ABSTRACT
Intravesical Bacillus-Calmette-Guérin (BCG) immunotherapy can reduce recurrence/progression of non-muscle-invasive bladder cancer (NMIBC), although significant adverse events and treatment failure argue for alternative options. Here, we examined whether another attenuated live vaccine, Vivotif/Ty21a, used since more than 30 y against typhoid fever, may be safely used intravesically to improve bladder-tumor treatment. Mice-bearing MB49 orthotopic bladder-tumors treated with intravesical Ty21a or BCG were compared for survival and bacteria recovery. Both Ty21a and BCG enhanced mice survival when treating just after tumor implantation for 4 weeks (p = 0.008 and 0.04, respectively), but only Ty21a was effective when treating once mice with larger already established bladder-tumors (p = 0.0003). In contrast to BCG, no Ty21a bacteria survived in mouse bladder, human urothelial cell-lines or human peripheral blood mononuclear cells. However, Ty21a was as potent as BCG to induce tumor-cell death in vitro. In a human, 3D-bladder-tissue ex-vivo assay, Ty21a bacteria, still not surviving, induced a panel of cytokines associated with effective BCG-treatment in patient's urine. Overall, our pre-clinical data demonstrate that intravesical Ty21a is more effective than BCG for bladder-tumor treatment. Absence of surviving Ty21a bacteria and the excellent safety-record of the typhoid vaccine support its testing in NMIBC patients.
KEYWORDS: BCG, immunotherapy, non-muscle invasive bladder cancer, Ty21a vaccine
Introduction
Bladder cancer is the fourth and eighth most common malignancy among men and women, respectively.1,2 Seventy percent of tumors present as non-muscle-invasive bladder cancer (NMIBC) at initial diagnosis with variable risk of recurrence and progression to invasive disease after transurethral tumor resection (TUR), thus requiring long-term surveillance.3 Immunotherapy with intravesical Bacillus-Calmette-Guerin (BCG) after TUR is the standard treatment since 30 y to limit recurrence/progression of high risk of recurrence NMIBC disease, including especially carcinoma in situ (CIS), but also high-grade papillary (Ta) or lamina-propria-invasive lesions (T1).4 However, BCG immunotherapy is associated with significant adverse events, which may lead to treatment discontinuation in up to 20% of the patients,4 as well as treatment failure, with 20–30% of patients experiencing early recurrence despite BCG treatment.4,5 Although the precise mechanisms of action of BCG are not fully understood, they involve induction of both innate and adaptive immune responses, one of the primary events being infection of the urothelium by BCG bacteria.6 Persistent BCG infection is also the cause of the most severe, but rare, adverse effects that may be associated with BCG immunotherapy.4 Thus, use of safer attenuated bacteria, while retaining the ability to induce a similar cascade of inflammatory cytokines and efficient anti-tumor immune responses, is highly desirable. Like BCG, the attenuated Salmonella enterica typhi Ty21a live vaccine-strain against typhoid fever induces Th1-type immune responses in human after oral ingestion.7 However, Ty21a, due to several attenuating mutations, has a poor capacity to survive in cells, with only a very low shedding detected in the first 24 h. This results in a vaccine strain with an excellent safety profile confirmed worldwide in more than 200 millions vaccines over the last 30 y.7
Here, we examined the immunotherapeutic intravesical potential of Ty21a in an immunocompetent orthotopic MB49-bladder cancer model, which closely reproduces NMIBC in mice.8 Our data show that Ty21a was more effective than BCG to induce regression of established bladder tumors in absence of bacterial survival. This was confirmed by the ability of Ty21a to kill tumor cells in vitro and induce, in a novel human 3D-bladder-tissue ex-vivo assay, an array of cytokines/chemokines/growth factors associated to efficient bladder-treatment. Altogether, our data provide an encouraging premise for the use of the typhoid vaccine for intravesical immunotherapy of NMIBC patients.
