Abstract
The polydnavirus Campoletis sonorensis virus (CsV) is present in the oviducts of all adult C. sonorensis female wasps and appears to be required for these wasps to parasitize hosts successfully. Physical mapping, Southern blot analysis, and nucleotide sequence analysis demonstrate that the viral DNA B-specific sequences in cloned wasp DNA are colinear with viral genomic segment DNA B from nucleocapsids and are covalently linked to nonviral wasp sequences. Integrated DNA B terminates in 59-nucleotide imperfect direct repeats, but a single repeat exists in the extrachromosomal superhelical viral DNA B. Sequences near each junction form imperfect inverted repeats with sequences near the ends of an internal viral 540-base-pair repeat element gene. CsV appears to be the first documented integrated, nonretroviral DNA virus of insects and probably is vertically transmitted as a provirus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blissard G. W., Smith O. P., Summers M. D. Two related viral genes are located on a single superhelical DNA segment of the multipartite Campoletis sonorensis virus genome. Virology. 1987 Sep;160(1):120–134. doi: 10.1016/0042-6822(87)90052-3. [DOI] [PubMed] [Google Scholar]
- Blissard G. W., Vinson S. B., Summers M. D. Identification, Mapping, and In Vitro Translation of Campoletis sonorensis Virus mRNAs from Parasitized Heliothis virescens Larvae. J Virol. 1986 Jan;57(1):318–327. doi: 10.1128/jvi.57.1.318-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson K. M., Vinson S. B., Stoltz D. B., Summers M. D. Virus in a parasitoid wasp: suppression of the cellular immune response in the parasitoid's host. Science. 1981 Feb 6;211(4482):582–583. doi: 10.1126/science.7455695. [DOI] [PubMed] [Google Scholar]
- Fleming J. A., Blissard G. W., Summers M. D., Vinson S. B. Expression of Campoletis sonorensis Virus in the Parasitized Host, Heliothis virescens. J Virol. 1983 Oct;48(1):74–78. doi: 10.1128/jvi.48.1.74-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
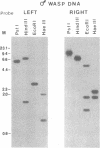
- Fleming J. A., Summers M. D. Campoletis sonorensis Endoparasitic Wasps Contain Forms of C. sonorensis Virus DNA Suggestive of Integrated and Extrachromosomal Polydnavirus DNAs. J Virol. 1986 Feb;57(2):552–562. doi: 10.1128/jvi.57.2.552-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jones D., Sreekrishna S., Iwaya M., Yang J. N., Eberely M. Comparison of viral ultrastructure and DNA banding patterns from the reproductive tracts of Eastern and Western hemisphere Chelonus sp. (Braconidae: Hymenoptera). J Invertebr Pathol. 1986 Jan;47(1):105–115. doi: 10.1016/0022-2011(86)90168-0. [DOI] [PubMed] [Google Scholar]
