Abstract
Imprinted genes display biased expression of paternal and maternal alleles and are only found in mammals and flowering plants. Compared to several hundred imprinted genes that are functionally characterized in mammals, very few imprinted genes were confirmed in plants and even fewer of them have been functionally investigated. Here, we report a new imprinted gene, NUWA, in plants. NUWA is an essential gene, because loss of its function resulted in reduced transmission through the female gametophyte and defective cell/nuclear proliferation in early Arabidopsis embryo and endosperm. NUWA is a maternally expressed imprinted gene, as only the maternal allele of NUWA is transcribed and translated from gametogenesis to the 16-cell globular embryo stage after fertilization, and the de novo transcription of the maternal allele of NUWA starts from the zygote stage. Different from other identified plant imprinted genes whose encoded proteins are mostly localized to the nucleus, the NUWA protein was localized to the mitochondria and was essential for mitochondria function. Our work uncovers a novel imprinted gene of a previously unidentified type, namely, a maternal-specific expressed nuclear gene with its encoded protein localizing to and controlling the function of the maternally inherited mitochondria. This reveals a unique mechanism of maternal control of the mitochondria and adds an extra layer of complexity to the regulation of nucleus-organelle coordination during early plant development.
Author Summary
Imprinted genes are only found in mammals and flowering plants, and they express with parent-of-origin-specific patterns. Unlike in mammals, only a few imprinted genes was identified and functionally characterized in plants, and almost all of them encode nuclear proteins. Here, we identified NUWA as a new type of imprinted gene in Arabidopsis. NUWA has maternal-specific expression and controls the early seed development in Arabidopsis, with its encoded protein localizing to and functioning in the maternally inherited mitochondria. The discovery of NUWA reveals a unique mechanism of maternal control of mitochondrial function and adds an extra layer of complexity to nucleus-organelle coordination. It further indicates that, similar to mammals, imprinted genes in plants are also involved in various subcellular biological processes.
Introduction
Genomic imprinting is a phenomenon in which somatic cells express some genes only from the maternal or paternal chromosome [1]. Genes with parent-of-origin-specific allele-biased expression patterns are called imprinted genes [2,3]. Imprinted genes have only been discovered and confirmed in placental mammals and flowering plants and are thought to have evolved independently through convergent evolution in these two groups [4,5]. Imprinting is regulated epigenetically and plays important roles in mediating complex traits in both mammals and plants [6–8]. In mammals, imprinted genes are essential not only in embryonic development and placental development of the fetus but also in sensory function and behavior in adults [9–11]. Loss-of-function of these genes always results in severe disease [12,13]. In plants, imprinted genes are also involved in early development, and the loss-of-function of these genes can lead to seed-lethal phenotypes [2,3].
In comparison with the hundreds of functional imprinted genes identified in mammals, confirmed imprinted genes in plants are rare, and many of them do not have obvious functions, because no phenotypes were observed in the loss-of-function mutants [1,13–17]. In addition, products of the imprinted genes in mammals have a variety of subcellular localizations and numerous molecular functions [18], whereas in plants, products of most of the confirmed imprinted genes are localized in the nucleus, and many are presumed to function in chromatin remodeling [19–28]. Although high-throughput analyses revealed many putative imprinted genes in Arabidopsis [29,30], maize [31] and rice [32,33], most of them have not been confirmed. It is not known whether the imprinted genes in plants are important in many aspects of subcellular and biological processes, as in mammals, and the driving force of the convergent evolution of genomic imprinting therefore remains a mystery.
In this study, we have identified a novel maternally expressed imprinted gene NUWA in Arabidopsis, which is named after the well-known goddess in Chinese ancient mythology who created humans by molding figures from earth. NUWA is an essential gene. Loss of NUWA function resulted in defects in cell/nuclear proliferation in early embryogenesis and endosperm development. Moreover, NUWA is a maternally expressed imprinted gene, because de novo transcription of maternal allele-specific NUWA was detected in the embryo sac after fertilization. Different from those previously-identified plant imprinted genes whose products were localized to the nucleus, the NUWA protein was targeted to the mitochondria and was essential for development and proper function of the mitochondria. These results indicate that NUWA is a new type of imprinted gene that maternally controls early embryo and endosperm development through regulating the function of the maternally inherited mitochondria. The discovery of NUWA also reveals a new aspect of subcellular and metabolic processes in which plant imprinted genes are involved.
Results
nuwa is a mutant with defects in early embryogenesis and endosperm development
The mutant nuwa/+ was obtained from genetic screening of a mutant collection with a Basta/Phosphinothricin (PPT)-resistant gene in the T-DNA [34]. In nuwa/+ siliques, 37.5% of seeds aborted very early; 1.6% of seeds aborted somewhat later (n = 6685) (Fig 1A). In selfed nuwa/+ progeny, the PPT-resistant (PPTr) to PPT-sensitive (PPTs) ratio was approximately 1.33:1 (n = 4387). No nuwa/- homozygous mutant plants were acquired.
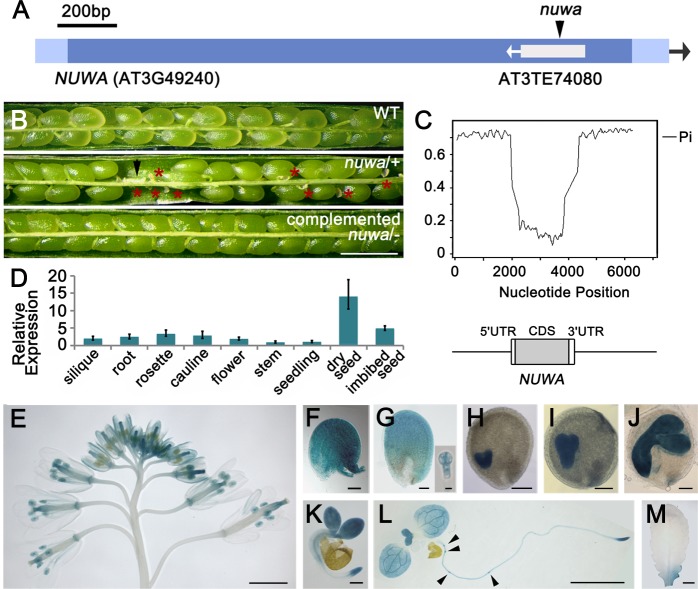
Fig 1. nuwa is a mutant with early embryo and endosperm developmental defects.
(A) Seed set of wild type and nuwa/+ siliques. Red stars and black arrows indicate earlier and later aborted seeds, respectively. Bar = 1 mm. (B) Early-stage embryos observed in wild type plant (upper row) and in nuwa/+ (lower row). Cells with abnormal morphology are marked by pseudo-colors. Percentages of aborted embryos are shown. Bars = 10 μm. (C) Endosperm nuclei development in 26 HAP ovules of wild type (upper row) and selfed nuwa/+ plants (lower row). Percentages of endosperm are shown. Red stars indicate embryo nuclei; yellow arrowheads mark endosperm nuclei. EnN, endosperm nuclei. Bars = 20 μm. (D) Ovules of nuwa/+ with the endosperm nuclei marked with FIS2-GUS. EnN, endosperm nuclei. Bars = 20 μm.
When we pollinated nuwa/+ with pollen of a fertilization indicator line FAC1-GUS, whose GUS signal appears 3 hours after fertilization in embryo sacs [35], the percentage of ovules with GUS signals was indistinguishable between wild type and nuwa/+ (|u| = 0.248 < u0.05 = 1.96, P > 0.05) (S1A to S1C Fig), suggesting that all of the defective female gametophytes of nuwa/+ could be fertilized. Therefore, we hypothesized that the reduction in the seed setting rate in nuwa/+ probably resulted from a defect in the embryo and/or endosperm after fertilization.
Using differential interference contrast (DIC) microscopy, we found that mutant embryos of nuwa/+ were arrested before the 16-cell stage. Most mutant embryos were arrested at the first three rounds of cell division, and some displayed abnormal cell shapes and irregular cell division patterns (Fig 1B). This result suggested that the mutant embryos were arrested at very early developmental stage, with cell division defects. Then, we analyzed endosperm development using confocal laser scanning microscopy. Only 42.7% of nuwa/+ ovules at 26 hours after pollination (HAP) were at the 8-endosperm-nuclei stage, which was significantly lower than the percentage in wild-type ovules (78.6%) (|u| = 3.91 > u0.01 = 2.58, P < 0.01) (Fig 1C). The percentage of ovules at 4-endosperm-nuclei stage and 2-endosperm-nuclei stage in nuwa were significantly higher than those in wild type (Fig 1C), indicating that the endosperm nuclei proliferations were slower in the mutant. After we crossed nuwa/+ with FIS2-GUS [36], we found that fewer spherical GUS signals indicating endosperm nuclei were observed in mutant ovules compared with normal ovules (Fig 1D, S1D to S1I Fig), further supporting the notion that endosperm nucleus proliferation is delayed in mutant ovules. The facts that both embryo and endosperm of nuwa/+ developed slowly after fertilization and were arrested at early stages demonstrate that NUWA is an essential gene.
NUWA encodes a conserved protein which is highly expressed in reproductive organs
Using TAIL-PCR, we found that the T-DNA in nuwa/+ mutant was inserted into the single exon of the gene At3g49240, which contains a transposable element, AT3TE74080 (Fig 2A). When the full-length genomic DNA of At3g49240 was introduced into nuwa/+, homozygous mutant plants with normal fertility were obtained (Fig 2B), indicating that the phenotypes of nuwa/+ were caused by loss of At3g49240 function. At3g49240 was designated as NUWA, the name of the Chinese goddess for human creation.
Fig 2. NUWA is mainly expressed in reproductive organs.