Result
Intravesical Ty21a induces bladder-tumor regression
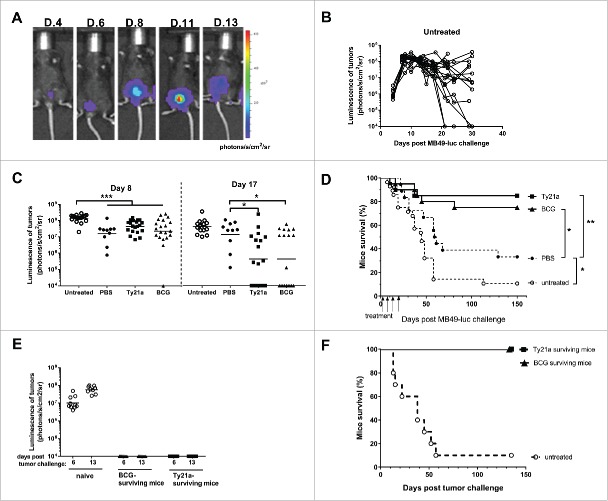
In order to test the capacity of intravesical Ty21a to induce antitumor effects, we used a mouse MB49 orthotopic bladder cancer model which is commonly utilized to investigate the mechanisms of BCG therapy.9,10 To better monitor bladder-tumor establishment and growth, luciferase-expressing MB49-cells were used. This allowed in vivo bioluminescent imaging of bladder-tumor growth (Fig. 1A) during the first 3 weeks, after which reliability to tumor-size was lost (Fig. 1B) due to presence of necrotic tumors and/or low tumor-perfusion, preventing tumor-penetration of the injected luciferin.13 A classical schedule for intravesical treatment in this model start 1 day after tumor implantation and is given four-times once a week.12 The first intravesical instillation results in an initially delayed tumor-growth (see tumor bioluminescence at day 8 in Fig. 1C), which is however independent from the treatment used (PBS, Ty21a, or BCG) and leads to a slightly enhanced mice survival (30% after PBS in Fig. 1D) as compared to untreated mice, likely due to a washing effect of the instillation. More interestingly, intravesical treatments with 3 × 107 CFU of Ty21a or BCG substantially induced tumor-regression after 2 weeks as assessed by bioluminescence at day 17 (i.e., 1 d after the 3rd instillation, Fig. 1C) and resulted in significant long-term mice survival after a full four-dose treatment (85 and 75%, respectively, as compared to 30% in PBS-instilled-mice, p = 0.008 and p = 0.04, Fig. 1D). To examine long-term protection, the mice that were cured by BCG or Ty21a treatment and that had survived for 150 d were intravesically re-challenged with new MB49-luc tumor cells. However, no MB49 tumor-take was visible, in contrast to naive mice challenged in parallel (Fig. 1E) and all the previously cured mice survived for 140 additional days (Fig. 1F). These data show that Ty21a-treatment, similarly to BCG, induced an antitumor immune response evidenced after 2 weeks by the regression of the growing tumors, together with an adaptive immunity responsible for long-term tumor-protection.
Figure 1.
Orthotopic MB49 bladder-cancer model and intravesical treatments. (A) Bioluminescence Xenogen imaging of one representative bladder-tumor bearing mice is shown at the indicated days after tumor instillation. (B) Bioluminescence quantification of bladder-tumors upon time in untreated mice (n = 19). Note that tumors can lose bioluminescence after day 20. (C) Bioluminescence of bladder-tumors in mice that had received intravesical instillations (four times, once a week starting 1 d after tumor administration, i.e., days 2, 9, 16, and 23) with PBS (n = 10), 3 × 107 CFU of Ty21a (n = 20) or 3 × 107 CFU of BCG (n = 20), as compared to untreated mice (n = 19). Bioluminescence is shown at day 8 (6 d after the first intravesical instillation) and at day 17 (1 d after the 3rd intravesical instillation). Note at day 8 the significant tumor-growth delay in mice that received instillation as compared to untreated mice; and at day 17 the significant tumor-regression in mice treated with Ty21a and BCG as compared to PBS-instilled mice. *p < 0.05, ***p < 0.001 following one-way Anova and Tukey's post-test. (D) Survival upon time of groups of mice that received intravesical instillations of PBS (plain circles, n = 18), BCG (triangles, n = 20), Ty21a (squares, n = 20) and untreated mice (empty circle, n = 28) and were compared. *p < 0.05 following an adjusted log-rank test. (E) Bladder-tumor bearing mice that had survived thanks to the intravesical treatment with BCG (triangle, n = 9) or Ty21a (square, n = 8) were re-challenged 150 d after the initial tumor-implantation with new intravesical MB49-luc cells. A control group of naive mice (empty circle, n = 10) was challenged in parallel. Bioluminescence of the growing tumors is shown at days 6 and 13 post tumor-challenge. No bioluminescence was detected in BCG and Ty21a surviving mice, indicating the absence of tumor-take in those mice, in contrast to the naive control mice. (F) Mice survival curves showing that all previously BCG (triangle, n = 9) or Ty21a (square, n = 8) treated mice survived at long-term as compared to naive-tumor-challenged mice (empty circle, n = 10). Two independent experiments for B, C, and D and a single experiment for E and F were performed.
