ABSTRACT
There is a critical unmet clinical need for bladder cancer immunotherapies capable of inducing durable antitumor immunity. We have shown that four intravesical treatments with a simple co-formulation of interleukin-12 and the biopolymer chitosan not only destroy orthotopic bladder tumors, but also promote a potent long-lasting systemic immune response as evidenced through tumor-specific in vitro killing assays, complete protection from rechallenge, and abscopal antitumor responses at distant non-treated tumors. This study investigates the immunological kinetics underlying these results. We show through depletion studies that CD8+ T cells are required for initial tumor rejection, but CD4+ T cells protect against rechallenge. We also show that even a single intravesical treatment can eliminate tumors in 50% of mice with 6/9 and 7/8 mice eliminating tumors after three or four treatments respectively. We then performed immunophenotyping studies to analyze shifts in immune cell populations after each treatment within the tumor itself as well as in secondary lymphoid organs. These studies demonstrated an initial infiltration of macrophages and granulocytes followed by increased CD4+ and CD8+ effector-memory cells. This was coupled with a decreased level of regulatory T cells in peripheral lymph nodes as well as decreased myeloid-derived suppressor cell infiltration in the bladder. Taken together, these data demonstrate the ability of properly delivered interleukin-12-based therapies to engage adaptive immunity within the tumor itself as well as throughout the body and strengthen the case for clinical translation of chitosan/interleukin-12 as an intravesical treatment for bladder cancer.
KEYWORDS: BCG, bladder cancer, chitosan, immunotherapy, interleukin-12, intratumoral, intravesical, MDSC, TILS, TReg
Introduction
In the United States alone more than 76,000 new cases of bladder cancer are expected this year with more than 16,000 deaths associated with the disease.1,2 The majority of new cases are of non-muscle invasive disease, accounting for 70–80% of diagnoses.3 For 40 y, the standard of care for high-grade, non-muscle invasive disease has been intravesical therapy with Mycobacterium bovis bacillus Calmette–Guerin (BCG) after a transurethral resection of the tumor. Despite years of clinical immunotherapy with BCG, a specific antitumor memory response as a result of BCG therapy has not been demonstrated.4 This has led to bladder cancer recurrence rates of 50–80%, the highest of any major malignancy.5 Although there has been recent progress in treatment of some metastatic cases with checkpoint inhibitors,6 there remains an urgent need for novel treatments for both muscle invasive and early stage disease.
Our laboratory has developed an immunotherapy composed of interleukin-12 co-formulated with the biopolymer chitosan (CS/IL-12).7-9 In CS/IL-12 immunotherapy, IL-12 acts as a powerful immune stimulant, whereas chitosan enhances IL-12's penetration into the urothelium.7 Interleukin-12 is a TH1 polarizing cytokine, capable of reversing an immunosuppressive environment within tumors. We have shown that four intravesical instillations of CS/IL-12 not only eliminate up to 90% of bladder tumors in two orthotopic murine bladder tumor models, but also induce a powerful memory response capable of complete systemic protection that remains durable for the remaining lifespan of the mice.7,9 We have also shown that a similar co-formulation delivered intratumorally has potent effects against other tumors of non-bladder origin.8,10,11 But the immunological mechanisms underlying this effectiveness, especially with regards to bladder tumors have not been elucidated.
A number of studies have shown that IL-12-based therapies delivered intratumorally act in an IFNγ dependent manner to increase CD3+, CD4+, and CD8+ T-cell infiltration while activating existing tumor infiltrating lymphocytes (TILs) and reducing the frequencies of regulatory T cells (TRegs) and myeloid-derived suppressor cells (MDSC).8,12,13 However, the means by which IL-12 induces an effective immune response varies by tumor type and even by the same tumor type in different tissues.14 Furthermore, the kinetics of an IL-12-based immunotherapy within the bladder has not been documented.
The purpose of the current study is to build on our knowledge of IL-12-based therapies by asking three questions regarding the immunological mechanisms and kinetics of intravesical CS/IL-12. First, which immune cells are most vital to both the initial treatment and the subsequent protection? Second, what is the effect of number of treatments on elimination of bladder tumors? Third, how does the response to intravesical CS/IL-12 immunotherapy evolve throughout the course of treatment both at the treatment site and in secondary lymphoid organs?
Results
Initial tumor rejection is primarily driven by CD8+ T cells
To determine the role of immune cell subsets on the efficacy of intravesical CS/IL-12 immunotherapy, we depleted tumor-bearing mice of CD4+, CD8+, or NK1.1+ cells prior to treatment. Each cell type was revealed to play a role in the effectiveness of CS/IL-12 immunotherapy with 4/8 NK-depleted, 4/8 CD4+-depleted, 0/9 CD8+-depleted, mice surviving tumor free (Fig. 1A). In contrast, 7/9 mice that were not depleted completely eliminated their tumors. Despite all succumbing to their tumors, mice depleted of CD8+ cells experienced extended median survival by 6 d when compared with phosphate buffered saline (PBS) treated mice (p < 0.05). All other treated mice regardless of depletion status also extended survival (p < 0.05) beyond PBS treated as well as CD8+ T-cell depleted mice.
Figure 1.
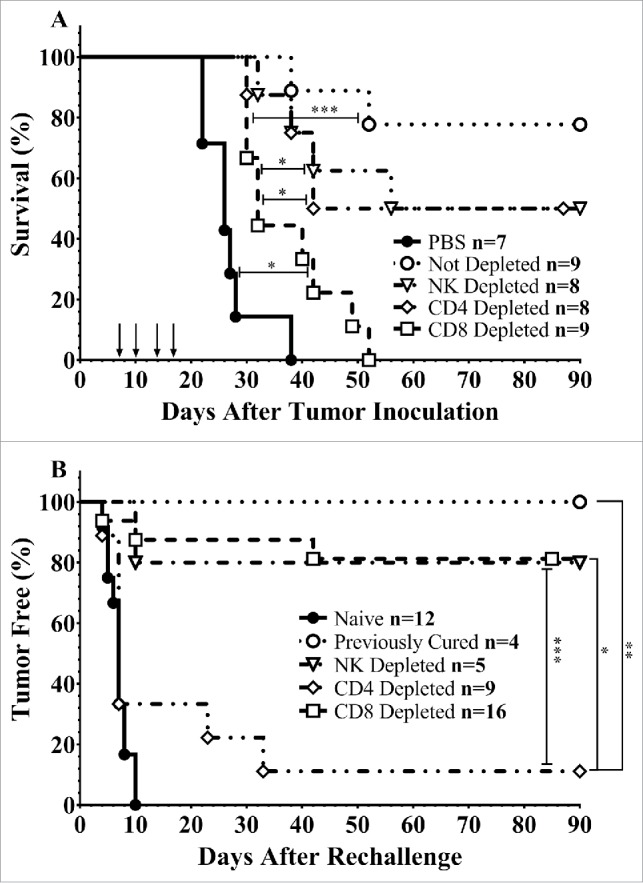
Immune cell depletion during treatment and rechallenge. (A) Mice were depleted of CD4+, CD8+, or NK1.1+ lymphocytes prior to implantation of 75,000 MB49 cells in the bladder and throughout twice-weekly application of intravesical CS/IL-12 immunotherapy (arrows) begun 7 d post-implantation. Mice were monitored for hematuria and survival. All CS/IL-12 treated groups, regardless of depletion status, significantly (p < 0.05) prolonged survival over PBS treated mice. (B) Mice which had previously eradicated their tumors were depleted of CD4+, CD8+, or NK1.1+ lymphocytes prior to subcutaneous rechallenge with 300,000 MB49 cells. Onset of tumor growth was monitored and measured. With the exception of the CD4+ depleted group all curves were significantly different (p < 0.05) than naive mice. Asterisks indicate differences between groups determined via the log-rank test: *p < 0.05 or ***p < 0.0005.
