Abstract
The recent cloning of a second member of the nerve growth factor family, brain-derived neurotrophic factor (BDNF), has prompted investigation into the cells that express this factor's mRNA and protein. In the present study, antibodies raised against unique peptide sequences within the porcine BDNF protein detect BDNF-like immunoreactivity in neurons in rat hippocampal and cortical areas consistent with the distribution of BDNF mRNA as detected with in situ hybridization. Within these neurons, BDNF-like immunoreactivity was observed in the cytoplasm, dendrites, and nuclei. In addition, BDNF immunoreactivity was observed in the cytoplasm of cholinergic neurons that do not express detectable levels of BDNF mRNA. Thus, anti-peptide antibodies can be used to detect this neurotrophic factor protein in cytoplasmic sites of synthesis and in areas of probable action. We propose that one form of the BDNF protein enters the nucleus and may directly influence transcription, while another fraction of the protein is transported out of the synthesizing cell and can be detected, after retrograde axonal transport, in cytoplasmic granules in the perikarya of cholinergic neurons. These basal forebrain cholinergic neurons project to regions enriched in BDNF-synthesizing cells and are known to be responsive to BDNF in vitro. Our data provide information regarding the cellular distribution of BDNF protein in vivo and suggest a dendro-axonic interneuronal transfer of BDNF as well as an additional, intracellular signaling pathway not previously thought to occur in postmitotic neurons in brain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Alderson R. F., Alterman A. L., Barde Y. A., Lindsay R. M. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990 Sep;5(3):297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- Andres R. Y., Jeng I., Bradshaw R. A. Nerve growth factor receptors: identification of distinct classes in plasma membranes and nuclei of embryonic dorsal root neurons. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2785–2789. doi: 10.1073/pnas.74.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidart J. M., Troalen F., Ghillani P., Rouas N., Razafindratsita A., Bohuon C., Bellet D. Peptide immunogen mimicry of a protein-specific structural epitope on human choriogonadotropin. Science. 1990 May 11;248(4956):736–739. doi: 10.1126/science.1692160. [DOI] [PubMed] [Google Scholar]
- Cao Y. H., Pettersson R. F. Human acidic fibroblast growth factor overexpressed in insect cells is not secreted into the medium. Growth Factors. 1990;3(1):1–13. doi: 10.3109/08977199009037497. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Ebendal T., Olson L., Seiger A. The level of nerve growth factor (NGF) as a function of innervation. A correlation radio-immunoassay and bioassay study of the rat iris. Exp Cell Res. 1983 Oct 15;148(2):311–317. doi: 10.1016/0014-4827(83)90155-6. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Ibáez C. F., Ebendal T., Olson L., Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P., Wetmore C., Olson L., Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990 Oct;5(4):511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Martin-Zanca D., Kaplan D. R., Parada L. F., Chao M. V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991 Apr 25;350(6320):678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Hofer M., Pagliusi S. R., Hohn A., Leibrock J., Barde Y. A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990 Aug;9(8):2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn A., Leibrock J., Bailey K., Barde Y. A. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990 Mar 22;344(6264):339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- Hyman C., Hofer M., Barde Y. A., Juhasz M., Yancopoulos G. D., Squinto S. P., Lindsay R. M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991 Mar 21;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Fuxe K., Goldstein M., Joh T. H. Immunohistochemical localization of three catecholamine synthesizing enzymes: aspects on methodology. Histochemie. 1973;33(3):231–254. doi: 10.1007/BF00274236. [DOI] [PubMed] [Google Scholar]
- Imamura T., Engleka K., Zhan X., Tokita Y., Forough R., Roeder D., Jackson A., Maier J. A., Hla T., Maciag T. Recovery of mitogenic activity of a growth factor mutant with a nuclear translocation sequence. Science. 1990 Sep 28;249(4976):1567–1570. doi: 10.1126/science.1699274. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kardami E., Fandrich R. R. Basic fibroblast growth factor in atria and ventricles of the vertebrate heart. J Cell Biol. 1989 Oct;109(4 Pt 1):1865–1875. doi: 10.1083/jcb.109.4.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Jing S. Q., Nanduri V., O'Rourke E., Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991 Apr 5;65(1):189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Klein R., Martin-Zanca D., Barbacid M., Parada L. F. Expression of the tyrosine kinase receptor gene trkB is confined to the murine embryonic and adult nervous system. Development. 1990 Aug;109(4):845–850. doi: 10.1242/dev.109.4.845. [DOI] [PubMed] [Google Scholar]