In a more stringent setting, a single intravesical instillation of BCG or of Ty21a was given at day 5, when bladder-tumors were already well established (Fig. 2A). In this setting, only Ty21a-treatment was able to induce significant bladder-tumor regression 2 weeks later (see bioluminescence at day 21 in Fig. 2A, p < 0.01 as compared to PBS or BCG, while no effect was visible at day 15), which resulted in a significant long-term mice survival (50%, as compared to 10% for BCG or PBS-instilled mice, p = 0.004 and p = 0.0003, respectively, Fig. 2B). These data suggest that intravesical treatment with Ty21a may be more efficient than BCG for inducing bladder-tumor regression.
Figure 2.
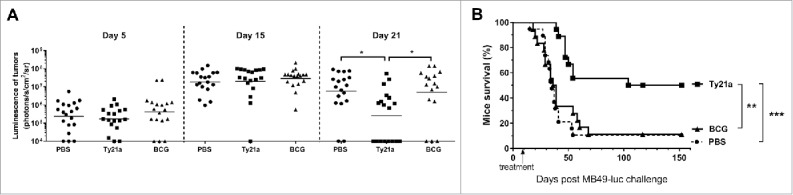
Ty21a immunotherapy is superior to BCG for regressing bladder-tumor. Groups of mice received a single intravesical instillation with PBS (plain circle, n = 20), 3×107CFU of Ty21a (black square, n = 20) or 3 × 107 CFU of BCG (black triangle, n = 20) at day 5 after tumor administration. (A) Bioluminescence of the bladder tumors is shown at days, 5, 15, and 21 in the three treatment groups. Note that bladder tumors are established before intravesical treatments (day 5) and that significant tumor-regression is only visible in mice treated with Ty21a at day 21. (B) Mice survival curves comparison show that only Ty21a provided significant long-term survival in 50% of the mice. **p < 0.01 and ***p < 0.001 following an adjusted log-rank test. Two independent experiments were performed.
Ty21a bacteria do not persist in mice tissues or human cells
Although wild-type or attenuated S. typhi are restricted to human host, intranasal administration of Ty21a to mice can lead to bacterial infection and persistence in the lung,14 without deeper invasion to the spleen.15 It is therefore important to examine the fate of Ty21a bacteria after intravesical instillation. Notably, no Ty21a bacteria were detected 7 d after administration, neither in healthy bladder (Fig. 3A) nor in bladder-tumors (Fig. 3B), despite the high dose of bacteria used (3 × 108 CFU). In addition, no bacteria were recovered in draining lymph nodes or in the spleen (data not shown). In contrast, and as expected,6 BCG bacteria were recovered from bladder or from bladder-tumor (2–3 × 102 CFU, 7 days following a 3 × 107 CFU inoculation, Fig. 3 A and B).
Figure 3.
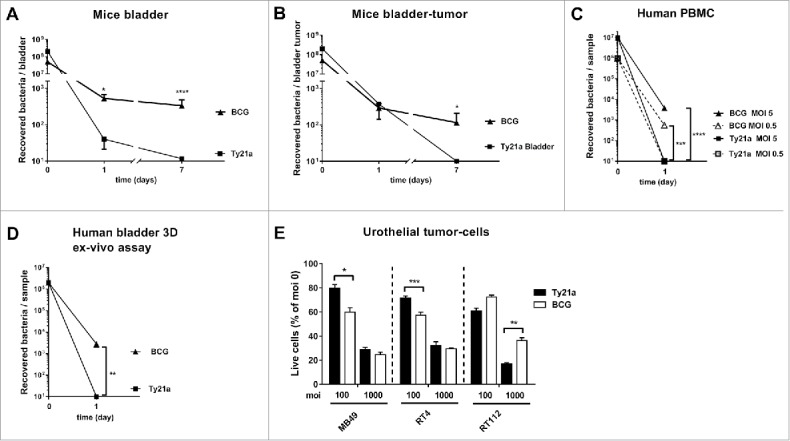
Bacteria and live cells recovery. Mean numbers ± SEM of BCG (triangle) or Ty21a (square) bacteria recovered from mouse bladder, n = 8 (A) or bladder-tumors, n = 7 (B), 1 and 7 d after intravesical instillation of 3 × 108 CFU for Ty21a or 3 × 107 CFU for BCG. (C) Mean numbers ± SEM of BCG (triangle) or Ty21a (square) bacteria recovered 1 d after human PBMC infection with 106 CFU (empty symbols and dashed lines, MOI 0.5, n = 7) or 107 CFU (plain symbols and lines, MOI 5, n = 7). (D) Mean numbers ± SEM of BCG (triangle, n = 6) or Ty21a (square, n = 6) bacteria recovered 1 d after infection of human-bladder 3D specimen 24 h after infection with 2 × 106 CFU. (E) Percentage of live cells (normalized to MOI 0) recovered 24 h after infection of MB49 (n = 6), RT4 (n = 6), or RT112 (n = 6) cells with Ty21a (black bars) or BCG (white bars) at MOI 100 or MOI 1000. Significant differences between groups are indicated by *p < 0.05, **p < 0.01, and ***p < 0.001 after unpaired t-test (A, B, D) or one-way Anova followed by a Sidak's post-test (C, E). Two independent experiments for A, B, C, and E were performed. All experiments in D are independent (with always PBS, Ty21a, and BCG in parallel).