The memory response is dependent on CD4+ T cells
We have previously shown that mice that have eliminated their orthotopic bladder tumors after treatment with CS/IL-12 were able to reject subsequent tumor rechallenge even at a distant (subcutaneous) site.9 To identify the immune cell subtypes required for this systemic memory, we depleted immune cell subsets in mice which had previously eliminated their tumors and then rechallenged them subcutaneously in the right flank with MB49 (Fig. 1B). All (4/4) non-depleted mice rejected rechallenge and all naive mice (12/12) grew tumors. Depletion of NK cells had little effect on tumor protection with 4/5 mice rejecting their rechallenge. Surprisingly, depletion of CD8+ cells also had a minimal effect with 81% (13/16) mice rejecting rechallenge. The main effectors of the subsequent rejection were found to be CD4+ cells with only 1/9 mice rejecting rechallenge. Non-depleted, CD8+ depleted, and NK1.1+ depleted groups were all significantly different (p < 0.05) than both naive and CD4+ depleted groups.
Number of treatments impact tumor rejection
The decision to use four intravesical treatments of CS/IL-12 in our previous publications was largely determined through experimental observation. Nearly all treated mice tend to be hematuria free after the fourth treatment. We sought to better explore the significance of the number of administrations by applying either 1, 2, 3, or 4 treatments while tracking survival (Fig. 2). As expected, mice given the full course of four treatments showed the greatest ability to eradicate their tumors with 7/8 surviving long term. However, fewer treatments were also effective with 6/9, 2/7, and 4/8 mice surviving long term when given 3, 2, or 1 treatment respectively. All curves were significantly different than PBS treated controls (p < 0.005) and both 3 and 4 treatments were significantly different than two treatments (p < 0.005) but were not statistically different than a single treatment. However, giving three or four treatments did significantly prolong survival over 1 or 2 treatments (p < 0.05) (Fig. 2B).
Figure 2.
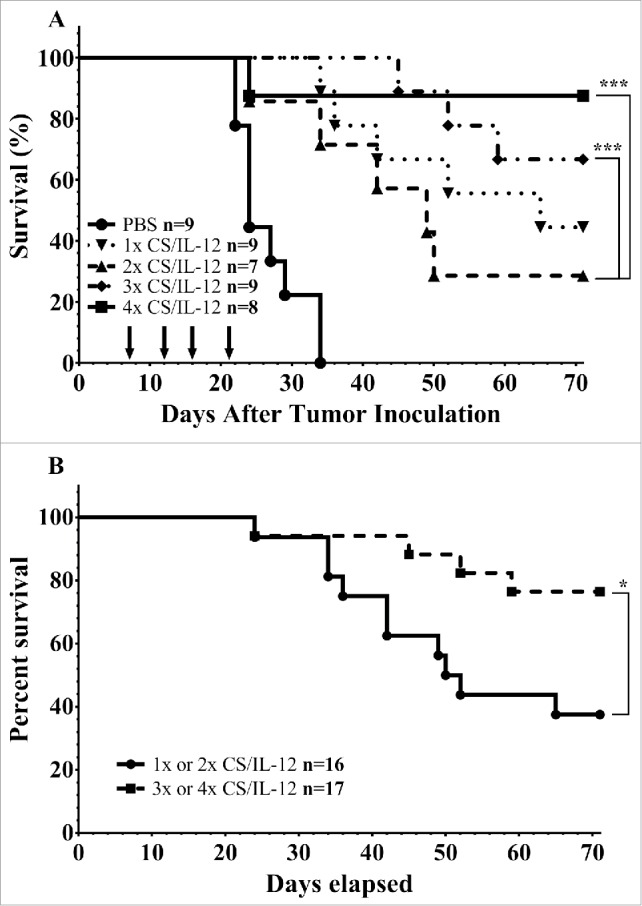
Number of treatments impact initial tumor rejection. (A) Mice were implanted with 75,000 MB49 cells in the bladder and given 1, 2, 3, or 4 intravesical treatments with CS/IL-12 on days 7, 11, 15, and 18, respectively. They were then tracked for hematuria and survival. (B) Survival curves from the same experiment as (A), but with the combined 1 and 2 treatments against the combined 3 and 4 treatments. Asterisks indicate differences between groups determined via the log-rank test: *p < 0.05 or ***p < 0.0005.
In a separate experiment, bladders were harvested 1 d after each treatment, weighed (Fig. 3A) and photographed (Fig. 3B). Bladder weight in CS/IL-12 treated mice began to separate from PBS controls (p = 0.056) by the third treatment. Bladders of CS/IL-12 treated mice were significantly smaller (p < 0.0005) after the fourth treatment. There was also a noticeable difference in the volume and redness of the bladders by the third treatment as seen in Fig. 3B.
Figure 3.
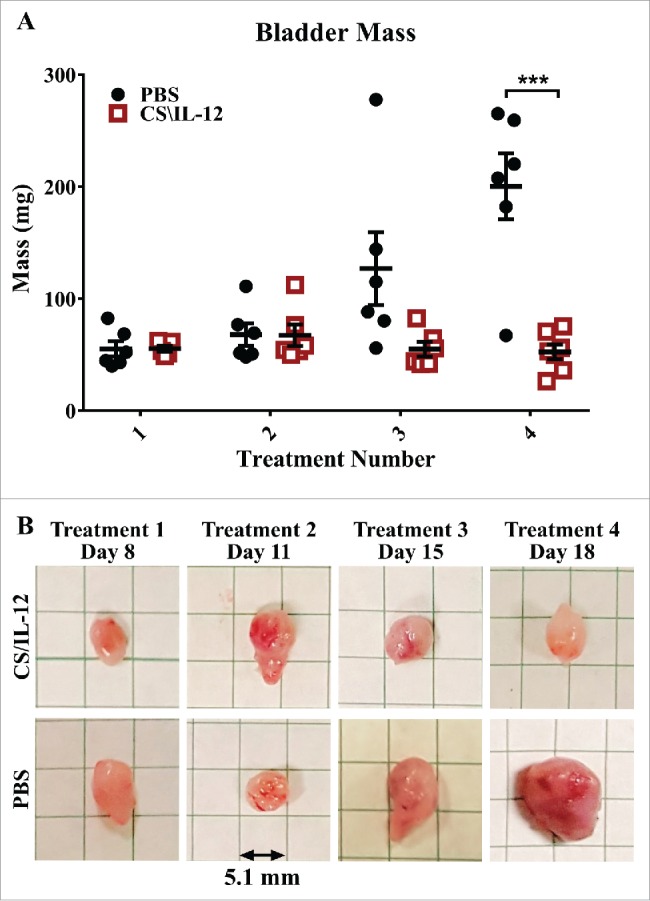
Bladder weight decreases with treatment. Mice were implanted with 75,000 MB49 cells in the bladder and given 1, 2, 3, or 4 intravesical treatments with CS/IL-12 on days 7, 11, 15, and 18, respectively (n = 5–7 per group). One day after each treatment, the mice were sacrificed and their bladders were removed, weighed (A), and photographed (B) before further processing for analysis. The representative bladders in (B) are placed on a 5.1-mm (0.2 in) grid paper. Error bars indicate mean with SEM. Asterisks indicate differences between PBS and CS/IL-12 treated mice at the indicated time point as determined through Student's t-test: ***p < 0.0005.
