Abstract
Nitric oxide (NO) vascular signaling has long been considered an independent, self-sufficient pathway. However, recent data indicate that the novel gaseous mediator, hydrogen sulfide (H2S), serves as an essential enhancer of vascular NO signaling. The current article overviews the multiple levels at which this enhancement takes place. The first level of interaction relates to the formation of biologically active hybrid S/N species and the H2S-induced stimulation of NO release from its various stable “pools” (e.g., nitrite). The next interactions occur on the level of endothelial calcium mobilization and PI3K/Akt signaling, increasing the specific activity of endothelial NO synthase (eNOS). The next level of interaction occurs on eNOS itself; H2S directly interacts with the enzyme: sulfhydration of critical cysteines stabilizes it in its physiological, dimeric state, thereby optimizing eNOS-derived NO production and minimizing superoxide formation. Yet another level of interaction, further downstream, occurs at the level of soluble guanylate cyclase (sGC): H2S stabilizes sGC in its NO-responsive, physiological, reduced form. Further downstream, H2S inhibits the vascular cGMP phosphodiesterase (PDE5), thereby prolonging the biological half-life of cGMP. Finally, H2S-derived polysulfides directly activate cGMP-dependent protein kinase (PKG). Taken together, H2S emerges an essential endogenous enhancer of vascular NO signaling, contributing to vasorelaxation and angiogenesis. The functional importance of the H2S/NO cooperative interactions is highlighted by the fact that H2S loses many of its beneficial cardiovascular effects when eNOS is inactive.
Keywords: angiogenesis, cGMP, hydrogen sulfide, nitric oxide, vascular
The Vascular eNOS/sGC/cGMP/PKG Pathway May Not Be Completely Self-Sufficient
Vascular NO production (overviewed in 12, 39, 43, 53, 74, 89, 90, 107) is predominantly due to endothelial NO synthase (eNOS), a calcium-dependent enzyme constitutively expressed in vascular endothelial cells.1 Various vasorelaxant and angiogenic hormones and factors, as well as shear stress, lead to calcium mobilization in the endothelial cells, which activates eNOS in a calmodulin-dependent manner. In the presence of various co-factors (e.g., NADPH and BH4), eNOS converts its physiological substrate l-arginine to NO and l-citrulline. In addition to calcium, eNOS is also regulated by phosphorylation/dephosphorylation at several critical regulatory amino acid residues. NO, produced by eNOS, either reaches its targets within the endothelial cell itself, or diffuses to the underlying vascular smooth muscle cells. In turn, NO binds to the heme group of its target enzyme, soluble guanylate cyclase (sGC), and activates it. The sGC-mediated production of cGMP, via stimulation of downstream enzymes (cGMP-dependent protein kinases, PKGs) is primarily responsible for the biological effects of eNOS, such as vascular relaxation and angiogenesis. Vascular cGMP levels are physiologically degraded by phosphodiesterase 5 (PDE5) (19, 26, 105). The vascular eNOS/sGC/cGMP/PKG pathway, one of the most intensively studied signaling pathways in biology, is generally considered a stand-alone, self-sufficient pathway that does not rely on external biochemical enhancers.
Several decades after the discovery of the essential role of the NO/sGC/cGMP pathway in the control of the cardiovascular system, the regulatory roles of another gaseous mediator, hydrogen sulfide (H2S), started to emerge (overviewed in 55, 57, 60, 61, 66, 85, 108, 109, 125–129, 139, 143, 145). In brief, H2S is produced in the vascular system by three distinct enzymes, cystathionine-gamma-lyase (CSE), cystathionine-beta-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST). The substrates of CBS and CSE are l-cysteine and homocysteine; the substrate of 3-MST is 3-mercaptopyruvate which is produced from l-cysteine. H2S exerts its biological effects via a variety of mechanisms including posttranscriptional modification of critical cysteines in various enzymes via a novel process entitled S-sulfhydration. Similar to NO, H2S causes vasorelaxation (143, 144), participates in the physiological maintenance of blood pressure (149), and serves an endogenous stimulator of angiogenesis (15, 106, 124). In addition, similar to NO, which converts into various stable or semi-stable “pools” (e.g., nitrite) and can be regenerated from it under certain conditions (59, 76, 77), H2S converts into thiosulfate, which can regenerate biologically active H2S (79, 117, 141).
Not only do NO and H2S exhibit biological and functional similarities in the cardiovascular system, but several lines of data, most of which has emerged over the last 5 years, indicate that the two pathways, in fact, cooperate with each other. In the vascular system, H2S, in many respects, is now viewed as an enhancer of the NO/cGMP/sGC/PKG pathway, without which eNOS cannot function to its fullest physiological extent. The biosynthesis, biological effects, metabolism, and physiological and pathophysiological roles of H2S in a variety of diseases are subject to separate review articles (55, 57, 60, 61, 66, 85, 108, 109, 125, 127, 128, 139, 143, 145). The sole focus of the current review is to summarize the mechanisms by which H2S acts as an enhancer of the vascular eNOS/sGC/cGMP/PKG system.
H2S Stimulates NO Release from Its Stable or Semi-Stable Pools
Starting with the work of Moore, Whiteman, and coworkers (1, 147, 150) the notion began to emerge that the vascular effects of NO and H2S may be interdependent, and may be, at least in part, related to the formation of a combined NO/H2S species, i.e., a nitrosothiol (147). These findings, together with earlier observations of Kimura and coworkers who demonstrated that H2S enhances the vascular relaxant effect of NO (47), suggested that H2S may act as an enhancer of vascular eNOS/sGC/cGMP/PKG signaling, an effect, which, perhaps, is most relevant in the microvasculature (143, 144).
There are multiple levels of direct chemical interactions between NO (and its various metabolites) and H2S (and its various metabolites) such that the field of NO/H2S “cross-talk” has emerged as a separate area of biochemistry, which investigates (among others) the reaction products of H2S with nitrosothiols, peroxynitrite, and nitrite (8, 17, 22–25, 37, 63, 73, 134, 146, 147). The biological responses induced by HNO (nitroxyl), one of the products of the H2S/NO interactions, has recently been reviewed by Nagpure and Bian (91). There are several open areas in this rapidly growing field, which are overviewed in several recent articles (24, 41, 63, 67, 73, 78, 91).
Although the goal of this article is not to outline the complex chemistry of H2S-related biological species, it must be mentioned that in biological systems, at or near physiological pH, in the presence of various biologically relevant and redox active molecules (e.g., thiols, transition metals, proteins), even when starting out from “pure” H2S gas (e.g., by “bubbling” H2S into the culture medium of cells), a complex mixture of sulfur species will be created. Because the dissociation constants of H2S are pKa1 = 6.8 and pKa2 >12, at pH 7.4, only about a quarter of H2S gas will remain as dissolved gas: H2S will be predominantly present in the form of hydrosulfide anion (HS−). At physiological pH, only minute amounts of H2S will convert into S2− (73, 92). In addition to these (primary) H2S-related species, in biological systems, due to a complex sequence of (bio)chemical interactions that are presently only partially understood, H2S will also lead to the formation of a complex mixtures of organic persulfides and polysulfides (52, 100). While H2S and HS− are generally believed to have similar (although probably not identical) biological character, the chemical reactions elicited by polysulfides are drastically different (6, 52, 61, 100, 135). Downstream from H2S, the biological liberation of sulfur (thiyl) radical, the highly unstable intermediate HS molecule, the reactive S− molecule, as well as the formation of other reactive sulfur species including S2−, is also chemically feasible (24, 92, 94, 103). When this “soup” of H2S-derived reactive species reacts with NO (or with a “soup” of NO-derived species), a wide variety of bioactive intermediates can form, including nitrosopersulfide (SSNO−), and SULFI/NO [ON(NO)SO3−] (24). These molecules are biologically active: therefore, via this interaction, H2S may create complex NO metabolites that modify (perhaps enhance) the biological action of NO (8, 23) (Fig. 1, arrow 1). The exact (patho)physiological role of the various S/N hybrid species remains to be further elucidated.
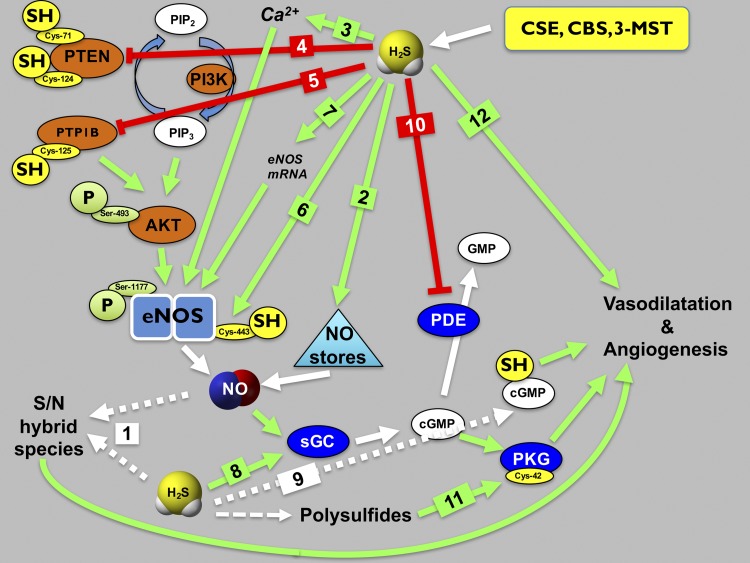
Fig. 1.