- Krell P. J., Stoltz D. B. Unusual Baculovirus of the Parasitoid Wasp Apanteles melanoscelus: Isolation and Preliminary Characterization. J Virol. 1979 Mar;29(3):1118–1130. doi: 10.1128/jvi.29.3.1118-1130.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell P. J., Summers M. D., Vinson S. B. Virus with a Multipartite Superhelical DNA Genome from the Ichneumonid Parasitoid Campoletis sonorensis. J Virol. 1982 Sep;43(3):859–870. doi: 10.1128/jvi.43.3.859-870.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung P. C., Teplow D. B., Harshey R. M. Interaction of distinct domains in Mu transposase with Mu DNA ends and an internal transpositional enhancer. Nature. 1989 Apr 20;338(6217):656–658. doi: 10.1038/338656a0. [DOI] [PubMed] [Google Scholar]
- Norton W. N., Vinson S. B. Correlating the initiation of virus replication with a specific pupal developmental phase of an ichneumonid parasitoid. Cell Tissue Res. 1983;231(2):387–398. doi: 10.1007/BF00222189. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz D. B. Evidence for chromosomal transmission of polydnavirus DNA. J Gen Virol. 1990 May;71(Pt 5):1051–1056. doi: 10.1099/0022-1317-71-5-1051. [DOI] [PubMed] [Google Scholar]
- Stoltz D. B., Guzo D., Cook D. Studies on polydnavirus transmission. Virology. 1986 Nov;155(1):120–131. doi: 10.1016/0042-6822(86)90173-x. [DOI] [PubMed] [Google Scholar]
- Stoltz D. B., Vinson S. B. Viruses and parasitism in insects. Adv Virus Res. 1979;24:125–171. doi: 10.1016/s0065-3527(08)60393-0. [DOI] [PubMed] [Google Scholar]
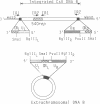
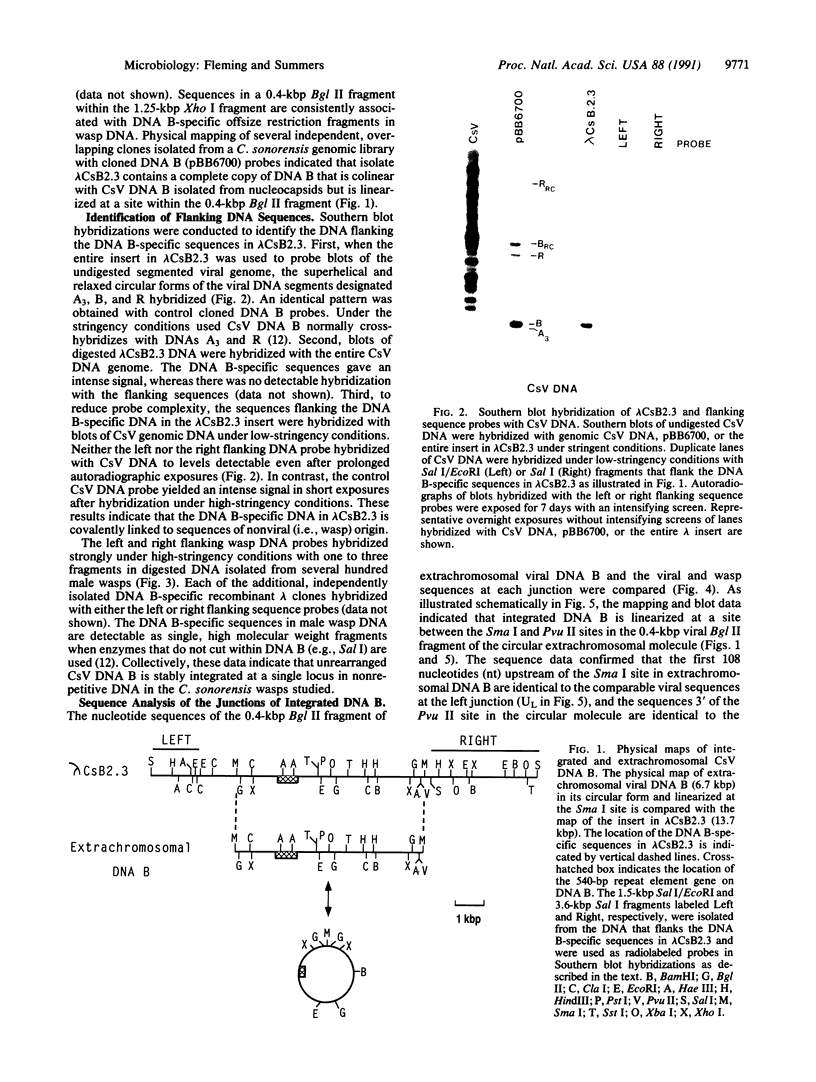
- Theilmann D. A., Summers M. D. Physical Analysis of the Campoletis sonorensis Virus Multipartite Genome and Identification of a Family of Tandemly Repeated Elements. J Virol. 1987 Aug;61(8):2589–2598. doi: 10.1128/jvi.61.8.2589-2598.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson S. B. Microplitis croceipes: inhibitions of the Heliothis zea defense reaction to Cardiochiles nigriceps. Exp Parasitol. 1977 Feb;41(1):112–117. doi: 10.1016/0014-4894(77)90136-9. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]