Abstract
Tau is a neuronal microtubule binding protein that, in Alzheimer’s disease and other neurodegenerative diseases, can form oligomeric and large fibrillar aggregates, which deposit in neurofibrillary tangles. Tau’s physiological state of multimerization appears to vary across conditions, and a stable dimeric form of soluble tau has been suggested from experiments using recombinant tau in vitro. We tested if tau dimerization, or oligomerization, also occurs in cells, and if soluble tau oligomers are relevant for the release and internalization of tau. We developed a sensitive tau split-luciferase assay to show the rapid intracellular formation of stable tau dimers that are released and taken up by cells. Our data further suggest that tau dimerization can be accelerated slightly by aggregation catalysts. We conclude that tau oligomers are a stable physiological form of tau, and that tau oligomerization does not necessarily lead to tau aggregation.
Keywords: tau protein, dimerization, oligomers, Gaussia Luciferase assay
Graphical Abstract
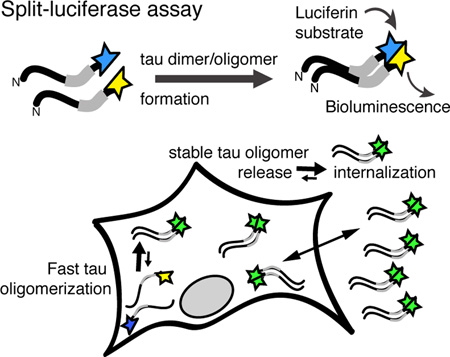
Tau dimers and oligomersare intermediates in vitro, but their formation in cells is not established. To study the formation of tau oligomers, we designed a split-luciferase assay enabling the sensitive detection of tau oligomerization in cells: dimerization or oligomerization of full-length tau fused to either hemisphere of Gaussia luciferase reconstitutes luciferase activity. We found that upon expression in cells, tau rapidly forms stable intracellular oligomers that are released and can be internalized by cells. These oligomeric species may or may not be on-pathway with tau aggregation.
BACKGROUND
The progressive accumulation of tau proteins is a pathological hallmark of different neurodegenerative diseases including Alzheimer's diseases (AD) and frontotemporal dementia (FTD), collectively termed tauopathies. In the human central nervous system, six tau isoforms are generated by alternative splicing (Goedert et al. 1989), of which the longest isoform (2N4R tau) contains 441 amino acids with two N-terminal inserts and four pseudo-repeats in the C-terminus. Tau is a neuronal protein that regulates microtubule stability and dynamics (Weingarten et al. 1975, Lindwall & Cole 1984, Drechsel et al. 1992, Gigant et al. 2014) and axonal transport (Trinczek et al. 1999). The binding of tau to microtubules is mediated by the C-terminal part of tau and regulated by phosphorylation in regions adjacent to the microtubule binding sites (Preuss et al. 1997). Tau aggregation is facilitated by hexapeptide motifs in the C-terminal repeat domain (TauRD), and the repeat domain is sufficient to form the core of amyloid-like paired helical filaments in vitro (von Bergen et al. 2005). The long N-terminal part of tau projects from the surface of both microtubules (Preuss et al. 1997) and tau fibril (Wegmann et al. 2013, Sillen et al. 2005).