Enhancement of the eNOS-mediated vascular signaling by H2S. On the level of direct biochemical interactions, H2S reacts with NO to produce biologically active S/N species (arrow 1). In addition, H2S has the capacity to release NO from stable and semi-stable “NO storage pools” (e.g., nitrite, nitrosothiols, peroxynitrite) (arrow 2). In addition, H2S can stimulate intracellular Ca2+ mobilization in endothelial cells (arrow 3), which stimulates eNOS via the calcium-calmodulin system. Importantly, H2S can activate the PI3K pathway by either by sulfhydrative inactivation of PTEN (arrow 4) or by sulfhydrative inactivation of PTP1B (arrow 5). These processes lead to the enhancement of the PI3K pathway, culminating in the elevation of intracellular PIP3 levels. PIP3, in turn, activates Akt, which, then, acts on various downstream kinases and phosphatases (not shown). The end-result is the phosphorylation on an activating serine of eNOS (Ser1177). Calcium mobilization and Akt activation both stimulate eNOS, resulting in increased eNOS-dependent endothelial NO production upon eNOS-stimulating factors, e.g., binding of various calcium-mobilizing hormones acetylcholine or VEGF to their receptors on the endothelial cell membrane (not shown). H2S also induce a direct posttranslational modification of eNOS via sulfhydration on Cys443 (arrow 6). This action, in turn, further activates and stabilizes eNOS by promoting its dimerization. There is also some evidence that in some experimental systems H2S can stimulate the expression of eNOS mRNA (arrow 7), putatively increasing eNOS protein levels. Further downstream, NO produced by eNOS binds to soluble guanylate cyclase (sGC), and induces the formation of cGMP. In endothelial cells, in the context of endothelial cell proliferation and migration, the sGC target of NO is in the endothelial cell itself. In the context of the endothelium-dependent relaxation, NO diffuses from the endothelial cell to the underlying vascular smooth muscle cell, where it binds to its sGC target in the vascular smooth muscle cytosol. (The endothelial cell and the smooth muscle cell sGC targets are not separated in the current scheme). H2S stimulates the sGC/cGMP pathway via several distinct mechanisms. As a reducing agent, H2S can induce the stabilization of sGC in its reduced, NO-responsive form (arrow 8). H2S can also react with cGMP to form 8-SH-cGMP (arrow 9), which is biologically active and less sensitive to enzymatic degradation by PDE5. In addition, H2S, by inhibiting PDE5 (arrow 10), suppresses the degradation of cGMP, thereby ensuring that the cGMP levels remain high for a longer period of time, thereby enhancing the activation of its downstream protein kinase, PKG. Finally, the degradation of H2S to intracellular polysulfides can induce the oxidative activation of PKG (arrow 11). These processes culminate in the prototypical vascular biological responses induced by both NO and H2S such as angiogenesis and vasorelaxation. Depending on the biological context, H2S may also stimulate vascular responses via pathways that do not involve the eNOS pathway (arrow 12).
For the purpose of the current review, the next relevant reaction is that of H2S and various semistable pools of NO (e.g., nitrite) leading to the generation of NO (11) (Fig. 1, arrow 2). As overviewed elsewhere (59, 76, 77), nitrite was initially believed an inactive, stable metabolite of NO, but it is now recognized as an important constituent of a biologically active, physiological “NO pool”; in fact, via administration of nitrite, therapeutically relevant, NO-mediated biological responses can be achieved (such as vascular relaxation, cardioprotection, and angiogenesis). The fact that H2S can stimulate NO release from nitrite, as shown in hypoxic endothelial cells in vitro (11), and furthermore, the fact that the reaction of H2S with peroxynitrite can produce the NO donor sulfinyl nitrite (37) may suggest that in the presence of H2S, nitrite may become more biologically active and/or perhaps therapeutically more efficacious. Moreover, peroxynitrite, a reactive NO-derived species (7, 123), may be rendered less noxious by H2S. In cultured endothelial cells the H2S-induced release of NO from nitrite is, at least in part, catalyzed by xanthine oxidase (11). In addition to nitrite and peroxynitrite, H2S can also induce NO release from nitrosothiols and metal nitrosyl complexes (99). It is worth mentioning that not only H2S can stimulate NO production, but also nitrite can stimulate H2S production: in a mouse model of chronic heart failure, treatment of the animals with sodium nitrite was found to induce the upregulation of the H2S-producing enzymes CSE and CBS in the heart; consequently, nitrite treatment was found to elevates circulating H2S levels (31).
H2S Stimulates eNOS Activity via Stimulation of Calcium Mobilization
Since eNOS is a calcium-dependent enzyme, intracellular calcium mobilization, in response to endothelium-dependent vasodilatators and angiogenic hormones, is a rapid, potent activator of eNOS. Several lines of studies indicate that this process can be stimulated in the presence of H2S. First, in rat aorta endothelial cells, Moccia and colleagues demonstrated that H2S stimulates Ca2+ entry in a tetraethylammonium- and glybenclamide inhibitable manner, suggesting the involvement of reverse-mode of KATP channels (84). H2S may also recruit the Na+/Ca2+ exchanger in a reverse mode (84). Subsequent studies showed that H2S increased intracellular Ca2+ levels in cultured endothelial cells, and this response was inhibitable by dantrolene or xestospongin C (58). Finally, in the HUVEC-derived cell line Ea.hy926, Potenza and colleagues demonstrated H2S elicited a time- and concentration-dependent intracellular Ca2+ response, which was attributed to inositol-1,4,5-trisphosphate-dependent Ca2+ release (111). The findings of Potenza and colleagues (showing that the endothelial calcium signal is identical in the presence or absence of extracellular calcium) are in disagreement with the findings of Moccia (suggesting that endothelial cell calcium mobilization in response to H2S requires the influx of extracellular calcium). Whether the source of calcium is extra- or intracellular, the findings discussed above identify endothelial Ca2+ mobilization as the first, most upstream level at which H2S may enhance the activity of the eNOS/sGC/cGMP/PKG system (84) (Fig. 1, arrow 3). It must be pointed out, nevertheless, that the above-referenced studies investigate calcium mobilization (in response to H2S), and present functional outcome variables (e.g., endothelial cell migration), but do not directly (e.g., by measuring l-arginine to l-citrulline conversion) show in the endothelial cells that the increased Ca2+ signal, is, indeed, directly responsible for the activation of eNOS; further studies, directly testing the link of the H2S-induced Ca2+ signal with eNOS activity, remain to be conducted.
H2S Stimulates eNOS Activity via AKT-Mediated Phosphorylation
A key level of the regulation of eNOS activity occurs at the level of phosphorylation/dephosphorylation of its critical regulatory amino acids. The best established regulatory amino acid residue is Ser1177 (the phosphorylation of which stimulates eNOS activity). Ser1177 phosphorylation, which increases the specific activity of eNOS, are facilitated by Akt activation in endothelial cells (29, 42, 82). Upstream, the activation of Akt in endothelial cells, is regulated by intracellular PIP3 levels, which are under the control of the PI3 kinase (PI3K) system (92).
The H2S-induced cytoprotection and cell migration involves the PI3K system which stimulates its downstream effectors such as Akt and Rac-1 (15, 152). In various cultured endothelial cell systems, several lines of studies demonstrate that H2S stimulates Akt by promoting its phosphorylation at its activating site (Ser493) (15, 21). H2S also induces Akt activation in other cells types (50, 78, 132). Whether the H2S-mediated increase in Akt is due to a direct effect of H2S on PI3K remains to be determined. Part of the stimulatory effect on AKT activity is likely to be related to H2S-mediated inhibition of Phosphatase and Tensin homolog (PTEN), an essential counterregulatory enzyme of the PI3K pathway, via sulfhydration of Cys124 (78, 98) (Fig. 1, arrow 4). The activity of PTP1B (another counterregulatory enzyme in the PI3K pathway) is also inhibited by H2S, via sulfhydration at Cys215 (71) (Fig. 1, arrow 5).
Whatever the upstream processes are (direct stimulation of PI3K and/or indirect effects via PTEN or PTP1B or via other mechanisms), elevated PIP3 levels by H2S would be expected to induce Akt activation, which, in turn, would be expected to produce the characteristic changes in the phosphorylation of regulatory amino acids on eNOS. Indeed, this is the case, as demonstrated by multiple studies in response to administration of various H2S donors in vitro and in vivo (2, 3, 6, 11, 18, 21, 49, 56, 62, 75, 80, 102, 112, 113). For instance, in endothelial cells incubated with H2S donors, the activation of Akt (evidenced by phosphorylation at Ser473) was accompanied by the phosphorylation of Ser1177 on eNOS (21). In endothelial cells subjected to shear stress, H2S was found to stimulate eNOS phosphorylation at Ser1177 (49). Similarly, H2S was shown in multiple studies (in the heart as well as in the coronary arterioles) to stimulate Ser1177 phosphorylation of eNOS in vivo (62, 103, 113). In endothelial cells subjected to shear stress, Ser1177 phosphorylation was diminished after siRNA-mediated silencing of either CSE, CBS, or 3-MST genes (49); likewise, in CSE−/− mice the degree of basal eNOS phosphorylation at Ser1177 is markedly lower than the corresponding basal Ser 1177 phosphorylation in wild-type mice (62). These findings indicate that basal, physiological Ser1177 phosphorylation of eNOS is maintained by endogenous H2S through the basal physiological actions of various H2S-producing enzymes. The functional relevance of the H2S-mediated eNOS phosphorylation is highlighted by the results Lefer and co-workers who demonstrated that that the protective effect of H2S was markedly lower in eNOS phosphomutant S1179A mice, than in the corresponding wild-type control mice (62).2
There may be additional levels at which H2S stimulates the generation of PIP3, even further upstream from PI3K and PTEN. One example relates to VEGF (a proangiogenic and vasorelaxant hormone). The endothelial cell receptor of VEGF (VEGFR2) is a strong activator of PI3K through VEGRF autophosphorylation (150). Tao and coworkers demonstrated that H2S can reduce the intramolecular Cys1024-Cys1045 disulfide bond in VEGFR2, which, in turn, increases the PI3K stimulating activity of this receptor, culminating in an increased angiogenic response (133).
