Abstract
Effects of black raspberry (BRB) extract and protocatechuic acid (PCA) on DNA adduct formation and mutagenesis induced by metabolites of dibenzo[a,l]pyrene (DBP) were investigated in rat oral fibroblasts (OFB). The DBP metabolites, (±)-anti-11,12-dihydroxy -11,12,-dihydrodibenzo[a,l]pyrene (DBP-diol) and 11,12-dihydroxy-13,14-epoxy-11,12,13,14-tetrahydrodibenzo[a,l]pyrene (DBPDE) induced dose-dependent DNA adducts and mutations. DBPDE was considerably more potent, while the parent compound had no significant effect. Treatment with BRB extract (BRBE) and PCA resulted in reduced DBP-derived DNA adduct levels and reduced mutagenesis induced by DBP-diol, but only BRBE was similarly effective against (DBPDE). BRBE did not directly inactivate DBPDE, but rather induced a cellular response – enhanced DNA repair. When BRBE was added to cells one day after the DBP-diol, the BRBE greatly enhanced removal of DBP-derived DNA adducts. As oxidative stress can contribute to several stages of carcinogenesis, BRBE and PCA were investigated for their abilities to reduce oxidative stress in a human leukoplakia cell line by monitoring the redox indicator, 2',7'-dichlorodihydrofluorescein diacetate (H2DCF) in cellular and acellular systems. BRBE effectively inhibited the oxidation, but PCA was only minimally effective against H2DCF. These results taken together provide evidence that BRBE and PCA can inhibit initiation of carcinogenesis by polycyclic aromatic hydrocarbons; and in addition, BRBE reduces oxidative stress.
Keywords: Black raspberries, DNA adducts, mutagenesis, oxidative stress, DNA repair
Introduction
Cancers of the oral cavity and pharynx comprise the sixth most common malignancies worldwide, representing a major international health problem (1). The five-year survival rate for patients with HNSCC is only about 50% (2). In the United States over 45,000 cases and about 11,000 deaths from HNSCC occur annually (3). Worldwide, the annual incidence of new cases exceeds 300,000 (1). The number of annual deaths from oral cancer is similar to that from melanoma (3). Patients with resected oral cancers have an increased incidence of second primary tumors of the oral cavity (4, 5). Epidemiological data provides strong support for exogenous factors such as tobacco, alcohol use and HPV infection as being major causative agents (1, 3). In general avoidance of risk factors has only been partially successful, as many individuals cannot change behavior – largely because smoking and alcohol have addictive effects.
As prevention is preferable to treatment, chemoprevention is a desirable approach. Numerous sources of phytochemicals have been proposed (6) and one that has shown promise in inhibiting head and neck cancers is freeze-dried black raspberry (BRB) (7). Many studies on black raspberries and their components have reported cancer preventive activity (8, 9). Topical treatment of dysplastic lesions in the oral cavity of humans with a 10% BRB gel, four times per day for six weeks, led to histologic regression of about 60% of the lesions; and several animal models have reported effectiveness of BRB against certain cancers (7–9). While studies with whole BRB powder and with different BRB formulations are promising, there are multiple concerns regarding the “standardization” of whole berry powders. In view of this, Stoner’s lab has conducted bio-fractionation studies of black raspberry powder to identify the bioactive constituents and has shown that the chemopreventive activity in multiple assays can be attributed significantly to the ACs, the most abundant class of compounds in BRBs; but they are expensive for routine chemoprevention. In addition, they are difficult to synthesize. Protocatechuic acid (PCA), which is commercially available and relatively inexpensive, accounts for about 70% of the metabolites of BRB ACs in humans (10), and is an inhibitor of chemically-induced cancers in rodents when administered subsequent to the carcinogen (11).
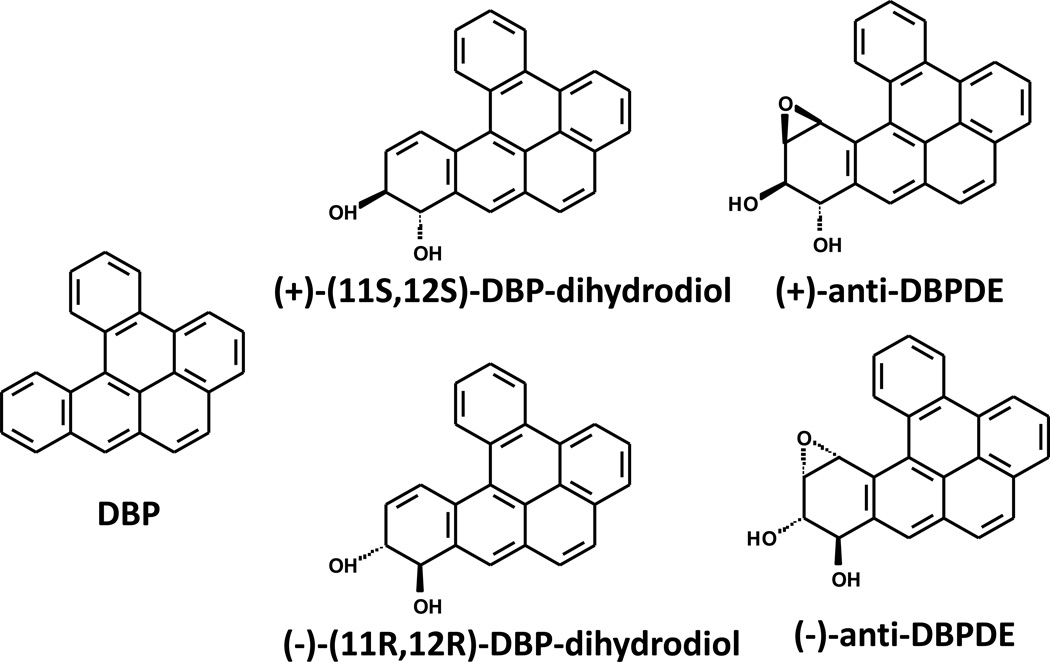
Previously we reported that the tobacco smoke carcinogen, dibenzo[a,l]pyrene (DBP) (also known as Dibenzo[def,p]chrysene) and its ultimate carcinogenic metabolite, (±)-anti-11,12-dihydroxy-13,14-epoxy-11,12,13,14-tetrahydrodibenzo[a,l]pyrene (DBPDE), are mutagenic and carcinogenic in the mouse oral cavity (12, 13) (see Fig. 1). We also reported that DBP and DBPDE form DNA adducts, preferentially with adenine (12, 13). Here we report on the mutagenic effects and DNA adduct formation from the DBP metabolite, (±)-anti-11,12-dihydroxy -11,12,-dihydrodibenzo[a,l]pyrene (DBP-diol), and DBPDE in rat oral fibroblasts (OFB) containing a mutagenesis reporter gene (14). We also determined the effects of a BRBE and protocatechuic acid (PCA), on mutagenesis and DNA adduct levels in OFB treated with DBP-diol and DBPDE. In addition, we report the effects of BRBE and PCA on oxidative stress, as assayed by their abilities to inhibit the oxidation of 2',7'-dichlorodihydrofluorescein diacetate (H2DCF) to its fluorescent product, DCF in a precancerous human oral epithelial cell line.