(A) The T-DNA insertion sites in nuwa/+. (B) Siliques of wild type (upper), nuwa/+ (middle) and complemented nuwa/- (lower). Red stars and the black arrow indicate early and late aborted seeds, respectively. Bar = 1 mm. (C) Nucleotide diversity in the genomic region centered on NUWA among 5 Brassicaceae species, i.e., A. thaliana, A. lyrata, C. rubella, B. rapa and E. salsugineum. (D) Real-time qPCR analysis of NUWA in various tissues. Error bars, mean ± SD. (E-M) GUS staining patterns in inflorescence (E), ovule before fertilization (F), ovule at 2–4 cell embryo stage (G), ovules at heart (H), torpedo (I), walking-stick (J) embryo stages, germinated seeds (K), 10-day-old seedlings (L) and rosette leaf (M) in pNUWA:NUWA-GFP-GUS transgenic plants. Arrowheads indicate lateral root primordia. Bars = 2 mm (E, L, M), 50 μm (F, G), 100 μm (H-J), 200 μm (M) or 10 μm (small figure in G).
Sequence similarity and synteny analysis showed that NUWA is a single copy gene with high sequence similarity in different plant species (S2 Fig). We then investigated the nucleotide diversity of a 6-Kb region centered around NUWA in five Brassicaceae species, and found that the nucleotide diversity of the coding region was significantly lower than that of the promoter region and its 3’ downstream sequence; and also lower than genome-wide average (0.007) [37] (Fig 2C; S2 Fig). This relative low level of nucleotide diversity supports that NUWA is conserved, with its evolution driven by purifying selection.
We investigated NUWA expression patterns at both transcriptional and protein levels. First, real-time quantitative RT-PCR (qRT-PCR) analysis showed that NUWA was transcribed in all organs tested and was highly expressed in dry seeds and imbibed seeds (Fig 2D). We then generated ProNUWA:NUWA-GFP-GUS transgenic plants to test the protein distribution pattern of NUWA. In the inflorescence, GUS signals appeared at the tips of pistils and the petals of young flowers (Fig 2E). In ovules, GUS activity was detected on integuments before (Fig 2F) or early after fertilization (Fig 2G), in embryos at 2–4 cell stage (Fig 2G), heart stage (Fig 2H), torpedo stage (Fig 2I) and walking stick stage (Fig 2J) and later. In seedlings, GUS activity was detected in cotyledons, young leaves, root tips and lateral root primordia (Fig 2K and 2L). Little GUS activity was observed in mature leaves (Fig 2M). These results suggest that NUWA is highly expressed in the reproductive organs of Arabidopsis.
NUWA is a maternal effect gene with its maternal allele specifically expressed before and after fertilization
When reciprocal crosses were conducted between nuwa/+ and wild-type plants, male transmission was normal, while female transmission was severely reduced to 15.6% (Fig 3A). The decreased female gametophyte transmission rate, together with the observed embryo and endosperm defects, implies that nuwa/+ is a maternal effect mutant. To verify this hypothesis, we pollinated nuwa/+ with either wild-type or nuwa/+ pollen. Seed-lethal phenotypes were observed in siliques (Fig 3B), and the percentage of aborted ovules were identical between the two crosses (|u| = 0.3255 < u0.05 = 1.96, P > 0.05) (S3A Fig), suggesting that the main phenotypes of the mutant seeds were controlled by the maternal genotype rather than that of both parents. These results confirmed that nuwa/+ is a maternal effect mutant.
Fig 3. NUWA is a maternal effect gene that predominantly expresses its maternal allele before and after fertilization.
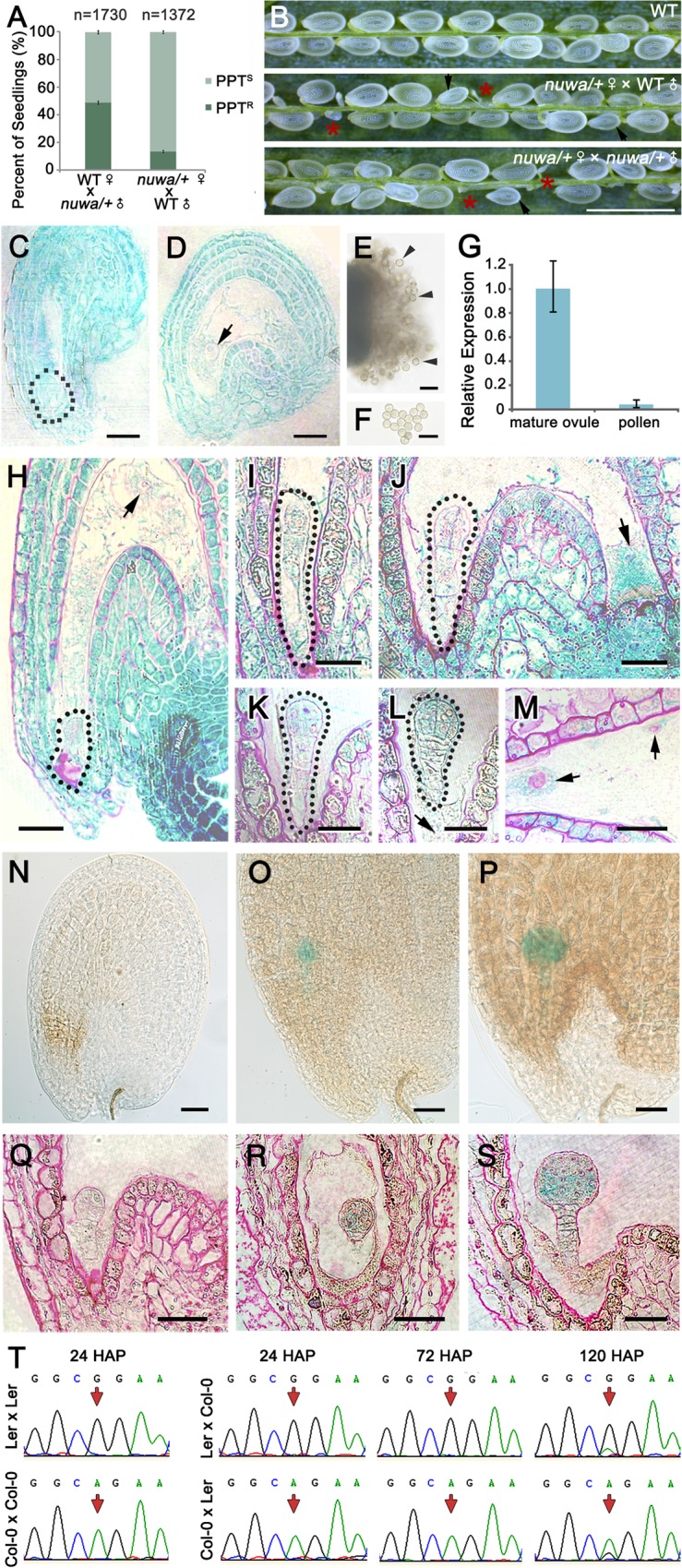
(A) Reciprocal crosses between wild type and nuwa/+. Error bars, mean ± SEM. (B) Siliques of selfed wild type (upper), nuwa/+ pollinated by wild type pollen (middle) and selfed nuwa/+ (lower). Red stars and black arrows indicate early and late aborted seeds, respectively. Bar = 1 mm. (C-F) GUS staining in mature female (C-D) and male gametophytes (E-F) in pNUWA:NUWA-GFP-GUS transgenic plants. The dotted line and the arrow indicate the egg apparatus (C) and the central cell nucleus (D), respectively. Bars = 20 μm. No GUS activity was detected in pollen grains (E-F). Arrowheads indicate pollen grains. Bars = 50 μm. (G) Transcript levels of NUWA in mature ovules and mature pollen revealed by real-time qPCR. Error bars, mean ± SD. (H-M) GUS-stained ovules of wild-type pollen-pollinated homozygous pNUWA:NUWA-GFP-GUS transgenic plants at zygote stage (H), 1-cell embryo stage (I), 2/4-cell embryo stage (J), 8-cell embryo stage (K), 16-cell embryo stage (L and M). Basic fuchsin dye was used to mark the boundary of cells. The dotted lines and arrowheads mark the embryos and the endosperm or endosperm nuclei. Bars = 20 μm. (N-S) GUS-stained ovules of homozygous pNUWA:NUWA-GFP-GUS transgenic pollen-pollinated wild-type plants at the 8-cell (N and Q), the 16-cell (O and R) and the 64-cell stages (P and S). The ovules were observed by tissue clearing (N-P) or resin sections stained with basic fuchsin (Q-S). Bars = 30 μm. (T) Parental origin of NUWA transcripts in fertilized ovules at different developmental stages analyzed by allele-specific RT-PCR and Sanger sequencing. The polymorphism between Col-0 and Ler are indicated from self-crosses as a control in the far left column. The polymorphic nucleotide in each cross is indicated by a red arrow.
To elucidate the cause of the maternal effect in nuwa/+, we first investigated the expression of NUWA before fertilization. We transformed the ProNUWA:NUWA-GFP-GUS construct into nuwa/+ to genetically complement the mutant and to investigate NUWA protein expression patterns in mature gametophytes in the wild-type background. GUS activity was observed in the egg apparatus (Fig 3C) and central cells (Fig 3D), but not in mature pollen located on the stigma (Fig 3E) or dispersed (Fig 3F). Real-time qPCR analysis showed that NUWA transcription levels were much lower in pollen than in mature ovules (Fig 3G). These results suggest that NUWA is predominantly expressed in female gametophytes before fertilization, with sparse expression occurring in male gametophytes.
We next analyzed the parent-of-origin-dependent expression patterns of the NUWA protein in ovules after fertilization. We reciprocally crossed homozygous ProNUWA:NUWA-GFP-GUS transgenic plants with wild-type ones. Maternal-specific GUS signal was detected in embryos and endosperm from the zygote to the 16-cell-embryo stage (Fig 3H to 3M). In contrast, a paternal-specific, embryo-specific GUS signal was not observed in early seeds (Fig 3N and 3Q) until the 16-cell-embryo stage (Fig 3O to 3P and 3R to 3S). These results indicate that only maternal-allele-specific expression of the NUWA protein is detected in early seeds.