In AD, tau aggregates intracellularly into paired helical filaments, accumulating in neurofibrillary tangles and neuropil threads, and forms high molecular weight (HMW) soluble oligomeric species containing hyper-phosphorylated tau (Lasagna-Reeves et al. 2012, Takeda et al. 2015). In FTDs, tau aggregates deposit in neurofibrillary tangles, Pick bodies, glial fibrillary tangles, and/or in small silver grains, although the majority of misfolded tau remains soluble in these diseases. While a substantial number of studies have explored the biophysical properties of paired helical filaments from recombinant tau that were assembled in vitro with the help of poly-anionic pro-aggregation molecules such as heparin (Daebel et al. 2012, Jeganathan et al. 2008, von Bergen et al. 2006, Bibow et al. 2011, Mukrasch et al. 2007, Kumar et al. 2014, Wegmann et al. 2013, Wegmann et al. 2010), the biology and biochemical characterization of soluble multimeric tau species in a cellular context is far less studied, in part because of the lack of tools available for such studies. Importantly, soluble tau also is released by neurons in vitro and in vivo (Pooler et al. 2013, Yamada et al. 2011), and is found even in normal healthy people in the cerebrospinal fluid (CSF) (Yamamori et al. 2007). Oligomeric HMW tau has also been identified in the interstitial fluid of tau overexpressing animals (Takeda et al. 2015), raising the possibilities that soluble tau species can aggregate in the extracellular space, or that aggregated tau species can be secreted from tau expressing cells.
The very low concentration of extracellular tau in the ng/ml-range (for example ~0.16 ng/ml tau in CSF of healthy controls and ~0.85 ng/ml tau in CSF of AD patients (Johnson et al. 1997); ~30–40 ng/ml in wild-type mouse ISF (Yamada et al. 2011) is near the detection limit of most tau ELISA assays, and the detection with such sandwich ELISA assays takes several hours to days. But most importantly, measuring total tau levels by ELISA does not access the oligomerization state of tau, and thus does not inform about the functional state of the protein. These confounds make it difficult to study the cellular release, uptake, and the aggregation state of tau at physiological concentrations and in real-time. To overcome these limitations, we developed an ultrasensitive tau-luciferase assay, in which full-length human tau (2N4R) is fused to either the N- or the C-terminal part of Gaussia luciferase (gLuc), and tau oligomer formation allows for complementation of split-gLuc parts tau-L1 and tau-L2, which then reconstitutes gLuc activity (Remy & Michnick 2006, Hashimoto et al. 2011, Kalia et al. 2011). The detected luciferase activity thus derives from tau dimers or multimers; to simplify we refer to all these species as oligomers. One benefit of the split-gLuc assay over split-fluorescent protein (split-FP) assays is the dynamic reversibility of the complementation (Remy & Michnick 2006). Using this tau-gLuc assay, we were able to study the generation, release, and uptake properties of oligomeric soluble tau species. For example, the ultrasensitive assay can detect tau concentrations of 0.01–1000 ng/ml and show that oligomeric tau can be actively released and taken up by cells and neurons in vitro. Furthermore, agents seeding the aggregation of tau did barely increase gLuc activity, suggesting that factors that promote tau aggregation of recombinant species may differ from those that govern the formation of soluble oligomeric tau species in cells. Naturally occurring tau dimerization and oligomerization may thus not necessarily be “on pathway” with tau aggregate formation.
METHODS
Plasmids
The split luciferase constructs (L1, aa 1–92; L2, aa 93–163), as previously described (Hashimoto et al. 2011), were each amplified and ligated into the HindIII (5’) and EcoRV (3’) restrictions sites in an AAV-CBA-4RTau-WPRE vector previously described (de Calignon et al. 2010), adding the luciferase fragments in frame to the C-terminus of full-length (2N, 4R) human Tau. The luciferase fragments were also added to the N-terminus of the Tau using amplification of each fragment and ligating into the XhoI site of the AAV-CBA-4RTau-WPRE vector. All plasmids were confirmed by sequencing.
HEK293 cells culture
HEK293 cells (ATCC) were maintained in OPTI-MEM+ 5% fetal bovine serum (FBS) following standard cell culture procedures. For experiments, cells were plated ~30% confluent in opaque white 96-well tissue culture plates with glass bottom. Next day, cells were transfected for 3 hours with tau-gLuc constructs, or with GFP or full-length tau as controls, using ~1% Lipofectamine 2000 (Life technologies), and subsequently cultivated in serum-free OPTI-MEM. At designated time points post transfection, 80 µl culture medium was separated into fresh culture plates, cells were rinsed once and then overlayed with 80 µl fresh pre-warmed OPTI-MEM for immediate luciferase activity measurement; for immunostaining, cells were fixed with PFA, for protein analysis, cells were harvested in RIPA buffer. Extracellular vesicles were enriched by ultracentrifugation of conditioned medium at 100,000g for 1 hour at 4°C and subsequent resuspension of pelleted membranes in PBS.