Fig. 1.
Structures of DBP, DBP-diol and two isomers of anti-DBPDE.
Materials and Methods
Chemicals
DBP-diol and (±)-anti-DB[a,l]PDE were prepared according to a published method by our group (15). BRB powder and BRBE were prepared as described and provided by Dr. Stoner (11). The solvent used to obtain the BRBE from BRB powder was ethanol/H2O (80:20). Using high-performance liquid chromatography, the extract was shown to be composed of approximately 70% anthocyanins (ACs)(16). The remaining compounds have not been identified. H2DCF was purchased from Biotium Inc. PCA was purchased from Thermo Sci. Acros Organics.
Cell Lines
OFB and rat epithelial cell lines were derived from a lacI (BigBlue™) Fisher 344 rat and were kindly provided by David Josephy (University of Guelph, Guelph, Canada). The preparation, characterization and maintenance of these lines has been described (14). Cells were grown in DMEM/F12 medium (Corning cellgro) containing 5% fetal calf serum (Hyclone), Glutamine (0.29 mg/mL), and G418-sulfate and penicillin-streptomycin (all from Mediatech) at 0.22 mg/mL. Cells were passaged weekly.
MSK-Leuk1 cells were established from a premalignant leukoplakic lesion adjacent to a squamous cell carcinoma of the tongue (17, 18). The cells were obtained from Dr. Peter Sacks, who is emeritus faculty member in the same department as JBG. The cells were authenticated by Genetica DNA Laboratories (Burlington, NC) using short tandem repeat DNA profiling on Dec. 8, 2015. Sequencing studies indicated that a GC>AT transition in exon 8 in one allele of p53, resulting in a glu to lys mutation in codon 286, was present in the MSK-Leuk1 cells (18). This cell line was routinely maintained in Keratinocyte Growth Medium (Lonza Bioresearch) grown to 70% confluence, and trypsinized with 0.125% trypsin-2 mmol/L EDTA solution before passage.
DNA adduct assays and mutagenesis studies
The OFB cell line was employed. Cells were grown in 1:1 DMEM/F12 (Corning cellgro) containing 5% fetal calf serum (Hyclone) to about 20% confluence and 50% for DNA adduct analysis. When the cells reached the desired confluencies, the medium was replaced with 10 ml of DMEM/F12 medium without serum and 2 hr later, the cells were treated with BRBE or PCA and four hr later, DBP-diol or DBPDE was added to the desired concentrations. The concentrations of BRBE, PCA, DBP-diol and DBPDE used are given in Figs. 1–6. One hr later the serum concentration was brought to 5%. For mutagenesis, which requires replication, the cells were incubated at 37°C for 72 hr before harvesting. For DNA adduct measurements, cells were treated as above, but harvested only 24 hr after addition of DBP-diol or DBPDE, as replication was not necessary. Longer incubation periods would dilute DNA adducts with new DNA and allow more time for DNA repair.
Effect of time-after-treatment on DNA adduct levels
Three groups of OFB were treated with DBP-diol. One day later DNA adducts were analyzed in one group and BRBE was added to one of the remaining two groups of cells. One day subsequently (48 hr after addition of DBP-diol) DNA adducts were analyzed in both groups of cells.
Analysis of DNA adducts
As reported by us, DBPDE-dA is the major DNA adduct detected in mice treated with DBP (19). The method used for analysis of the DBPDE-dA adducts has been published (19–21). In brief, DNA was isolated from cells using the Qiagen DNA easy kit as described by the manufacturer. Prior to enzymatic digestion, 150 pg of each [15N5]-(−)-anti-trans- and [15N5]-(−)-anti-cis-DBPDE-dA adducts were added to ~100 µg DNA. DNA was hydrolyzed in the presence of 1M MgCl2 (10 µL/mg DNA) and DNase I (0.2 mg/mg DNA) at 37 °C for 1.5 h. Subsequently, nuclease P1 (20 µg/mg DNA) snake venom phosphodiesterase (0.08 unit/mg DNA) and alkaline phosphatase (2 units/mg DNA) were used. An aliquot of the DNA hydrolysate was subjected to dA base analysis by HPLC. The remaining supernatant was partially purified by solid phase extraction using an Oasis HLB column (1 cm3, 30 mg, Waters Ltd.). Then, the analysis was carried out on an API 3200™ LC/MS/MS triple quadrupole mass spectrometer interfaced with an Agilent 1200 series HPLC using an Agilent extend-c18 5 µm 4.6 × 150 mm column. Adducts were monitored in multiple reaction monitoring (MRM) mode. The MS/MS transitions of m/z 604→ m/z 335, and m/z 609→ m/z 335 were monitored for targeted adducts and internal standards, respectively. Experiments using DBP-diol and DBPDE with and without BRBE or PCA were performed on different days and the absolute values of DNA adduct levels without inhibitor varied from experiment to experiment, but were internally consistent. Each condition was assayed from triplicate plates except where there are no error bars; these represent single plates and were used to establish a dose range. Results in the figures represent the major adduct, (−) anti-trans-DBP-dA.
Mutagenesis assay
After treatment of the cells, with mutagen in the presence or absence of BRBE or PCA DNA was extracted using a Recoverase kit (Agilent Technologies) as per manufacturer’s instructions, which involve isolation of nuclei, cell lysis, digestion with protease K, RNAse, and dialysis on a membrane. Phage packaging was carried out using a phage packaging mix prepared from bacterial strains E coli NM759 and BHB2688, generously supplied by Dr. Peter Glazer (Yale, Univ. School of Medicine, New Haven, CT) according to published methods (22).
The cII mutagenesis assay was then employed (23). Briefly, the isolated DNA was treated with the phage packaging extract, which contains all the components necessary for the in vitro assembly of a lambda phage containing in the phage head a vector that includes the bacterial lacI locus and the cII gene, the target for the mutagenesis assay (23–27). In appropriate E. coli (E. coli 1250) host cells, under specified conditions (25°C) only mutants give rise to phage plaques, whereas at 37°C all infected cells give rise to plaques, providing a phage titer (23–27). The mutant fraction (MF) is the ratio of mutant to non-mutant plaques. Plates for each condition were done in triplicate. Experiments using DBP-diol and DBPDE with and without BRBE or PCA were performed on different days and the absolute values for mutagenesis with and without inhibitor vary from experiment to experiment, but are internally consistent.