To further analyze the parent-of-origin-dependent expression pattern of NUWA transcripts in ovules after fertilization, we adopted allele-specific RT-PCR. A single-nucleotide polymorphism (SNP) between Arabidopsis ecotypes Col-0 and Ler in the exon of NUWA was used to distinguish parental origins of endogenous NUWA transcripts in seeds resulting from reciprocal crosses. We found that, for Col-0×Ler cross, all the NUWA transcripts detected in 24 and 72 HAP were from Col-0 allele, and a small proportion of the transcripts detected in 120 HAP were from Ler allele. Similarly, for Ler×Col-0 cross, all the NUWA transcripts detected in 24 HAP and 72 were from Ler allele, and a small proportion of the transcripts detected in 120 HAP were from Col-0 allele. These results indicate that, while transcripts derived from the maternal allele were detected in seeds from 24 HAP to 120 HAP, transcripts derived from the paternal allele were not detected in seeds until 120 HAP (Fig 3T). This result suggests that in the early seeds, only maternal-allele-specific NUWA transcripts are present in ovules, with transcripts of paternal allele first emerging between 72 HAP and 120 HAP. These data indicate that the maternal allele of NUWA is specifically detected at both transcriptional and translational levels before and shortly after fertilization.
NUWA is an imprinted gene
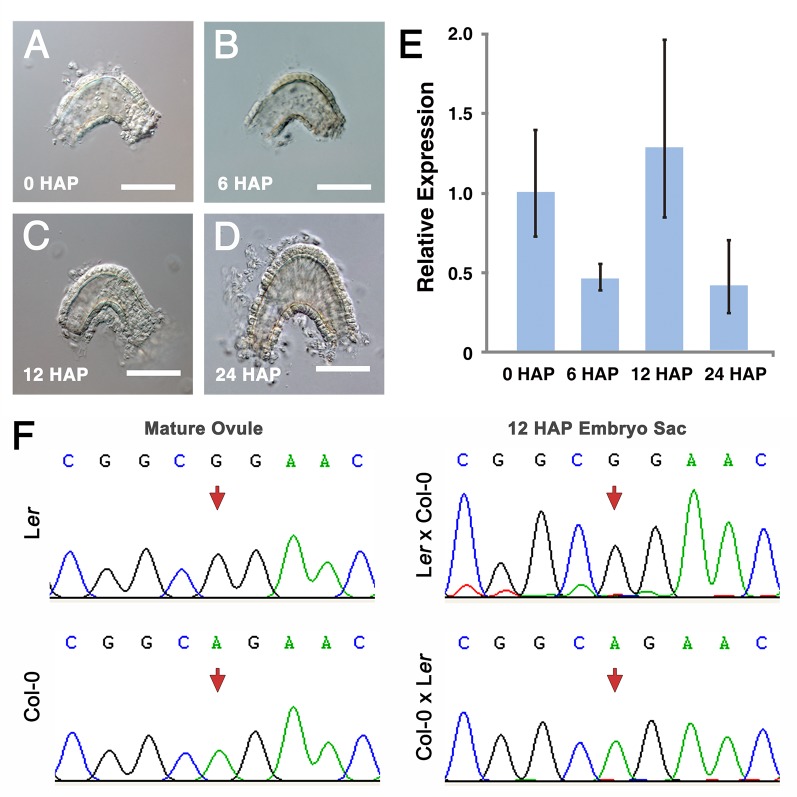
To precisely investigate the maternal expression pattern of NUWA, we analyzed our RNA-seq data of ovules at different developmental stages. We found that the amount of NUWA transcripts reduced at 6 HAP comparing to 0 HAP, then increased at 12 HAP, and decreased again at 24 HAP (S3B Fig). Because fertilization is completed within 6 HAP in Arabidopsis [38], this result reveals that NUWA is transcribed in ovules after fertilization. As NUWA expresses on integuments at early development stages, we then isolated wild type embryo sacs at 0 HAP, 6 HAP, 12 HAP and 24 HAP (Fig 4A to 4D) and examined transcripts of NUWA. The qRT-PCR results showed the transcript level of NUWA in embryo sacs decreased at 6 HAP before increasing at 12 HAP (Fig 4E), which confirmed that NUWA is transcribed de novo in embryo sacs after fertilization. Because the paternal allele of NUWA transcript is not detected before 120 HAP (Fig 3T), this result suggests that the de novo expressed NUWA transcripts are predominantly from the maternal allele. Since imprinted genes are known as genes expressed predominantly from one of their parental alleles during a period of diploid stage [2], our results also suggests that NUWA is an imprinted gene. To confirm this hypothesis, we isolated the embryo sacs at 12 HAP from progeny plants of reciprocal crosses between wild type Col-0 and Ler, extracted RNA and performed allele-specific RT-PCR. The results showed that in 12 HAP embryo sacs, only maternal-allele-specific NUWA transcripts were detected (Fig 4F), which supports that the de novo expression of NUWA transcripts detected in early developmental stage are predominantly from the maternal allele. These results indicate that NUWA is a maternally expressed imprinted gene.
Fig 4. NUWA is an imprinted gene.
(A-D) isolated embryo sacs at 0 HAP (A) (before fertilization), 6 HAP (B) (around fertilization), 12 HAP (C) and 24 HAP (D) (after fertilization). Bars = 50 μm. (E) Transcription level of NUWA in isolated embryo sacs by real-time qRT-PCR. Error bars, mean ± SD. (F) Parental origin of NUWA transcripts in isolated embryo sacs at 12 HAP analyzed by allele-specific RT-PCR and Sanger sequencing. The polymorphic nucleotides between Col-0 and Ler are indicated by red arrows.
NUWA is localized to mitochondria and is required for mitochondrial development and function
When we investigated the subcellular localization of NUWA, the GFP fluorescence signal was found to co-localize with the mitochondria stained with the specific dye MitoTracker Orange in seedling cells (Fig 5A). The similar GFP signal was also observed in the heart-stage embryo, and the signal did not co-localize with the plastid auto-fluorescence (S4 Fig). Additionally, a putative mitochondrial-targeting peptide (mTP) was predicted in the N-terminus of NUWA [39]. These results suggest that NUWA is localized in mitochondria. When we introduced a ProNUWA:NUWAΔmTP-GFP into nuwa/+, the mitochondrial-co-localized GFP signal disappeared although the GFP transcript was detected (S5 Fig), implying that mitochondrial localization of NUWA is mediated by the mTP. In addition, no homozygous nuwa was identified among 180 T2 transformants, demonstrating that the NUWAΔmTP-GFP fragment could not rescue nuwa. These results suggest that the mitochondrial localization of NUWA is essential for its proper function.
Fig 5. NUWA is a mitochondrial-localized protein that is required for mitochondrial development in the early embryo and endosperm.
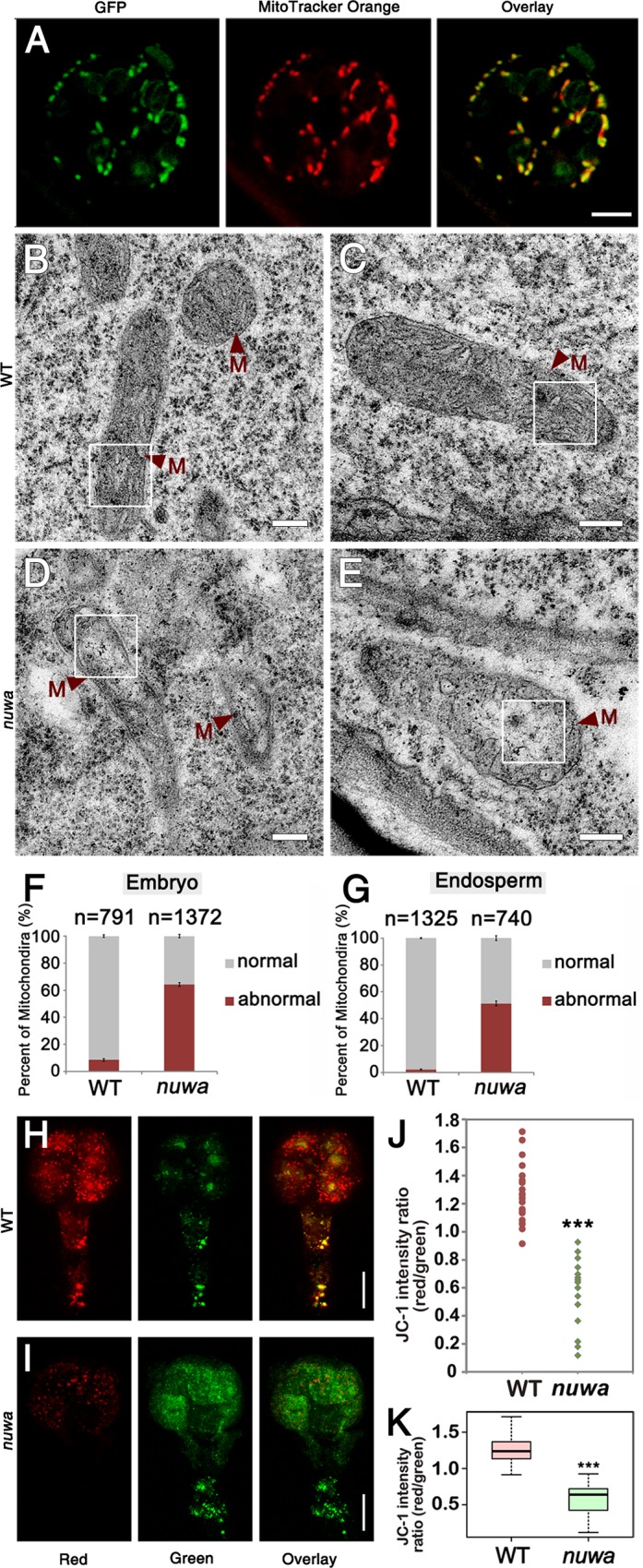
(A) GFP signals, MitoTracker Orange signals and the overlay of both signals in a guard cell of pNUWA:NUWA-GFP-GUS transgenic seedling. Bar = 5 μm. (B-C) Mitochondria in embryos (B) and endosperm (C) in ovules of wild-type plant. M, mitochondria. Bars = 200 nm. (D-E) Mitochondria in embryos (D) and endosperm (E) in aborted ovules of nuwa/+ plant. M, mitochondria. Bars = 200 nm. (F-G) Percentage of normal and abnormal mitochondria in embryos (F) and endosperm (G) in wild-type and aborted nuwa/+ ovules. Error bars, mean ± SEM. (H-I) JC-1 fluorescence in isolated wild type embryo (H) and nuwa/+ mutant embryo (I) at the 8-cell stage. Bars = 10 μm. (J-K) Dispersion graph (J) and box-plot (K) showing the red/green JC-1 fluorescence ratios measured in isolated wild type embryos (n = 22) and nuwa/+ embryos (n = 15) at various developmental stages (from the 2-cell stage to the 16-cell stage). Asterisks indicate statistically significant differences compared with the wild type. (***, P<0.01). White frames are the regions been enlarged in S6 Fig.
