 Stay | 44 | 5.7% |
| 2+ Stays | 15 | 2.0% |
In order to ensure that all self-reported statins were obtained within the 12-month baseline period prior to statin initiation, we examined only eligible interview windows. On average, 234 days out of the 365-day baseline were covered in interview windows completely within the baseline period. Those with index claims in January and February had an average of 220 days of coverage, slightly less compared to those with index claims later in the calendar year, with averages ranging from 232 to 238 days of coverage in all other index months.
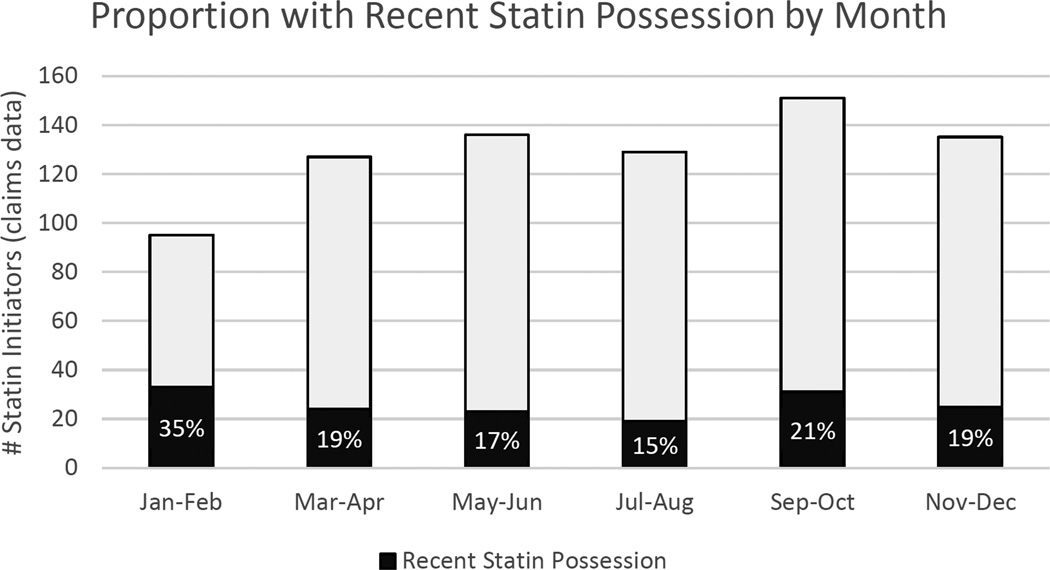
Out of the 766 statin initiators identified in the prescription claims, 155 (20.2%, 95%CI: 17.5% - 23.2%) had obtained statins during the 12-month baseline period (Table 2). Recent statin possession was higher among males (25%) than females (17%), and higher among those who did not qualify for LIS (22%) compared to those who did qualify (14%). Individuals who had no Part D claims in the 30 days prior to index date were more likely to have recent statin possession than those with at least one prescription claim (29% vs 17%). Similarly, individuals with no recorded inpatient, outpatient or physician office visits in the past 30 days were more likely to have recent statin possession than those with at least one encounter (26% vs 15%). Individuals with an index claim for a brand name statin were more likely to have recent statin possession than those receiving a generic statin (26% vs 18%), and 35% of those with index claims in the months of January or February had recent statin possession compared to 21% of those with index claims in September or October (the 2-month period with the most identified initiators). Overall, we identified the fewest statin initiators in January and February, however those identified as new users in this period were most likely to have recent statin possession (Figure 3).
Table 2.