Oxidative stress
The oxidative stress assays are dependent on the oxidation of H2DCF to DCF - the latter compound being much more fluorescent than the reduced form. In the cellular assays, 5 – 10 thousand MSK-Leuk1 cells were plated in 96 well plates in keratinocyte growth medium, and the next day BRBE or PCA was added at the concentrations mentioned above. Four hr later, H2DCF was added to a concentration of 5 uM. Fluorescence was read in a Molecular Devices SpectraMax-M5 plate reader using 495 nm excitation and 525 nm emission wavelengths at 30 min intervals up to 90 min. For the acellular assay 0.1mM hydrogen peroxide, with and without BRBE or PCA in KGM was incubated for 4 hr at 37°C. H2DCF was then added and fluorescence was read at 60 min. The background fluorescence of cells alone was about 3% of the final reading and was not subtracted. For the acellular assay without hydrogen peroxide, DCF with or without BRBE or PCA was incubated overnight with shaking at 37°C and fluorescence was read 16 hr after the start of the incubation. H2DCF in PBS or KGM without cells was essentially non fluorescent after 90 minutes. BRBE and PCA at the concentrations employed did not affect the fluorescence of DCF, and were not significantly fluorescent at the concentrations employed.
Analysis of DBP-tetrols
DBP tetrols were analyzed by an HPLC method previously described by us (18, 28). Elution was performed using a Shimadzu LC20AD system and a Waters C18 Symmetry column (2.1 × 150mm, 3.5 micron particle size) at a flow rate of 0.17 ml/min in a pH 4.0, 10 mM ammonium phosphate buffer containing 45% acetonitrile. A fluorescence detector (Shimadzu, RF10Axl) was set at 344 nm excitation and 400 emission wavelengths.
Results
Dose response for DNA adducts resulting from treatment with DBP, DBP-diol and DBPDE
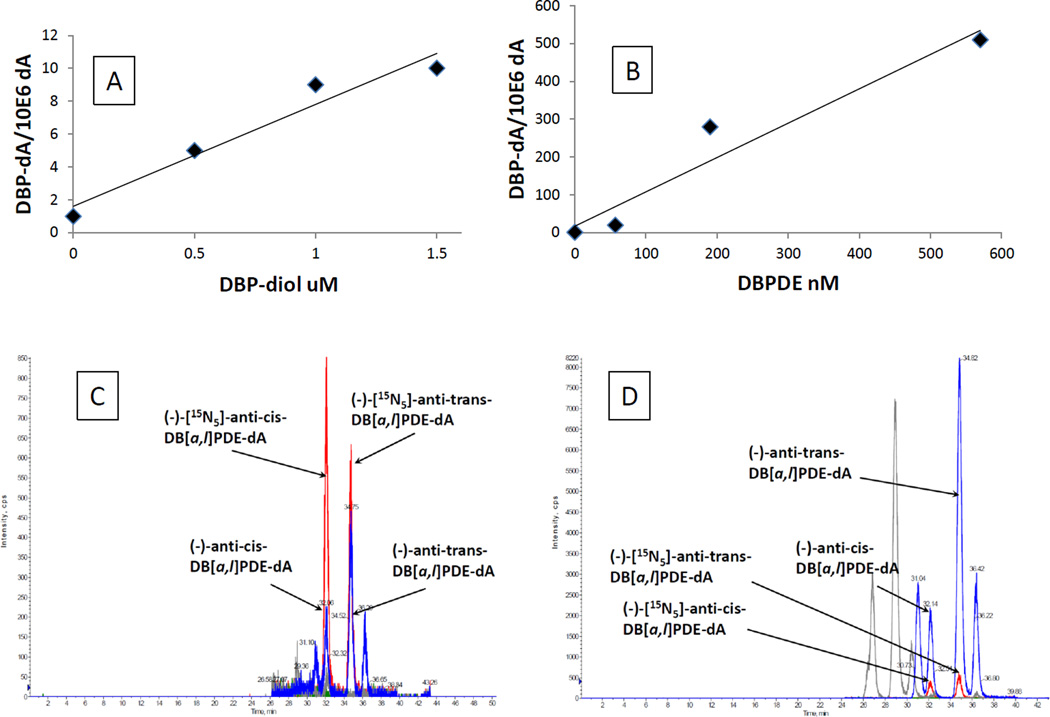
In order to determine an appropriate dose range for experiments using DBP, DBP-diol and DBPDE, DNA adducts produced in OFB and epithelial cells by these compounds were measured. No measurable adducts were produced by DBP. The major DNA adducts produced by both DBP-dihydrodiol and DBPDE were the cis and trans anti-DBPDE-dA (Fig 2A–D). In Fig. 2D, peaks with RT 26.5 and 29 min are DBPDE-dG adducts; peaks with RT 30.5 and 36.4 are (+)-DBPDE-dA adducts. They are all correlated with the doses of DBPDE, but we only quantified (−)-DBPDE-dA adducts because they are more important to DBP-induced carcinogenesis as reported in our previous publications (21, 29). DBPDE was much more effective at producing adducts than DBP-diol. The dose-response results were then used to determine concentrations of DBP-diol and DBPDE for subsequent experiments.
Fig. 2.
Dose response for DNA adducts.
A. Dependence of DNA adduct level on concentration of DBP-diol in OFB.
B. Dependence of DNA adduct level on concentration of DBPDE in OFB.
C. A representative HPLC-MS/MS chromatogram of DBP-diol-induced dA adducts.
D. A representative HPLC-MS/MS chromatogram of DBPDE-induced dA adducts.
These values in Figs. 2 A and B represent DNA adducts from single plates and were used to establish a dose range.
Mutagenesis induced by DBP, DBP-diol, and DBPDE
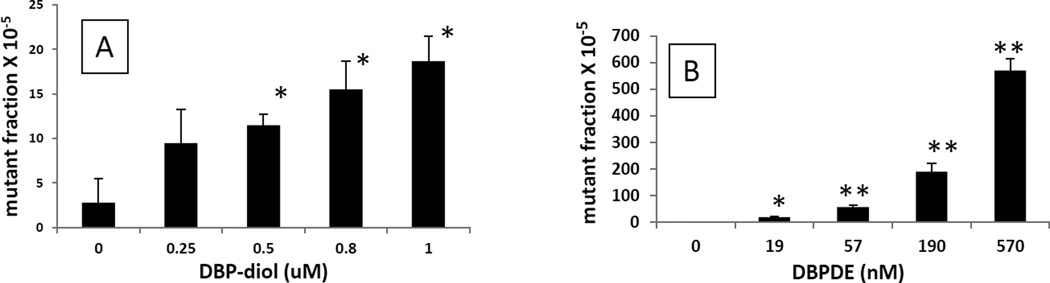
Mutagenesis induced by DBP, DBP-diol and DBPDE was monitored in the lacI rat oral epithelial and fibroblast cells. DBP was at best very weakly mutagenic (results not shown) and was not studied further. Preliminary studies revealed that DBP-diol and DBPDE were similarly mutagenic in both cell lines but background mutation levels were greater in the epithelial cell line, resulting in the fibroblast line being more sensitive to mutagenesis. Therefore, the fibroblast cell line was used for further experiments. Results of the mutagenesis assay are shown in Figure 3A,B. DBPDE was much more potent than DBP-diol. The relative potencies were: DBPDE, 9,600; DBP-diol, 14.7; DBP, 1.
Fig. 3.
A. Dependence of mutagenesis on concentration of DBP-diol in OFB.
B. Dependence of mutagenesis on concentration of DBPDE in OFB.
*, P < 0.05; **, P <0.01 in a one – tailed t-test vs control (no DBP metabolite).