Treatment number affects immune cell infiltration locally and systemically
Since the number of treatments impacts the therapeutic efficacy of intravesical CS/IL-12, we decided to perform a study of the immune kinetics over the full course of treatment. We also knew from previous studies that an intravesical treatment of CS/IL-12, though confined to the bladder, induces a systemic immune response capable of eliminating distant tumors.4 To characterize this response, we performed a time-course study in which the bladder tumors as well as the spleen and bladder-draining lymph nodes (BDLNs) were harvested 24 h after each treatment and analyzed for shifts in a number immune cell populations via flow cytometry (Fig. S2). All results are of 4–5 mice from duplicate experiments. Statistical comparisons are either between immune cell populations within the CS/IL-12 and PBS treated groups at the same time point via Student's t-test or differences in those populations within the CS/IL-12 treatment group with respect to treatment number as determined via one-way ANOVA.
Shifts in lymphocyte populations are most pronounced in the bladder after the third treatment
CD3+ Lymphocytes: The proportion of live leukocytes consisting of CD3+ T cells varied by tissue, treatment, and treatment number. T-cell infiltration in treated mice rose in the bladder after the third and fourth treatments with mean infiltrations more than twice that of PBS treated mice (p < 0.05, Fig. 4, first row). These differences were less pronounced in distant organs with a small decrease in the spleen from 25.5% (PBS) to 19.5% (CS/IL-12) after the third treatment (p < 0.05) as well as a slight increase from 34.6% (PBS) to 41.6% (CS/IL-12) in the BDLNs at the fourth treatment (p < 0.05). Overall, CS/IL-12 treatment number affected CD3+ infiltration in the bladder (p < 0.05 via ANOVA), but not in the spleen or BDLNs.
Figure 4.
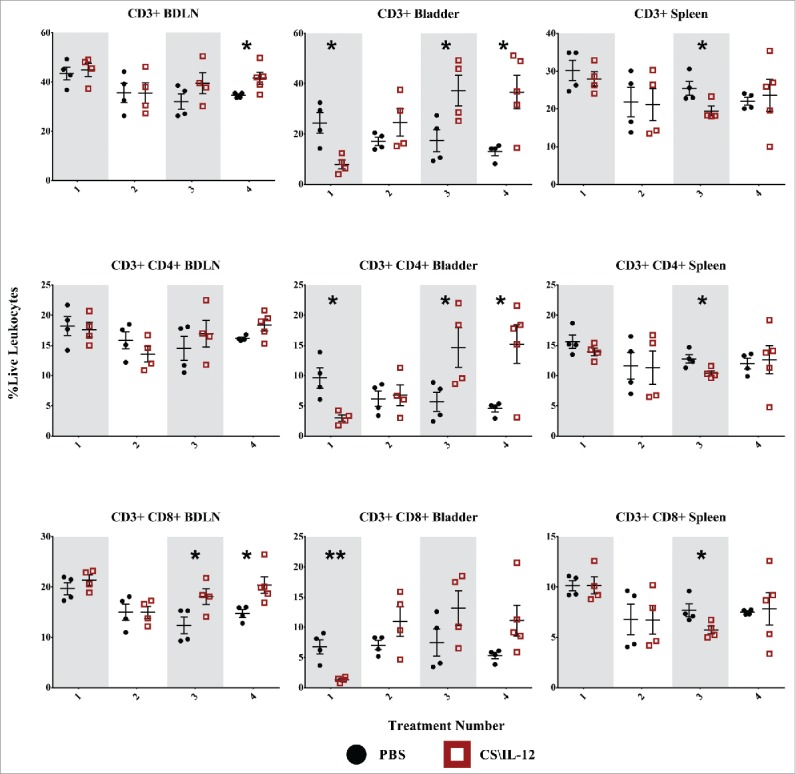
T-cell infiltration by treatment number and tissue. Mice were implanted with 75,000 MB49 cells in the bladder and given 1, 2, 3, or 4 intravesical treatments with CS/IL-12 or PBS on days 7, 11, 15, and 18, respectively (n = 4–5 per group). Bladder draining lymph nodes (BDLNs), bladders, and spleens were harvested 24 h after each treatment and analyzed via flow cytometry. Error bars indicate mean with SEM. Asterisks indicate significant differences between PBS and CS/IL-12 treatment groups at the same treatment number by Student's t-test: *p < 0.05 or **p < 0.005.
CD4+ lymphocytes: CD3+CD4+ helper T-cell fluctuation was most pronounced in the bladder tumors (Fig. 4, second row) and varied with treatment type as well as treatment number (p < 0.05 via ANOVA). T-cell infiltration initially dropped (perhaps as an artifact of intense macrophage and granulocyte inflammation) before recovering by the third treatment and exceeding that of PBS treated tumors by a factor of 3 (p < 0.05). There were only minor effects at distant lymphoid sites with a small drop in the spleen at the third treatment (p < 0.05). Overall, CS/IL-12 caused increased CD4+ infiltration in the bladder, but not at distant sites regardless of treatment number.
CD8+ lymphocytes: There was a drop in CD3+CD8+ cytotoxic T cells (Fig. 4, third row) in the bladder corresponding with the first treatment. Similar to CD4+ decreases after one treatment, this may be an artifact of intense monocytic and granulocytic inflammation. However, an eventual recovery by the second treatment led to levels statistically indistinguishable from PBS treated mice. There were more substantial effects at the systemic level with increased infiltration in the BDLNs after the third treatment as well as the same small drop observed in the spleen for both CD3+ and helper T cells at the third treatment. Overall, CS/IL-12 treatment number affected CD3+CD8+ infiltration in the bladder and BDLNs (p < 0.05 via ANOVA), but not in the spleen.
Shifts in T-cell effector/memory phenotypes
We used CD44 and CD62L to monitor shifts in T-cell phenotypes. CD44 is upregulated on effector (TEff), effector-memory (TEM), and central memory (TCM) cells, and promote the genesis and survival of TH1 memory cells.15 CD62L, also known as L-Selectin, is a surface receptor known to increase homing to secondary lymphoid tissues and is associated with naive T cells (Tnaive) and TCM cells. Although additional markers such as CD107 and CCR7 are often included to get a more precise definition of memory phenotypes, we chose a more general approach by monitoring shifts in populations defined as Tnaive (CD44lowCD62L+), TEff (CD44lowCD62L−), TEM (CD44highCD62L−), and TCM (CD44highCD62L+) for both CD4+ (Fig. 5) and CD8+ T cells (Fig. 6).16-18 The following statistical comparisons refer to differences in the proportion of the parent population (CD4+ or CD8+ T cells) between PBS and CS/IL-12 treated mice at a given time point in the same tissue via Student's t-test or differences in those populations within the CS/IL-12 treatment group with respect to treatment number determined via one-way ANOVA.
Figure 5.