Crude and adjusted* prevalence ratios of recent statin possession during the 12-month baseline period among new users identified in claims data
| Total Patients |
% With Recent Statin Possession |
Crude Prevalence Ratio |
95% Confidence Interval |
Adjusted* Prevalence Ratio |
95% Confidence Interval |
|
|---|---|---|---|---|---|---|
| Total Population | 766 | 20.2% | - | - | - | - |
| Mean Age | 75.8 | - | - | - | 1.01 | (0.99, 1.03) |
| Sex | ||||||
| Female | 483 | 17.2% | 1.00 | - | 1.00 | - |
| Male | 283 | 25.4% | 1.48 | (1.12, 1.96) | 1.23 | (0.93, 1.53) |
| Race | ||||||
| White | 617 | 21.1% | 1.00 | - | 1.00 | - |
| Black | 80 | 18.8% | 0.89 | (0.55, 1.44) | 0.99 | (0.62, 1.60) |
| Other | 69 | 14.5% | 0.69 | (0.38, 1.24) | 0.77 | (0.43, 1.38) |
| Low Income Subsidy | ||||||
| Receive LIS | 191 | 14.1% | 1.00 | - | 1.00 | - |
| No LIS | 575 | 22.3% | 1.57 | (1.08, 2.31) | 1.37 | (0.93, 2.03) |
| Healthcare Past 30 Days | ||||||
| 1+ Rx | 579 | 17.4% | 1.00 | - | 1.00 | - |
| No Rx | 187 | 28.9% | 1.66 | (1.24, 2.20) | 1.49 | (1.13, 1.96) |
| 1+ Inpatient or Office Visit | 404 | 15.1% | 1.00 | - | 1.00 | - |
| No Inpatient or Office Visit | 362 | 26.0% | 1.72 | (1.29, 2.30) | 1.50 | (1.11, 2.03) |
| Index Prescription | ||||||
| Generic | 527 | 17.5% | 1.00 | - | 1.00 | - |
| Brand | 239 | 26.4% | 1.51 | (1.14, 2.19) | 1.55 | (1.19, 2.02) |
| Calendar Month of Initiation | ||||||
| January–February | 95 | 34.7% | 1.68 | (1.11, 2.55) | 1.50 | (1.00 2.26) |
| March–April | 124 | 19.4% | 0.94 | (0.58, 1.51) | 0.94 | (0.59, 1.50) |
| May–June | 134 | 17.2% | 0.83 | (0.51, 1.35) | 0.96 | (0.59, 1.55) |
| July–August | 129 | 14.7% | 0.71 | (0.42, 1.20) | 0.79 | (0.47, 1.32) |
| September–October | 150 | 20.7% | 1.00 | - | 1.00 | - |
| November–December | 134 | 18.7% | 0.90 | (0.56, 1.45) | 0.91 | (0.57, 1.45) |
Estimates adjusted for age in years, sex, race, LIS status, healthcare utilization in the past month, brand name vs. generic index prescription, and calendar month of initiation
Figure 3.
Proportion of statin initiators with recent statin possession by month. The bars represent the number of statin initiators identified from Part D claims data in two-month intervals. Black represents the number of users with self-reported statins obtained during the baseline period, with white representing no self-reported statins obtained in the baseline period. Percentages within each 2 month category are provided.
After examining distributions of potential covariates, we removed copay (highly collinear with LIS) and census region (little variability between groups), and limited our candidate predictors to a more parsimonious model including age in years (centered at the mean, 76), sex, race (white, black, and other race), brand name vs. generic drug as the index statin claim, number of inpatient, outpatient, or physician office visits in the past 30 days (no visits, or 1+), number of prescriptions filled in the past 30 days (no prescriptions, or 1+), and calendar month of index claim (categorized into six 2-month categories).
After adjustment, beneficiaries with no Part D claims for any prescriptions in the past 30 days (adjPR=1.49, 95% CI: 1.13, 1.96, referent=1+ Rx), those with no inpatient, outpatient or physician visits in the past 30 days (adjPR=1.50, 95% CI: 1.11, 2.03, referent=1+ visit), those with a brand name index claim (adjPR=1.55, 95% CI: 1.19, 2.02, referent=generic index claim), and those with an index claim in January or February (adjPR=1.55, 95% CI: 1.00, 2.26, referent= September or October) had an increased probability of recent statin possession (Table 2). The c-statistic associated with this model was 0.68.
We evaluated the degree to which additional inclusion criteria might improve the selection of a cohort of new users. In a cohort of patients required to have at least one inpatient, outpatient, or physician office visit and at least one prescription claim in the 30 days prior to index date, the sample size decreased to 42% of the original cohort and the proportion of claims-based statin initiators with recent statin possession decreased from 20.2% to 13.7% (Table 3). If we also required the index claim to occur in March-December and selected only those initiating generic drugs, the proportion who reported obtaining statins during baseline dropped to 11.8% and the sample size decreased to 24% of the original cohort.
Table 3.
Number of claims-based statin initiators and proportion with recent statin possession in the baseline period after implementing further exclusion criteria.
| Study Exclusion Criteria | Number of Claims- Based Statin Initiators |
% of Cohort Retained |
% With Recent Statin Possession |
|---|---|---|---|
| Original cohort | 766 | 100% | 20.2% |
| + At least one inpatient/outpatient/office visit in the prior 30 days |
404 | 52.7% | 15.1% |
| + At least one Rx in the past 30 days | 322 | 42.0% | 13.7% |
| + Index date not in January or February |
292 | 38.1% | 12.7% |
| + Generic index prescription | 195 | 25.5% | 11.8% |
The sensitivity analysis using only data from 2007–2011 had similar results with 21.5% (95%CI: 18.3% - 25.0%) of statin initiators identified in the claims data having recent statin. Patterns of recent statin possession and adjusted prevalence ratios were similar (data not shown).