Effects of BRBE and protocatetuic acid on DNA adduct formation by DBP-diol and DBPDE
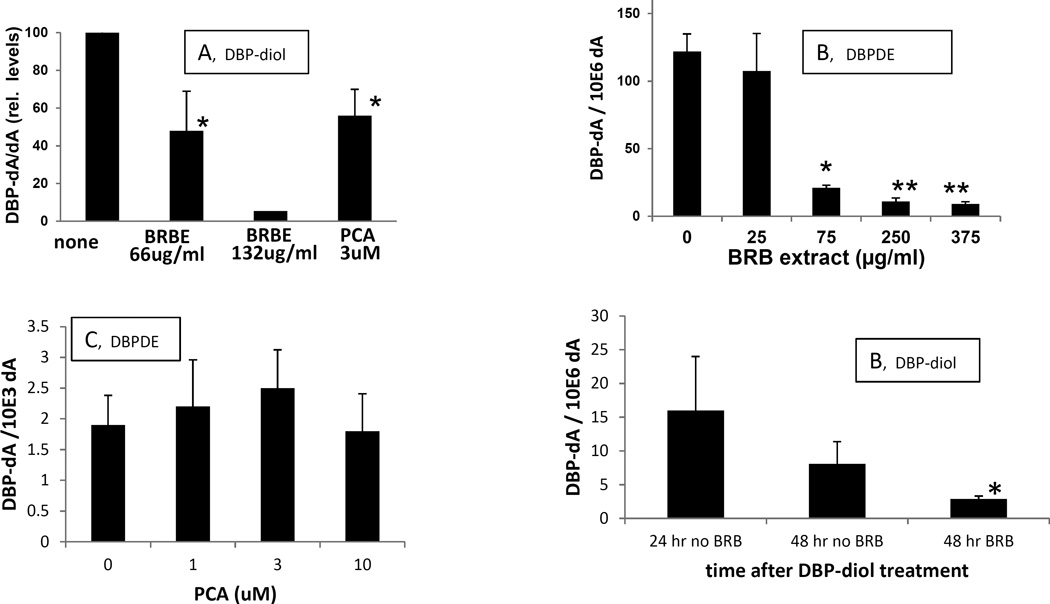
BRBE produced a significant inhibition (about 50%) of DBP-diol induced DNA adduct formation at 66 ug/ml medium and greater inhibition at higher doses (Fig 4A). It also inhibited DNA adduct formation by DBPDE; similar to the effects on adduct formation by DBP-diol, it was very effective at concentrations of 75 ug/ml and above (Fig 4B). PCA also inhibited DNA adduct formation by DBP-diol (Fig 4A), but in contrast to BRBE, it had no effect on adduct formation by DBPDE at the concentrations employed (Fig 4C).
Fig. 4.
A. Effects of BRBE and PCA on DNA adducts produced by DBP-diol in OFB.
DBP-diol, 0.5 uM.
B. Effect of BRBE on DNA adducts produced by DBPDE in OFB.
DBPDE, 70 nM. For the highest dose of DBPDE there was only one sample that yielded sufficient DNA for adduct analysis; the single result is plotted in the figure.
C. Effect of PCA on DNA adducts produced by DBPDE in OFB.
DBPDE, 140 nM. *, P < 0.05; **, P <0.01 in a one – tailed t-test vs control (no DBP metabolite).
a. Experiments using BRBE and PCA in the presence of DBP-diol were performed on different days and are expressed as a percentage of the control (DBP-alone).
D. Effect of BRBE on removal of DBPDE-dA adducts. OFB were treated with 1.5 uM DBP-diol. One day later DNA adducts were analyzed in one group and 75 ug/ml BRBE was added to a second group of cells; while a third group of DBP-diol-treated cells was incubated without further additions. One day later (48 hr after addition of DBP-diol) adducts were analyzed in DBP-diol treated OFB, with and without BRBE. *, P ≤ 0.05 in a Mann-Whitny U-test, and 0.055 in a t-test with unequal variances, vs. 48 hr group with no BRBE.
Effect of BRBE on removal of DBP-diol induced DNA adducts
When BRBE was added to cells one day after the DBP-diol, it greatly enhanced removal of DBP-derived DNA adducts over the next 24 hr (Fig. 4D). One day after treatment with 1.5 uM DBP-diol, levels of the DBP-dA adduct were about 16 × 10−6 adducts/dA. One day later they declined to 8.1 adducts × 10−6 adducts/dA. However, when the OFB were treated with 75 ug/ml BRBE one day after DBP-diol treatment, the adduct level declined to 2.9 adducts × 10−6 adducts/dA, a major enhancement in removal.
Effects of BRBE and PCA on mutagenesis by DBP diol and DBPDE
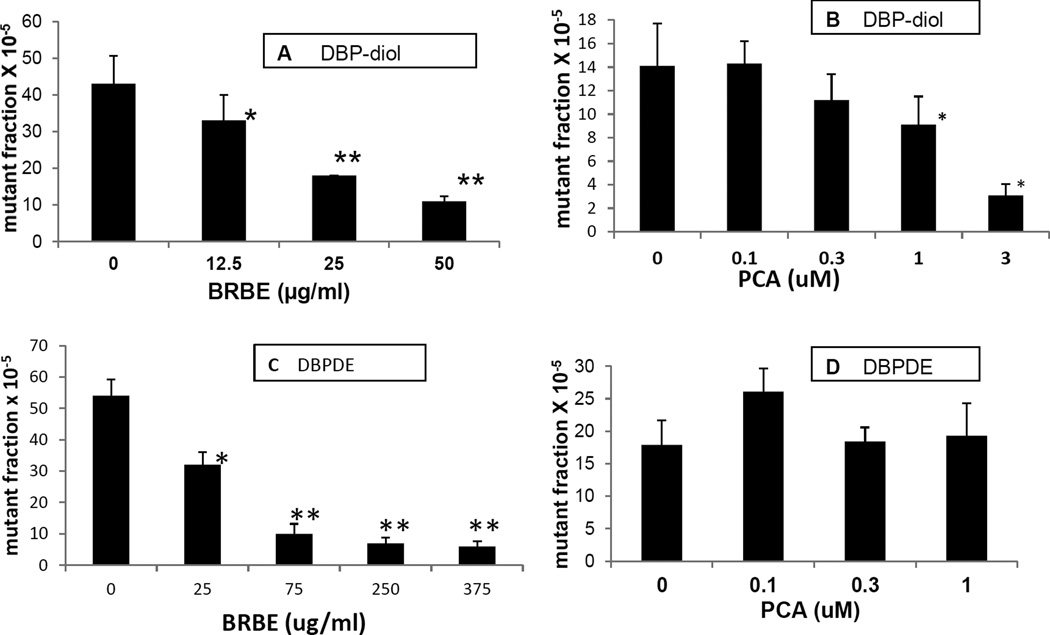
BRBE was an effective inhibitor of mutagenesis induced by DBP-diol at the concentrations employed (Fig 5A). The inhibition was dose-responsive with slightly above 50% inhibition at 25 ug/ml. BRBE was also effective at reducing mutagenesis induced by DBPDE (Fig 5C). PCA was moderately effective at inhibiting mutagenesis induced by DBP-diol, showing a dose-response, although the inhibition only reached significance at one uM and above (Fig 5B). PCA had no noticeable effect on mutagenesis induced by DBPDE at the concentrations employed (Fig 5D).