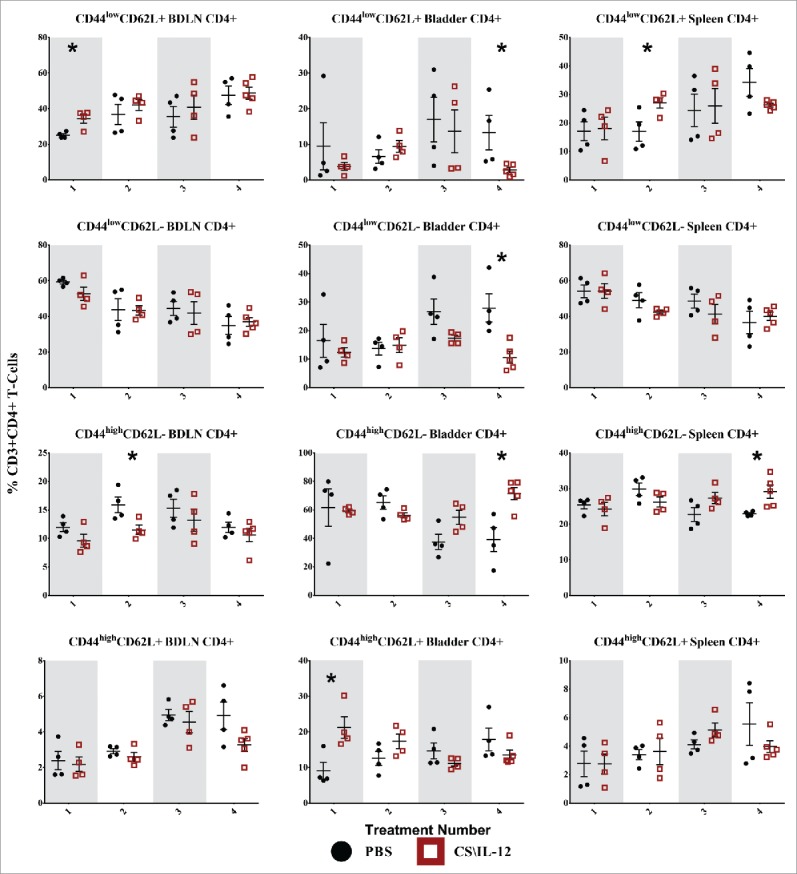
CD4+ T-cell effector/memory phenotypes by treatment number and tissue. Mice were implanted with 75,000 MB49 cells in the bladder and given 1, 2, 3, or 4 intravesical treatments with CS/IL-12 or PBS on days 7, 11, 15, and 18, respectively (n = 4–5 per group). Bladder draining lymph nodes (BDLNs), bladders, and spleens were harvested 24 h after each treatment and analyzed via flow cytometry. Error bars indicate mean with SEM. Asterisks indicate significant differences between PBS and CS/IL-12 treatment groups at the same treatment number by Student's t-test: *p < 0.05.
Figure 6.
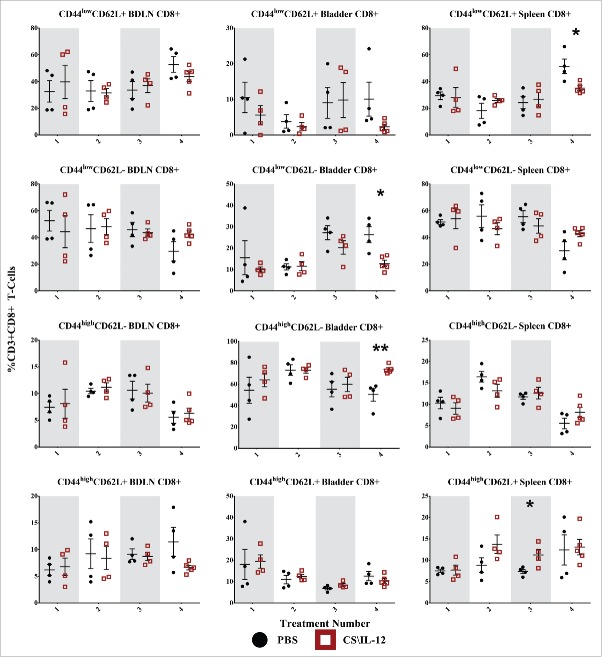
CD8+ T-cell effector/memory phenotypes by treatment number and tissue. Mice were implanted with 75,000 MB49 cells in the bladder and given 1, 2, 3, or 4 intravesical treatments with CS/IL-12 or PBS on days 7, 11, 15, and 18, respectively (n = 4–5 per group). Bladder-draining lymph nodes (BDLNs), bladders, and spleens were harvested 24 h after each treatment and analyzed via flow cytometry. Error bars indicate mean with SEM. Asterisks indicate significant differences between PBS and CS/IL-12 treatment groups at the same treatment number by Student's t-test: *p < 0.05 or **p < 0.005.
CD4+ T-cell effector/memory phenotypes
CD4+ CD44lowCD62L+ Tnaive cells were increased in the BDLNs following the first treatment, but this difference was not sustained after subsequent treatments (Fig. 5, first row). There was also a general (but not significant) trend toward increased proportions of Tnaive in the BDLNs for both CS/IL-12 and PBS treatments. The proportion of CD4+ bladder-infiltrating lymphocytes (BILs) that were Tnaive cells remained small (< 15%) throughout the treatments with a significantly (p < 0.05) smaller proportion in the CS/IL-12 treatment group by the fourth treatment. There was a 60% (p < 0.05) increase of Tnaive in the spleens of treated mice after the second treatment when compared with PBS at the same time point, but this difference was not maintained.
CD4+ CD44lowCD62L− TEff cells began as the dominant phenotype in both the BDLNs and spleen after the first treatment, but decreased over time (Fig. 5, second row). There was no difference in treatment groups in either the spleen or BDLNs at any time point. However, there was a decrease (p < 0.05) of TEff cells in CS/IL-12 treated bladders versus PBS treated bladders after the fourth time point as well as a trend toward fewer TEff over treatment number in the spleen (p < 0.05 via ANOVA).
CD4+ CD44highCD62L− TEM cells were the dominant phenotype in the bladder comprising approximately 60% of CD4+ cells after the first CS/IL-12 treatment with a significant climb (p < 0.05 via ANOVA) to 71% after the fourth treatment (Fig. 5, third row). After the fourth treatment, there was also a significant increase (p < 0.05) between treatment groups from 39.2% (PBS) to 71.4% (CS/IL-12). TEM infiltration in the BDLNs or spleen was less pronounced throughout the duration of treatment comprising 10–15% (BDLNs) and 20–30% (spleen) of CD4+ T cells. There was a higher (p < 0.05) proportion of TEM in the BDLNs of PBS treated mice when compared with CS/IL-12 treated mice after the second treatment. There was also an increase (p < 0.05) in the spleens of CS/IL-12 versus PBS treated mice, but the differences and proportions remained small.
CD4+ CD44highCD62L+ TCM cells increased significantly (p < 0.05) in proportion in the bladder after the first treatment from PBS (9.1%) to CS/IL-12 (21.2%), but this difference was not maintained after additional treatments (Fig. 5, fourth row). Although there was a trend toward increasing this population over time in both the spleen (NS) and BDLNs (p < 0.05 via ANOVA), the total proportion remained small (<10%) and there were no significant differences between treatment types in these tissues.
Overall, treatment number and type impacted CD4+ memory phenotypes. CD4+ T cells were characterized by an early jump (treatments 1 and 2) in naive phenotypes at systemic sites. This was followed by a late shift toward effector/memory phenotypes after the fourth treatment both in the bladder and in the spleen.