Immunostaining of cells
After removal of culture medium, cells were rinsed with warm PBS once and then fixed in 4% PFA/PBS for 15 min at room temperature, permeabilized with 0.2% TritonX-100/PBS, blocked with 3–5%normal goat serum (NGS)/PBS, and incubated in primary antibodies (mouse anti-human Tau13, Biolegends, 1:1000, and rabbit anti-β-actin, 1:1000, Abcam) in 3%NGS/PBS overnight at 4 °C. Secondary antibodies (Alexa488-anti-mouse and Cy3 anti-rabbit, Life technologies, 1:1000) in 3%NGS/PBS were applied for 1 h at room temperature. After washing in PBS, and incubation with DAPI, cells were imaged with a Zeiss Axiovert 200 inverted confocal microscope.
Primary cortical neurons
Primary cortical neurons were prepared from cerebral cortices of embryonic day E14–E15 CD1 mice embryos (Charles River Laboratories) as described previously (Wu et al. 2012). In brief, cortices were mechanically dissociated in Neurobasal medium (Life Technologies) including with 10%FBS, 2 mM Glutamate, 100 U ml−1 penicillin, and 100 g ml−1 streptomycin, then pelleted at ~150 g for 5 min, resuspended in the same medium, and plated in 96-well or 6-well plates coated with poly-d-lysine (50 µg/ml Sigma) for 1 hour. Cultures were maintained at 37 °C with 5%CO2 in Neurobasal medium with 2%(v/v) B27 nutrient, 2 mM Glutamate, 100 U ml−1 penicillin and 100 g ml−1 streptomycin. Neuronal transfection was done as described for HEK293 cells at day 7 in vitro (DIV7). All experiments were performed under national (United States National Institutes of Health) and institutional (Massachusetts General Hospital Subcommittee for Research Animal Care and the Institutional Animal Care and Use Committee at Harvard Medical School) guidelines. All animal experiments were approved by the Massachusetts General Hospital and McLaughlin Research Institute Institutional Animal Care and Use Committees.
Luciferase assay
Gaussia luciferase (gLuc) activity of adherent cells were measured directly in opaque plastic glass bottom 96-well culture plates, culture medium, or cell lysates and centrifugation fractions were added to the same plates and measured by adding luciferase substrate, 50 µl per well of 10 µM coelenterazine (Nanolight) diluted in culture medium or PBS, and counting emitted photons 1 s after substrate injection for the duration of 2 s. Measurements and substrate injections were performed on a semi-automated plate reader (Wallac) and raw values were transformed into photons per second.
Immunoblot analysis and human tau ELISA
For Western blot analysis, proteins in cell lysates and centrifugation fractions were separated by SDS-PAGE in 4–12% Bis-Tris gels (NuPAGE, Invitrogen) and blotted onto nitrocellulose membrane (Amersham) for 2 hours at 90 V. After blocking the membrane in blocking buffer (LICOR PBS-based blocking buffer) for 1 hour at room temperature, primary antibodies mouse anti-human tau specific Tau13 (Biolegends) and rabbit anti-tubulin (Abcam) were diluted 1:1000 in blocking buffer and applied over night at 4°C. After three washes with TBS+0.2% Tween-20 (TBS-T), secondary antibodies (anti-mouse-800, anti-rabbit-700, LICOR) diluted 1:5000 in blocking buffer were applied for 2 hours at room temperature, then the membrane was washed three times in TBS-T and imaged using a LICOR infrared imaging setup. Human tau levels in conditioned medium of HEK293 cells and primary neurons and in size exclusion chromatography (SEC) fractions were measured by total Human Tau ELISA Kit (Life Technologies).