Fig. 5.
A. Effect of BRBE on mutagenesis induced by DBP-diol in OFBs, DBP diol, 0.5 uM
B. Effect of PCA on mutagenesis induced by DBP-diol in OFBs, DBP diol, 0.5uM.
C. Effect of BRBE on mutagenesis induced by DBP-diol in OFB, DBPDE 70 nM.
D. Effect of PCA on mutagenesis induced by DBPDE in OFB, DBPDE, 70 nM.
*, P < 0.05; **, P <0.01 in a one – tailed t-test vs control (no DBP metabolite).
Mutagenesis experiments depicted in each panel in Figs 3 and 5 were all performed on different days, so the absolute mutant fraction values for a particular mutagen may differ from day to day, but results are internally consistent.
Investigation of possible direct effects of BRBE on DBPDE
In contrast to DBP-diol, which must be further metabolized to exert its mutagenic and carcinogenic effects, fiord-region diol-epoxides like DBPDE are short-lived highly reactive electrophiles that bind to DNA without further metabolism(30). Because BRBE inhibited DNA adduct formation by DBPDE, we considered the possibility that there was a direct reaction between the extract and DBPDE. If some component (s) of BRBE reacted with DBPDE before the DBPDE could permeate the cells or when DBPDE was within the cells, this could explain the mechanism of the observed inhibitory effects. As DBP-diol is metabolized to DBPDE this mechanism could also apply to inhibition of DNA adduct formation and mutagenesis by DBP-diol. To test this possibility, we investigated whether the products from the spontaneous decomposition of the DBPDE were affected by the presence of BRBE. Upon spontaneous hydrolysis of DBPDE in aqueous medium, two products are formed – the cis and trans (−) anti-11,12,13,14,-tetrahydro-11,12,13,14-tetrahydroxy-DBP (DBP-tetrols). DBPDE was incubated in keratinocyte growth medium for 30 minutes, one hr, 2 hr and overnight at 25°C. After incubation, the solutions were applied to an HPLC and the two expected peaks were observed (Fig. 1, Supplemental Data). The concentrations of the products increased from 30 to 60 minutes, but did not change after that – even after an overnight incubation. A second solution containing the same concentration of DBPDE plus 50 ug/ml BRBE and incubated in parallel, resulted in the same two products at the same final concentrations, indicating that BRB components do not react with DBPDE and prevent it from reacting with DNA and other molecules.
Effects of BRBE and PCA on oxidative stress
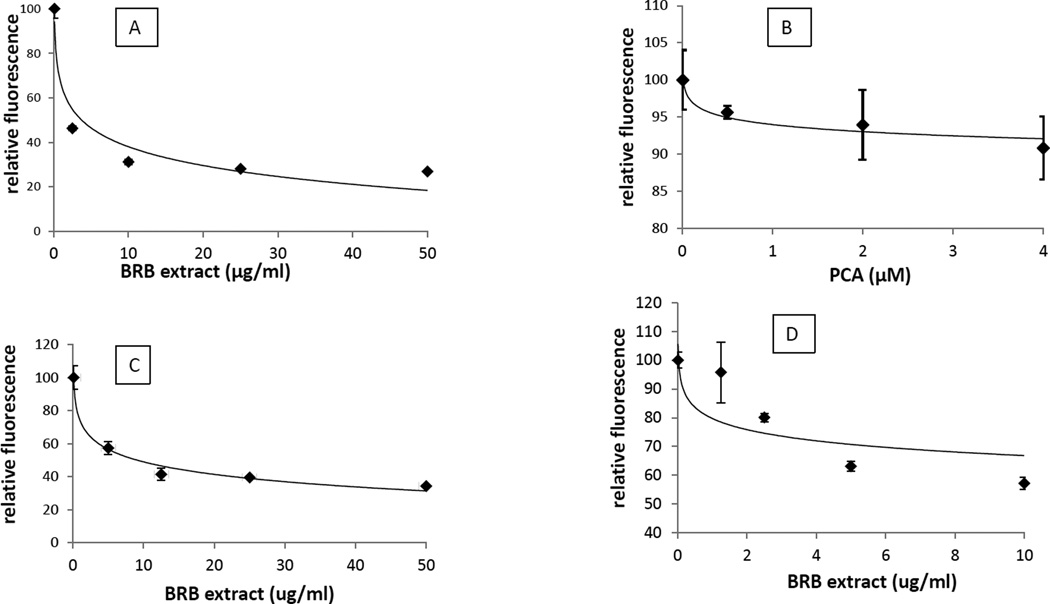
As oxidative stress can play a role in initiation of carcinogenesis, as well as during the promotion and progression stages (31, 32) we investigated whether BRBE and PCA could inhibit oxidative stress. To determine whether BRBE affects intracellular oxidative stress, we monitored the ability of the precancerous human oral epithelial cell line, MSK Leuk1 to oxidize H2DCF to DCF in the presence and absence of BRBE. The oxidized form (DCF) is highly fluorescent, in contrast to the reduced form (H2DCF). BRBE was very effective at reducing the rate of oxidation. Even at a relatively low dose (2.5 ug/ml) it reduced the rate of oxidation by over 50%, and the effect was even more pronounced at higher concentrations (Fig 6A). In contrast, PCA exhibited only a minor effect on oxidative stress, with a statistically significant inhibition reached at the relatively high dose of 4 uM (Fig 6B).
Fig. 6.
A. Effect of BRBE on fluorescence intensity of DCF intensity in human oral epithelial cells.
B. Effect of PCA acid on fluorescence intensity of DCF in human oral epithelial cells
C. Effect BRBE on fluorescence intensity of DCF intensity in the presence of hydrogen peroxide in the absence of cells.
D. Effect BRBE on fluorescence intensity of DCF intensity in the absence of cells when incubated overnight at 37° C.
Results are expressed as relative fluorescence intensity, where the control (no BRBE) is 100%.
The trend lines are logarithmic.
For all points with BRBE, P <0.01 in a one – tailed t-test vs control (no BRBE) except the 2ug/ml point in D, which was not significantly different than control. There was no statistically significant effect of PCA on fluorescence intensity at any of the concentrations tested.
We also tested whether BRB could inhibit the oxidation of H2DCF extracellularly. We pre-incubated H2DCF with 1 mM hydrogen peroxide in the presence and absence of BRBE for three hr in PBS before addition of DCF. BRB-extract reduced the rate of oxidation by about 50% at 5 ug/ml, indicating it need not generate an intracellular response to act as an antioxidant (Fig 6C). This experiment demonstrated that a component of BRB-extract reacted with preformed hydrogen peroxide. We also investigated whether BRB-extract could inhibit the ambient atmospheric oxidation of H2DCF. H2DCF is oxidized to DCF in aqueous solution, but less rapidly than in the presence of hydrogen peroxide or when taken up by the MSK cells. We incubated H2DCF with and without BRBE, in the absence of any added oxidant for 16 hr at 37°C and then measured fluorescence. Similar to the results with hydrogen peroxide, BRBE inhibited the oxidation of H2DCF by about 50% at a concentration of 5ug/ml (Fig 6D).