CD8+ T-cell effector/memory phenotypes
CD8+ CD44lowCD62L+ Tnaive cells showed minimal variation by treatment type or number (Fig. 6, first row), but did make up a large (40–60%) proportion of CD8+ cells in both the BDLNs and spleen. The sole significant difference was a decrease (p < 0.05) in the spleen for treated mice after the fourth treatment.
CD8+ CD44lowCD62L− TEff cells were also present in large (40–60%) proportions in both the BDLNs and spleen without any significant differences between treatment groups (Fig. 6, second row). They comprised a smaller portion (10–30%) of CD8+ cells in the bladder with an increased (p < 0.05) proportion for PBS (26.4%) over treated (12.8%) mice after the fourth treatment. There was also a significant effect (p < 0.05 via ANOVA) of CS/IL-12 treatment number on TEff infiltration in the bladder.
CD8+ CD44highCD62L− TEM cells were the largest (50–75%) proportion of CD8+ cells in the bladder with the proportion in treated mice (73.8%) reaching significantly (p < 0.005) higher than PBS (50.3%) after the fourth treatment (Fig. 6, third row). Their proportion in the BDLNs and spleen were smaller (5–15%) without any significant differences between treatments types. However, there was a significant effect on CS/IL-12 treatment number on TEM proportions in the spleen (p < 0.05 via ANOVA).
CD8+ CD44highCD62L+ TCM cells exhibited little variation with treatment type in any of the tissues with the sole significant difference between treatment types (p < 0.05) an increase after the third treatment in the spleen (Fig. 6, fourth row). There was, however, a significant effect of treatment number on their proportion in the bladder (p < 0.005 via ANOVA). They consistently comprised a small (5–20%) overall percentage of CD8+ T cells in each tissue.
Overall, treatment number and type impacted CD8+ memory phenotypes. However, changes were only found in the bladder and spleen, but not in the BDLNs. These effects were characterized by a late shift (treatments 3 and 4) toward effector/memory phenotypes.
Regulatory T-cells decline in the lymph nodes after the first treatment
Differences in TRegs (CD3+CD4+CD25+Foxp3+) were most pronounced after the first treatment with a 30% decrease (p <0.005) in the BDLNs. This was reflected in the CD8+:TReg ratio as well, with ratios of 19 (PBS) and 29.7 (CS/IL-12). However, these differences were not sustained, nor were they found in the bladder or spleen at any other time point. However, CS/IL-12 treatment number did decrease infiltration in the spleen (p < 0.05 via ANOVA), but not in the bladder or BDLNs.
Natural killer cell infiltration is minimally affected
We identified natural killer (NK) cells as CD3−CD49b+ leukocytes (Fig. 8, third row). Despite the depletion studies (Fig. 1A) demonstrating a role for NK cells in tumor rejection, we observed few significant differences in their infiltration with treatment type or number. In the BDLNs, these changes included a small increase after the first treatment as well as a decrease (p < 0.05) after the fourth treatment. While in the bladder, there was a small decrease after the first treatment in CS/IL-12 versus PBS treated mice. Notably, the tumor itself seemed to cause substantial NK infiltration as seen by the large proportion of NK cells (∼10%) in bladders of PBS treated mice. While not assayed in the current study, the role of CS/IL-12 may be to activate these resident NK cells in lieu of increasing their infiltration.
Figure 8.
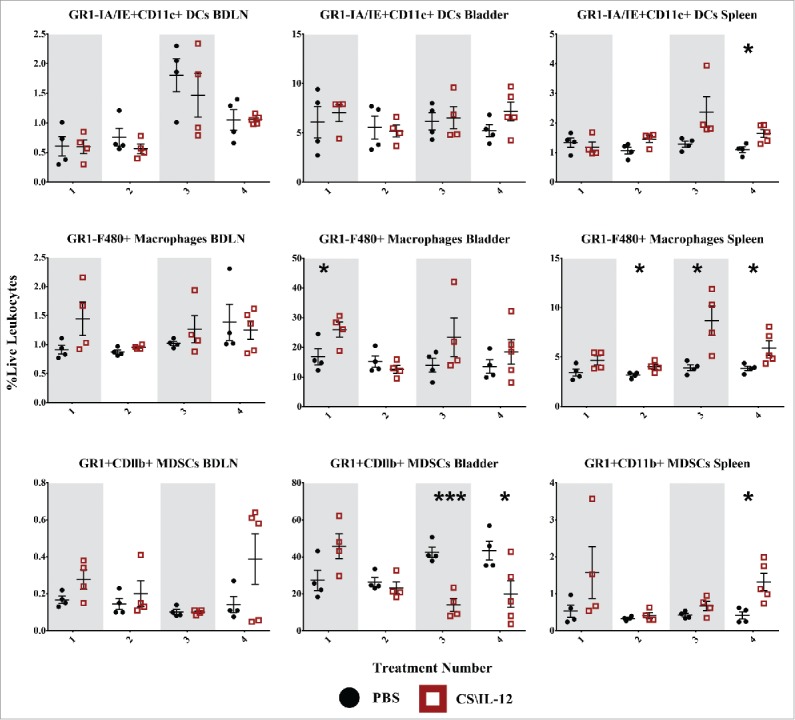
Myeloid cell infiltration by treatment number and tissue. Mice were implanted with 75,000 MB49 cells in the bladder and given 1, 2, 3, or 4 intravesical treatments with CS/IL-12 or PBS on days 7, 11, 15, and 18, respectively (n = 4–5 per group). Bladder draining lymph nodes (BDLNs), bladders, and spleens were harvested 24 h after each treatment and analyzed via flow cytometry. Error bars indicate mean with SEM. Asterisks indicate significant differences between PBS and CS/IL-12 treatment groups at the same treatment number by Student's t-test: *p < 0.05 or ***p < 0.0005.
Dendritic cell populations are minimally affected
To identify a potential source of antigen presenting cells (APCs) as drivers of systemic immunity we gated on GR1-MHCII+CD11C+ dendritic cells (DCs) at each time point (Fig. 6, first row). However, there were no obvious changes in DC populations between treatment types with the exception of a slight increase after the final treatment in the spleen for CS/IL-12 versus PBS treated mice (p < 0.05). There was also a significant (p < 0.05 via ANOVA) increase in DC infiltration in the BDLNs with respect to treatment number. Overall, CS/IL-12 had minimal effects on DC infiltration.
Macrophages drive initial infiltration and are likely sources of antigen presentation
Another possible source of antigen presentation, macrophages (GR1−F4/80+) were significantly (p < 0.05) increased in the bladder following the first treatment with 26.0% (CS/IL-12) and 16.8% (PBS) of all live leukocytes staining as macrophages (Fig. 8, second row). There was also an up to 2-fold increase of macrophages in the spleens of CS/IL-12 versus PBS treated mice that was sustained after the second treatment. There was also a significant increase (p < 0.05 via ANOVA) of macrophages in the spleen with treatment number. Overall, CS/IL-12 caused early macrophage infiltration in the bladder, later infiltration in the spleen, and no effects on macrophages in the BDLNs.