H2S Stimulates eNOS Activity via Direct Sulfhydration
eNOS homodimers represent the physiological state of the enzyme, where it produces NO in the most efficient manner, as well as the least amount of superoxide. On the other hand, monomeric eNOS is prone to superoxide generation (33, 114, 131). Wang and colleagues have demonstrated that H2S sulfhydrates Cys443 of eNOS, which, in turn, stimulates the catalytic activity of eNOS, thereby keeping the enzyme in the dimeric state and suppressing superoxide generation by eNOS and maximizing physiological NO generation (2) (Fig. 1, arrow 6).
H2S Stimulates eNOS mRNA Synthesis
H2S was found to increase the expression of eNOS (Fig. 1, arrow 7) in some systems, for instance in EAhy926 cells in vitro (18). In contrast, in other experimental settings only a slight and nonsignificant trend towards increased eNOS protein expression was noted in response to H2S exposure (3). In some experimental systems, H2S can also induce an upregulation of eNOS mRNA in vivo. For instance, in a murine model of thrombus formation induced by phototoxic light/dye-injury, H2S administration caused the upregulation of eNOS in venules of the ear of hairless SKH1-hr mice (69). Moreover, in kidney reperfusion model in the rat, H2S administration maintained eNOS expression levels during ischemia (while eNOS was downregulated in the ischemic control group that did not receive H2S) (51). eNOS mRNA expression also increased after H2S treatment in the corpus cavernosum (81). While H2S donation can stimulate eNOS mRNA expression, basal physiological production of H2S does not appear to be an absolute requirement for eNOS expression: eNOS expression levels were comparable in wild-type mice and CSE−/− mice (62).
H2S Maintains sGC in an NO-Activatable State
Cells contain two distinct sGC pools: the reduced, ferrous heme group of the enzyme (Fe2+) is the physiological form, which is responsive to NO in the classical NO/sGC/cGMP/PKG pathway. However, the heme group of sGC can also be oxidized (e.g., in various pathophysiological states) to yield the ferric (Fe3+) that is no longer NO-activatable (9, 28, 34, 38, 105). Recent studies show that H2S can act as a reducing agent for the heme group of sGC, catalyzing a ferric to ferrous heme transition (154). ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one) is a sGC inhibitor that acts via oxidizing the heme group (46). Treatment of recombinant sGC with ODQ reduced the responsiveness of the enzyme to NO, an effect that was attenuated if in the presence of H2S. On the other hand, H2S attenuated the response to the heme-independent activator BAY58-2667 that targets oxidized sGC. The beneficial effect of H2S on maintaining an NO-activatable state of sGC (Fig. 1, arrow 8) has been confirmed in oxidatively stressed endothelial cells as well as in isolated vascular rings (154). The above-described redox effect of H2S on the heme group of sGC appears to be most relevant under conditions of oxidative stress, because in the absence of oxidative stimuli, H2S does not influence sGC activity, neither under basal conditions, nor in response to NO donors (21, 154).
H2S Reacts with cGMP to Yield 8-SH-cGMP
The next level of cooperation between H2S and the vascular NO/sGC/cGMP/PKG pathway is at the level of cGMP itself. Modified cyclic nucleotides, including 8-nitro-cGMP, play key roles in vascular regulation (95, 96). Recent data show that H2S directly reacts with 8-nitro-cGMP, yielding 8-SH-cGMP. This molecule appears to be more resistant to PDE5 than “normal” cGMP (95, 96) and, in turn stimulates PKG activity (Fig. 1, arrow 9). 8-Nitro-cGMP/8-SH-cGMP imbalance has been implicated in a variety of pathophysiological conditions including cardiac ischemia and remodeling (95, 96, 100).
H2S Inhibits PDE5 Activity
Vascular cGMP phosphodiesterases (PDEs), predominantly PDE5, are essential negative regulators of cGMP levels. Inhibition of PDE5 activity suppresses cGMP degradation and, by maintaining/prolonging intracellular cGMP levels, enhances downstream signaling. In effect, in the presence of PDE inhibitors, the same amount of NO gets a “bigger bang for the buck” in terms of cGMP-dependent downstream vascular responses. PDE5 inhibitors, by boosting cGMP signaling, have successfully been employed not only in the therapy of male erectile dysfunction, but also pulmonary hypertension and other cardiovascular diseases (19, 26, 105). Two independent laboratories have demonstrated that H2S inhibits PDE activity (13, 21), which, in turn, boosts NO-stimulated cGMP levels in vascular endothelial cells and vascular smooth muscle cells (Fig. 1, arrow 10). Although H2S inhibits all known isoforms of both cAMP- and cGMP phosphodiesterases, it is most potent in inhibiting PDE5 (104). The mechanism of the inhibition does not appear to involve PDE sulfhydration (9, 10). The functional consequence of this interaction is that H2S elevates intracellular cGMP in vascular cells and tissues, thereby promoting and enhancing NO-mediated vascular responsiveness. This is also evidenced by H2S-induced increases in the phosphorylation of the PKG target VASP (13, 14, 21). There is also evidence for inhibition of the mitochondrial cAMP PDE isoform (PDEA2) by H2S (88); the functional consequence of this response is the stimulation of mitochondrial electron transport; this effect cooperates with the other mechanisms, including direct electron donation, and activation of ATP synthase (44, 88, 87, 122, 125), by which physiological concentrations of H2S stimulate mitochondrial electron transport and cellular bioenergetics.
Polysulfides Directly Activate PKG
Another layer of interaction between H2S and the NO/sGC/cGMP/PKG pathway occurs at the level of protein kinase G (PKG) activation. PKG is the essential effector of eNOS-mediated vasorelaxant, angiogenic, and cardioprotective responses (40, 115). Greiner and coworkers demonstrated that polysulfides (molecules that are formed from H2S intracellularly via a variety of mechanisms, see above) are capable of catalyzing the formation of an activating interprotein disulfide within PKG Iα, thereby stimulating the activity of PKG (45) (Fig. 1, arrow 11). This has important consequences for the cardiovascular regulatory effect of H2S: while in control mice H2S lowers blood pressure, in genetically engineered mice in which PKG's Cys42 redox sensor has been rendered redox-insensitive, the blood pressure effect of H2S was attenuated (120). PKG may also have other levels of interaction with the NO or H2S systems, although the information on this subject remains somewhat fragmented. In some cases, evidence of a positive interaction has been presented, for instance PKG was reported to induce CSE expression in the heart (27), whereas in other experimental systems PKG-induced phosphorylation and consequent inhibition of CSE have been observed (4).
Consequences of the NO-H2S Cooperation for the Control of the Cardiovascular System
If the cooperative biochemical interactions between H2S and the NO/sGC/cGMP system outlined above and summarized in Fig. 1 are physiologically or pathophysiologically relevant, then the following functional consequences would be expected: 1) H2S administration would be expected to increase vascular NO production, with a consequent boost of vascular cGMP levels; 2) H2S administration would be expected to potentiate the vasorelaxant and angiogenic effect of NO; 3) if endogenously produced H2S plays a cooperative role with the NO system, then inhibition of vascular H2S biosynthesis would be expected to attenuate the vasorelaxant and angiogenic effect of NO; 4) if the cardiovascular effect of H2S primarily occurs via enhancement of the NO pathway, then in the absence of functional NO/sGC/cGMP/PKG system the vasorelaxant and angiogenic effects of H2S would be expected to be diminished. There is, indeed, direct experimental evidence for all of the above-mentioned scenarios, as outlined below.
1) H2S administration elevates vascular NO and cGMP levels.
In various models of cultured endothelial cells, H2S has been found to both increase NO levels (3, 18, 112) and to elevate cGMP levels (9, 21). These effects were also associated with an increase in VASP phosphorylation (21), indicative of the fact that H2S exposure results in the stimulation of the full NO/sGC/cGMP pathway, culminating in an increase in PKG activity. Not only authentic H2S, but also the CBS/CSE substrate l-cysteine, was found to increase endothelial NO levels, in a manner that was reduced by the CSE inhibitor (5) PAG (3), confirming the ability of the endogenous endothelial H2S biosynthesis to contribute to NO production. In a cultured rat corpus cavernosum preparation, various H2S donors were also found to increase NO levels (81). Importantly, the vasorelaxant and angiogenic responses to H2S are attenuated by the PKG-Iα inhibitor DT-2 (14, 21). In addition, l-cysteine- and H2S-induced relaxations are less pronounced in aortae isolated from PKG-I KO mice than the corresponding control relaxations in aortae isolated from wild-type mice (14). These data, taken together, support the notion that stimulation of NO production and/or enhancement of the cGMP/sGC system, culminating on PKG activation, represent essential components of the vascular signaling induced by H2S. There are examples of H2S donation increasing circulating NO levels as well. For instance, it was demonstrated that the H2S donor diallyl trisulfide increases circulating NO levels in a pressure overload-induced heart failure model in mice (107). Moreover, Bir and colleagues demonstrated that H2S donor therapy increases circulating NO levels in mice (11). Finally, Wang and colleagues found that plasma NO levels are lower in CSE-deficient mice than in wild-type mice (2), concluding that endogenous H2S production contributes to the physiological maintenance of circulating NO levels.
2) H2S administration potentiates the vascular effect of NO.