Discussion
BRB powder and the ethanol/H2O-soluble (80:20) extract have been effective at inhibiting carcinogenesis in a number of in vitro and in vivo experimental systems (7, 9, 11). Exposure to BRB results in a reduced rate of growth of precancerous cells, reduced inflammation, oxidative stress and angiogenesis, increased apoptosis and a reduced tumor multiplicity in initiated animals. In those studies, BRB is functioning to inhibit the promotion and progression stages of carcinogenesis. Here we investigated the effects of an ethanol/H2O soluble (80:20) extract of BRB and a major in vivo metabolite, PCA, on processes important for initiation of carcinogenesis – DNA damage, mutagenesis, and potentially, oxidative stress. In experimental animals, DBP is metabolized to (among other metabolites) DBP-epoxide, then to DPB-diol and finally to DBPDE (20). The diol is thought to represent the proximate mutagenic and carcinogenic metabolite of DBP, and DBPDE is the ultimate mutagen and carcinogen (20). We have previously reported that in mice, DBP and DBPDE form DNA adducts in oral tissue - predominantly with adenine (21) and a much higher percentage of the mutations were found at A:T base pairs than in untreated mice (12, 13). Significant fractions of mutations at G:C pairs were also observed.
Consistent with the above metabolic pathway for DBP, DBPDE was much more potent than DBP-diol, and DBP-diol was more potent than DBP in the induction of DNA adducts and mutations. Because the OFB were unable to metabolize sufficient DBP to produce detectable levels of DNA adducts or lead to mutagenesis, further experiments on inhibition of DNA adduct formation and mutagenesis were carried out using DBP-diol and DBPDE. We then investigated inhibition of these processes by the BRBE and PCA. BRB contains about 4–5 % by dry weight of ACs and they are thought to be responsible for many of the reported effects of BRB on carcinogenesis and inflammation (11, 16). PCA is a major metabolite of ACs, and is produced by microbial metabolism of ACs in the gut (11, 33). There is evidence that some inhibitory effects of BRB and ACs on carcinogenesis can be attributed to PCA (11).
BRBs may act on different stages of carcinogenesis, and in a number of experimental models of carcinogenesis, BRB and ACs were shown to inhibit growth of implanted tumor cells, or retard growth of tumors in animals predisposed for cancer (34). In some cases they were administered in the diet after treatment with a carcinogen (11). In such models their effects were on promotion and progression of carcinogenesis. There have been studies where they were administered before, during and after treatment with a carcinogen, but in those cases it was not possible to determine at which stage of carcinogenesis inhibition was occurring (34). Here we investigated effects of BRB on processes involved in tumor initiation, using an in vitro system, in which formation of DNA adducts by a proximate and ultimate carcinogen were measured, and mutagenesis (a consequence of DNA damage) was also monitored. In addition, effects of BRBE on oxidative stress were examined, as oxidative stress contributes to several stages of carcinogenesis. Pretreatment of cells with BRBE followed by treatment with DBP-diol led to a reduction in DBPDE-induced DNA adducts and mutagenesis, compared with cells treated with DBP-diol alone. Presumably, the reduction in adducts was responsible for the reduction in mutagenesis. Potential mechanisms of inhibition of initiating effects of DBP-diol are, 1) direct inhibition of its metabolism to DBPDE or altering its metabolism so that less DBPDE is produced, and/or 2) trapping or detoxifying DBPDE before it reacts with DNA. BRBE also inhibited mutagenesis and DNA adduct formation by DBPDE. In contrast to DBP-diol, DBPDE is the ultimate mutagenic metabolite of DBP diol, and inhibition of the reaction of DBPDE with DNA cannot be attributed to inhibition of its metabolism to a more potent mutagen. The inhibitory effects of BRBE on adduct formation by DBPDE likely result from trapping or enzymatic detoxification of DBPDE.
To investigate the possibility that BRBE can trap DBPDE and prevent it from reacting with nucleophiles (including DNA) we incubated DBPDE in culture medium in the presence and absence of 50 ug/ml BRBE. DBPDE spontaneously decomposed to form two DBP-tetrols. We allowed the incubation proceed to completion and then measured the concentrations of the two DBP-tetrols. The concentration of tetrols produced was unchanged by the presence of BRBE in the incubation solution. If a component of the BRBE reacted with DBPDE before it could hydrolyze, then the concentration of the tetrols would have been reduced. This was not the case, and we conclude that the inhibitory effects of BRB on DNA adduct formation and mutagenesis likely results from a cellular response to DBPDE. One possibility is that ACs induce the expression of NAD(P)H dehydrogenase [quinone] (NQO1) which is a sentinel for induction of phase 2 enzymes (35). Several of these enzymes such as the glutathione transferases, may conjugate DBPDE and thus reduce its concentration. It has already been reported that several glutathione transferases are capable of catalyzing the conjugation of DBPDE with glutathione (36, 37). We also show here that BRBE enhances the removal of DBPDE-DNA adducts. The mechanism for inhibition by BRB of DBP-diol-induced adduct formation and mutagenesis may be the same as that for its inhibition of DBPDE, as DBP-diol is metabolized to DBPDE. However, the conversion of DBP-diol to DBPDE may also be subject to enzyme inhibition.
We also investigated the effects of PCA, a major metabolite of ACs on DNA damage and mutagenesis by DBP-diol and DBPDE. Pretreatment with PCA also led to inhibition of DNA adduct formation and mutagenesis by DBP-diol, but not by DBPDE. Hence the reason for its inhibitory effects likely involves its inhibition of the formation of DBPDE, either by enzyme inhibition or induction of phase 2 enzymes. PCA is readily available and would represent a convenient substitute for BRBE; the latter must be produced by more involved processing.
An important consideration is whether the levels of inhibitors employed here are relevant to in vivo levels. Plasma levels of PCA in volunteers consuming berry powder were 1–2 orders of magnitude below those used here (38). However in mice fed PCA in food, higher plasma levels (38) were achieved. In principle then, blood levels of PCA similar to those used here in cell culture could be achievable by use of supplemental dietary PCA.
For ACs it has been reported that intraoral delivery of a 10% (w/w) bioadhesive freeze-dried BRB gel to human subjects led to a mean oral tissue concentration of about 0.5 ug AC /mg protein five minutes after administration of the gel (39) although there were wide inter-individual variations. As typical tissue concentrations of proteins are about 15%, an average tissue concentration would be 75 ug/ml (150 mg/g tissue × 0.5 ug AC/mg = 75ug AC/g tissue). This is only for a single time point, but suggests the concentrations employed here are consistent with levels delivered to humans. Also, levels in saliva are in the same range as employed here, but again with very wide inter-individual variations (39). The results reported here, taken together with the previous report, suggest that topical application of an BRBE or AC enriched gel could inhibit DNA damage and mutagenesis induced by PAH’s in the human oral cavity.