Discussion
In a cohort of Medicare beneficiaries identified as new users of statins based on their prescription claims, we found that 1 out of five initiators had evidence of statin possession during the baseline period. These results are concerning and suggest current methods used in claims-based pharmacoepidemiologic research may result in misclassification of the timing of treatment initiation of statins, a common class of medications, in a substantial portion of the population.
This study leveraged Medicare Part D claims for all beneficiaries involved in the MCBS from 2006 to 2011, as well as all interview reported prescriptions obtained in that time frame. The availability of claims data and self-reported data allows for the unique opportunity to evaluate the current standards employed in pharmacoepidemiologic studies that have access to only the claims data.
This is not the first study to examine the reliability of prescription claims data. Roberto 3 and Stuart evaluated the extent of out-of-plan medication use in Medicare Part D beneficiaries and found that in 2009, 6.8% of reported statin prescriptions were unadjudicated (generated no Part D payment) with cash prescriptions accounting for the majority of out-of-plan use.3 They also found that 44% of unadjudicated fills were for brand name medicines.3 Li et.al studied statin initiation using commercial claims in the United States (2007–2010) and found that an estimated 13.4% of the 9,256 patients using brand name statins had evidence of prior use not captured in the claims, and concluded that this was most likely due to medication samples dispensed by physicians.20 Another study directly comparing extent of sample use between brand name and generic formulations across different drug classes consistently found higher rates of sample use in branded drugs.4
Our results indicate that individuals with no recorded inpatient, outpatient, or physician office visits, and no recorded prescription claims in the 30 days prior to drug initiation were more likely to have recent statin possession. These findings highlight the importance of assessing healthcare utilization during the baseline period, and also provide support for including sensitivity analyses excluding those with minimal claims-based evidence of recent interactions with the healthcare system, indicating a potential lack of ability to accurately ascertain medical history, therefore increasing the probability of misclassifying drug initiation time.
The findings of increased misclassification for those with index dates in January and February may be driven by the Part D coverage gap, and the calendar-based insurance benefit periods. If a beneficiary enters the coverage gap and does not expect to reach the catastrophic coverage phase, they may be less incentivized to go through their Part D benefit, as there is no financial benefit. However, when the calendar year resets, there is an incentive to use their Part D benefit, so we may begin observing prescriptions in the beginning of the year, when in fact the beneficiary has received earlier prescriptions that were not submitted through Part D.
In studies comparing drugs which may differ on brand/generic availability, it is plausible that there would be differential misclassification of the timing of initiation, with brand name medications having a higher rate of misclassification compared with generic medications. Of note, higher rates of sample usage in rosuvastatin compared to simvastatin have recently been reported, suggesting that studies comparing these treatments may be subject to differential misclassification of drug initiation time.4
In addition to differences in brand and generic availability, our findings indicate potential differential rates of misclassification if sex or LIS status distribution differ among treatment arms. Studies aiming to assess presence of heterogeneous treatment effects are similarly subject to biases resulting from misclassification of the timing of initiation, as analyses in sex-specific strata or income strata may result in comparisons between populations with differential rates of misclassification, either creating the false appearance of heterogeneity, or obscuring actual heterogeneity.
This study has several limitations. The interview aims to collect information on prescriptions obtained or filled, however it is likely that sample use is not uniformly captured, thus underestimating recent statin possession.4 Evidence of recent statin possession does not necessarily imply medication consumption. This is also a limitation of claims data, where prescription claims may not translate directly to consumption, or per-protocol treatment adherence. Additionally, as with all interview data, there is the potential for both over- and underreporting of prescription fills. A prior study compared MCBS self-report to pharmacy profiles, and found that in 1999, on average, Medicare beneficiaries underreported prescriptions by 17.7 percent.15 The same study found that 23 percent of beneficiaries over-report drug utilization, and those with a large number of prescriptions were more likely to over-report. There is no date associated with self-reported prescriptions, as such, interview windows were the most granular time period available. On average, 234 days out of the 365-day baseline was covered in interview windows falling completely within the baseline period, suggesting that the current result of 20% having recent statin possession is likely an underestimate.