To investigate whether NUWA affects mitochondrial development, we adopted transmission electron microscopy (TEM) to analyze ultrastructure in early embryos and endosperm. Compared with mitochondria in wild-type embryos (Fig 5B) and endosperm (Fig 5C), the mitochondrial matrix of nuwa mutant embryos and endosperm was less dense, with the cristae composed of structurally looser inner membranes (Fig 5D and 5E, S6 Fig). Statistical analysis showed that the percentages of abnormal mitochondria in nuwa/+ mutant embryos and endosperm were 64.3% and 51.4%, respectively, both significantly higher than those in wild-type plants (8.60% and 2.19%, respectively) (Fig 5F and 5G) (|u| = 25.15 > u0.01 = 2.58, P <0.01; |u| = 26.88 > u0.01 = 2.58, P < 0.01). This result indicates that abnormal mitochondria were significantly more abundant in nuwa mutant ovules.
We then investigated mitochondrial functional status in isolated early embryos by using the mitochondrial trans-membrane potential indicator JC-1. In healthy cells with mitochondria at high membrane potential, JC-1 exhibits red fluorescence; in abnormal cells with mitochondria at low membrane potential, JC-1 exhibits green fluorescence [40,41]. Results show that red signals of JC-1 were visibly stronger in many wild type embryos (Fig 5H and S7 Fig), whereas JC-1 exhibited much stronger green signals in many nuwa mutant embryos at the same development stages (Fig 5I and S7 Fig). Quantitative analysis showed that the red-to-green fluorescence ratios of nuwa embryos were significantly lower than those of wild type embryos (|t| = 9.277 > t0.005 = 3.591, P < 0.005) (Fig 5J and 5K), indicating that the mitochondrial membrane potential levels of the mutant embryos were decreased compared to the wild type embryos. This result suggests that the mutant embryos of nuwa/+ have defective mitochondria, and the abnormal phenotypes of mutant embryos and endosperm might be attributed to mitochondrial dysfunction.
Because PPR proteins are widely involved in regulating the post-transcriptional processing of organelle encoded genes through affecting splicing, editing and translation of organelle transcripts [42,43], we intend to investigate whether NUWA is involved in regulating the expression level and splicing of mitochondrial genes. We extracted RNA from isolated endosperm of nuwa mutant and wild type plant at the same developmental stage, and analyzed, by using real-time qPCR, the expression level of those intron-containing mitochondrial genes and of those ovule-highly expressed genes chosen based on our preliminary RNA-seq data and the expression pattern data in Arabidopsis eFP Browser Database [44]. We also analyzed the trans splicing of the three NAD1 genes, two NAD2 genes and three NAD5 genes. The result showed that the expression levels of these genes were higher in the nuwa mutant, and no defect in splicing was detected (S8 Fig), suggesting that the expression level of mitochondria encoded genome is abnormal in the mutant, and that NUWA is probably not involved in splicing and stabilization processes of mRNA encoded by mitochondria.
The first PPR motif and the coiled-coil domain are essential to the function of NUWA during embryogenesis and endosperm development
NUWA encodes a P-subfamily pentatricopeptide repeat (PPR) protein of 629 amino acids with 11 PPR motifs (Fig 6A). PPR proteins, which form one of the largest protein families in higher plants, are named after the eukaryote-specific PPR repeat that typically comprises 35 amino acid residues, and have RNA-binding activities [45,46]. Not all PPR motifs contribute directly to the RNA-binding activity of PPR proteins, and some PPR proteins have protein-binding activity [46–48]. We therefore investigated whether all the PPR motifs in NUWA are equally important to its function. We also examined the importance of the predicted coiled-coil domain, which usually functions in protein-protein binding. We created four GUS-tagged deletion variants of NUWA, each expressed with the NUWA promoter, for genetic complementation (Fig 6A). We observed that NUWAΔ1stPPR, NUWAΔ1st-6thPPR and NUWAΔcoiled-coil failed to genetically complement nuwa/+ (S1 Table). No homozygous nuwa mutant was identified out of 200 or 180 T2 resistance-pre-selected transformants of each variant; in contrast, a NUWA-GUS fusion protein generated in the same manner completely rescued nuwa. These results indicate that both the first PPR motif and the coiled-coil domain are crucial to NUWA protein functions during early embryogenesis and endosperm development.
Fig 6. The 1st PPR Motif and the Coiled-Coil Domain Are Critical for the Proper Function of NUWA.
(A) The deletion variants of NUWA. (B) The wild type and nuwa/- plants complemented by NUWAΔ11thPPR. Bar = 2 cm. (C-D) Flowers of wild-type (C) and nuwa/- plants complemented by NUWAΔ11thPPR (D). Bars = 500 μm. The small pictures at the corners are enlarged images of anthers. Bar = 100 μm. (E) GUS signal of pNUWA::NUWA-GFP-GUS was detected in the joint region of anther and filament, and the guard cells on the anther wall. Black arrow points to the joint region of anther and filament, black arrowheads point to the guard cells, white arrowhead points to the pollen. Bar = 100 μm. (F) Enlarged view of the region surrounded by the white dotted line in (E). Black arrowheads point to the guard cells. Bar = 20 μm. (G) GUS signal of pNUWA::NUWA-GFP-GUS was not observed in guard cells on mature leafs. Black arrowheads point to the guard cells. Bar = 20 μm. (H) Seed sets of wild type, nuwa/+ and nuwa/+ plants transformed with four deletion variants. Blue stars and purple arrows indicate earlier and much later aborted seeds, respectively. Bar = 1 mm. (I) Seed abortion rate of wild type, nuwa/+ and nuwa/+ plants transformed with four deletion variants.
When we used NUWAΔ11thPPR to complement nuwa/+, however, 10 homozygous nuwa were detected out of 200 T2 resistance-pre-selected transformants (S1 Table). These homozygous nuwa that were complemented by NUWAΔ11thPPR-GUS had longer stems, undeveloped siliques (Fig 6B) and flowers featuring shortened stamens and shriveled anthers with undeveloped pollen (Fig 6C and 6D). These results indicate that the 11th PPR motif is not critical to NUWA function during embryogenesis and endosperm development. However, NUWA may have functions beyond embryogenesis and endosperm development, as the GUS signals were detected in the junction region of filament anthers (Fig 6E) and specifically in stomatal guard cells on anther walls (Fig 6E to 6G) of proNUWA::NUWA-GFP-GUS transgenic plants.
We next examined seed-setting rates and arrested seed developmental stages in the T2 transgenic plants in the nuwa/+ background. Seeds aborted as early as those of nuwa/+ were observed in siliques of these transgenic plants, whereas later-aborted seeds were found in siliques of transgenic plants expressing NUWAΔ11thPPR or NUWAΔcoiled-coil fragments (Fig 6H). Deeper analysis revealed that rates of seed abortion in heterozygous transgenic plants expressing NUWAΔ11thPPR and NUWAΔcoiled-coil were 13.0% and 23.9%, respectively, which is significantly lower than the abortion rate of nuwa/+ in this experiment (Fig 6I). This result confirms that the 11th PPR motif of NUWA is not as important as the other motifs and domains that were analyzed for their biological functions in the protein. These data also indicate that the coiled-coil domain is functionally less crucial than the first PPR motif during embryogenesis and endosperm development.
Discussion
In this study, we have identified the expression pattern and essential role of a novel imprinted gene, NUWA, in Arabidopsis early seed development. First, NUWA is an essential gene that controls early embryogenesis and endosperm development in Arabidopsis, because loss of function of NUWA resulted in embryo and endosperm lethality. Second, NUWA is a maternal effect gene, because control of seed development is only through the maternal allele of NUWA, and wild-type pollen could not rescue the defective phenotypes. Third, NUWA is imprinted, as only the maternal allele-specific NUWA transcripts and proteins were identified before and early after fertilization, and the maternal allele of NUWA is transcribed de novo after fertilization. Fourth, NUWA is a new type of imprinted gene, because NUWA is localized in the mitochondria and is essential for the proper function of the mitochondria.
Only the maternal allele-specific NUWA transcripts and encoded proteins were detectable during a period of several days after fertilization, and de novo transcription of NUWA started shortly after fertilization. As imprinted genes feature parent-of-origin specific allele biased expression patterns [2,7], or, in particular, parental allele-specific de novo transcription after fertilization [16], we concluded that NUWA is an maternally expressed imprinted gene. In addition, a TE is inserted in the coding sequence of NUWA, and most of the identified imprinted genes in Arabidopsis have TEs flanked by or harbored in coding sequences [29,49]. The mechanism of how NUWA imprinting is regulated, however, is not yet clear, and needs to be further investigated.
NUWA is a new type of imprinted gene. It is not because that NUWA is not only expressed in the endosperm but also expressed in many other tissues (Fig 3H to 3M). In fact, although some of the plant imprinted genes are only expressed in endosperm (such as FWA), a lot of plant imprinted genes are expressed in multiple tissues. For instance, MEADA, one of the most famous imprinted genes in plant, was expressed in embryo and in many other vegetative tissues from both paternal and maternal allele [20,50]. In addition, genes expressed and imprinted in embryo were also discovered in maize [51], rice [32] and Arabidopsis [16]. NUWA is special because its expression starts before fertilization, and the maternal control of it in early seed development is mediated by both maternally-deposited products and genomic imprinting. Maternal-specific NUWA products (including both RNA and protein) are detected in mature gametes before pollination, suggesting that maternal-deposited products of NUWA exist in embryo sac shortly after fertilization. Because NUWA encodes a PPR protein and mRNAs of PPR protein-encoding genes were reported to be rather unstable with a much shorter half-life (i.e., less than 6 hours) than the average mRNA half-lives of other genes [52], it is possible that the maternal allele of the zygotic NUWA is specifically activated soon after fertilization to make up the shortage of maternally-deposited NUWA products degraded during the maternal-to-zygotic transition [53–55]. Therefore, the products of NUWA detected in embryo sac early after fertilization are composed of the maternally-deposited NUWA products expressed in female gametophyte before fertilization and the maternal-allele specific NUWA products de novo synthesized after fertilization. That is, the parental effect of NUWA is controlled by parental-allele-specific expression not only before but also after fertilization, which is different from other reported imprinted genes in plants.