Evidence for a potentiating effect of H2S on vascular relaxations induced by exogenous NO was first provided by Kimura and co-workers in 1997: H2S donation was found to enhance the relaxant effect of various NO donors (Na-nitroprusside and morpholinosydnonimine) in rat portal vein and thoracic aortic ring preparations, in an endothelium-independent fashion (47). We have demonstrated in 2012 that the acetylcholine-induced, endothelium-dependent relaxations, as well as the relaxant responses to the NO donor DEA/NO, are enhanced (concentration-response curves shifted to the left) in the presence of exogenously administered H2S, and this functional synergy was accompanied with a synergistic enhancement of vascular cGMP levels (21). With respect to angiogenesis, vascular overexpression of CSE was found to enhance the stimulating effect of both DEA/NO and VEGF in an aortic ring sprouting assay (21). Moreover, a recent study demonstrated that ZYZ-803 (a novel synthetic H2S/NO hybrid molecule) induces endothelial cell proliferation, migration, and tube-like structure formation and angiogenesis in rat aortic rings and Matrigel plug assay: all of these actions occur at greater potency than the effect of either H2S or a NO donor on its own (48). ZYZ-803 also exerts vasorelaxant effects via stimulation of the cGMP pathway (148).
3) Inhibition of vascular H2S biosynthesis attenuates the vascular effect of NO.
Although the eNOS/sGC/cGMP/PKG system is generally believed to be an independent signaling pathway, surprisingly, it relies, at least in part, on endogenous H2S production. This component is relatively smaller in the context of vascular relaxation, but it is surprisingly marked in the context of angiogenesis. Wang and Snyder have observed that vasorelaxant effect of metacholine (which, similar to acetylcholine, relaxes blood vessels via activation of cholinergic receptors and stimulation of the eNOS/sGC/cGMP/PKG system) is lower in blood vessels of CSE−/− mice than the corresponding response in wild-type mice (149). In addition, the vasorelaxant effect of the NO donor SNAP is less pronounced in vascular rings of CSE−/− mice than the vasorelaxation observed in rings of wild-type control mice (149). Confirming and extending these observations, we have demonstrated that acetylcholine, as well as DE/NO-induced vascular relaxation dose-responses, are shifted to the right in thoracic aortic rings after vascular CSE silencing; this was also associated with a reduction in vascular cGMP levels (21). As far as interactions in angiogenesis: DEA/NO-induced in vitro angiogenic activity (as well as the ability of VEGF to induce angiogenesis) was markedly attenuated after silencing of CSE in endothelial cells; once again, after CSE silencing, the NO donors failed to induce elevations in endothelial cGMP levels (21). H2S-induced angiogenesis (which served as the control group in this experiment) remained unaffected after CSE silencing. In line with these findings, Wang and coworkers have demonstrated that the angiogenic effect of the eNOS substrate l-arginine is markedly less pronounced in aortic rings of CSE−/− mice compared with the responses in wild-type mice (3).
4) Inhibition of eNOS attenuates the vascular effect of H2S.
Moore and colleagues observed in 2006 that the hypotensive effect of intravenous H2S infusion in rats is suppressed by treatment with the NOS inhibitor l-NAME (1), and similar findings were recently reported in human volunteers: the local vasodilatory response to administration of H2S was reduced by l-NAME (73). The in vitro vascular relaxant effect of H2S is also reduced by pretreatment with the NOS inhibitor l-NAME, and it is shifted to the right in vascular rings from eNOS-deficient mice (21), indicating that part (but not all) of the vascular relaxations induced by H2S require the eNOS/sGC/cGMP/PKG pathway. Since H2S exerts vascular relaxant effects via a number of additional mechanisms, including KATP channel opening, as well as, at higher concentrations, by direct metabolic effects (64, 66), it is not surprising that NOS inhibition only abrogates part of its vascular relaxant responses. However, it appears that the NO system is obligatory for H2S-induced in angiogenesis: H2S mediated in vitro angiogenic activity (as well as the ability of H2S to induce an elevation of endothelial cGMP levels) was completely abrogated by pretreatment of the endothelial cell cultures with the NOS inhibitor l-NAME (21); likewise, the stimulatory effect of H2S donors in the Matrigel plug assay in vivo was absent in eNOS−/− mice (21). In agreement with these findings, Wang and colleagues demonstrated that l-NAME, as well as vascular eNOS silencing, abrogates H2S-induced endothelial cell proliferation, tube formation, and aortic ring sprouting responses in vitro, whereas eNOS overexpression facilitates the angiogenic effect of H2S (3).3
5) H2S-induced cardiovascular and therapeutic actions are abrogated in eNOS-deficient systems.
Examples for this are shown in Table 1. Given the fact that (as discussed above) H2S-induced angiogenic responses (Matrigel plug responses) are suppressed in eNOS-deficient mice (21), it should not come as a surprise that the stimulating effect of H2S on wound healing (an effect, which, at least in part, relies on the stimulation of angiogenesis) is also suppressed after pretreatment of the animals with the NOS inhibitor l-NAME (21). In isolated perfused hearts, the protective effect of H2S against isoproterenol-induced cardiac injury is attenuated by l-NAME pretreatment (118). In addition, the well-established (32) ability of H2S pretreatment to reduce infarct size in a murine model of ischemia-reperfusion injury is absent in eNOS deficient mice (62). Similarly, chronic treatment with SG-1001 (a novel H2S donor) was no longer protective against the development of heart failure, when the experiments were conducted in eNOS deficient mice (68). There may be some species differences in the extent to which the H2S-mediated cardioprotection relies on the NOS system: the cardioprotective effect of H2S pretreatment prior to coronary occlusion/reperfusion is suppressed by l-NAME in a mouse model of myocardial ischemia-reperfusion, while in a similar model, performed in rabbits, the protective effect of H2S is maintained even after pretreatment with l-NAME (10). In a mouse cardiac arrest/resuscitation model, the H2S treatment, administered shortly prior to the start of the CPR procedure, prolongs survival in wild-type animals, but is without significant therapeutic effect in eNOS deficient mice (83). Finally, the H2S-induced preconditioning responses on leukocyte rolling, but not on leukocyte adhesion, are reduced in eNOS−/− mice, compared with wild-type mice (151). There are, however, examples when eNOS deficiency does not abrogate the beneficial effect of H2S. For instance, in a model of angiogenesis induced by permanent femoral artery ligation, the beneficial effects of H2S are maintained even on eNOS-deficient background (11).
Table 1.
Examples for abrogation of H2S-induced cardiovascular responses in eNOS-deficient systems
| Experimental System | Mode of eNOS Inhibition | Biological Response to H2S That Is Impaired in the Absence of Functional eNOS | Ref. No. |
|---|---|---|---|
| Blood pressure measurement in anesthetized rats | l-NAME | Hypotension | 1 |
| Cultured bEnd3 endothelial cell angiogenesis | l-NAME | Migration, proliferation, tube formation | 21 |
| Cultured bEnd3 endothelial cells | l-NAME | cGMP formation | 21 |
| Cultured bEnd3 endothelial cell angiogenesis | eNOS silencing | Migration, proliferation, tube formation | 3 |
| Aortic ring sprouting assay | eNOS−/− | Endothelial cell migration | 21 |
| Isolated murine aortic rings | l-NAME | Vasorelaxation | 21 |
| Isolated murine aortic rings | eNOS−/− | Vasorelaxation | 21 |
| Normothermic cardiac arrest, followed by resuscitation by cardiac compression and respiration in mice | eNOS−/− | Prolongation of survival | 81 |
| Matrigel plug assay in mice | eNOS−/− | Angiogenesis | 21 |
| Burn induced wound healing in rats | l-NAME | Wound closure | 21 |
| Isoproterenol-induced cardiac injury in mice | l-NAME | Cardiac lactate dehydrogenase and creatine kinase release, cardiac TBARS, glutathione, SOD and catalase activity, TNF expression, histopathological alterations | 118 |
| Myocardial infarction induced by transient LAD occlusion in mice | eNOS−/− | Infarct size | 62 |
| Myocardial infarction induced by transient LAD occlusion in mice | l-NAME | Infarct size | 10 |
| Chronic heart failure induced by aortic constriction in mice | eNOS−/− | Cardiac hypertrophy, BNP plasma levels, myocardial contractility | 68 |
| Intestinal ischemia-reperfusion in mice, intravital microscopy | l-NAME | Leukocyte rolling | 151 |
BNP, B-type natriuretic peptide; LAD, left anterior descending coronary artery; LDH, lactate dehydrogenase; l-NAME: NG-nitro-l-arginine methyl ester; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substrates; TNF, tumor-necrosis factor alpha.
Implications
There are several practical/translational implications of the above outlined multiple layers of intricate interplay between the vascular NO and H2S systems. The first one relates to the fact that the eNOS/sGC/cGMP/PKG system becomes impaired in a variety of pathophysiological conditions (from hypertension to atherosclerosis and diabetic complications). As reviewed elsewhere in more detail (121, 129, 138, 142, 143, 145), in many forms of vascular disease (diabetic vascular complications, preeclampsia, vascular aging, atherosclerosis) H2S levels are impaired; the absence of endogenous H2S production (e.g., CSE deficient systems) exacerbates the onset and severity of the vascular dysfunction, while H2S donation improves vascular function. Under conditions of vascular disease, given the partial or absolute requirement of the NO system for H2S to be fully effective, one would expect that the therapeutic benefit provided by H2S donation would be diminished. Under such conditions, one would also expect that simultaneous substitution of both NO and H2S may be more beneficial than the effect of H2S alone. H2S levels are dynamically and delicately regulated by production and consumption (30, 121, 140). Since 1) H2S levels are diminished in various pathophysiological states such as in diabetic complications (121, 129, 138), and 2) the NO system, in part, relies on the presence of H2S, one would expect that NO-based therapies will not be fully efficacious, unless not only NO, but also H2S is also substituted. Multiple mechanisms contribute to the loss of vascular H2S levels in diabetic animals: part of it relates to mitochondrial dysfunction, excessive ROS production, and consequent excessive H2S consumption (121); another part relates to the oxidative inactivation of the H2S-producing enzyme 3-MST (20). Under such conditions, appropriately selected antioxidant therapies may be useful to restore biologically active H2S (as well as NO) levels.