The current study has limited generalizability as it includes only Medicare beneficiaries with fee-for-service coverage, excluding those enrolled in Medicare Advantage plans and residing in nursing homes and other long-term care facilities in the year prior to statin initiation. Accuracy of claims data will likely vary by insurance program and population. Furthermore, within those participating in the Medicare Part D program, completeness of insurance claims may vary by drug type, formulary structures, amount of copay, availability of generics, and characteristics of the patients studied, including comorbidity. Future research assessing the completeness of claims for various drug classes would be helpful in identifying common trends predicting potential prevalent use, and identifying specific factors that may lead to better prediction of prevalent use. Lastly, the sample size included in the current analysis was relatively small (766 claims-based statin initiators), and several of the predictors were exploratory in nature, and were identified through descriptive analyses, warranting future research.
We conclude that among 766 statin initiators identified using Medicare Part D claims, 1 out of five had reported obtaining statins during a 12-month baseline period. Predictors of recent statin possession included lack of inpatient, outpatient, or physician office visits, and lack of prescription drug fills, in the prior 30 days, a brand name (rather than generic) formulation as the index claim, and index claims in January or February.
Our results suggest settings in which misclassification of prevalent users as new users might lead to bias. Pharmacoepidemiologic new user studies may benefit by including sensitivity analyses within subgroups less likely to include prevalent users such as those with evidence of prescription claims and physician contact in the past 30 days, and those initiating on generics, to assess the sensitivity of results to inclusion of prevalent users in the study. Studies of Medicare Part D beneficiaries may additionally benefit from analyses focused on those initiating later in the calendar year.
Key-points.
This study uses current methods to identify statin initiators in healthcare claims data and estimates the prevalence of prior drug use based on interview-reported data.
Findings raise concern that misclassification of the medication initiation date occurs in a substantial proportion of statin initiators identified in Medicare Part D claims.
In circumstances where study cohorts compared vary in brand/generic availability, recent healthcare utilization, or calendar month of initiation, researchers should consider subgroup analyses to assess the sensitivity of study results to inclusion of prevalent users.
Acknowledgments
Conflict-of-interest:
JY is a recipient of the UNC Center for Pharmacoepidemiology Fellowship, which received funding from GlaxoSmithKline, UCB BioSciences, and Merck.
TS receives investigator-initiated research funding and support as Principal Investigator (R01 AG023178) from the National Institute on Aging (NIA), and as Co-Investigator (R01 CA174453; R01 HL118255, R21-HD080214), National Institutes of Health (NIH). He also receives salary support as Director of the Comparative Effectiveness Research (CER) Strategic Initiative, NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR001111) and as Director of the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck) and research support from pharmaceutical companies (Amgen, AstraZeneca) to the Department of Epidemiology, University of North Carolina at Chapel Hill. Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca, and Johnsen & Johnsen. JL receives research funding through the UNC K12 Oncology Clinical Translational Research Training Program (5K12CA120780), as well as salary support from the PhRMA Foundation to the Department of Epidemiology, UNC Gillings School of Global Public Health.
JL receives research funding through the UNC K12 Oncology Clinical Translational Research Training Program (5K12CA120780), as well as salary support from the PhRMA Foundation to the Department of Epidemiology, UNC Gillings School of Global Public Health.
MJF receives investigator-initiated research funding and support as a Co-Investigator from the NIH National Institute on Aging (NIA, R01 AG023178), the NIH National Center for Advancing Translational Sciences (NCATS, 1UL1TR001111), AstraZeneca, and the Patient Centered Outcomes Research Institute (PCORI, 1IP2PI000075). Dr. Jonsson Funk does not accept personal compensation of any kind from any pharmaceutical company, though she receives salary support from the Center for Pharmacoepidemiology in the Department of Epidemiology, Gillings School of Global Public Health (current members: GlaxoSmithKline, UCB BioSciences, Merck).
This research was supported by the National Institutes of Health (NIH), National Heart Lung and Blood Institute (NHLBI, R01 HL118255).
The database infrastructure used for this project was funded by the Pharmacoepidemiology Gillings Innovation Lab (PEGIL) for the Population-Based Evaluation of Drug Benefits and Harms in Older US Adults (GIL 200811.0010), the Center for Pharmacoepidemiology, Department of Epidemiology, UNC Gillings School of Global Public Health; the CER Strategic Initiative of UNC’s Clinical & Translational Science Award (UL1TR001111); the Cecil G. Sheps Center for Health Services Research, UNC; and the UNC School of Medicine.