BRBE was an effective inhibitor of cellular oxidative stress as measured by its ability to reduce the rate of oxidation of H2DCF to DCF. Significant inhibition was observed at concentrations well below those necessary to inhibit DNA adduct formation. It has been reported that BRB can induce the antioxidant response element in liver cells, resulting in increased levels of antioxidant enzymes and glutathione (40). Also, grape seed ACs induce phase II enzymes in MCF10A breast cells, and inhibit H2DCF oxidation (41). These reports suggest that cellular responses to AC treatment can lead to the antioxidant effect of BRB. However, we observed that BRB inhibited the oxidation of DCF by hydrogen peroxide or molecular oxygen in the absence of cells. Our results are consistent with either inhibition resulting from a cellular response to BRBE or direct reduction of intracellular oxidants by BRBE. The results thus far do not allow us to choose between the two possibilities, or a combination of the two.
In conclusion, BRBE at concentrations leading to AC levels achievable by topical application of BRB, led to inhibition of DNA adduct formation resulting from the DBP metabolites, DBP diol and DBPDE in a rat oral cell model. Similar inhibition of mutagenesis induced by DBP-diol and DBPDE was also observed. PCA was an inhibitor of DNA adduct formation and mutagenesis induced by DBP-diol, but not DBPDE. BRBE but not PCA reduced oxidative stress. BRB is potentially an inhibitor of carcinogenesis initiation by tobacco smoke PAH’s, and its antioxidant ability may play a role in inhibiting several steps in multi-stage carcinogenesis.
Supplementary Material
Acknowledgments
Supported by NIH grant # R01-CA173465.
JBG thanks Ms. Vera Golgotiu for assistance in the performing the oxidative stress assays and Ms. Tia Han for cell maintenance.
Abbreviations
- AC
anthocyanin
- BRB
black raspberries
- BRBE
BRB extract
- DBP
dibenzo[,a,l]pyrene
- DBP-diol
(±)-anti-11,12-dihydroxy -11,12,-dihydrodibenzo[a,l]pyrene
- DBPDE
(±)-anti-11,12-dihydroxy-13,14-epoxy-11,12,13,14-tetrahydrodibenzo[a,l]pyrene
- DBP tetrols
cis and trans (−) -anti-11,12,13,14,-tetrahydro-11,12,13,14-tetrahydroxy-DBP
- H2DCF
2',7'-dichlorodihydrofluorescein diacetate
- HNSCC
head and neck squamous cell carcinoma
- PAH
polycyclic aromatic hydrocarbon
- PCA
protocatechuic acid
- OFB
rat oral fibroblasts
Footnotes
The author and coauthors have no conflicts of interest to report.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a Cancer Journal for Clinicians. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Fuller CD, Wang SJ, Thomas CR, Jr, Hoffman HT, Weber RS, Rosenthal DI. Conditional survival in head and neck squamous cell carcinoma: results from the SEER dataset 1973–1998. Cancer. 2007;109:1331–1343. doi: 10.1002/cncr.22563. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a Cancer Journal for Clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.Franco EL, Kowalski LP, Kanda JL. Risk factors for second cancers of the upper respiratory and digestive systems: a case-control study. J Clin Epidemiol. 1991;44:615–625. doi: 10.1016/0895-4356(91)90021-z. [DOI] [PubMed] [Google Scholar]
- 5.Licciardello JT, Spitz MR, Hong WK. Multiple primary cancer in patients with cancer of the head and neck: second cancer of the head and neck, esophagus, and lung. Int J Radiat Oncol Biol Phys. 1989;17:467–476. doi: 10.1016/0360-3016(89)90096-5. [DOI] [PubMed] [Google Scholar]
- 6.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nature Reviews Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 7.Mallery SR, Tong M, Shumway BS, Curran AE, Larsen PE, Ness GM, et al. Topical application of a mucoadhesive freeze-dried black raspberry gel induces clinical and histologic regression and reduces loss of heterozygosity events in premalignant oral intraepithelial lesions: results from a multicentered, placebo-controlled clinical trial. Clinical Cancer Research. 2014;20:1910–1924. doi: 10.1158/1078-0432.CCR-13-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao AV, Snyder DM. Raspberries and human health: a review. J Agric Food Chem. 2010;58:3871–3883. doi: 10.1021/jf903484g. [DOI] [PubMed] [Google Scholar]
- 9.Stoner GD, Wang LS, Zikri N, Chen T, Hecht SS, Huang C, et al. Cancer prevention with freeze-dried berries and berry components. Seminars in Cancer Biology. 2007;17:403–410. doi: 10.1016/j.semcancer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitaglione P, Donnarumma G, Napolitano A, Galvano F, Gallo A, Scalfi L, et al. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr. 2007;137:2043–2048. doi: 10.1093/jn/137.9.2043. [DOI] [PubMed] [Google Scholar]
- 11.Peiffer DS, Zimmerman NP, Wang LS, Ransom BW, Carmella SG, Kuo CT, et al. Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prevention Research. 2015;7:574–584. doi: 10.1158/1940-6207.CAPR-14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guttenplan JB, Kosinska W, Zhao ZL, Chen KM, Aliaga C, DelTondo J, et al. Mutagenesis and carcinogenesis induced by dibenzo[a,l]pyrene in the mouse oral cavity: a potential new model for oral cancer. Int J Cancer. 2012;130:2783–2790. doi: 10.1002/ijc.26344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen KM, Guttenplan JB, Zhang SM, Aliaga C, Cooper TK, Sun YW, et al. Mechanisms of oral carcinogenesis induced by dibenzo[a,l]pyrene: an environmental pollutant and a tobacco smoke constituent. Int J Cancer. 2013;133:1300–1309. doi: 10.1002/ijc.28152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDiarmid HM, Douglas GR, Coomber BL, Josephy PD. Epithelial and fibroblast cell lines cultured from the transgenic BigBlue rat: an in vitro mutagenesis assay. Mutat Res. 2001;497:39–47. doi: 10.1016/s1383-5718(01)00245-5. [DOI] [PubMed] [Google Scholar]
- 15.Sharma AK, Kumar S, Amin S. A Highly Abbreviated Synthesis of Dibenzo[def,p]chrysene and Its 12-Methoxy Derivative, a Key Precursor for the Synthesis of the Proximate and Ultimate Carcinogens of Dibenzo[def,p]chrysene. Journal of Organic Chemistry. 2004;69:3979–3982. doi: 10.1021/jo0303822. [DOI] [PubMed] [Google Scholar]
- 16.Wang LS, Hecht SS, Carmella SG, Yu N, Larue B, Henry C, et al. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prevention Research. 2009;2:84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacks PG. Cell, tissue and organ culture as in vitro models to study the biology of squamous cell carcinomas of the head and neck. Cancer and Metastasis Reviews. 1996;15:27–51. doi: 10.1007/BF00049486. [DOI] [PubMed] [Google Scholar]