Recognizing the simultaneous need for the restoration of vascular NO and H2S homeostasis, several groups started to design and test various “hybrid” molecules. H2S-donating groups have been placed on a large number of existing drugs (reviewed in 119, 142), and some of these combinations may simultaneously enhance NO and H2S signaling. For instance, ACS6, a H2S-donating derivative of sildenafil (116), boosts both NO-induced cGMP signaling and H2S signaling (via PDE5 inhibition and direct chemical H2S donation, respectively). The angiogenic and vascular effects of the combined NO/H2S donor ZYZ-803 (48, 148) have already been discussed above.
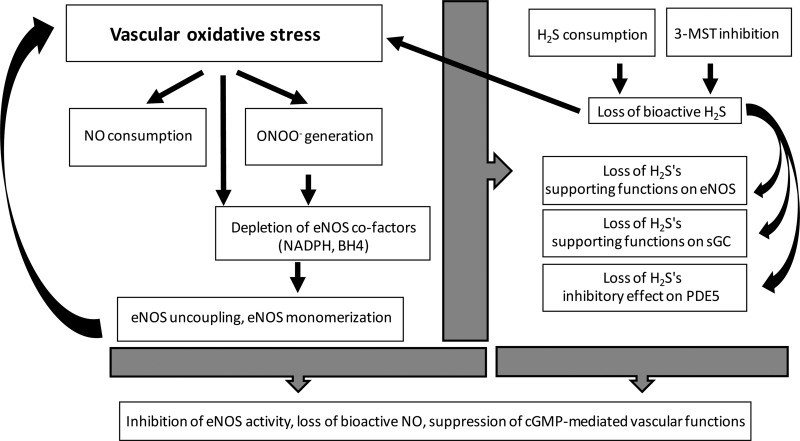
H2S has been demonstrated to protect against the development of endothelial dysfunction in various pathophysiological conditions including ischemia-reperfusion, diabetes, and preeclampsia (32, 102, 124, 143). Under conditions of vascular oxidative stress, the biological profile of both NO and H2S becomes drastically different. For instance, as discussed earlier, NO can form the cytotoxic oxidant peroxynitrite as well as a variety of other potentially cytotoxic or detrimental species, and H2S can also convert into a variety of reactive molecules. Under such conditions, the antioxidant character of H2S may become more important. To some extent, such antioxidant effects may be related to direct interactions with ROS species, even though the corresponding reaction rate constants, as determined by stopped flow studies, are widely variable, some of them being rather low (17, 25, 93). Probably more important is the fact that H2S can also exert antioxidant effects through various indirect mechanisms, including the stimulation of the antioxidant vascular “master switch” Nrf2 and stimulation of intracellular glutathione generation; these effects may also contribute to the phenomenon of H2S-induced preconditioning (4, 16, 54, 112). Importantly, the absence of vascular H2S biosynthesis, on its own, induces an elevation of oxidative stress (61). When considering the somewhat controversial and fragmented body of evidence, the role of H2S/NO interactions, on the background of vascular oxidative stress, may be summarized in the following working hypothesis (Fig. 2). 1) Vascular oxidative stress increases the consumption of NO, and converts it into more deleterious species (e.g., peroxynitrite). 2) Oxidative stress may also consume cofactors of eNOS (e.g., tetrahydrobiopterin, NADPH), which impairs eNOS activity and leads to further oxidant generation from eNOS. 3) Vascular oxidant generation may also oxidize sGC, increasing the pool of sGC that is no longer activatable by NO. 4) These processes may form self-amplifying cycles of injury, increasing oxidant generation from various sources including mitochondrial electron transport chain. 5) The vascular oxidative stress response diminishes cellular H2S levels, either by directly interacting with them, or by inactivating some of the physiological enzymatic sources of H2S (e.g., 3-MST). 6) When ambient H2S levels are diminished, vascular oxidative stress further increases (62, 121). Moreover, the stimulatory effect of H2S on the eNOS/sGC/cGMP/PKG pathway are diminished, because aging, in turn, causes further impairment in the biological effectiveness of the vascular NO system. 7) All of the above processes may be even further exacerbated in aging blood vessels, which appears to suppress H2S levels, and H2S-dependent bioenergetic responses in various tissues (125, 154) (although this has not yet been sufficiently investigated in vascular tissues). Aged blood vessels also have lower levels of endogenous antioxidants and have a diminished ability to mobilize antioxidant responses (e.g., aging blood vessels have a markedly diminished ability to mount an Nrf2-mediated antioxidant response) (136, 137). 8) Antioxidant therapy and/or replacement therapy with H2S donors, including mitochondrially targeted H2S donors, which have been shown to protect endothelial cells from oxidative injury (130), may be useful to restore the functionality of the eNOS/sGC/cGMP/PKG pathway. It must be reiterated that the above chain of processes represents a working hypothesis, which remains to be experimentally evaluated in the future.
Fig. 2.
Potential mechanisms of impairment of vascular NO and H2S signaling processes during oxidative stress: a working hypothesis. Vascular oxidative stress increases the consumption of NO and converts it into more deleterious species (e.g., peroxynitrite). Oxidative stress also consumes various eNOS cofactors (e.g., tetrahydrobiopterin, NADPH), which impairs eNOS activity and leads to further oxidant generation from NOS. Vascular oxidant generation may also oxidize sGC, increasing the pool of sGC that is no longer activatable by NO. These processes may form self-amplifying cycles of injury, leading to more oxidant generation from various sources including mitochondrial electron transport chain. The vascular oxidative stress response diminishes cellular H2S levels, either by directly interacting with H2S, or by inactivating its enzymatic sources (e.g., 3-MST). When ambient H2S levels are diminished, vascular oxidative stress is exacerbated and the stimulatory/balancing roles of H2S on the vascular eNOS/sGC/cGMP/PKG pathway are reduced, which, in turn, further impairs eNOS-mediated vascular responses.
Some Open Questions
There are numerous areas, some more theoretical, some more practical from either the standpoint of physiology or pathophysiology, that remain severely underdeveloped and open for further investigation. We briefly summarize some of these areas, and hope that the current review will stimulate thought and future experimentation in these areas. The first theoretical area focuses around the following question: what (evolutionary) purpose does it serve to “double down” on NO signaling with H2S-associated enhancers and facilitators? A related question relates to the relative importance of H2S in regulating various proteins that are traditionally thought to be regulated by ROS-sensitive mechanisms. Several authors speculate on the evolutionary roots of NO, H2S, and ROS, and attempt to explain the necessity of these interactions (e.g., 35, 99), but the topic, in the specific context of vascular regulation, remains to be further explored. Another topic relates to the currently unknown levels (and possible regional differences) of H2S in various parts of the vasculature, as well as the potential regional differences in the expression and activity of the various H2S-producing enzymes. The studies investigating the role of endogenous H2S production in various vascular function have, so far, typically only focused on one particular enzyme, such as, in some cases, e.g., the coronary arterial bed, where 3-MST has been proposed as the main H2S-dependent regulatory system (71). However, systematic studies comparing the relative importance of the three H2S-producing enzymes in regulating various vascular responses in various vascular regions remain to be conducted. This subject remains to be studied, both as a stand-alone matter as well as in relation to eNOS signaling. Another area that remains to be studied is the role of H2S in the regulation of vascular permeability (and the potential interplay between NO and H2S in regulating it). Yet another area that remains to be studied much more extensively is the interactions between the H2S pathways and the various constituents of the ROS “world,” including the regulation by H2S of the expression and activity enzymes involved in ROS production and ROS neutralization; the responses of the antioxidant systems to various levels of H2S exposure, and the pathophysiological interplay between the H2S and ROS systems. Once again, this subject remains to be studied, both as a stand-alone matter as well as in relation to eNOS signaling. A working hypothesis related to the interactions of NO and H2S in the context of vascular overproduction is presented in Fig. 2 in the context of vascular dysfunction; however, the various steps of this working hypothesis remain to be further studied.
GRANTS
The work of C. Szabo on various aspects of H2S biology is supported by grants from the National Institutes of Health (R01-GM-107846, R01-CA-178803, and R21-TR-001734), the Shriners of North America (Grant 85800), and the Cancer Prevention and Research Institute of Texas (CPRIT, Grant DP150074).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.S. interpreted results of experiments; C.S. prepared figures; C.S. drafted manuscript; C.S. edited and revised manuscript; C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The editorial assistance of Dr. Anita Marton in the preparation of this manuscript is appreciated.
Footnotes
Please note that this study used a bovine construct: the Ser1177 site in rodent and human eNOS corresponds to Ser1179 in bovine eNOS.
Please note that l-NAME, similar to most pharmacological agents, can have independent pharmacological actions unrelated to NOS inhibition. The interrelationships between NO and H2S that are based solely on pharmacological evidence are, therefore, less robust than those interrelationships that are both supported by pharmacological (NOS inhibitors) as well as genetic (siRNA-mediated silencing and/or knockout mice) proof.