Footnotes
Presentations: The abstract for this manuscript was presented as an oral presentation at the Triangle Comparative Effectiveness Research Symposium on May 14, 2015 in Research Triangle Park, NC, and at the 31st Annual International Conference on Pharmacoepidemiology & Therapeutic Risk Management (ICPE) on August 26, 2015 in Boston, Massachusetts.
References
- 1.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. Journal of clinical epidemiology. 2005;58:323–337. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Bruxvoort K, Festo C, Cairns M, Kalolella A, Mayaya F, Kachur SP, et al. Measuring Patient Adherence to Malaria Treatment: A Comparison of Results from Self-Report and a Customised Electronic Monitoring Device. PLoS One. 2015;10:e0134275. doi: 10.1371/journal.pone.0134275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberto PN, Stuart B. Out-of-plan medication in Medicare Part D. Am J Manag Care. 2014;20:743–748. [PubMed] [Google Scholar]
- 4.Hampp C, Greene P, Pinheiro S. [Abstract] Use of Prescription Drug Samples in the United States and Implications for Pharmacoepidemiologic Studies. Pharmacoepidemiology and drug safety. 2015:24. [Google Scholar]
- 5.Kaiser Family Foundation. Medicare Part D Spotlight: Part D Plan Availablity in 2010 and Key Changes since 2006. [updated November 2009]; Available from: https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7986.pdf.
- 6.Hales JW, George S. How the doughnut hole affects prescription fulfillment decisions involving cardiovascular medications for Medicare Part D enrollees. Manag Care. 2010;19:36–44. [PubMed] [Google Scholar]
- 7.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. American journal of epidemiology. 2003;158:915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 8.Kramer MS, Lane DA, Hutchinson TA. Analgesic use, blood dyscrasias, and case-control pharmacoepidemiology. A critique of the International Agranulocytosis and Aplastic Anemia Study. J Chronic Dis. 1987;40:1073–1085. doi: 10.1016/0021-9681(87)90073-7. [DOI] [PubMed] [Google Scholar]
- 9.Best Practices for Conducting and Reporting Pharmacoepidemiologic Safety Studies Using Electronic Healthcare Data. 2013 Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm243537.pdf.
- 10.Lund J, Richardson D, Stürmer T. The Active Comparator, New User Study Design in Pharmacoepidemiology: Historical Foundations and Contemporary Application. Curr Epidemiol Rep. 2015:1–8. doi: 10.1007/s40471-015-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suissa S, Azoulay L. Metformin and cancer: mounting evidence against an association. Diabetes Care. 2014;37:1786–1788. doi: 10.2337/dc14-0500. [DOI] [PubMed] [Google Scholar]
- 12.Parker J. Data Evaluation and Methods Research Paper #1: New Administrative Data Source for Identifying Medicare-Medicaid Enrollees in the Medicare Current Beneficiary Survey. 2015 Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Research/MCBS/Downloads/Data_Brief_003.pdf.
- 13.Centers for Medicare & Medicaid Services. Appendix A. Technical documentation for the Medicare Current Beneficiary Survey. Available from: http://www.cms.hhs.gov/Research-Statistics-Data-and-Systems/Research/MCBS/downloads//HHC2003appendixA.pdf.
- 14.Parker J. Data Evaluation and Methods Research Paper #1: New Administrative Data Source for Identifying Medicare-Medicaid Enrollees in the Medicare Current Beneficiary Survey. Center for Medicare and Medicaid Services. 2015 Available from: http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/MCBS/Downloads/Data_Brief_003.pdf.
- 15.Poisal JA. Reporting of drug expenditures in the MCBS. Health Care Financ Rev. 2003;25:23–36. [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Medicare & Medicaid Services. Questionnaires. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Research/MCBS/Questionnaires.html.
- 17.Layton JB, Brookhart MA, Jonsson Funk M, Simpson RJ, Jr, Pate V, Sturmer T, et al. Acute kidney injury in statin initiators. Pharmacoepidemiology and drug safety. 2013;22:1061–1070. doi: 10.1002/pds.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham DJ, By K, McKean S, Mosholder A, Kornegay C, Racoosin JA, et al. Cardiovascular and mortality risks in older Medicare patients treated with varenicline or bupropion for smoking cessation: an observational cohort study. Pharmacoepidemiology and drug safety. 2014;23:1205–1212. doi: 10.1002/pds.3678. [DOI] [PubMed] [Google Scholar]
- 19.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. American journal of epidemiology. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Sturmer T, Brookhart MA. Evidence of sample use among new users of statins: implications for pharmacoepidemiology. Med Care. 2014;52:773–780. doi: 10.1097/MLR.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]