MDSCs decline in the bladders of treated mice
We were also curious about the role of GR1+CDllb+ MDSCs on tumor rejection or growth (Fig. 8, third row). We found that MDSCs were very prevalent in the bladder tumors, comprising more than 50% of all live cells in the case of some PBS treated tumors. We found that levels of MDSCs in CS/IL-12 treated mice seemed to increase in all tissues after the initial treatment though not in a statistically significant manner. There was a significant effect (p < 0.05 via ANOVA) of treatment number on MDSC infiltration in the bladder. This was coupled with a decrease in MDSCs in the bladder for CS/IL-12 versus PBS treated mice after the third(p < 0.0005) and fourth (p < 0.05) treatments with levels reaching less than a third of those in PBS treated mice. By the fourth treatment, there was also a 3-fold increase (p < 0.05) of MDSCs in the spleen, although the overall percentage remained small. There was no significant effect of treatment number or type on MDSCs in the BDLNs. Overall, CS/IL-12 caused a substantial depopulation of MDSCs in the bladder, a small uptick of MDSCs in the spleen, and did not impact MDSCs in the BDLNS.
Discussion
These are the first studies to elucidate the local and systemic immunological mechanisms of intravesical IL-12-based immunotherapy. Our depletion studies (Fig. 1A) demonstrated the importance of cytotoxic CD8+ effector cells in eliminating an initial bladder tumor, a finding that is unsurprising as cytotoxic T cells are known to directly engage tumor cells. However, distant (subcutaneous) rechallenge of previously treated mice which had eliminated their orthotopic tumors revealed that CD4+ helper T cells are vital for protective systemic memory (Fig. 1B). Indeed, these cells were able to eradicate new tumors even during depletion of cytotoxic T cells.
While surprising at first glance, antitumor CD4+ helper T cells activity has been demonstrated previously, although their role as antitumor immune cells is still being unraveled.19,20 It has been established that CD4+ T cells help recruit and activate tumor specific CD8+ T cells and promote their survival,16,21,22 but studies have also begun to show the ability of CD4+ T cells to kill tumor cells in the absence of CD8+ T cells.20 One example related to our current study showed that adoptively transferred, monoclonal, antigen-specific CD4+ T cells were more efficient than CD8+ T cells at rejecting subcutaneous MB49 bladder tumors, even when the tumors did not express MHC-II.23 That study also identified NK cells as important effectors in the absence of CD8+ T cells. However, unlike our own study and likely due to limitations of their model system, they did not demonstrate the role of CD4+ cells in a memory response, nor in an orthotopic model. Thus our study provides additional evidence into the important role that CD4+ cells play in antitumor immunity and in memory. However, the mechanistic way in which they do so has not been fully explored here and should be the subject of future investigations.
The second question we addressed was the role of the number of treatments on successful therapy. Somewhat surprisingly, we found that even a single treatment was effective at eliminating tumors in 50% of mice, but it required 3 or 4 treatments to reach higher levels of cure (Fig. 2). Nonetheless, four treatments are a vast improvement over BCG, the current standard of care, which shows no efficacy against MB49 after four treatments7 and in the clinic maximal efficacy, requires an induction course of 6 weekly treatments followed by 7 maintenance courses of 3 applications each for a total of 27 applications.24
Building upon those results we asked our third major question: how are immune cell kinetics within the tumor and in secondary lymphoid organs affected by each treatment. This is not the first study to investigate the immune infiltration of tumors caused by an IL-12-based treatment, but it is the first to do so in a quantitative manner using an orthotopic model of bladder cancer and the first to look at the infiltration over the course of multiple treatments.8,9,11,12,25 Our investigation revealed a role for each of the treatments in reversing immunosuppression and promoting a long-term immune response.
The first treatment was found to be critical for inducing macrophage and granulocyte inflammation, reducing TReg infiltration in the BDLNS, and increasing the CD8+:TReg ratio in the BDLNs (Fig. 7). Curiously, the first treatment also demonstrated increased infiltration of TCM CD4+ T cells in the bladder perhaps as an augmentation of the resident TCM population induced by MB49 implantation. The second treatment appeared to be a turning point of sorts at which several populations were in flux, but with a few detectible shifts in populations. At this stage, cells appeared ready to divide and infiltrate (as seen through increased naïve CD4+ T cells in Fig. 5) while macrophage expansion occurs in the spleen (Fig. 8). After the third treatment, the differences in immune infiltration become more apparent with increased CD4+ and CD8+ T-cell infiltration in the bladder (Fig. 4) as well as decreased MDSC infiltration (Fig. 8). Changes on the periphery included increased CD8+ T cells in the BDLNs coupled by a decrease of T cells in the spleen, possibly a result of their exfiltration to the tumor site as effectors (Fig. 4). The fourth treatment seemed to solidify the change from an immunosuppressive to an active phenotype. In the bladder, this change was characterized by most immune cells becoming effector-memory T cells (Figs. 5 and 6) with minimal lingering innate inflammation (Fig. 8). In the BDLNs, it was shown by continued naive/effector CD8+ T-cell infiltration, whereas in the spleen it was evidenced by increased macrophages, DCs, and granulocytes (Fig. 8) as well as a shift in CD4+ cells to an effector-memory phenotype (Fig. 5).
Figure 7.
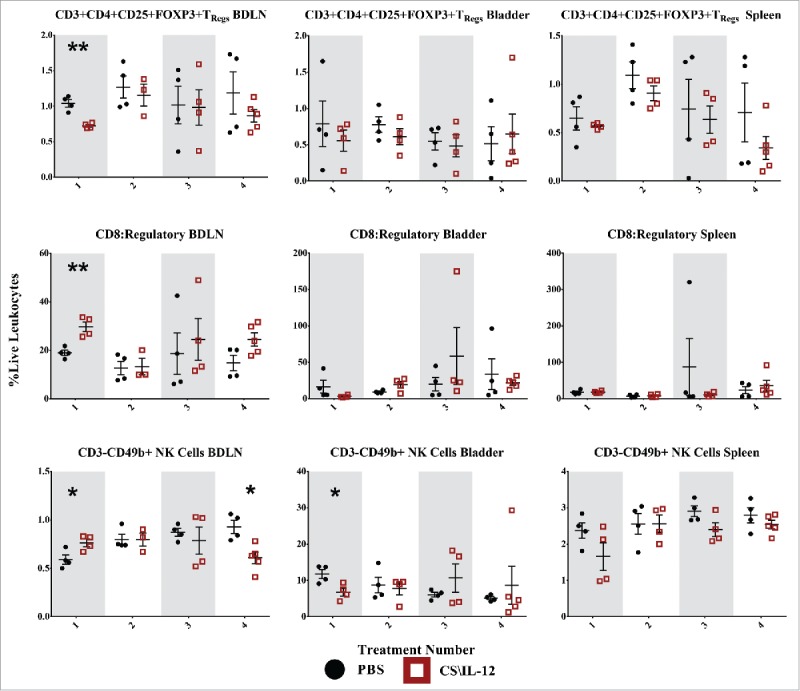
Regulatory T-cell and natural killer cell infiltration by treatment number and tissue. Mice were implanted with 75,000 MB49 cells in the bladder and given 1, 2, 3, or 4 intravesical treatments with CS/IL-12 or PBS on days 7, 11, 15, and 18, respectively (n = 4–5 per group). Bladder draining lymph nodes (BDLNs), bladders, and spleens were harvested 24 h after each treatment and analyzed via flow cytometry. Error bars indicate mean with SEM. Asterisks indicate significant differences between PBS and CS/IL-12 treatment groups at the same treatment number by Student's t-test: **p < 0.005.