- Klein R., Parada L. F., Coulier F., Barbacid M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 1989 Dec 1;8(12):3701–3709. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knüsel B., Winslow J. W., Rosenthal A., Burton L. E., Seid D. P., Nikolics K., Hefti F. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):961–965. doi: 10.1073/pnas.88.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer L. F. Nerve growth factor treatment after brain injury prevents neuronal death. Science. 1987 Jan 9;235(4785):214–216. doi: 10.1126/science.3798108. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y. A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989 Sep 14;341(6238):149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor: thirty-five years later. Biosci Rep. 1987 Sep;7(9):681–699. doi: 10.1007/BF01116861. [DOI] [PubMed] [Google Scholar]
- Maher D. W., Lee B. A., Donoghue D. J. The alternatively spliced exon of the platelet-derived growth factor A chain encodes a nuclear targeting signal. Mol Cell Biol. 1989 May;9(5):2251–2253. doi: 10.1128/mcb.9.5.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Friedman B., Alderson R. F., Wiegand S. J., Furth M. E., Lindsay R. M., Yancopoulos G. D. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990 Oct;5(4):501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Squinto S., Ip N. Y., Furth M. E., Lindsay R. M., Yancopoulos G. D. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990 Mar 23;247(4949 Pt 1):1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips H. S., Hains J. M., Laramee G. R., Rosenthal A., Winslow J. W. Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science. 1990 Oct 12;250(4978):290–294. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- Rakowicz-Szulczynska E. M., Rodeck U., Herlyn M., Koprowski H. Chromatin binding of epidermal growth factor, nerve growth factor, and platelet-derived growth factor in cells bearing the appropriate surface receptors. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3728–3732. doi: 10.1073/pnas.83.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renko M., Quarto N., Morimoto T., Rifkin D. B. Nuclear and cytoplasmic localization of different basic fibroblast growth factor species. J Cell Physiol. 1990 Jul;144(1):108–114. doi: 10.1002/jcp.1041440114. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tébar A., Dechant G., Barde Y. A. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990 Apr;4(4):487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Rohrer H., Schäfer T., Korsching S., Thoenen H. Internalization of nerve growth factor by pheochromocytoma PC12 cells: absence of transfer to the nucleus. J Neurosci. 1982 Jun;2(6):687–697. doi: 10.1523/JNEUROSCI.02-06-00687.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A., Goeddel D. V., Nguyen T., Lewis M., Shih A., Laramee G. R., Nikolics K., Winslow J. W. Primary structure and biological activity of a novel human neurotrophic factor. Neuron. 1990 May;4(5):767–773. doi: 10.1016/0896-6273(90)90203-r. [DOI] [PubMed] [Google Scholar]
- Schwab M., Thoenen H. Selective trans-synaptic migration of tetanus toxin after retrograde axonal transport in peripheral sympathetic nerves: a comparison with nerve growth factor. Brain Res. 1977 Feb 25;122(3):459–474. doi: 10.1016/0006-8993(77)90457-7. [DOI] [PubMed] [Google Scholar]
- Selby M. J., Edwards R., Sharp F., Rutter W. J. Mouse nerve growth factor gene: structure and expression. Mol Cell Biol. 1987 Sep;7(9):3057–3064. doi: 10.1128/mcb.7.9.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P., Goodson H. Nuclear protein transport. Crit Rev Biochem Mol Biol. 1989;24(4):419–435. doi: 10.3109/10409238909082557. [DOI] [PubMed] [Google Scholar]
- Soppet D., Escandon E., Maragos J., Middlemas D. S., Reid S. W., Blair J., Burton L. E., Stanton B. R., Kaplan D. R., Hunter T. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991 May 31;65(5):895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- Squinto S. P., Stitt T. N., Aldrich T. H., Davis S., Bianco S. M., Radziejewski C., Glass D. J., Masiakowski P., Furth M. E., Valenzuela D. M. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991 May 31;65(5):885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- Tessler S., Neufeld G. Basic fibroblast growth factor accumulates in the nuclei of various bFGF-producing cell types. J Cell Physiol. 1990 Nov;145(2):310–317. doi: 10.1002/jcp.1041450216. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Wetmore C., Elde R. Detection and characterization of a sensory microganglion associated with the spinal accessory nerve: a scanning laser confocal microscopic study of the neurons and their processes. J Comp Neurol. 1991 Mar 1;305(1):148–163. doi: 10.1002/cne.903050114. [DOI] [PubMed] [Google Scholar]
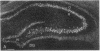
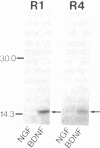
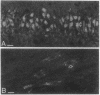
- Wetmore C., Ernfors P., Persson H., Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990 Aug;109(2):141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- Williams L. R., Varon S., Peterson G. M., Wictorin K., Fischer W., Bjorklund A., Gage F. H. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9231–9235. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner B. A., Shooter E. M. Nerve growth factor in the nucleus: interaction with receptors on the nuclear membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1269–1273. doi: 10.1073/pnas.76.3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H. J., Pierce G. F., Deuel T. F. Ultrastructural localization of a platelet-derived growth factor/v-sis-related protein(s) in cytoplasm and nucleus of simian sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2317–2321. doi: 10.1073/pnas.84.8.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]