Size exclusion chromatography (SEC)
HEK tau-L1/L2 conditioned medium (500–600 µl), rTg4510 brain PBS-extracts (300–400 µl), and monomeric/oligomeric recombinant human tau (hTau-441, 500–600 µl, ~3 mg/ml in PBS with 2 mM DTT) were separated by SEC using a Superdex200 10/300GL column (#17-5175-01, GE Healthcare) mounted on an AKTA purifier 10 (GE Healthcare) in phosphate buffered saline (Sigma-Aldrich) at a flow rate of 0.5 ml/min; all samples were filtered through a membrane filter (pore size 0.2 µm) before loading onto the column. Human tau concentrations in the collected SEC fractions (0.5 ml) were determined by human total tau ELISA (Life Technologies).
Atomic force microscopy (AFM)
Isolation of tau from HEK tau-L1/L2 conditioned medium for AFM analysis was performed as described previously (Takeda et al. 2015). Briefly, tosyl-activated magnetic Dynabeads (Life Technologies) were coated with human tau-specific Tau13 antibody (Biolegend). Beads were washed (0.2 M Tris, 0.1%bovine serum albumin, pH 8.5) and incubated with HEK tau-L1/L2 conditioned medium for 1 h at room temperature. After the beads were washed three times with PBS, human tau was eluted using 0.1 M glycine, pH 2.8 for ~1 min, and the pH of the eluted fraction was immediately adjusted using 1 M Tris pH 8.0. For AFM imaging, the isolated tau was adsorbed onto freshly cleaved muscovite mica and imaged using oscillation mode AFM (Nanoscope III, Di-Veeco, Santa Barbara, CA) and Si3N4 cantilevers (NPS series, Di-Veeco) in PBS, as described previously (Wegmann et al. 2010). Most probable particle sizes (=AFM heights) of tau-L1/L2 monomers and oligomers were determined from Gaussian fits to AFM height distribution histograms of 269 particles imaged in six randomly picked areas (1.0 × 1.0 µm).
Thioflavine-T assay
To test the presence of fibrillar tau aggregates, 20 µl of HEK tau-L1/L2 conditioned medium were transferred into opaque glass bottom 96-well plates and incubated with 180 µl of Thioflavne-T solution (50 mM Sodium Acetate, pH 6.8, containing 3 µM Thioflavine-T) for 15 min in the dark. The amount of Thioflavne-T bound to fibrillar tau was measured as emission at 510 nm after excitation at 430 nm using a plate reader (Wallac). Samples were measured in duplicates and pre-aggregated recombinant huTau-441 was used as positive control.
Recombinant human tau
Human full-length wildtype tau (huTau-441, 2N4R, 441 aa) was expressed in Escherichia coli BL21 DE3 using tau/pET29b plasmid (Addgene). Expression was induced by 1 mM IPTG for 3.5 h at 37 °C. Tau purification was performed by heat treatment and FPLC Mono S chromatography (Amersham Biosciences) as described previously (Barghorn et al. 2005). In brief, cells were boiled in 3 ml buffer (50 mM MES, pH 6.8, 500 mM NaCl, 1 mM MgCl2, 5 mM DTT) for 20 min, then the whole cell lysate was ultracentrifuged at 125,000 g for 45 min, and the supernatant was dialyzed (MW cut-off =20 kDa) against 20 mM MES, pH 6.8, 50 mM NaCl, 2 mM DTT overnight at 4 °C. Protein content was analyzed by BCA assay (Pierce) and Western blot. Tau oligomer mixture solution was prepared by incubating ~3 mg/ml recombinant human tau with 2 mM DTT for 2 days at 37 °C.
Brain extracts
Brain lysates from 12 month-old rTg4510 mice expressing human tauP301L under the CamK2alpha promotor {SantaCruz, 2005 #22}, and from age-matched controls, were prepared as followed: mice were perfused with PBS, their brains were snap-frozen and stored at −80 °C or directly homogenized in five volumes (w/v) of ice-cold PBS containing protease inhibitors (Complete protease cocktail; Roche) using a Teflon-glass homogenizer. The homogenate was centrifuged at 3,000 g for 10 min at 4 °C, and the supernatant was stored at −80 °C.