It is not yet known whether the imprinting of NUWA occurs in embryo, or in endosperm or in both. The imprinting of NUWA occurs very early, i.e., at about zygote stage, and NUWA is expressed in integument and seed coat. Unfortunately isolation and collection of enough amounts of Arabidopsis zygote and endosperm at zygote stage are extremely challenging, making it difficult to investigate the region in NUWA where imprinting occurs and the imprinting mechanism. Even so, we still think that NUWA is very likely to be imprinted in embryo. The expression of maternal allele of NUWA (i.e., the GUS signal) could always be detected in embryos staged from zygote stage to later stages, whereas the expression of paternal allele of NUWA could not be detected in embryo until the 16-cell stage, suggesting that the NUWA products in early embryo are predominantly derived from its maternal allele. From the TEM result, we could observe severe defective phenotypes in mitochondria in nuwa embryos, suggesting an important function of NUWA in mitochondria and in early embryogenesis. Meanwhile, NUWA belongs to PPR family, proteins from which all have very short half-lives [52]. The de novo expression of the maternal allele of NUWA is likely to be present in the embryo to make up the shortage of maternally-deposited NUWA products degraded during the maternal-to-zygotic transition. Therefore it is very possible that NUWA is imprinted in embryo. On the other hand, NUWA is also very possible to be imprinted in endosperm. The products of the paternal allele of NUWA were never detected in endosperm. It is likely that the products of NUWA in early endosperm are also predominantly derived from its maternal allele. Similarly, from the TEM results we could also see that NUWA has important function in early endosperm development. As NUWA protein could be detected in endosperm from zygote stage to 16-cell stage, the de novo expression of the maternal allele of NUWA is also likely to be present in the endosperm. Thus NUWA is also likely to be imprinted in endosperm.
NUWA is a new type of imprinted gene, also because it functions in mitochondria. In plants, although many imprinted genes have been identified, functional imprinted genes are still rarely discovered [17]. NUWA is a functional imprinted gene identified in Arabidopsis which controls early embryo and endosperm development. In addition, NUWA is the first discovered imprinted gene in plants whose protein product has an essential function in mitochondria. In mammals, imprinted genes that function in mitochondria were also identified, loss of function of which lead to severe diseases [56–58]. The discovery of NUWA indicates that, like the imprinted genes in mammals, imprinted genes in plants are also involved in many aspects of subcellular biological processes during early embryo development.
The specific mitochondria RNAs that are regulated by NUWA remain to be characterized. As we did not find reduced expression level and defected splicing in mitochondrial mRNA of the mutant (S8 Fig), it is possible that NUWA might be involved in regulating translation of mitochondrial mRNA, possibly through binding to mitochondrial mRNA and facilitating the release of a hairpin structure without changing RNA sequence and RNA copy number [59,60]. Another possibility is that NUWA may participate in the post-transcriptional processing of tRNAs or rRNAs. However, it is extremely challenging to detect the structural change of mitochondrial mRNAs, and the sequence and expression level changes of mitochondrial tRNAs and rRNAs, especially from the materials of early embryo, endosperm or ovules of Arabidopsis. New technology advances would be required to investigate the specific molecular function of NUWA in mitochondria.
The mitochondrion is an important organelle for energy and metabolism in eukaryotic cells. Coordination between mitochondrial and nuclear genomes is important for the efficiency of mitochondrial function, because mitochondrial proteins encoded by both genomic and mitochondrial DNA varies significantly in different cells [61–63]. In most species of higher eukaryotes, mitochondria are maternally inherited after fertilization. In Arabidopsis, mitochondrial DNA in sperm cells is almost completely degraded before fertilization [63]. Moreover, very few mitochondria from Arabidopsis sperm cells are able to enter the embryo sac during double fertilization, and all are degraded shortly after gamete fusion, as in animals [64,65]. Therefore, all the mitochondria and mitochondrial DNA in the fertilized Arabidopsis embryo sac are maternally inherited. After fertilization, a series of remarkable changes take place in newly formed zygotes and endosperm, including recombination of the two differentiated nuclear genomes, remodeling of parental chromatin, and fusion and coordination of parental nuclear and cytoplasmic components [54,55,66]. Consequently, regulating maternally inherited essential mitochondria by nuclear-encoded maternal allele-specific NUWA products could possibly improve the implementation efficacy of the NUWA function and increase the efficiency of mitochondria-nuclear interaction in the complex post-fertilization environment, which might be the advantage for having an essential gene NUWA to be imprinted and selected during evolution. Therefore, the imprinting of NUWA would be an adaptation and enhancement to the maternal control of mitochondrial development. The discovery of NUWA reveals a unique mechanism of maternal control of the mitochondria and adds an extra layer of complexity to the regulation of nucleus-organelle coordination during early plant development.
Methods
Plant materials and growth conditions
Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used as wild type plant. All the transgenic lines used in this study were in the Columbia ecotype. Wild type Arabidopsis thaliana Landsberg ereta (Ler-0) was only used in the allele specific RT-PCR. The nuwa mutant was obtained from the T-DNA insertion mutant library (in Col background) of our lab [34]. Plants were grown under long-day conditions (16 hr light/8 hr dark) at 22°C. For crosses, maternal partners were emasculated, and pollinated 2 or 3 days after emasculation.
TAIL-PCR and genotype analysis
The flanking sequence of the T-DNA insertion site in nuwa was determined by TAIL-PCR with the specific and arbitrary degenerate primers described previously [34]. For genotypic analysis of nuwa, primers nuwa_CS_F and nuwa_CS_R were used to amplify the wild-type allele of NUWA. Primers nuwa_CS_R and DL3 were used to amplify the insertion allele of nuwa and described in the S2 Table.
RNA extraction and qPCR analysis
Total RNA of most tissues was extracted from liquid nitrogen frozen tissues using TRIzol (Invitrogen) according to manufacturer’s instructions. Total RNA extraction of seeds and stem was performed with phenol extraction buffer (50% Tris saturated phenol, 0.5% SDS, 50 mM LiCl, 50 mM Tris-HCl at pH8.0, 50 mM EDTA at pH8.0) at room temperature. Residual DNA was removed using the RNase-free DNase (TaKaRa), and cDNA was synthesized using the M-MLV kit (Invitrogen/Fermentas) according to the manufacturer’s instructions.
In real-time qPCR, diluted cDNA was used as a template, and three biological repeats was performed using SYBR Green real-time PCR Master Mix (TOYOBO) as described previously [67] on an ABI 7500 Real-Time PCR System. The relative expression level of each gene was calculated with the cycle threshold (CT) 2-ΔΔCT method [68]. Gene expression values were standardized to TUB2 (AT5G62690) or PP2AA3 (AT1G13320).
Microscopy analysis
DIC observation of cleared ovules and bright field observation of GUS stained ovules were performed using Olympus BX51 microscope. Images of other GUS stained samples were taken using a Leica M205C FA stereoscope.
Subcellular localization observation was performed using a confocal laser scanning microscope (Leica TCS SPE confocal microscope).
To determine the phenotype of endosperm nuclei, confocal observation of ovules was modified from previously described [69]. Artificial pollinated selfed 26 HAP siliques were harvested and fixed in 4% glutaraldehyde (in 12.5 mM cacodylate, pH 6.9), and a vacuum was applied for the initial 20 min, after which they were in fixative overnight at room temperature. After fixation, samples were dehydrated through a conventional ethanol series with 20 min per step, then cleared in 2:1 (v/v) benzyl benzoate:benzyl alcohol for a minimum of 1 hr. Siliques were dissected, mounted with immersion oil, and observed with a Leica TCS SPE confocal microscope.
For transmission electron microscopy, protocol for sample preparation was modified from previously described [70]. Developing seeds at specific stages were collected, vacuumed and fixed in 5% glutaraldehyde and 3% paraformaldehyde in 0.1 M sodium cacodylatetrihydrate buffer (pH7.2) at room temperature overnight and then at 4°C for 24 hrs. Ovules were then washed four times with 0.1 M sodium cacodylatetrihydrate buffer (more than 30 min for each time) and fixed in 2% OSO4 at room temperature for 4 hrs and then at 4°C over night, followed by dehydration in a graded ethanol series. Seeds were embedded in a complete resin mixture (Spi-chem Spurr) and incubated in 65°C for 16 hrs. Samples were sectioned using a Leica EM UC6 ultramicrotome and stained with 2% uranyl acetate and lead stain solutions. Sections were examined with a FEI Tecnai G2 20 Twin electron microscope at 200 kV.
Plasmids construction and plant transformation
For genetic complementation, we used GW_NUWA_COMP_16_F and GW_NUWA_COMP_R primers to amplify the NUWA genomic DNA and generated the pNUWA::NUWA genomic DNA constructs through BP clonase and subsequent LR clonase (Invitrogen). The amplified fragments were cloned into a reconstructed vector pK7FWG0, which was made from the GATEWAY-compatible destination vector pB7RWG2 (Department of Plant Systems Biology, VIB-Ghent University, Ghent, Belgium) by digesting the 35S promoter via Spe I and Sac I sites.
To generate the pNUWA::NUWA-EGFP-GUS constructs, the NUWA genomic DNA was amplified using TOPO_NUWA_GUS_F and TOPO_NUWA_pg+GUS_R primers. This genomic sequence was cloned into pBGWFS7 vector (Department of Plant Systems Biology, VIB-Ghent University, Ghent, Belgium) through pENTR/D-TOPO kit (Invitrogen) and LR reactions.