Absence of Ty21a bacterial survival in human cells was further confirmed after infection of PBMC from healthy donors (Fig. 3C) and human urothelial cell-lines (RT4 and RT112, data not shown). For a better pre-clinical assessment, we exposed healthy human urothelium (obtained from nine cystectomies, see Table S1 for patient details) to Ty21a or BCG in this novel human 3D-bladder-tissue ex-vivo assay. Again, Ty21a bacteria were not recovered from this tissue 24 h after infection, in contrast to BCG bacteria (ca. 3 × 103 CFU, Fig. 3D). These data demonstrate the inability of Ty21a bacteria to survive in human bladder tissues and thus suggest a better safety-profile than BCG for bladder-treatment.
Ty21a can induce tumor-cell death and cytokines/chemokines/growth factors associated with successful bladder-treatment
Despite the absence of Ty21a bacterial survival, infection of tumor-cells (mouse MB49, or human RT4 and RT112) in vitro resulted in significant cell-death 24 h later, similarly to what was observed after BCG infection (ca. 2/3 and 1/3 of the tumor-cells surviving at multiplicity of infection (MOI) of 100 and 1000, respectively, Fig. 3E), with some variations in efficacy pending on the cell line and/or the MOI considered. This shows that one of the primary events of bladder-immunotherapy, i.e., local urothelial/tumor cell-killing,6 can be mediated by Ty21a without the risk of bacterial persistence associated to BCG.
A second important event in bladder-immunotherapy is the induction of inflammatory cytokines/chemokines/growth factors6 that we have thus analyzed both after infection of human tumor cell-lines and in the 3D-bladder-tissue ex-vivo assay. We anticipated that the presence of both the urothelium and the underlying submucosa in this assay would be closer to what occurs in vivo in the patient. Indeed, among a panel of 30 cytokines/chemokines/growth factors, 22 were detected at 24 h in the tissue supernatants of both Ty21a and BCG infected-samples (fold-increases are shown in Table 1 and raw data in Fig. S1). Most of them were increased by BCG and/or Ty21a as compared to PBS (significantly for IL-1β, IL-8, IL-10, IL12, IL-15, IL-2R, MIP-1α, MIP-1β, MCP-1, GM-CSF, FGF-basic, VEGF, G-CSF, and HGF, Fig. S1). All these analytes were reported to be increased in urine upon BCG treatment in NMIBC patients,4,6,16,17 suggesting that our assay was recapitulating at least some of the events occurring in vivo. The presence of the submucosa with infiltrated immune cells in the bladder-tissue specimen possibly allowed secretion of IL-1β, IL-10, IL-15, MIP-1α, TNF-α, IP-10, and MIG, that are not detected upon infection of urothelial cells (RT4 and/or RT112) alone (Table 1 and raw data in Fig. S2).
Table 1.
Increased Inflammatory cytokine/chemokine growth factors upon BCG or Ty21a infection of human bladder explants and/or urothelial cell lines.
| Human bladder 3D-assay (n = 9) |
RT4 (n = 3) |
RT112 (n = 3) |
||||
|---|---|---|---|---|---|---|
| Ty21aa | BCGa | Ty21ab | BCGb | Ty21ab | BCGb | |
| IL-1β | +++ | ++++ | ||||
| IL-10 | ++ | + | ||||
| IL-15 | + | +/− | ND | |||
| MIP-1α | +++ | ++ | ||||
| TNF-α | ++ | + | ||||
| IP-10 | +/− | + | ||||
| MIG | + | ++ | ||||
| IL1-RA | +/− | ∼ | ||||
| FGF-basic | +/− | +/− | +/− | +/− | ∼ | ∼ |
| IL-2R | +/− | + | ∼ | ∼ | ∼ | ∼ |
| Rantes | +/− | +/− | +++ | ND | ND | ND |
| IL-6 | +/− | +/− | ++++ | ++ | ++ | + |
| GM-CSF | +++ | + | +++ | ++ | ++ | +/− |
| IL-8 | ++ | + | ++ | + | ++++ | ++ |
| MIP-1β | ++ | ++ | + | ∼ | ND | ND |
| MCP-1 | + | +/− | +/− | ∼ | ++ | + |
| HGF | +/− | +/− | ∼ | ∼ | ++ | ++ |
| G-CSF | +/− | +/− | ++++ | ND | ND | ND |
| IFN-α | +/− | +/− | ∼ | ∼ | + | ND |
| IL-12 | +/− | +/− | + | +/− | + | +/− |
| VEGF | +/− | +/− | + | +/− | ++ | +/− |
| IL-7 | ∼ | ∼ | + | ∼ | ND | ND |
Geometric mean fold increases between Ty21a or BCG treated samples versus PBS are indicated:
∼ : 0.5–<1.3, +/−: 1.3–<2.5, +: 2.5–<4; ++: 4–<10; +++: 10–<20; ++++: >20. ND: not detected
a 2 × 106 CFU:, b moi 100.