Statistical analysis
All luciferase experiments were performed at least three times (experiments) with 3 to 6 repeats per experiments. All values are given as Mean±SEM, and the number of experiments is given in each figure legend. Statistical analysis was performed using GraphPad Prism6, with Student’s T test if comparing two groups, and one-way ANOVA for comparison of multiple groups.
RESULTS
Sensitive detection of tau oligomerization by split-luciferase complementation
In current experiments studying tau aggregation, there is a key need for assaying soluble multimeric species to understand the biology of these potentially important physiological and pathophysiological molecular conformations.
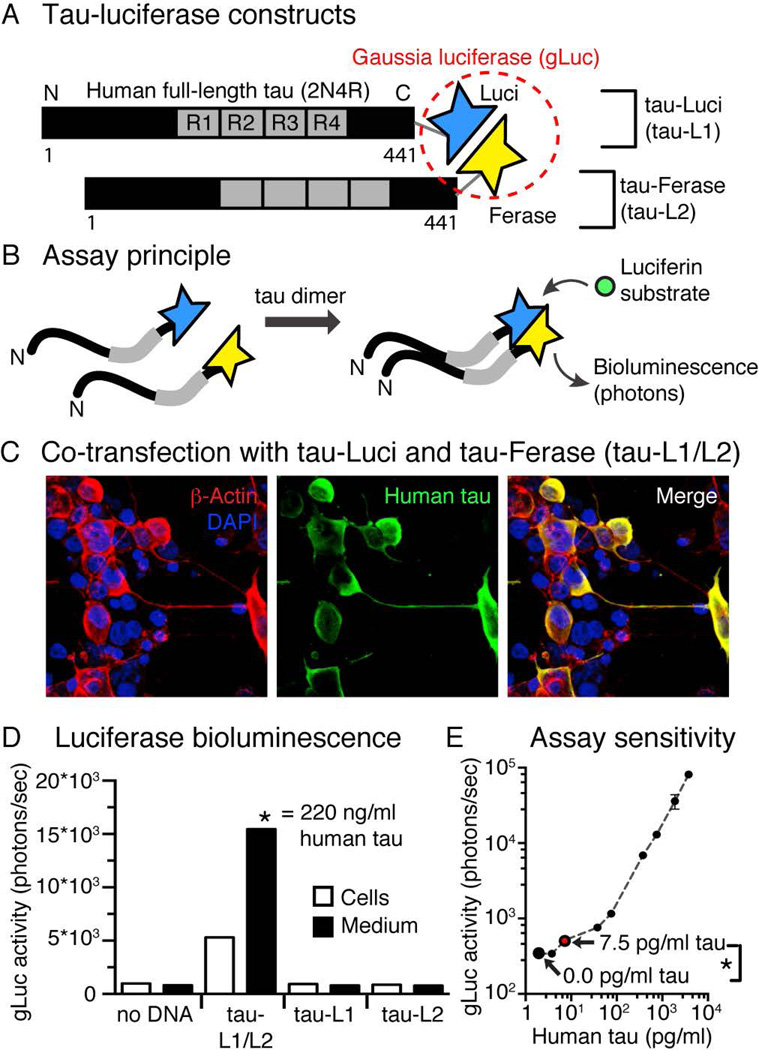
To enable the detection of tau oligomerization and aggregation in physiological relevant concentrations (0.01–100 ng/ml), we designed a split-luciferase assay, in which we fused full-length human tau (2N4R) to either the N-terminal (tau-L1) or the C-terminal (tau-L2) half of Gaussia luciferase (gLuc), which are both inactive in their isolated form (Figure 1A). Dimerization (n=2 tau molecules) or oligomerization (n>2 tau molecules) of tau can bring the two complementing gLuc parts in close proximity, allow their complementation, and restores gLuc activity and photon emission upon luciferase substrate presentation (Figure 1B). Because the assay cannot distinguish between tau dimers or larger multimers, we refer to tau species containing two or more tau monomers as tau oligomers. Co-expressing tau-L1 and tau-L2 in HEK293 cells (Figure 1C) leads to robust gLuc activity in cells and conditioned medium after 40 hours (Figure 1D). Co-expression of tau-L1 with gLuc2, tau-L2 with gLuc1, or gLuc1 with gLuc2 did not lead to luciferase activity, showing that oligomerization of tau was driving luciferase complementation, and not the presence of complements by themselves (Supplemental Figure 1). The gLuc activity, detected in the