In the genetic complementation assay with truncated NUWA, five groups of sequences were amplified to generate the pNUWA::NUWAΔmTP, pNUWA::NUWA Δ1st-PPR, pNUWA::NUWA Δ1stto6th-PPR, pNUWA::NUWA Δ11th-PPR and pNUWA::NUWA Δcoil constructs. As NUWA has no intron, genomic DNA was used as templates in the amplifications. P4P1R_NUWAΔX_F and P4P1R_NUWAΔX_R (X represents one of mTP, 1st-PPR, 1stto6th-PPR, 11th-PPR and coil) primers were used to amplify the sequence of the promoter. This sequence was then cloned into pDONRP4-P1r by BP reaction to generate pEN-L4-NUWAΔX-R1. Meanwhile, TOPO_NUWAΔX_F and TOPO_NUWAΔX_R primers were used to amplify the sequence from the base next to the coding region of the mTP to the base right before the terminator. This sequence was then cloned into pENTR/D-TOPO by TOPO reaction to generate pENTR-NUWAΔX. GUS and EGFP coding sequence were amplified by GUS-F_attB2r/ GUS-R_attB3 and EGFP-F_attB2r/ EGFP-R_attB3 primers, and cloned in to pDONRP2R-P3 respectively to generate pEN-R2-GUS-L3 and pEN-R2-EGFP-L3. The pNUWA::NUWAΔX constructs were generated by LR reaction of four plasmids, including pK7m34GW, pEN-L4-NUWAΔX-R1, pENTR-NUWAΔX, and pEN-R2-EGFP-L3.
Constructs were transformed into Agrobacterium tumefaciens GV3101, using a freeze-thaw procedure. Arabidopsis transformation and transgenic plant screening were conducted as reported [71].
Staining and tissue clearing
The histochemical GUS staining was modified from described previously [72]. After per-fixation by pure acetone at -20°C for 1 hr, tissues immerged in a staining solution containing 0.5 mg ml-1 X-gluc (Sigma) in 100 mM Na2HPO4/NaH2PO4 (pH7), 2 mM K3Fe(CN)6, 2 mM K4Fe(CN)6, 10 mM EDTA and 0.1% Triton X-100, modified from [35]. Samples were vacuumed for 10 min and then put in a 37°C incubator overnight. The staining buffer was removed, and the samples were fixed in FAA for 1 or 2 hrs before cleared and embedded.
For fuchsin basic staining, resin sections on the glass slides was covered by drops of 1% fuchsin basic solution and incubated at 60°C for 6 min. Then wash away the dye by flowing sterile water. Slices were sealed with 50% glycerine before microscopy.
MitoTracker Orange (Invitrogen) staining of the seedlings was performed according to manufacturer’s instructions, as described before [73].
The GUS stained samples were cleared using 70% ethanol before microscopy.
For whole-mount preparations, ovules at different development stages were mounted in diluted Hoyer’s solution, which was introduced on http://www.seedgenes.org/Tutorial.html [74], and cleared for 5 to 30 min before microscopy analysis.
Mitochondrial membrane potential analysis
Isolated early embryos were incubated in 12% (w/v) mannitol with 10 μg/mL JC-1 (Molecular Probes, Invitrogen) for 30 min at room temperature, and then washed with 12% (w/v) mannitol. Images were collected using the confocal microscope mentioned above. JC-1 aggregates were detected with red fluorescence (excitation, 532 nm; emission, 575–590 nm), and JC-1 monomers were detected with green fluorescence (excitation, 488 nm; emission, 515–545). The ratio of red to green fluorescence of JC-1 images was calculated by Imaris 7.5.0 software (Bitplane). R software was used for the box-plot.
Allele specific RT-PCR
cDNA from ovules (or isolated embryo sacs) at different development stages from self and crossed Col-0 and Ler was used for allele-specific RT-PCR (primers and PCR parameters in S2 Table). PCR products were sequenced by the Sanger Chain Termination Method.
Embryo sac isolation, RNA extraction and cDNA synthesis of isolated embryo sacs
The isolation of embryo sacs at different stages and the extraction of RNA from the isolated embryo sacs were performed according to modified versions of protocols described previously [75]. Embryo sacs were isolated by brief manual dissection combined with enzymatic maceration. Enzymatic solution is composed of 1% cellulose (Yakult Honsha Co. Ltd, Tokyo, Japan) and 0.8% Macrozyme R-10 (Yakult Honsha). Before manual dissection, ovules at different development stages were incubated in the enzyme solution for 0.5 h. The Dynabeads mRNA DIRECT Micro Kit (Invitrogen) was used for mRNA isolation. cDNA was synthesized using the SuperScript III kit (Invitrogen) according to the manufacturer’s instructions. For each experiment (or each time point), three independent biological replicates were performed. For each independent biological replicate, 11–16 embryo sacs were isolated for RNA extraction.
Supporting Information
(A and B) Ovules with GUS signal resulting from pollination of emasculated wild type (A) and nuwa-1/+ (B) by pollen of FAC1-GUS marker line. Bars = 100 μm. (C) Statistic analysis of ovules with GUS signal resulting from pollination of emasculated wild type and nuwa-1/+ by pollen of FAC1-GUS marker line. (D-I) FIS2-GUS marker line in wild type background. GUS signals indicate the endosperm nuclei. Bars = 50 μm.
(TIF)
(A) Synteny analysis of NUWA in plant species. Colors of the arrows represent for the various genes in the flanking regions of NUWA. Syntenic orthologs are depicted in the same color, while white arrows are functionally uncharacterized genes. The grey arrows aligned in the middle are NUWA gene and its orthologs in the same direction in each genome. Topology shown here is a species tree based on Phytozome v10. (B) Phylogenetic tree of NUWA and its orthologs. Scale bar, 0.2 amino acid substitutions per site. Ath, Arabidopsis thaliana; Aly, Arabidopsis lyrata; Cru, Capsella rubella; Bra, Brassica rapa; Esa, Eutrema salsugineum; Gra, Gossypium raimondii; Tca, Theobroma cacao; Ccl, Citrus clementina; Egr, Eucalyptus grandis. (C) Nucleotide diversity in the genomic region centered on NUWA among 19 A. thaliana accessions: Col-0, Bur-0, Can-0, Ct-1, Edi-0, Hi-0, Kn-0, Ler-0, Mt-0, No-0, Oy-0, Po-0, Rsch-4, Sf-2, Tsu-0, Wil-2, Ws-0, Wu-0 and Zu-0. (D) Tajima’s D value along the 6 kb genomic region centered on NUWA based on the same set of 19 A. thaliana accessions.
(TIF)
(A) Percentage of seeds in wild type and nuwa-1/+ self crossed siliques and siliques resulted from emasculated nuwa-1/+ pollinated by wild type pollen. (B) RNA-seq data of the transcription level of NUWA in ovules at different developmental stages.
(TIF)
(A-D) GFP signals (A), autofluorescence of chloroplast (B), the overlay of both signals (C) and bright field (D) in heart-stage embryo of pNUWA:NUWA-GFP transgenic plant. Bar = 20 μm.
(TIF)
(A-C) GFP signals (A), MitoTracker Orange signals (B) and the overlay of both signals (C) in root cells of pNUWA:NUWA-GFP transgenic seedling. Bar = 5 μm. (D-F) GFP signals (D), MitoTracker Orange signals (E) and the overlay of both signals (F) in root cells of pNUWA:NUWAΔmTP-GFP transgenic seedling. Bar = 5 μm. (G) The expression level of GFP in seedling of five pNUWA:NUWAΔmTP-GFP transgenic lines and one of the pNUWA::NUWA-GFP-GUS transgenic lines. The genomic GFP sequence and the tubulin2 sequences were amplified as controls.
(TIF)
(A) Enlarged part of mitochondria in Fig 5B marked by the white frame. (B) Enlarged part of mitochondria in Fig 5C marked by the white frame. (C) Enlarged part of mitochondria in Fig 5D marked by the white frame. (D) Enlarged part of mitochondria in Fig 5E marked by the white frame. White arrowheads indicate the inner membranes.
(TIF)
(A-B) JC-1 fluorescence in isolated wild type embryos (A) and nuwa-1/+ mutant embryos (B) at different developmental stages. For each embryo, green fluorescence, red fluorescence, overlay the two fluorescence and bright field are shown. Bars = 10 μm.
(TIF)
Error bars, mean ± SD.