Although this study has added to the understanding of intravesically delivered IL-12 immunotherapies, it does have limitations. First, in order to plumb the depths of immune cell changes, we focused on a single tumor model. Future studies ought to explore immune infiltration in additional orthotopic tumor models and in additional species. Second, this study focused on immune cell phenotype and not on function. Future studies ought to investigate cytokine release, antigen presentation, and cellular activation. This is especially true in the case of NK cells, which were not shown in this current study to infiltrate into the bladder in response to CS/IL-12, but did play a role in therapy during depletion studies and comprised a substantial population in the bladder even in the absence of therapy. Third, we did not look at immune populations in the blood which could be useful as a more clinically palatable monitor of treatment efficacy. Fourth, the current study suggests that treatments with CS/IL-12 have effects long after their initial application as evidenced by the delay in tumor weight (Fig. 3) in relation to survival benefit (Fig. 2) as well as the surprising effectiveness of a single CS/IL-12 treatment. Thus, this study opens the potential for additional investigations into the state of bladder tumor suppression, tumor escape, and immune cell fluctuations after a single treatment of CS/IL-12.
Nevertheless, this study provides another stepping stone on the path to IL-12-based immunotherapies for bladder cancer. The current studies could provide the basis for correlative studies in planned Phase I and II clinical trials. Likely readouts would include a shift in the CD8+:TReg ratio from a draining lymph node (or potentially blood), or a demonstrated increase in macrophage infiltration in the bladder tumor after the first treatment with continued monitoring of T-cell infiltration throughout treatment.
Methods
Animals, materials, and cell lines
The University of Arkansas Institutional Animal Care and Use Committee approved of all animal protocols in compliance with The Guide for Care and Use of Laboratory Animals (National Research Council). Female C57BL/6J mice were purchased from Jackson Laboratory, housed in microisolator cages, and placed on study at 9–15 weeks of age.
Dulbecco's Phosphate Buffered Saline (DPBS), Roswell Park Memorial Institute Medium (RPMI), Dulbecco's Modified Eagle's Medium (DMEM), and Fetal Bovine Serum (FBS) were purchased from HyClone Laboratories. Chitosan glutamate (Protosan G213, 200–600 kDa, 75–90% deacetylated) was obtained from Novamatrix (4210306). Recombinant murine IL-12 was provided by the University of Arkansas Biologics Center. Triple enzyme digestive solution was prepared from collagenase type IV (10 mg/mL, MP Biomedicals, 0219511090), Hyaluronidase (1 mg/mL, MP Biomedicals, 0210074080), and DNase I (200 mg/ml, VWR, 97062-108) in Hanks Balanced Salt Solution (Corning). Lympholyte-M was obtained from CedarLane (CL5031).
MB49, a C57BL/6 syngeneic transitional cell carcinoma, was kindly provided by Dr. Jeffrey Schlom, Laboratory of Tumor Immunology and Biology, National Cancer Institute. MB49 was maintained in DMEM supplemented with 10% FBS, 2 mM L-glutamine, and 1% penicillin/streptomycin. Complete media for the culture of cells isolated from tissues was composed of RPMI supplemented with 10% FBS, 2mM β-Mercaptoethanol (55 µM), penicillin/streptomycin (100 U/mL), L-glutamine (2 mM), Sodium Pyruvate (1 mM), and non-essential amino acids.
Tumor model and therapy
Orthotopic bladder tumors were implanted via intravesical instillation of 75,000 MB49 cells as described previously.7 Intravesical tumor burden was monitored by development of hematuria and palpable tumors. Intravesical treatments were given using the same catheterization technique. Briefly, the therapy was prepared by first dissolving chitosan glutamate in DPBS at 10 mg/mL in a shaker (1400 RPM, 55 °C, 1 h) and then adding IL-12 to a final concentration of 10 µg/mL. The mice were catheterized and 100 µL of therapy allowed to dwell for 35–45 min. All administration of CS/IL-12 described in this manuscript consisted of a 1 µg dose of IL-12.
All subcutaneous implantations were of 300,000 MB49 cells administered in the right flank and the tumor volume calculated as Volume = 0.5∗Length ∗Width.2Mice were euthanized when moribund or when tumor volume reached 2,000 mm3.
Depletion studies
Depletion antibodies for CD4+ (BE0003), CD8+ (BE0061), or NK1.1 (BE0036) were obtained from BioXCell. Mice were depleted prior to starting the experiment via four once-daily administrations of 100 µg depletion antibodies given intraperitoneally. This depletion was maintained throughout the study by twice weekly administrations of 100 µg of antibody. The depletion protocol was verified via flow cytometry (Fig. S1).
Tissue homogenization and flow cytometry
Bladder tumors, BDLNs, and spleens were obtained for single cell analysis of immune cell infiltrates via flow cytometry. Bladder and tumor tissues were weighed and imaged before being cut into small pieces in RPMI, allowed to digest in triple enzyme concoction for 30 min at 37 °C, passed through a 60-µm filter, separated via a Lympholyte gradient, and subjected to ACK lysis prior to staining. BDLNs were taken to be the lumbar lymph nodes.26 The spleen as well as the BDLNs were harvested into complete media and homogenized using the 60-µm filter. Splenocytes were also subjected to an ACK lysis step. Prior to staining, cell concentrations and numbers were determined via a manual hemacytometer.
Cells were stained with a fixable live/dead discriminator, treated with FC Block (BD 553142), and then surface stained with pre-titrated amounts of antibodies prior to fixation, permeabilization, and intracellular staining. Fixation/permeabilization was implemented with either BD Fix/Perm (5230583) or for panels containing FoxP3 BD Fix/Perm TF (562574). Samples were then acquired on a Becton Dickinson (Franklin Lakes, NJ) FACSCanto II cytometer equipped with red (633 nm) and blue (488 nm) lasers. Data analysis was performed using FlowJo (Ashland, Oregon).
All antibodies and stains were purchased from Becton Dickinson: Live/Dead fixable viability dye-FITC (564407), CD3-PE (553064), CD4-PE-Cy7 (552775), CD8-Percp-Cy5.5 (551162), CD44-APC (559250), CD62L-APC-Cy7 (560514), CD49b-APC (560628), CD25-APC-Cy7 (557658), FoxP3-PerCp-Cy5.5 (563902), GR-1-PE-Cy7 (560601), F4/80-PE (565410), CDllb-APC-Cy7 (557657), CDllc-APC (550261), and I-A/I-E-Percp-Cy5.5 (562363). Overall, analysis was performed on three panels (Fig. S2) as well as isotype controls (Fig. S3) and fluorescence minus-one (FMO) controls for CD44 and CD62L (Fig. S3).
Statistical analysis
Data was plotted and statistical analysis performed with Graphpad Prism with data pre-processing implemented in MATLAB. For survival curves, significance was determined via the log-rank test. An unpaired Student's t-test was used to compare PBS and CS/IL-12 treatments at each time point for bladder weights and immunophenotyping studies. An ordinary one-way ANOVA was used to examine the effects of CS/IL-12 treatment number on immunophenotypes. Significance levels denoted as not significant (NS), *p < 0.05, **p < 0.005, or ***p < 0.0005.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the National Institutes of Health, National Cancer Institute (R01CA172631, R15CA176648).