tau-L1/L2 culture medium, corresponded to 220 ng/ml total human tau as determined by human tau specific ELISA. Dilutions of tau-L1/L2 conditioned medium revealed a linear correlation between tau concentration and gLuc activity (Figure 1E) with an assay sensitivity (=significant luciferase bioluminescence difference between buffer and sample) of 7.5 pg/ml tau-L1/L2 (~0.16 nM full-length tau). One may keep in mind that the tau ELISA measures the total tau concentration (pg/ml) in the medium, whereas the tau-L1/L2 assay detects both dimeric (n=2) and oligomeric (n>2) but not monomeric (n=1) tau. Our assay may thus allow detection of even lower concentrations of tau-L1/L2.
Figure 1. Split-Luciferase assay for the sensitive detection of tau oligomerization.
A) Human full-length wildtype tau was fused to the N-terminal half (L1, Luci) or the C-terminal half (L2, Ferase) of Gaussia luciferase (gLuc).
B) Dimerization and oligomerization of individual tau molecules results in complementation of gLuc and restores luciferase activity, which is detected as bioluminescence (emitted photons) after addition of the Luciferin substrate (coelenterazine).
C) HEK293 cells transfected with tau-L1 and tau-L2 show robust human tau expression after 40 hours.
D) Luciferase activity can be measured in double transfected (tau-L1/L2) cells and conditioned medium, but not in single transfected (tau-L1 and tau-L2) after 48 hours of expression. gLuc activity measured in tau-L1/L2 medium compares to 220 ng/ml human tau, as determined by human total tau ELISA.
E) The split-gLuc/tau oligomerization assay sensitivity was determined by using of HEK tau-L1/L2 conditioned medium of known human tau concentration (by human total tau ELISA), and gLuc activity was measured on serial dilutions of the medium (three replicates). A significant difference (p=0.0159, two-tailed Student’s t-test) in gLuc activity could be detected between 0.0 and 7.5 pg/ml human tau content. Mean±SEM, n=3 repeats.