REFERENCES
- 1.Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, Bhatia M, Moore PK. Regulation of vascular nitric oxide in vitro and in vivo: a new role for endogenous hydrogen sulphide? Br J Pharmacol 149: 625–634, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altaany Z, Ju Y, Yang G, Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal 7: ra87, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Altaany Z, Yang G, Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J Cell Mol Med 17: 879–888, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreadou I, Iliodromitis EK, Szabo C, Papapetropoulos A. Hydrogen sulfide and PKG in ischemia-reperfusion injury: sources, signaling, accelerators and brakes. Basic Res Cardiol 110: 510, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br J Pharmacol 169: 922–932, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey TS, Zakharov LN, Pluth MD. Understanding hydrogen sulfide storage: probing conditions for sulfide release from hydrodisulfides. J Am Chem Soc 136: 10573–10576, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87: 1620–1624, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berenyiova A, Grman M, Mijuskovic A, Stasko A, Misak A, Nagy P, Ondriasova E, Cacanyiova S, Brezova V, Feelisch M, Ondrias K. The reaction products of sulfide and S-nitrosoglutathione are potent vasorelaxants. Nitric Oxide 46: 123–130, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Bibli SI, Andreadou I, Chatzianastasiou A, Tzimas C, Sanoudou D, Kranias E, Brouckaert P, Coletta C, Szabo C, Kremastinos DT, Iliodromitis EK, Papapetropoulos A. Cardioprotection by H2S engages a cGMP-dependent protein kinase G/phospholamban pathway. Cardiovasc Res 106: 432–442, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bibli SI, Yang G, Zhou Z, Wang R, Topouzis S, Papapetropoulos A. Role of cGMP in hydrogen sulfide signaling. Nitric Oxide 46: 7–13, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, Kevil CG. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1α and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc 1: e004093, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryan NS, Bian K, Murad F. Discovery of the nitric oxide signaling pathway and targets for drug development. Front Biosci (Landmark Ed) 14: 1–18, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol 30: 1998–2004, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Zaid A, Giannogonas P, Cantalupo A, Dhayade S, Karalis KP, Wang R, Feil R, Cirino G. cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS One 7: e53319, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 76: 29–40, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105: 365–374, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carballal S, Trujillo M, Cuevasanta E, Bartesaghi S, Möller MN, Folkes LK, García-Bereguiaín MA, Gutiérrez-Merino C, Wardman P, Denicola A, Radi R, Alvarez B. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic Biol Med 50: 196–205, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Chen PH, Fu YS, Wang YM, Yang KH, Wang DL, Huang B. Hydrogen sulfide increases nitric oxide production and subsequent S-nitrosylation in endothelial cells. Sci World J 2014: 480387, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cockrill BA, Waxman AB. Phosphodiesterase-5 inhibitors. Hand Exp Pharmacol 218: 229–255, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Coletta C, Módis K, Szczesny B, Brunyánszki A, Oláh G, Rios EC, Yanagi K, Ahmad A, Papapetropoulos A, Szabo C. Regulation of vascular tone, angiogenesis and cellular bioenergetics by the 3-mercaptopyruvate sulfurtransferase/H2S pathway: functional impairment by hyperglycemia and restoration by DL-α-lipoic acid. Mol Med 21: 1–14, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA 109: 9161–9166, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortese-Krott MM, Fernandez BO, Kelm M, Butler AR, Feelisch M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide 46: 14–24, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Cortese-Krott MM, Fernandez BO, Santos JL, Mergia E, Grman M, Nagy P, Kelm M, Butler A, Feelisch M. Nitrosopersulfide (SSNO-) accounts for sustained NO bioactivity of S-nitrosothiols following reaction with sulfide. Redox Biol 2: 234–244, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortese-Krott MM, Kuhnle GG, Dyson A, Fernandez BO, Grman M, DuMond JF, Barrow MP, McLeod G, Nakagawa H, Ondrias K, Nagy P, King SB, Saavedra JE, Keefer LK, Singer M, Kelm M, Butler AR, Feelisch M. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc Natl Acad Sci USA 112: E4651–E4660, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuevasanta E, Zeida A, Carballal S, Wedmann R, Morzan UN, Trujillo M, Radi R, Estrin DA, Filipovic MR, Alvarez B. Insights into the mechanism of the reaction between hydrogen sulfide and peroxynitrite. Free Radic Biol Med 80: 93–100, 2015. [DOI] [PubMed] [Google Scholar]
- 26.Das A, Durrant D, Salloum FN, Xi L, Kukreja RC. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol Ther 147: 12–21, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das A, Samidurai A, Hoke NN, Kukreja RC, Salloum FN. Hydrogen sulfide mediates the cardioprotective effects of gene therapy with PKG-Ia. Basic Res Cardiol 110: 42, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Dasgupta A, Bowman L, D'Arsigny CL, Archer SL. Soluble guanylate cyclase: a new therapeutic target for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Clin Pharmacol Ther 97: 88–102, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR Jr, Darley-Usmar VM, Kraus DW. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem 341: 40–51, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Donnarumma E, Bhushan S, Bradley JM, Otsuka H, Donnelly EL, Lefer DJ, Islam KN. Nitrite therapy ameliorates myocardial dysfunction via H2S and nuclear factor-erythroid 2-related factor 2 (Nrf2)-dependent signaling in chronic heart failure. J Am Heart Assoc 5: e003551, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 104: 15560–15565, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erwin PA, Mitchell DA, Sartoretto J, Marletta MA, Michel T. Subcellular targeting and differential S-nitrosylation of endothelial nitric-oxide synthase. J Biol Chem 281: 151–157, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HHHW, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 5: 755–768, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feelisch M, Martin JF. The early role of nitric oxide in evolution. Trends Ecol Evol 10: 496–499, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Filipovic MR, Eberhardt M, Prokopovic V, Mijuskovic A, Orescanin-Dusic Z, Reeh P, Ivanovic-Burmazovic I. Beyond H2S and NO interplay: hydrogen sulfide and nitroprusside react directly to give nitroxyl (HNO). A new pharmacological source of HNO. J Med Chem 56: 1499–1508, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Filipovic MR, Miljkovic J, Allgäuer A, Chaurio R, Shubina T, Herrmann M, Ivanovic-Burmazovic I. Biochemical insight into physiological effects of H2S: reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochem J 441: 609–621, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Follmann M, Griebenow N, Hahn MG, Hartung I, Mais FJ, Mittendorf J, Schäfer M, Schirok H, Stasch JP, Stoll F, Straub A. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew Chem Int Ed Engl 52: 9442–9462, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62: 525–563, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuto JM, Carrington SJ, Tantillo DJ, Harrison JG, Ignarro LJ, Freeman BA, Chen A, Wink DA. Small molecule signaling agents: the integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide, and their derived species. Chem Res Toxicol 25: 769–793, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furchgott RF. Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci Rep 19: 235–251, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Goubern M, Andriamihaja M, Nübel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J 21: 1699–1706, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Greiner R, Pálinkás Z, Bäsell K, Becher D, Antelmann H, Nagy P, Dick TP. Polysulfides link H2S to protein thiol oxidation. Antioxid Redox Signal 19: 1749–1765, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffmann LS, Schmidt PM, Keim Y, Schaefer S, Schmidt HH, Stasch JP. Distinct molecular requirements for activation or stabilization of soluble guanylyl cyclase upon haem oxidation-induced degradation. Br J Pharmacol 157: 781–795, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Hu Q, Wu D, Ma F, Yang S, Tan B, Xin H, Gu X, Chen X, Chen S, Mao Y, Zhu YZ. Novel angiogenic activity and molecular mechanisms of ZYZ-803, a slow-releasing hydrogen sulfide-nitric oxide hybrid molecule. Antioxid Redox Signal 25: 498–514, 2016. [DOI] [PubMed] [Google Scholar]
- 49.Huang B, Chen CT, Chen CS, Wang YM, Hsieh HJ, Wang DL. Laminar shear flow increases hydrogen sulfide and activates a nitric oxide producing signaling cascade in endothelial cells. Biochem Biophys Res Commun 464: 1254–1259, 2015. [DOI] [PubMed] [Google Scholar]
- 50.Huang Y, Li F, Tong W, Zhang A, He Y, Fu T, Liu B. Hydrogen sulfide, a gaseous transmitter, stimulates proliferation of interstitial cells of Cajal via phosphorylation of AKT protein kinase. Tohoku J Exp Med 221: 125–132, 2010. [DOI] [PubMed] [Google Scholar]
- 51.Ibrahim MY, Aziz NM, Kamel MY, Rifaai RA. Sodium hydrosulphide against renal ischemia/reperfusion and the possible contribution of nitric oxide in adult male Albino rats. Bratisl Lek Listy 116: 681–688, 2015. [DOI] [PubMed] [Google Scholar]
- 52.Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, Ono K, Devarie-Baez NO, Xian M, Fukuto JM, Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci USA 111: 7606–7611, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ignarro LJ. Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling. Pharmacol Toxicol 67: 1–7, 1990. [DOI] [PubMed] [Google Scholar]