(TIF)
(XLSX)
(XLSX)
Acknowledgments
We thank Dr. Fuchou Tang (Peking University, China) for valuable discussions and helpful suggestions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Basic Research Program of China (Grant No. 2012CB944801) and partially by 111 Project (Grant No. B06001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Barlow DP (2011) Genomic imprinting: a mammalian epigenetic discovery model. Annu Rev Genet 45: 379–403. 10.1146/annurev-genet-110410-132459 [DOI] [PubMed] [Google Scholar]
- 2.Gehring M (2013) Genomic imprinting: parental lessons from plants. Annu Rev Genet 47: 205–226. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues JA, Zilberman D (2015) Evolution and function of genomic imprinting in plants. Genes Dev 29: 2517–2531. 10.1101/gad.269902.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickinson H, Scholten S (2013) And baby makes three: genomic imprinting in plant embryos. PLoS Genetics 9: e1003981 10.1371/journal.pgen.1003981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feil R, Berger F (2007) Convergent evolution of genomic imprinting in plants and mammals. Trends Genet 23: 192–199. 10.1016/j.tig.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 6.Feng S, Jacobsen SE, Reik W (2010) Epigenetic reprogramming in plant and animal development. Science 330: 622–627. 10.1126/science.1190614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohler C, Wolff P, Spillane C (2012) Epigenetic mechanisms underlying genomic imprinting in plants. Annu Rev Plant Biol 63: 331–352. 10.1146/annurev-arplant-042811-105514 [DOI] [PubMed] [Google Scholar]
- 8.Lawson HA, Cheverud JM, Wolf JB (2013) Genomic imprinting and parent-of-origin effects on complex traits. Nat Rev Genet 14: 609–617. 10.1038/nrg3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swaney WT, Curley JP, Champagne FA, Keverne EB (2007) Genomic imprinting mediates sexual experience-dependent olfactory learning in male mice. Proc Natl Acad Sci U S A 104: 6084–6089. 10.1073/pnas.0609471104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reik W, Walter G (2001) Genomic imprinting: parental influence on the genome. Nat Rev Genet 2: 21–32. 10.1038/35047554 [DOI] [PubMed] [Google Scholar]
- 11.Curley JP (2011) Is there a genomically imprinted social brain? Bioessays 33: 662–668. 10.1002/bies.201100060 [DOI] [PubMed] [Google Scholar]
- 12.Zoghbi HY, Beaudet AL (2016) Epigenetics and Human Disease. Cold Spring Harb Perspect Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JT, Bartolomei MS (2013) X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell 152: 1308–1323. 10.1016/j.cell.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 14.Shirzadi R, Andersen ED, Bjerkan KN, Gloeckle BM, Heese M, et al. (2011) Genome-wide transcript profiling of endosperm without paternal contribution identifies parent-of-origin-dependent regulation of AGAMOUS-LIKE36. PLoS Genet 7: e1001303 10.1371/journal.pgen.1001303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinoshita T, Miura A, Choi Y, Kinoshita Y, Cao X, et al. (2004) One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science 303: 521–523. 10.1126/science.1089835 [DOI] [PubMed] [Google Scholar]
- 16.Raissig MT, Bemer M, Baroux C, Grossniklaus U (2013) Genomic imprinting in the Arabidopsis embryo is partly regulated by PRC2. PLoS Genet 9: e1003862 10.1371/journal.pgen.1003862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haig D (2013) Kin conflict in seed development: an interdependent but fractious collective. Annu Rev Cell Dev Biol 29: 189–211. 10.1146/annurev-cellbio-101512-122324 [DOI] [PubMed] [Google Scholar]
- 18.Tycko B, Morison IM (2002) Physiological functions of imprinted genes. J Cell Physiol 192: 245–258. 10.1002/jcp.10129 [DOI] [PubMed] [Google Scholar]
- 19.Grossniklaus U, Vielle-Calzada J-P, Hoeppner MA, Gagliano WB (1998) Maternal control of embryogenesis by MEDEA, a polycomb group gene in Arabidopsis. Science 280: 446–450. [DOI] [PubMed] [Google Scholar]
- 20.Kiyosue T, Ohad N, Yadegari R, Hannon M, Dinneny J, et al. (1999) Control of fertilization-independent endosperm development by the MEDEA polycomb gene in Arabidopsis. Proc Natl Acad Sci U S A 96: 4186–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baroux C, Gagliardini V, Page DR, Grossniklaus U (2006) Dynamic regulatory interactions of Polycomb group genes: MEDEA autoregulation is required for imprinted gene expression in Arabidopsis. Genes Dev 20: 1081–1086. 10.1101/gad.378106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jullien PE, Kinoshita T, Ohad N, Berger F (2006) Maintenance of DNA methylation during the Arabidopsis life cycle is essential for parental imprinting. Plant Cell 18: 1360–1372. 10.1105/tpc.106.041178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiwari S, Schulz R, Ikeda Y, Dytham L, Bravo J, et al. (2008) MATERNALLY EXPRESSED PAB C-TERMINAL, a novel imprinted gene in Arabidopsis, encodes the conserved C-terminal domain of polyadenylate binding proteins. Plant Cell 20: 2387–2398. 10.1105/tpc.108.061929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohler C, Page DR, Gagliardini V, Grossniklaus U (2005) The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat Genet 37: 28–30. 10.1038/ng1495 [DOI] [PubMed] [Google Scholar]
- 25.Kradolfer D, Wolff P, Jiang H, Siretskiy A, Kohler C (2013) An imprinted gene underlies postzygotic reproductive isolation in Arabidopsis thaliana. Dev Cell 26: 525–535. 10.1016/j.devcel.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 26.Luo M, Platten D, Chaudhury A, Peacock WJ, Dennis ES (2009) Expression, imprinting, and evolution of rice homologs of the polycomb group genes. Mol Plant 2: 711–723. 10.1093/mp/ssp036 [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez-Marcos JF, Costa LM, Dal Pra M, Scholten S, Kranz E, et al. (2006) Epigenetic asymmetry of imprinted genes in plant gametes. Nat Genet 38: 876–878. 10.1038/ng1828 [DOI] [PubMed] [Google Scholar]
- 28.Haun WJ, Laoueillé-Duprat S, O'Connell MJ, Spillane C, Grossniklaus U, et al. (2007) Genomic imprinting, methylation and molecular evolution of maize Enhancer of zeste (Mez) homologs. The Plant Journal 49: 325–337. 10.1111/j.1365-313X.2006.02965.x [DOI] [PubMed] [Google Scholar]
- 29.Gehring M, Bubb KL, Henikoff S (2009) Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science 324: 1447–1451. 10.1126/science.1171609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gehring M, Missirian V, Henikoff S (2011) Genomic analysis of parent-of-origin allelic expression in Arabidopsis thaliana seeds. PLoS One 6: e23687 10.1371/journal.pone.0023687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waters AJ, Makarevitch I, Eichten SR, Swanson-Wagner RA, Yeh CT, et al. (2011) Parent-of-origin effects on gene expression and DNA methylation in the maize endosperm. Plant Cell 23: 4221–4233. 10.1105/tpc.111.092668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo M, Taylor JM, Spriggs A, Zhang H, Wu X, et al. (2011) A genome-wide survey of imprinted genes in rice seeds reveals imprinting primarily occurs in the endosperm. PLoS Genet 7: e1002125 10.1371/journal.pgen.1002125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xing MQ, Zhang YJ, Zhou SR, Hu WY, Wu XT, et al. (2015) Global Analysis Reveals the Crucial Roles of DNA Methylation during Rice Seed Development. Plant Physiol 168: 1417–1432. 10.1104/pp.15.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin G, Kang D, Dong Y, Shen Y, Zhang L, et al. (2003) Obtaining and analysis of flanking sequences from T-DNA transformants of Arabidopsis. Plant Sci 165: 941–949. [Google Scholar]
- 35.Xu J, Zhang HY, Xie CH, Xue HW, Dijkhuis P, et al. (2005) EMBRYONIC FACTOR 1 encodes an AMP deaminase and is essential for the zygote to embryo transition in Arabidopsis. Plant J 42: 743–756. 10.1111/j.1365-313X.2005.02411.x [DOI] [PubMed] [Google Scholar]
- 36.Luo M, Bilodeau P, Dennis ES, Peacock WJ, Chaudhury A (2000) Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proc Natl Acad Sci U S A 97: 10637–10642. 10.1073/pnas.170292997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmid KJ, Ramos-Onsins S, Ringys-Beckstein H, Weisshaar B, Mitchell-Olds T (2005) A multilocus sequence survey in Arabidopsis thaliana reveals a genome-wide departure from a neutral model of DNA sequence polymorphism. Genetics 169: 1601–1615. 10.1534/genetics.104.033795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faure JE, Rotman N, Fortune P, Dumas C (2002) Fertilization in Arabidopsis thaliana wild type: developmental stages and time course. Plant J 30: 481–488. [DOI] [PubMed] [Google Scholar]
- 39.Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300: 1005–1016. 10.1006/jmbi.2000.3903 [DOI] [PubMed] [Google Scholar]
- 40.Martin MV, Fiol DF, Sundaresan V, Zabaleta EJ, Pagnussat GC (2013) oiwa, a female gametophytic mutant impaired in a mitochondrial manganese-superoxide dismutase, reveals crucial roles for reactive oxygen species during embryo sac development and fertilization in Arabidopsis. Plant Cell 25: 1573–1591. 10.1105/tpc.113.109306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng Y, Zou W, Li G, Zhao J (2014) TRANSLOCASE OF THE INNER MEMBRANE9 and 10 are essential for maintaining mitochondrial function during early embryo cell and endosperm free nucleus divisions in Arabidopsis. Plant Physiol 166: 853–868. 10.1104/pp.114.242560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz-Linneweber C, Small I (2008) Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends Plant Sci 13: 663–670. 10.1016/j.tplants.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 43.Nakamura T, Yagi Y, Kobayashi K (2012) Mechanistic insight into pentatricopeptide repeat proteins as sequence-specific RNA-binding proteins for organellar RNAs in plants. Plant Cell Physiol 53: 1171–1179. 10.1093/pcp/pcs069 [DOI] [PubMed] [Google Scholar]
- 44.Baxter I, Vinegar B, Winter D, Nahal H, Ammar R, et al. (2007) An “Electronic Fluorescent Pictograph” Browser for Exploring and Analyzing Large-Scale Biological Data Sets. PLoS ONE 2: e718 10.1371/journal.pone.0000718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Small ID, Peeters N (2000) The PPR motif—a TPR-related motif prevalent in plant organellar proteins. Trends Biochem Sci 25: 46–47. [DOI] [PubMed] [Google Scholar]