Discussion
Here, we report preclinical evidences that intravesical instillations with the Ty21a vaccine strain may be more efficient and safer than BCG for treatment of NMIBC. Ty21a is an highly attenuated Salmonella that has lost the capacity to survive in cells, but has kept its potential to trigger danger signals leading to cell-death and cytokines secretion, as also shown in intestinal epithelial cells.18 This can be mediated by bacterial components acting as TLR-agonists (LPS/TLR4, flagellin/TLR5, DNA/TLR9,19 and/or the ability of Salmonella to inject effector proteins through its type III secretion system.20 In our assays, Ty21a bacteria induced a similar array of inflammatory cytokines as BCG, though more consistently for some of them (GM-CSF, IL-8, IL-15, MIP1α, MIP1β, and MCP-1 in the 3D-bladder assay, IL-6, IL-8, IL-12, GM-CSF, MCP-1, and VEGF in RT4 and RT112 cells). These molecules are involved in recruitment of neutrophils (IL-8), NK cells (MCP-1, MIP-1β), and monocytes and T cells (GM-CSF), as well as activation of NK and T cells (IL-15, IL-12), all of which may underlie the higher efficacy of Ty21a for inducing tumor-regression in our study. Indeed, some of these cytokines (IL-8, IL-12) were associated to positive responses to BCG immunotherapy21,22 and/or were eventually administered together with BCG or expressed by recombinant BCG (IL-12, GM-CSF) toward a higher efficacy of BCG-immunotherapy in bladder-cancer patients.23
Beside BCG, other bacteria, including Salmonella, but not Ty21a, have been tested in the past for their potential in anticancer treatment.24 However, the effects of these bacteria were rather associated to their reported capacities to target and multiply in the hypoxic tumor-microenvironment at the detriment of the tumor-cells themselves, than to a potent inflammatory reaction as could be observed with the historical Coley's toxin.24 Induction of tumor-cell death, innate and adaptive immune responses by Ty21a is thus in the same therapeutic line as BCG-immunotherapy. The absence of Ty21a bacterial survival in our experiments is in agreement with previous data reporting absence of Ty21a recovery and/or survival from human intestine and blood in vivo (reviewed in ref.7 and references therein) and rapid degradation of the bacteria in human blood in vitro.25 This makes it unlikely that accidental intravenous administration of Ty21a may result in the serious deleterious effects associated to incorrect catheterization with BCG. In contrast, the similar array of inflammatory cytokines induced by Ty21a and BCG in our 3D bladder assay may result after intravesical instillations in local inflammatory reactions alike those experienced upon BCG. However, the finding that Ty21a was more efficient than BCG in more stringent preclinical tumor-settings may be predictive of a higher efficacy in the patients. Thirty-to-forty percent of high risk of progression NMBIC patients, particularly those presenting with carcinoma in situ lesions, will not respond to BCG therapy and would thus greatly benefit from a more efficient drug, that could avoid radical cystectomy.26Altogether, our preclinical data demonstrate that intravesical Ty21a is more effective than BCG for bladder-tumor treatment. Absence of surviving Ty21a bacteria and the excellent safety-record of the typhoid vaccine support its further testing for intravesical immunotherapy of NMIBC patients.
Material and methods
Mouse and human cells
The MB49 cell-line (kindly provided by Prof. A. Loskog, Uppsala University, Sweden) is derived from a carcinogen-induced urothelial carcinoma in male C57Bl/6 mice.8 Luciferase-expressing MB49 cells (MB49-luc) were generated by transfection with lentiviral vectors encoding for firefly luciferase (kindly provided by Prof. D. Trono, EPFL, Lausanne, Switzerland). The human urothelial cell lines RT4 and RT112 were kindly provided by Prof. Thalman (Inselspital, Bern, Switzerland). Human peripheral blood mononuclear cells (PBMC) were purified by ficoll gradient from purchased buffy coats of healthy subjects (Blood Transfusion Center, Epalinges, Switzerland).