- 18.Kochhar A, Kopelovich L, Sue E, Guttenplan JB, Herbert BS, Dannenberg AJ, et al. p53 modulates Hsp90 ATPase activity and regulates aryl hydrocarbon receptor signaling. Cancer Prevention Research. 2014;7:596–606. doi: 10.1158/1940-6207.CAPR-14-0051. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Chen KM, Zhang SM, Aliaga C, Sun YW, Cooper T, Gowdahalli K, et al. Induction of ovarian cancer and DNA adducts by Dibenzo[a,l]pyrene in the mouse. Chemical Research in Toxicology. 2012;25:374–380. doi: 10.1021/tx2004322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang SM, Chen KM, Aliaga C, Sun YW, Lin JM, Sharma AK, et al. Identification and quantification of DNA adducts in the oral tissues of mice treated with the environmental carcinogen dibenzo[a,l]pyrene by HPLC-MS/MS. Chemical Research in Toxicology. 2011;24:1297–1303. doi: 10.1021/tx200188j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SM, Chen KM, Sun YW, Aliaga C, Lin JM, Sharma AK, et al. Simultaneous detection of deoxyadenosine and deoxyguanosine adducts in the tongue and other oral tissues of mice treated with Dibenzo[a,l]pyrene. Chemical Research in Toxicology. 2014;27:1199–1206. doi: 10.1021/tx5001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hohn B. In vitro Packaging of Lambda and Cosmid DNA. Methods in Enzymology. 1979;68:299–324. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- 23.Jakubczak JL, Merlino G, French JE, Muller WJ, Paul B, Adhya S, et al. Analysis of Genetic Instability During Mammary Tumor Progression Using a Novel Selection-Based Assay for In Vivo Mutations in a Bacteriophage Transgene Target. Proc Natl Acad Sci, USA. 1996;93:9073–9078. doi: 10.1073/pnas.93.17.9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gollapudi BB, Jackson KM, Stott WT. Hepatic lacI and cII mutation in transgenic (lambdaLIZ) rats treated with dimethylnitrosamine. Mutat Res. 1998;419:131–135. doi: 10.1016/s1383-5718(98)00132-6. [DOI] [PubMed] [Google Scholar]
- 25.Zimmer DM, Harbach PR, Mattes WB, Aaron CS. Comparison of mutant frequencies at the transgenic lambda LacI and cII/cI loci in control and ENU-treated Big Blue mice. Environ Mol Mutagen. 1999;33:249–256. [PubMed] [Google Scholar]
- 26.Watson DE, Cunningham ML, Tindall KR. Spontaneous and ENU-induced mutation spectra at the cII locus in Big Blue Rat2 embryonic fibroblasts. Mutagenesis. 1998;13:487–497. doi: 10.1093/mutage/13.5.487. [DOI] [PubMed] [Google Scholar]
- 27.Swiger RR. Just how does the cII selection system work in Muta Mouse? Environmental & Molecular Mutagenesis. 2001;37:290–296. doi: 10.1002/em.1035. [DOI] [PubMed] [Google Scholar]
- 28.Mohebati A, Guttenplan JB, Kochhar A, Zhao ZL, Kosinska W, Subbaramaiah K, et al. Carnosol, a constituent of Zyflamend, inhibits aryl hydrocarbon receptor-mediated activation of CYP1A1 and CYP1B1 transcription and mutagenesis. Cancer Prevention Research. 2012;5:593–602. doi: 10.1158/1940-6207.CAPR-12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Sun YW, El-Bayoumy K, Aliaga C, Awad AS, Gowda K, Amin S, et al. Tissue Distribution, Excretion and Pharmacokinetics of the Environmental Pollutant Dibenzo[def,p]chrysene in Mice. Chemical Research in Toxicology. 2015;28:1427–1433. doi: 10.1021/acs.chemrestox.5b00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips DH, Hewer A, Seidel A, Steinbrecher T, Schrode R, Oesch F, et al. Relationship between mutagenicity and DNA adduct formation in mammalian cells for fjord- and bay-region diol-epoxides of polycyclic aromatic hydrocarbons. Chemico-Biological Interactions. 1991;80:177–186. doi: 10.1016/0009-2797(91)90023-z. [DOI] [PubMed] [Google Scholar]
- 31.Dizdaroglu M. Oxidatively induced DNA damage and its repair in cancer. Mutation Research-Reviews in Mutation Research. 2015;763:212–245. doi: 10.1016/j.mrrev.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Cooks T, Harris CC, Oren M. Caught in the cross fire: p53 in inflammation. Carcinogenesis. 2014;35:1680–1690. doi: 10.1093/carcin/bgu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keppler K, Humpf HU. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorganic & Medicinal Chemistry. 2005;13:5195–5205. doi: 10.1016/j.bmc.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwak MK, Ramos-Gomez M, Wakabayashi N, Kensler TW. Chemoprevention by 1,2-dithiole-3-thiones through induction of NQO1 and other phase 2 enzymes. Methods in Enzymology. 2004;382:414–423. doi: 10.1016/S0076-6879(04)82022-6. [DOI] [PubMed] [Google Scholar]
- 36.Kabler SL, Seidel A, Jacob J, Doehmer J, Morrow CS, Townsend AJ. Differential protection by human glutathione S-transferase P1 against cytotoxicity of benzo[a]pyrene, dibenzo[a,l]pyrene, or their dihydrodiol metabolites, in bi-transgenic cell lines that co-express rat versus human cytochrome P4501A1. Chemico-Biological Interactions. 2009;179:240–246. doi: 10.1016/j.cbi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kushman ME, Kabler SL, Ahmad S, Doehmer J, Morrow CS, Townsend AJ. Cytotoxicity and mutagenicity of dibenzo[a,l]pyrene and (+/−)-dibenzo[a,l]pyrene-11,12-dihydrodiol in V79MZ cells co-expressing either hCYP1A1 or hCYP1B1 together with human glutathione-S-transferase A1. Mutation Research. 2007;624:80–87. doi: 10.1016/j.mrfmmm.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen W, Wang D, Wang LS, Bei D, Wang J, See WA, et al. Pharmacokinetics of protocatechuic acid in mouse and its quantification in human plasma using LC-tandem mass spectrometry. Journal of Chromatography B: Analytical Technologies in the Biomedical & Life Sciences. 2012;908:39–44. doi: 10.1016/j.jchromb.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ugalde CM, Liu Z, Ren C, Chan KK, Rodrigo KA, Ling Y, et al. Distribution of anthocyanins delivered from a bioadhesive black raspberry gel following topical intraoral application in normal healthy volunteers. Pharmaceutical Research. 2009;26:977–986. doi: 10.1007/s11095-008-9806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shih PH, Yeh CT, Yen GC. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J Agric Food Chem. 2007;55:9427–9435. doi: 10.1021/jf071933i. [DOI] [PubMed] [Google Scholar]
- 41.Singletary KW, Jung KJ, Giusti M. Anthocyanin-rich grape extract blocks breast cell DNA damage. Journal of Medicinal Food. 2007;10:244–251. doi: 10.1089/jmf.2006.258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.