References
- 1.American Cancer Society Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016 [Google Scholar]
- 2.Douglass L, Schoenberg M. The future of intravesical drug delivery for non-muscle invasive bladder cancer. Bladder Cancer 2016; 2:285-92; PMID:27500196; http://dx.doi.org/ 10.3233/BLC-160056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman D, Shipley W, Feldman A. Bladder cancer. Lancet 2009; 374:239-49; PMID:19520422; http://dx.doi.org/ 10.1016/S0140-6736(09)60491-8 [DOI] [PubMed] [Google Scholar]
- 4.Smith S, Zaharoff D. Future directions in bladder cancer immunotherapy: towards adaptive immunity. Immunotherapy 2016; 8(3):351-65; PMID:26860539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamie K, Litwin MS, Bassett JC, Daskivich TJ, Lai J, Hanley JM, Konety BR, Saigal CS. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer 2013; 119:3219-3227; PMID:23737352; http://dx.doi.org/ 10.1002/cncr.28147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bidnur Savdie, Black PC. Inhibiting immune checkpoints for the treatment of bladder cancer. Bladder Cancer 2016; 2:15-25; PMID:27376121; http://dx.doi.org/ 10.3233/BLC-150026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaharoff D, Hoffman B, Hooper B, Benjamin C, Khurana K, Hance K, Rogers C, Pinto P, Schlom J, Greiner J. Intravesical immunotherapy of superficial bladder cancer with chitosan/interleukin-12. Cancer Res 2009; 69:61926199; PMID:19638573; http://dx.doi.org/23453060 10.1158/0008-5472.CAN-09-1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Zaharoff D. Role of chitosan co-formulation in enhancing interleukin-12 delivery and antitumor activity. Biomaterials 2013; 34:3828-36; PMID:23453060; http://dx.doi.org/ 10.1016/j.biomaterials.2013.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith S, Koppolu B prasanth, Ravindranathan S, Kurtz S, Yang L, Katz M, Zaharoff D. Intravesical chitosan/interleukin-12 immunotherapy induces tumor-specific systemic immunity against murine bladder cancer. Cancer Immunol Immunother 2015; 64:689-96; http://dx.doi.org/ 10.1007/s00262-015-1672-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaharoff D, Hance K, Rogers C, Schlom J, Greiner J. Intratumoral immunotherapy of established solid tumors with chitosan/IL-12. J Immunother 2010; 33:697; PMID:20664357; http://dx.doi.org/ 10.1097/CJI.0b013e3181eb826d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vo J, Yang L, Kurtz S, Smith S, Koppolu B prasanth, Ravindranathan S, Zaharoff D. Neoadjuvant immunotherapy with chitosan and interleukin-12 to control breast cancer metastasis. OncoImmunol 2014; 3:e968001; PMID:25964864; http://dx.doi.org/ 10.4161/21624011.2014.968001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilinc M, Gu T, Harden J, Virtuoso L, Egilmez N. Central role of tumor-associated CD8+ T effector/memory cells in restoring systemic antitumor immunity. J Immunol Baltim Md 1950 2009; 182:4217-25; PMID:19299720; http://dx.doi.org/17082611 10.4049/jimmunol.0802793 [DOI] [PubMed] [Google Scholar]
- 13.Kilinc MO, Aulakh KS, Nair RE, Jones SA, Alard P, Kosiewicz MM, Egilmez NK. Reversing tumor immune suppression with intratumoral IL-12: activation of tumor-associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effectors. J Immunol 2006; 177:6962-73; PMID:17082611; http://dx.doi.org/ 10.4049/jimmunol.177.10.6962 [DOI] [PubMed] [Google Scholar]
- 14.vom Berg J, Vrohlings M, Haller S, Haimovici A, Kulig P, Sledzinska A, Weller M, Becher B. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell–mediated glioma rejection. J Exp Med 2013; 210:2803-11; http://dx.doi.org/ 10.1084/jem.20130678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baaten B, Li C-R, Deiro M, Lin M, Linton P, Bradley L. CD44 regulates survival and memory development in Th1 cells. Immunity 2010; 32:104-115; PMID:20079666; http://dx.doi.org/ 10.1016/j.immuni.2009.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Church SE, Jensen SM, Antony PA, Restifo NP, Fox BA. Tumor-specific CD4+ T cells maintain effector and memory tumor-specific CD8+ T cells. Eur J Immunol 2014; 44:69-79; PMID:24114780; http://dx.doi.org/ 10.1002/eji.201343718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiser J, Banerjee A. Effector, Memory, and Dysfunctional CD8. J Immunol Res 2016; 2016:1-14; PMID:27314056; http://dx.doi.org/ 10.1155/2016/8941260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan L, Gurnani K, Dicaire C, van Faassen H, Zafer A, Kirschning C, Sad S, Sprott D. Rapid clonal expansion and prolonged maintenance of memory CD8+ T cells of the effector (CD44highCD62Llow) and central (CD44highCD62Lhigh) phenotype by an archaeosome adjuvant independent of TLR2. J Immunol 2007; 178:2396-406; PMID:17277146; http://dx.doi.org/ 10.4049/jimmunol.178.4.2396 [DOI] [PubMed] [Google Scholar]
- 19. Kim H, Cantor H. CD4 T-cell Subsets and Tumor Immunity: The Helpful and the Not-so-Helpful. Cancer Immunol Res 2014; 2(2):91-8; http://dx.doi.org/10.1158/2326-6066.CIR-13-0216 [DOI] [PubMed] [Google Scholar]
- 20. Zanetti M. Tapping CD4 T cells for cancer immunotherapy: the choice of personalized genomics. J Immunol 2015; 194(5):2049-56; http://dx.doi.org/10.4049/jimmunol.1402669 [DOI] [PubMed] [Google Scholar]
- 21. Bos R, Sherman L. CD4+ T-Cell Help in the Tumor Milieu Is Required for Recruitment and Cytolytic Function of CD8+ T Lymphocytes. Cancer Res 2010; 70(21):8368-77; http://dx.doi.org/10.1158/0008-5472.CAN-10-1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong J, Bos R, Sherman L. Tumor-specific CD4+ T cells render the tumor environment permissive for infiltration by low-avidity CD8+ T cells. J Immunol 2008; 180(5):3122-31; http://dx.doi.org/10.4049/jimmunol.180.5.3122 [DOI] [PubMed] [Google Scholar]
- 23. Perez-Diez A, Joncker N, Choi K, Chan W, Anderson C, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 2007; 109(12):5346-54; http://dx.doi.org/10.1182/blood-2006-10-051318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kamat A, Flaig T, Grossman B, Konety B, Lamm D, O’Donnell M, Uchio E, Efstathiou J, Taylor J. Expert consensus document: Consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nat Rev Urol 2015; 12(4):225-35; http://dx.doi.org/10.1038/nrurol.2015.58 [DOI] [PubMed] [Google Scholar]
- 25. Egilmez N, Kilinc M, Gu T, Conway T. Controlled-release particulate cytokine adjuvants for cancer therapy. Endocr Metab Immune Disord Drug Targets 2007; 7(4):266-70; http://dx.doi.org/10.2174/187153007782794335 [DOI] [PubMed] [Google Scholar]
- 26. Collste L, Darzynkiewicz Z, Traganos F, Sharpless T, Whitmore W, Melamed M. Regional lymph node reactivity in explanted bladder cancer of mice as measured by flow cytometry. Cancer Res 1979; 39(6 Pt 1):2120-4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.