The MB49 orthotopic bladder tumor model
Seven-to-ten-week-old female C57Bl/6 wild type mice (Charles River) were used in compliance with ethical directives of the Swiss veterinary authorities. Bladder-tumors were established in deeply anesthetized mice that were urethrally catheterized using Introcan 24Gx3/4 catheters (Braun, Melsungen, Germany). A 15 min pre-treatment with 100 μL 22% ethanol was performed before instillation of 200′000 MB49-luc cells in 50 μL. Tumor growth was monitored by bioluminescence 15 min after intraperitoneal injection of D-luciferin (Promega, 150 μg/g of body weight) in the Xenogen imaging system (Xenogen/IVIS Caliper Life Science, kindly provided by cellular imaging facility, CIF/UNIL, Lausanne, Switzerland). Bioluminescence monitoring of MB49-luc tumor is very efficient for assessing tumor establishment and growth during the first 3 weeks, however, uncontrolled loss of luminescence of the growing tumors can then often appear,13 requiring additional monitoring by palpation, hematuria and overall health status of the mice, that were euthanized in case of >15% weight-loss.
Ty21a and BCG bacteria preparation
Ty21a bacteria were prepared by resuspension of the lyophilized content of a Vivotif ® capsule (PaxVax, Bern, Switzerland) into 750 μL of PBS, resulting in ca. 3 × 109 CFU/mL. BCG bacteria were prepared by re-suspension of one vial of oncoTICE™ (Essex Chemie SA, Luzern, Switzerland) in 1ml of PBS, resulting in ca. 3 × 108 CFU/mL. Further dilutions were made in PBS as required to achieve the indicated bacteria numbers used in the different experiments.
Intravesical treatments
75 μL bacterial suspensions volumes were instilled by urethral catheterization, as described above. The retention time in the bladder was ca. 1 h, until the mice awaking from the anesthesia will spontaneously urinate. Classical treatment (Fig. 1) start 1 day after intravesical tumor-cell instillation and is administered four-times at weekly intervals (day 2, 9,16, and 23). In a more stringent setting (Fig. 2), a single intravesical treatment-instillation was administered at day 5 after intravesical tumor-cell instillation.
Bacterial survival
Healthy- or tumor-bearing bladders, draining lymph nodes (iliac) and spleen recovered at mice sacrifice were homogenized in a sucrose solution as previously described27 and plated in Luria Bertani (LB) agar plates for 48 h or in M7H11 agar plates for 3–4 weeks, for Ty21a and BCG, respectively.
In vitro infections, bacterial and cell survival, and cytokine analysis
Cells were infected with Ty21a or BCG at the indicated multiplicity of infection (MOI) for 1.5 h at 37 °C, 5% CO2. Cells were washed and medium containing 50 μg/mL Gentamycin was added for 1 h to kill extracellular bacteria and then replaced by medium containing 15 μg/mL gentamycin for over-night incubation. Supernatant were recovered for analysis of a panel of cytokines/chemokines/growth factors using a 30-plex human-cytokines Luminex assay according to manufacturer's instructions (Thermo Fisher Scientific). Surviving cells were counted with Trypan blue and bacterial content was analyzed following plating in agar plate as described above.
3D-bladder-tissue ex-vivo assay
Bladder tissues from patients were obtained after written consent and full local State Ethics Committee approval (protocol #119/10). Fresh, healthy bladder tissue samples were carefully collected by pathologist from a surgical cystectomy specimen at a mean distance of 2 cm from a visible tumor lesion. In the following hour, urothelium and underlying chorion/submucosa specimen were dissected out from the muscle layer and surfaces > 8 × 8 mm were mounted in a 5-mm unjacketed Franz Cell (SES-Analysesysteme, Bechenheim, Germany) with the urothelium facing up (see Fig. S3). The pathological characteristics of the patients from which enough tissue was available to be mounted in three Franz Cells in parallel (i.e., for infection with PBS, Ty21a, and BCG) are summarized in Table S1. Upon bladder tissue mounting, DMEM medium without antibiotic was added in the lower and upper chambers. Then, bacteria were added in the upper chamber for 1.5 h at 37°C in 5% CO2 incubator. Upper chamber liquid was then removed and replaced by medium containing 50 μg/mL Gentamycin for 1 h to kill extracellular bacteria, and finally replaced by medium containing 15 μg/mL Gentamycin for over-night incubation. Supernatant were recovered for cytokine analysis. Bladder tissues were recovered and homogenized to assess bacterial survival as described for mouse organs above.