- 54.Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol 295: H801–H806, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem 285: 21903–21907, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karwi QG, Whiteman M, Wood ME, Torregrossa R, Baxter GF. Pharmacological postconditioning against myocardial infarction with a slow-releasing hydrogen sulfide donor, GYY4137. Pharmacol Res 111: 442–451, 2016. [DOI] [PubMed] [Google Scholar]
- 57.Katsouda A, Bibli SI, Pyriochou A, Szabo C, Papapetropoulos A. Regulation and role of endogenously produced hydrogen sulfide in angiogenesis. Pharmacol Res 113: 175–185, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kida M, Sugiyama T, Yoshimoto T, Ogawa Y. Hydrogen sulfide increases nitric oxide production with calcium-dependent activation of endothelial nitric oxide synthase in endothelial cells. Eur J Pharm Sci 48: 211–215, 2013. [DOI] [PubMed] [Google Scholar]
- 59.Kim-Shapiro DB, Gladwin MT. Mechanisms of nitrite bioactivation. Nitric Oxide 38: 58–68, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kimura H. Production and physiological effects of hydrogen sulfide. Antioxid Redox Signal 20: 783–793, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide 41: 4–10, 2014. [DOI] [PubMed] [Google Scholar]
- 62.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci USA 111: 3182–3187, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King BS. Potential biological chemistry of hydrogen sulfide (H2S) with the nitrogen oxides. Free Radic Biol Med 55: 1–7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiss L, Deitch EA, Szabo C. Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sci 83: 589–594, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koenitzer JR, Isbell TS, Patel HD, Benavides GA, Dickinson DA, Patel RP, Darley-Usmar VM, Lancaster JR Jr, Doeller JE, Kraus DW. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am J Physiol Heart Circ Physiol 292: H1953–H1960, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide 35: 5–20, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kolluru GK, Yuan S, Shen X, Kevil CG. H2S regulation of nitric oxide metabolism. Methods Enzymol 554: 271–297, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G Sr, Gojon G Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 127: 1116–1127, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kram L, Grambow E, Mueller-Graf F, Sorg H, Vollmar B. The anti-thrombotic effect of hydrogen sulfide is partly mediated by an upregulation of nitric oxide synthases. Thromb Res 132: e112–117, 2013. [DOI] [PubMed] [Google Scholar]
- 70.Krishnan N, Fu C, Pappin DJ, Tonks NK. H2S-induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal 4: ra86, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuo MM, Kim DH, Jandu S, Bergman Y, Tan S, Wang H, Pandey DR, Abraham TP, Shoukas AA, Berkowitz DE, Santhanam L. MPST but not CSE is the primary regulator of hydrogen sulfide production and function in the coronary artery. Am J Physiol Heart Circ Physiol 310: H71–H79, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kutz JL, Greaney JL, Santhanam L, Alexander LM. Evidence for a functional vasodilatatory role for hydrogen sulphide in the human cutaneous microvasculature. J Physiol 593: 2121–2129, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Q, Lancaster JR Jr. Chemical foundations of hydrogen sulfide biology. Nitric Oxide 35: 21–34, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liaudet L, Soriano FG, Szabo C. Biology of nitric oxide signaling. Crit Care Med 28: N37–N52, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Liu Z, Han Y, Li L, Lu H, Meng G, Li X, Shirhan M, Peh MT, Xie L, Zhou S, Wang X, Chen Q, Dai W, Tan CH, Pan S, Moore PK, Ji Y. The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E−/− mice. Br J Pharmacol 169: 1795–1809, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov 14: 623–641, 2015. [DOI] [PubMed] [Google Scholar]
- 77.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discovery 7: 156–167, 2008. [DOI] [PubMed] [Google Scholar]
- 78.Manna P, Jain SK. Hydrogen sulfide and l-cysteine increase phosphatidylinositol 3,4,5-trisphosphate (PIP3) and glucose utilization by inhibiting phosphatase and tensin homolog (PTEN) protein and activating phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase (AKT)/protein kinase Cζ/λ (PKCζ/λ) in 3T3l1 adipocytes. J Biol Chem 286: 39848–39859, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marutani E, Yamada M, Ida T, Tokuda K, Ikeda K, Kai S, Shirozu K, Hayashida K, Kosugi S, Hanaoka K, Kaneki M, Akaike T, Ichinose F. Thiosulfate mediates cytoprotective effects of hydrogen sulfide against neuronal ischemia. J Am Heart Assoc 4: e002125, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazza R, Pasqua T, Cerra MC, Angelone T, Gattuso A. Akt/eNOS signaling and PLN S-sulfhydration are involved in H2S-dependent cardiac effects in frog and rat. Am J Physiol Regul Integr Comp Physiol 305: R443–R451, 2013. [DOI] [PubMed] [Google Scholar]
- 81.Meng J, Ganesan Adaikan P, Srilatha B. Hydrogen sulfide promotes nitric oxide production in corpus cavernosum by enhancing expression of endothelial nitric oxide synthase. Int J Impot Res 25: 86–90, 2013. [DOI] [PubMed] [Google Scholar]
- 82.Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PR, Kemp BE, Pearson RB. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol 9: 845–848, 1999. [DOI] [PubMed] [Google Scholar]
- 83.Minamishima S, Bougaki M, Sips PY, Yu JD, Minamishima YA, Elrod JW, Lefer DJ, Bloch KD, Ichinose F. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation 120: 888–896, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moccia F, Bertoni G, Pla AF, Dragoni S, Pupo E, Merlino A, Mancardi D, Munaron L, Tanzi F. Hydrogen sulfide regulates intracellular Ca2+ concentration in endothelial cells from excised rat aorta. Curr Pharm Biotechnol 12: 1416–1426, 2011. [DOI] [PubMed] [Google Scholar]
- 85.Módis K, Bos EM, Calzia E, van Goor H, Coletta C, Papapetropoulos A, Hellmich MR, Radermacher P, Bouillaud F, Szabo C. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. II. Pathophysiological and therapeutic aspects. Br J Pharmacol 171: 2123–2146, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Módis K, Coletta C, Erdélyi K, Papapetropoulos A, Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J 27: 601–611, 2013. [DOI] [PubMed] [Google Scholar]
- 87.Módis K, Ju Y, Ahmad A, Untereiner AA, Altaany Z, Wu L, Szabo C, Wang R. S-sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol Res 113: 116–124, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Módis K, Panopoulos P, Coletta C, Papapetropoulos A, Szabo C. Hydrogen sulfide-mediated stimulation of mitochondrial electron transport involves inhibition of the mitochondrial phosphodiesterase 2A, elevation of cAMP and activation of protein kinase A. Biochem Pharmacol 86: 1311–1319, 2013. [DOI] [PubMed] [Google Scholar]
- 89.Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Hand Exp Pharmacol 176: 213–254, 2006. [DOI] [PubMed] [Google Scholar]
- 90.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, pharmacology. Pharmacol Rev 43: 109–142, 1991. [PubMed] [Google Scholar]
- 91.Nagpure BV, Bian JS. Interaction of hydrogen sulfide with nitric oxide in the cardiovascular system. Oxid Med Cell Longev 2016: 6904327, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nagy P, Pálinkás Z, Nagy A, Budai B, Tóth I, Vasas A. Chemical aspects of hydrogen sulfide measurements in physiological samples. Biochim Biophys Acta 1840: 876–891, 2014. [DOI] [PubMed] [Google Scholar]
- 93.Nagy P, Winterbourn CC. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem Res Toxicol 23: 1541–1543, 2010. [DOI] [PubMed] [Google Scholar]
- 94.Nagy P. Mechanistic chemical perspective of hydrogen sulfide signaling. Methods Enzymol 554: 3–29, 2015. [DOI] [PubMed] [Google Scholar]
- 95.Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, Ihara H, Motohashi H, Yamamoto M, Suematsu M, Kurose H, van der Vliet A, Freeman BA, Shibata T, Uchida K, Kumagai Y, Akaike T. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat Chem Biol 8: 714–724, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishida M, Toyama T, Akaike T. Role of 8-nitro-cGMP and its redox regulation in cardiovascular electrophilic signaling. J Mol Cell Cardiol 73: 10–17, 2014. [DOI] [PubMed] [Google Scholar]
- 97.Niwa K, Inanami O, Yamamori T, Ohta T, Hamasu T, Kuwabara M. Redox regulation of PI3K/Akt and p53 in bovine aortic endothelial cells exposed to hydrogen peroxide. Antioxid Redox Signal 5: 713–722, 2003. [DOI] [PubMed] [Google Scholar]
- 98.Ohno K, Okuda K, Uehara T. Endogenous S-sulfhydration of PTEN helps protect against modification by nitric oxide. Biochem Biophys Res Commun 456: 245–249, 2015. [DOI] [PubMed] [Google Scholar]
- 99.Olson KR. Mitochondrial adaptations to utilize hydrogen sulfide for energy and signaling. J Comp Physiol B 182: 881–897, 2012. [DOI] [PubMed] [Google Scholar]
- 100.Ondrias K, Stasko A, Cacanyiova S, Sulova Z, Krizanova O, Kristek F, Malekova L, Knezl V, Breier A. H2S and HS− donor NaHS releases nitric oxide from nitrosothiols, metal nitrosyl complex, brain homogenate and murine L1210 leukaemia cells. Pflügers Arch 457: 271–279, 2008. [DOI] [PubMed] [Google Scholar]
- 101.Ono K, Akaike T, Sawa T, Kumagai Y, Wink DA, Tantillo DJ, Hobbs AJ, Nagy P, Xian M, Lin J, Fukuto JM. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: implications of their possible biological activity and utility. Free Radic Biol Med 77: 82–94, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Osipov RM, Robich MP, Feng J, Liu Y, Clements RT, Glazer HP, Sodha NR, Szabo C, Bianchi C, Sellke FW. Effect of hydrogen sulfide in a porcine model of myocardial ischemia-reperfusion: comparison of different administration regimens and characterization of the cellular mechanisms of protection. J Cardiovasc Pharmacol 54: 287–297, 2009. [DOI] [PubMed] [Google Scholar]