- 46.Yin P, Li Q, Yan C, Liu Y, Liu J, et al. (2013) Structural basis for the modular recognition of single-stranded RNA by PPR proteins. Nature 504: 168–171. 10.1038/nature12651 [DOI] [PubMed] [Google Scholar]
- 47.Chi W, Mao J, Li Q, Ji D, Zou M, et al. (2010) Interaction of the pentatricopeptide-repeat protein DELAYED GREENING 1 with sigma factor SIG6 in the regulation of chloroplast gene expression in Arabidopsis cotyledons. Plant J 64: 14–25. 10.1111/j.1365-313X.2010.04304.x [DOI] [PubMed] [Google Scholar]
- 48.Uyttewaal M, Mireau H, Rurek M, Hammani K, Arnal N, et al. (2008) PPR336 is associated with polysomes in plant mitochondria. J Mol Biol 375: 626–636. 10.1016/j.jmb.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 49.Pignatta D, Erdmann RM, Scheer E, Picard CL, Bell GW, et al. (2014) Natural epigenetic polymorphisms lead to intraspecific variation in Arabidopsis gene imprinting. eLife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinoshita T, Yadegari R, Harada JJ, Goldberg RB, Fischer RL (1999) Imprinting of the MEDEA polycomb gene in the Arabidopsis endosperm. Plant Cell 11: 1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jahnke S, Scholten S (2009) Epigenetic resetting of a gene imprinted in plant embryos. Curr Biol 19: 1677–1681. 10.1016/j.cub.2009.08.053 [DOI] [PubMed] [Google Scholar]
- 52.Narsai R, Howell KA, Millar AH, O'Toole N, Small I, et al. (2007) Genome-wide analysis of mRNA decay rates and their determinants in Arabidopsis thaliana. Plant Cell 19: 3418–3436. 10.1105/tpc.107.055046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schier AF (2007) The maternal-zygotic transition: death and birth of RNAs. Science 316: 406–407. 10.1126/science.1140693 [DOI] [PubMed] [Google Scholar]
- 54.Baroux C, Autran D, Gillmor CS, Grimanelli D, Grossniklaus U (2008) The maternal to zygotic transition in animals and plants. Cold Spring Harb Symp Quant Biol 73: 89–100. 10.1101/sqb.2008.73.053 [DOI] [PubMed] [Google Scholar]
- 55.Xin HP, Zhao J, Sun MX (2012) The maternal-to-zygotic transition in higher plants. J Integr Plant Biol 54: 610–615. 10.1111/j.1744-7909.2012.01138.x [DOI] [PubMed] [Google Scholar]
- 56.Baysal BE, McKay SE, Kim YJ, Zhang Z, Alila L, et al. (2011) Genomic imprinting at a boundary element flanking the SDHD locus. Hum Mol Genet 20: 4452–4461. 10.1093/hmg/ddr376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su H, Fan W, Coskun PE, Vesa J, Gold JA, et al. (2011) Mitochondrial dysfunction in CA1 hippocampal neurons of the UBE3A deficient mouse model for Angelman syndrome. Neurosci Lett 487: 129–133. 10.1016/j.neulet.2009.06.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baysal BE (2013) Mitochondrial complex II and genomic imprinting in inheritance of paraganglioma tumors. Biochim Biophys Acta 1827: 573–577. 10.1016/j.bbabio.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 59.Pfalz J, Bayraktar OA, Prikryl J, Barkan A (2009) Site-specific binding of a PPR protein defines and stabilizes 5' and 3' mRNA termini in chloroplasts. EMBO J 28: 2042–2052. 10.1038/emboj.2009.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prikryl J, Rojas M, Schuster G, Barkan A (2011) Mechanism of RNA stabilization and translational activation by a pentatricopeptide repeat protein. Proc Natl Acad Sci U S A 108: 415–420. 10.1073/pnas.1012076108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shamsi MB, Govindaraj P, Chawla L, Malhotra N, Singh N, et al. (2013) Mitochondrial DNA variations in ova and blastocyst: implications in assisted reproduction. Mitochondrion 13: 96–105. 10.1016/j.mito.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 62.Wai T, Teoli D, Shoubridge EA (2008) The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet 40: 1484–1488. 10.1038/ng.258 [DOI] [PubMed] [Google Scholar]
- 63.Wang DY, Zhang Q, Liu Y, Lin ZF, Zhang SX, et al. (2010) The levels of male gametic mitochondrial DNA are highly regulated in angiosperms with regard to mitochondrial inheritance. Plant Cell 22: 2402–2416. 10.1105/tpc.109.071902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, et al. (1999) Ubiquitin tag for sperm mitochondria. Nature 402: 371–372. 10.1038/46466 [DOI] [PubMed] [Google Scholar]
- 65.Matsushima R, Hamamura Y, Higashiyama T, Arimura S, Sodmergen, et al. (2008) Mitochondrial dynamics in plant male gametophyte visualized by fluorescent live imaging. Plant Cell Physiol 49: 1074–1083. 10.1093/pcp/pcn084 [DOI] [PubMed] [Google Scholar]
- 66.Ostrup O, Andersen IS, Collas P (2013) Chromatin-linked determinants of zygotic genome activation. Cell Mol Life Sci 70: 1425–1437. 10.1007/s00018-012-1143-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tao Q, Guo D, Wei B, Zhang F, Pang C, et al. (2013) The TIE1 transcriptional repressor links TCP transcription factors with TOPLESS/TOPLESS-RELATED corepressors and modulates leaf development in Arabidopsis. Plant Cell 25: 421–437. 10.1105/tpc.113.109223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 69.Liu J, Zhang Y, Qin G, Tsuge T, Sakaguchi N, et al. (2008) Targeted degradation of the cyclin-dependent kinase inhibitor ICK4/KRP6 by RING-type E3 ligases is essential for mitotic cell cycle progression during Arabidopsis gametogenesis. Plant Cell 20: 1538–1554. 10.1105/tpc.108.059741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu R, Li S, He S, Wassmann F, Yu C, et al. (2011) CFL1, a WW domain protein, regulates cuticle development by modulating the function of HDG1, a class IV homeodomain transcription factor, in rice and Arabidopsis. Plant Cell 23: 3392–3411. 10.1105/tpc.111.088625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin G, Gu H, Zhao Y, Ma Z, Shi G, et al. (2005) An indole-3-acetic acid carboxyl methyltransferase regulates Arabidopsis leaf development. Plant Cell 17: 2693–2704. 10.1105/tpc.105.034959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu J, Zhong S, Guo X, Hao L, Wei X, et al. (2013) Membrane-bound RLCKs LIP1 and LIP2 are essential male factors controlling male-female attraction in Arabidopsis. Curr Biol 23: 993–998. 10.1016/j.cub.2013.04.043 [DOI] [PubMed] [Google Scholar]
- 73.Han L, Qin G, Kang D, Chen Z, Gu H, et al. (2010) A nuclear-encoded mitochondrial gene AtCIB22 is essential for plant development in Arabidopsis. J Genet Genomics 37: 667–683. 10.1016/S1673-8527(09)60085-0 [DOI] [PubMed] [Google Scholar]
- 74.Tzafrir I, Dickerman A, Brazhnik O, Nguyen Q, McElver J, et al. (2003) The Arabidopsis SeedGenes project. Nucleic Acids Research 31: 90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu JJ, Peng XB, Li WW, He R, Xin HP, et al. (2012) Mitochondrial GCD1 dysfunction reveals reciprocal cell-to-cell signaling during the maturation of Arabidopsis female gametes. Dev Cell 23: 1043–1058. 10.1016/j.devcel.2012.09.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A and B) Ovules with GUS signal resulting from pollination of emasculated wild type (A) and nuwa-1/+ (B) by pollen of FAC1-GUS marker line. Bars = 100 μm. (C) Statistic analysis of ovules with GUS signal resulting from pollination of emasculated wild type and nuwa-1/+ by pollen of FAC1-GUS marker line. (D-I) FIS2-GUS marker line in wild type background. GUS signals indicate the endosperm nuclei. Bars = 50 μm.
(TIF)
(A) Synteny analysis of NUWA in plant species. Colors of the arrows represent for the various genes in the flanking regions of NUWA. Syntenic orthologs are depicted in the same color, while white arrows are functionally uncharacterized genes. The grey arrows aligned in the middle are NUWA gene and its orthologs in the same direction in each genome. Topology shown here is a species tree based on Phytozome v10. (B) Phylogenetic tree of NUWA and its orthologs. Scale bar, 0.2 amino acid substitutions per site. Ath, Arabidopsis thaliana; Aly, Arabidopsis lyrata; Cru, Capsella rubella; Bra, Brassica rapa; Esa, Eutrema salsugineum; Gra, Gossypium raimondii; Tca, Theobroma cacao; Ccl, Citrus clementina; Egr, Eucalyptus grandis. (C) Nucleotide diversity in the genomic region centered on NUWA among 19 A. thaliana accessions: Col-0, Bur-0, Can-0, Ct-1, Edi-0, Hi-0, Kn-0, Ler-0, Mt-0, No-0, Oy-0, Po-0, Rsch-4, Sf-2, Tsu-0, Wil-2, Ws-0, Wu-0 and Zu-0. (D) Tajima’s D value along the 6 kb genomic region centered on NUWA based on the same set of 19 A. thaliana accessions.
(TIF)
(A) Percentage of seeds in wild type and nuwa-1/+ self crossed siliques and siliques resulted from emasculated nuwa-1/+ pollinated by wild type pollen. (B) RNA-seq data of the transcription level of NUWA in ovules at different developmental stages.
(TIF)
(A-D) GFP signals (A), autofluorescence of chloroplast (B), the overlay of both signals (C) and bright field (D) in heart-stage embryo of pNUWA:NUWA-GFP transgenic plant. Bar = 20 μm.
(TIF)
(A-C) GFP signals (A), MitoTracker Orange signals (B) and the overlay of both signals (C) in root cells of pNUWA:NUWA-GFP transgenic seedling. Bar = 5 μm. (D-F) GFP signals (D), MitoTracker Orange signals (E) and the overlay of both signals (F) in root cells of pNUWA:NUWAΔmTP-GFP transgenic seedling. Bar = 5 μm. (G) The expression level of GFP in seedling of five pNUWA:NUWAΔmTP-GFP transgenic lines and one of the pNUWA::NUWA-GFP-GUS transgenic lines. The genomic GFP sequence and the tubulin2 sequences were amplified as controls.
(TIF)
(A) Enlarged part of mitochondria in Fig 5B marked by the white frame. (B) Enlarged part of mitochondria in Fig 5C marked by the white frame. (C) Enlarged part of mitochondria in Fig 5D marked by the white frame. (D) Enlarged part of mitochondria in Fig 5E marked by the white frame. White arrowheads indicate the inner membranes.
(TIF)
(A-B) JC-1 fluorescence in isolated wild type embryos (A) and nuwa-1/+ mutant embryos (B) at different developmental stages. For each embryo, green fluorescence, red fluorescence, overlay the two fluorescence and bright field are shown. Bars = 10 μm.
(TIF)
Error bars, mean ± SD.
(TIF)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.