Statistics
Statistical analyses were performed using Prism 7.00 for Windows (GraphPad software). Single comparisons were performed using t-test. Multiple comparisons were performed using one-way Anova and Tukey's Multiple Comparison Test or adjusted log-rank test as indicated in the figure legends.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the pathologists of the CHUV for providing us the fresh healthy bladder samples from cystectomies.
Funding
The study was funded by Swiss National Funds (#32153201 and #CRII3 160742) and Swiss Cancer League #2808-08-2011 to DNH.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10-29; PMID:22237781; http://dx.doi.org/ 10.3322/caac.20138 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013; 49:1374-1403; PMID:23485231; http://dx.doi.org/ 10.1016/j.ejca.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E., Sylvester RJ, Kaasinen E, Bohle A, Palou Redorta J et al.. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013; 64:639-653; PMID:23827737; http://dx.doi.org/ 10.1016/j.eururo.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 4.Gontero P, Bohle A, Malmstrom PU, O'Donnell MA, Oderda M, Sylvester R, Witjes F. The role of bacillus Calmette-Guerin in the treatment of non-muscle-invasive bladder cancer. Eur Urol 2010; 57:410-429; PMID:19969411; http://dx.doi.org/ 10.1016/j.eururo.2009.11.023 [DOI] [PubMed] [Google Scholar]
- 5.Kamat AM, Sylvester RJ, Bohle A, Palou J, Lamm DL, Brausi M, Soloway M, Persad R, Buckley R, Colombel M et al.. Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: recommendations from the international bladder cancer group. J Clin Oncol 2016; 34:1935-44; PMID:26811532; http://dx.doi.org/ 10.1200/JCO.2015.64.4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer–a current perspective. Nat Rev Urol 2014; 11:153-162; PMID:24492433; http://dx.doi.org/ 10.1038/nrurol.2014.15 [DOI] [PubMed] [Google Scholar]
- 7.Guzman CA, Borsutzky S, Griot-Wenk M, Metcalfe IC, Pearman J, Collioud A, Favre D, Dietrich G. Vaccines against typhoid fever. Vaccine 2006; 24:3804-11; PMID:16278037; http://dx.doi.org/ 10.1016/j.vaccine.2005.07.111 [DOI] [PubMed] [Google Scholar]
- 8.Summerhayes IC, Franks LM. Effects of donor age on neoplastic transformation of adult mouse bladder epithelium in vitro. J Natl Cancer Inst 1979; 62:1017-23; PMID:107359 [PubMed] [Google Scholar]
- 9.Günther JH, Jurczok A, Wulf T, Brandau S, Deinert I, Jocham D, Böhle A. Optimizing syngeneic otrhotopic murine bladder cancer (MB49). Cancer Res 1999; 59:2834-37; PMID:10383142 [PubMed] [Google Scholar]
- 10.Suttmann H, Riemensberger J, Bentien G, Schmaltz D, Stöckle M, Jocham D, Böhle A, Brandau S. Neutrophil granulocytesare required for effective Bacilus Calmette-Guérin Imunotherapy of bladder cancer and orchestrate local immune reponses. Cancer Res 2006; 16:8250-57; PMID:16912205; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-1416 [DOI] [PubMed] [Google Scholar]
- 11.Loskog A, Fransson ME, Totterman TH. AdCD40L gene therapy counteracts T regulatory cells and cures aggressive tumors in an orhotopic bladder cancer model. Clin. Cancer Res 2005; 24:8816-21; PMID:16361570; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-1817 [DOI] [PubMed] [Google Scholar]
- 12.Mangsbo SM, Nanalga C, Essand M, Loskog A, Totterman TH. CpG therapy is superior to BCG in an otrhotopic bladder cancer model and generates CD4+ T -cell immunity. J. Immunother 2008; 31:34-42; PMID:18157010; http://dx.doi.org/ 10.1097/CJI.0b013e3181587d29 [DOI] [PubMed] [Google Scholar]
- 13.Jurczok A, Fornara P, Soling A. Bioluminescence imaging to monitor bladder cancer cell adhesion in vivo: a new approach to optimize a syngeneic, orthotopic, murine bladder cancer model. BJU Int 2008; 101:120-4; PMID:17888045; http://dx.doi.org/ 10.1111/j.1464-410X.2007.07193.x [DOI] [PubMed] [Google Scholar]
- 14.Galen JE, Gomezduarte OG, Losonsky GA, Halpern JL, Lauderbaugh CS, Kaintuck S, Reymann MK, Levine MM. A murine model of intranasal immunization to assess the immunogenicity of attenuated salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine 1997; 15:700-8; PMID:9178472; http://dx.doi.org/ 10.1016/S0264-410X(96)00227-7 [DOI] [PubMed] [Google Scholar]