- 103.Pálinkás Z, Furtmüller PG, Nagy A, Jakopitsch C, Pirker KF, Magierowski M, Jasnos K, Wallace JL, Obinger C, Nagy P. Interactions of hydrogen sulfide with myeloperoxidase. Br J Pharmacol 172: 1516–1532, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Panopoulos P, Yang G, Asimakopoulou A, Topouzis S, Wang R, Szabo C, Papapetropoulos A. Selectivity of hydrogen sulfide towards cyclic nucleotide phosphodiesterases. Nitric Oxide 47: S39, 2015. [Google Scholar]
- 105.Papapetropoulos A, Hobbs AJ, Topouzis S. Extending the translational potential of targeting NO/cGMP-regulated pathways in the CVS. Br J Pharmacol 172: 1397–1414, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA 106: 21972–21977, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Papapetropoulos A, Rudic RD, Sessa WC. Molecular control of nitric oxide synthases in the cardiovascular system. Cardiovasc Res 43: 509–520, 1999. [DOI] [PubMed] [Google Scholar]
- 108.Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13: 499–507, 2012. [DOI] [PubMed] [Google Scholar]
- 109.Paul BD, Snyder SH. Modes of physiologic H2S signaling in the brain and peripheral tissues. Antioxid Redox Signal 22: 411–423, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Polhemus DJ, Kondo K, Bhushan S, Bir SC, Kevil CG, Murohara T, Lefer DJ, Calvert JW. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail 6: 1077–1086, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Potenza DM, Guerra G, Avanzato D, Poletto V, Pareek S, Guido D, Gallanti A, Rosti V, Munaron L, Tanzi F, Moccia F. Hydrogen sulphide triggers VEGF-induced intracellular Ca2 signals in human endothelial cells but not in their immature progenitors. Cell Calcium 56: 225–234, 2014. [DOI] [PubMed] [Google Scholar]
- 112.Predmore BL, Julian D, Cardounel AJ. Hydrogen sulfide increases nitric oxide production from endothelial cells by an akt-dependent mechanism. Front Physiol 2: 104, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, Grinsfelder DB, Condit ME, Lefer DJ. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol 302: H2410–H2418, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci USA 101: 2619–2624, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sellak H, Choi CS, Dey NB, Lincoln TM. Transcriptional and post-transcriptional regulation of cGMP-dependent protein kinase (PKG-I): pathophysiological significance. Cardiovasc Res 97: 200–207, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shukla N, Rossoni G, Hotston M, Sparatore A, Del Soldato P, Tazzari V, Persad R, Angelini GD, Jeremy JY. Effect of hydrogen sulphide-donating sildenafil (ACS6) on erectile function and oxidative stress in rabbit isolated corpus cavernosum and in hypertensive rats. BJU Int 103: 1522–1529, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Snijder PM, Frenay AR, de Boer RA, Pasch A, Hillebrands JL, Leuvenink HG, van Goor H. Exogenous administration of thiosulfate, a donor of hydrogen sulfide, attenuates angiotensin II-induced hypertensive heart disease in rats. Br J Pharmacol 172: 1494–1504, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sojitra B, Bulani Y, Putcha UK, Kanwal A, Gupta P, Kuncha M, Banerjee SK. Nitric oxide synthase inhibition abrogates hydrogen sulfide-induced cardioprotection in mice. Mol Cell Biochem 360: 61–69, 2012. [DOI] [PubMed] [Google Scholar]
- 119.Sparatore A, Santus G, Giustarini D, Rossi R, Del Soldato P. Therapeutic potential of new hydrogen sulfide-releasing hybrids. Expert Rev Clin Pharmacol 4: 109–121, 2011. [DOI] [PubMed] [Google Scholar]
- 120.Stubbert D, Prysyazhna O, Rudyk O, Scotcher J, Burgoyne JR, Eaton P. Protein kinase G Iα oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension 64: 1344–1351, 2014. [DOI] [PubMed] [Google Scholar]
- 121.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gerö D, Szoleczky P, Chang T, Zhou Z, Wu L, Wang R, Papapetropoulos A, Szabo C. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci USA 108: 13829–13834, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Szabo C, Coletta C, Chao C, Módis K, Szczesny B, Papapetropoulos A, Hellmich MR. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci USA 110: 12474–12479, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6: 662–680, 2007. [DOI] [PubMed] [Google Scholar]
- 124.Szabo C, Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br J Pharmacol 164: 853–865, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Szabo C, Ransy C, Módis K, Andriamihaja M, Murghes B, Coletta C, Olah G, Yanagi K, Bouillaud F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. I. Biochemical and physiological mechanisms. Br J Pharmacol 171: 2099–2122, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Szabo C. Gaseotransmitters: New frontiers for translational science. Science Transl Med 2: 59ps54, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Szabo C. Gasotrans mitters in cancer: from pathophysiology to experimental therapy. Nat Rev Drug Discov 15: 185–203, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917–935, 2007. [DOI] [PubMed] [Google Scholar]
- 129.Szabo C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal 17: 68–80, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Szczesny B, Módis K, Yanagi K, Coletta C, Le Trionnaire S, Perry A, Wood ME, Whiteman M, Szabo C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide 41: 120–130, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taldone FS, Tummala M, Goldstein EJ, Ryzhov V, Ravi K, Black SM. Studying the S-nitrosylation of model peptides and eNOS protein by mass spectrometry. Nitric Oxide 13: 76–87, 2005. [DOI] [PubMed] [Google Scholar]
- 132.Tamizhselvi R, Sun J, Koh YH, Bhatia M. Effect of hydrogen sulfide on the phosphatidylinositol 3-kinase-protein kinase B pathway and on caerulein-induced cytokine production in isolated mouse pancreatic acinar cells. J Pharmacol Exp Ther 329: 1166–1177, 2009. [DOI] [PubMed] [Google Scholar]
- 133.Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ, Wang Y, Shen Q, Wang MJ, Chen Y, Zhang LJ, Zhu YZ, Zhu YC. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal 19: 448–464, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Teng X, Scott Isbell T, Crawford JH, Bosworth CA, Giles GI, Koenitzer JR, Lancaster JR, Doeller JE, Kraus DW, Patel RP. Novel method for measuring S-nitrosothiols using hydrogen sulfide. Methods Enzymol 441: 161–172, 2008. [DOI] [PubMed] [Google Scholar]
- 135.Toohey JI, Cooper AJ. Thiosulfoxide (sulfane) sulfur: new chemistry and new regulatory roles in biology. Molecules 19: 12789–12813, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol 301: H363–H372, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci 67: 821–829, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.van den Born JC, Hammes HP, Greffrath W, van Goor H, Hillebrands JL, DFG GRK International Research Training Group 1874 Diabetic Microvascular Complications (DIAMICOM). Gasotransmitters in vascular complications of diabetes. Diabetes 65: 331–345, 2016. [DOI] [PubMed] [Google Scholar]
- 139.Vandiver M, Snyder SH. Hydrogen sulfide: a gasotransmitter of clinical relevance. J Mol Med 90: 255–263, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vitvitsky V, Kabil O, Banerjee R. High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxid Redox Signal 17: 22–31, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vitvitsky V, Yadav PK, Kurthen A, Banerjee R. Sulfide oxidation by a noncanonical pathway in red blood cells generates thiosulfate and polysulfides. J Biol Chem 290: 8310–8320, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov 14: 329–345, 2015. [DOI] [PubMed] [Google Scholar]
- 143.Wang R, Szabo C, Ichinose F, Ahmed A, Whiteman M, Papapetropoulos A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol Sci 36: 568–578, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang R. Signaling pathways for the vascular effects of hydrogen sulfide. Curr Opin Nephrol Hypertens 20: 107–112, 2011. [DOI] [PubMed] [Google Scholar]
- 145.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92: 791–896, 2012. [DOI] [PubMed] [Google Scholar]
- 146.Wedmann R, Zahl A, Shubina TE, Dürr M, Heinemann FW, Bugenhagen BE, Burger P, Ivanovic-Burmazovic I, Filipovic MR. Does perthionitrite (SSNO−) account for sustained bioactivity of NO? A (bio)chemical characterization. Inorg Chem 54: 9367–9380, 2015. [DOI] [PubMed] [Google Scholar]
- 147.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun 343: 303–310, 2006. [DOI] [PubMed] [Google Scholar]
- 148.Wu D, Hu Q, Ma F, Zhu YZ. Vasorelaxant effect of a new hydrogen sulfide-nitric oxide conjugated donor in isolated rat aortic rings through cGMP pathway. Oxid Med Cell Longev 2016: 7075682, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yong QC, Cheong JL, Hua F, Deng LW, Khoo YM, Lee HS, Perry A, Wood M, Whiteman M, Bian JS. Regulation of heart function by endogenous gaseous mediators-crosstalk between nitric oxide and hydrogen sulfide. Antioxid Redox Signal 14: 2081–2091, 2011. [DOI] [PubMed] [Google Scholar]
- 151.Yusof M, Kamada K, Kalogeris T, Gaskin FS, Korthuis RJ. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO- and p38 MAPK-dependent mechanism. Am J Physiol Heart Circ Physiol 296: H868–H876, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang LJ, Tao BB, Wang MJ, Jin HM, Zhu YC. PI3K p110α isoform-dependent Rho GTPase Rac1 activation mediates H2S-promoted endothelial cell migration via actin cytoskeleton reorganization. PLoS One 7: e44590, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang X, Simons M. Receptor tyrosine kinases endocytosis in endothelium: biology and signaling. Arterioscler Thromb Vasc Biol 34: 1831–1837, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang Y, Tang ZH, Ren Z, Qu SL, Liu MH, Liu LS, Jiang ZS. Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases. Mol Cell Biol 33: 1104–1113, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou Z, Martin E, Sharina I, Esposito I, Szabo C, Bucci M, Cirino G, Papapetropoulos A. Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide. Pharmacol Res 111: 556–562, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]