- 15.Fraillery D, Baud D, Pang SY, Schiller J, Bobst M, Zosso N, Ponci F, Nardelli-Haefliger D. Ty21a expressing Human papillomavirus type 16 L1 as a potential live Salmonella vaccine against cervical cancer and typhoid fever. Clin. Vaccine Immunol 2007; 14:1285-95; PMID:17687110; http://dx.doi.org/ 10.1128/CVI.00164-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bisiaux A, Thiounn N, Timsit MO, Eladaoui A, Chang HH, Mapes J, Mogenet A, Bresson JL, Prie D, Bechet S. et al.. Molecular analyte profiling of the early events and tissue conditioning following intravesical bacillus calmette-guerin therapy in patients with superficial bladder cancer. J Urol 2009; 181:1571-80; PMID:19230924; http://dx.doi.org/ 10.1016/j.juro.2008.11.124 [DOI] [PubMed] [Google Scholar]
- 17.Askeland EJ, Newton MR, O'Donnell MA, Luo Y. Bladder Cancer Immunotherapy: BCG and Beyond. Adv Urol 2012; 2012:181987; PMID:22778725; http://dx.doi.org/ 10.1155/2012/181987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorentino M, Lammers KM, Levine MM, Sztein MB, Fasano A. In vitro intestinal mucosal epithelial responses to wild-type salmonella typhi and attenuated typhoid vaccines. Front Immunol 2013; 4:17; PMID:23408152; http://dx.doi.org/ 10.3389/fimmu.2013.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broz P, Ohlson MB, Monack DM. Innate immune response to Salmonella typhimurium, a model enteric pathogen. Gut Microbes 2012; 3:62-70; PMID:22198618; http://dx.doi.org/ 10.4161/gmic.19141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raymond B, Young JC, Pallett M, Endres RG, Clements A, Frankel G. Subversion of trafficking, apoptosis, and innate immunity by type III secretion system effectors. Trends Microbiol 2013; 21:430-441; PMID:23870533; http://dx.doi.org/ 10.1016/j.tim.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 21.Thalmann GN, Sermier A, Rentsch C, Mohrle K, Cecchini MG, Studer UE. Urinary Interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette-Guerin. J Urol 2000; 164:2129-33; PMID:11061941; http://dx.doi.org/ 10.1016/S0022-5347(05)66983-2 [DOI] [PubMed] [Google Scholar]
- 22.Zuiverloon TC, Nieuweboer AJ, Vekony H, Kirkels WJ, Bangma CH, Zwarthoff EC. Markers predicting response to bacillus Calmette-Guerin immunotherapy in high-risk bladder cancer patients: a systematic review. Eur Urol 2012; 61:128-145; PMID:22000498; http://dx.doi.org/ 10.1016/j.eururo.2011.09.026 [DOI] [PubMed] [Google Scholar]
- 23.Luo Y, Henning J, O'Donnell MA. Th1 cytokine-secreting recombinant Mycobacterium bovis bacillus Calmette-Guerin and prospective use in immunotherapy of bladder cancer. Clin Dev Immunol 2011; 2011:728930; PMID:21941579; http://dx.doi.org/ 10.1155/2011/728930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kucerova P, Cervinkova M. Spontaneous regression of tumour and the role of microbial infection–possibilities for cancer treatment. Anticancer Drugs 2016; 27:269-277; PMID:26813865; http://dx.doi.org/ 10.1097/CAD.0000000000000337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi H, Santander J, Brenneman KE, Wanda SY, Wang S, Senechal P, Sun W, Roland KL, Curtiss R. Live recombinant Salmonella Typhi vaccines constructed to investigate the role of rpoS in eliciting immunity to a heterologous antigen. PLoS One 2010; 5:e11142; PMID:20585446; http://dx.doi.org/ 10.1371/journal.pone.0011142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, Hernandez V, Kaasinen E, Palou J, Roupret M et al.. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2016. Eur Urol 2016; 30249-4; PMID:27324428; http://dx.doi.org/ 10.1016/j.eururo.2016.05.041 [DOI] [PubMed] [Google Scholar]
- 27.Nardelli-Haefliger D, Roden R, Benyacoub J, Sahli R, Kraehenbuhl JP, Schiller JT, Lachat P, Potts A, Degrandi P. Human papillomavirus type 16 virus-like particles expressed in attenuated salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infection & Immunity 1997; 65:3328-3336; PMID:9234794 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.