Intra- and extracellular tau oligomers
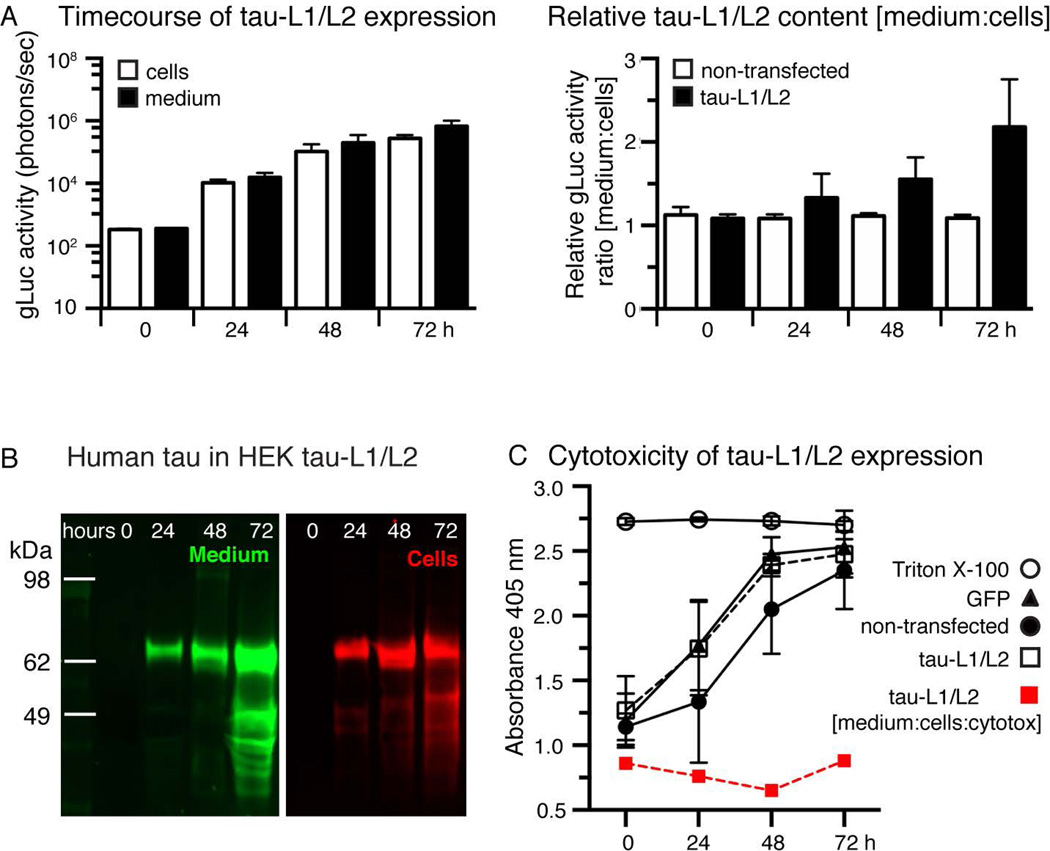
Next we examined the expression and release kinetics of tau-L1/L2 and characterized the released tau species. Co-expression of tau-L1 and tau-L2 in HEK cells revealed a continuous non-linear increase in intra- and extracellular tau-L1/L2 over a period of 72 hours (Figure 2A), whereby extracellular tau-L1/L2 progressively exceeded that of intracellular tau-L1/L2. The total tau concentration, determined by Western Blotting, was similar in cells compared to culture medium (Figure 2B). A similar increase of tau-L1/L2 in the culture medium was obtained when expressing tau-L1/L2 in primary cortical neurons over the course of 96 hours (Supplementary Figure 2A). These data indicate a preference for the release of gLuc activity-bearing oligomeric tau above monomeric tau. No difference in cell toxicity was observed between non-transfected cells and tau-L1/L2 expressing cells (Figure 2C), and the gLuc activity in tau-L1/L2 transfected cells decayed exponentially over multiple generations (Supplementary Figure 2B), suggesting no immediate cytotoxicity of tau-L1/L2 expression. Tau-L1/L2 in the culture medium is not an artifact of cell death in our assay, but is actively released.
Figure 2. Oligomeric tau is released by HEK cells.
A) The amount of tau-L1/L2 in HEK cells and the culture medium increases exponentially over time during the first ~48 hours. The amount of tau-L1/L2 in the culture medium relative to intracellular tau-L1/L2 (ratio [medium:cells]) increases linearly over time. Mean±SEM, n=6.
B) Western Blot analysis of human tau (Tau13 antibody) shows the presence of lower molecular weight fragments of tau-L1/L2, likely due to proteolytic degradation, in both culture medium (green) and cell lysates (red) after 72 hours.
C) LDH assay reveals no difference in cell death between GFP and tau-L1/L2 transfected HEK cells (black lines and markers). Normalizing the relative amount of gLuc activity in the medium [medium:cells] to LDH (red line and markers, [medium:cells:LDH]) suggests that tau-L1/L2 release may be partially caused by cell death. Mean±SEM, n=6 experiments.
Release of soluble tau oligomers into the culture medium
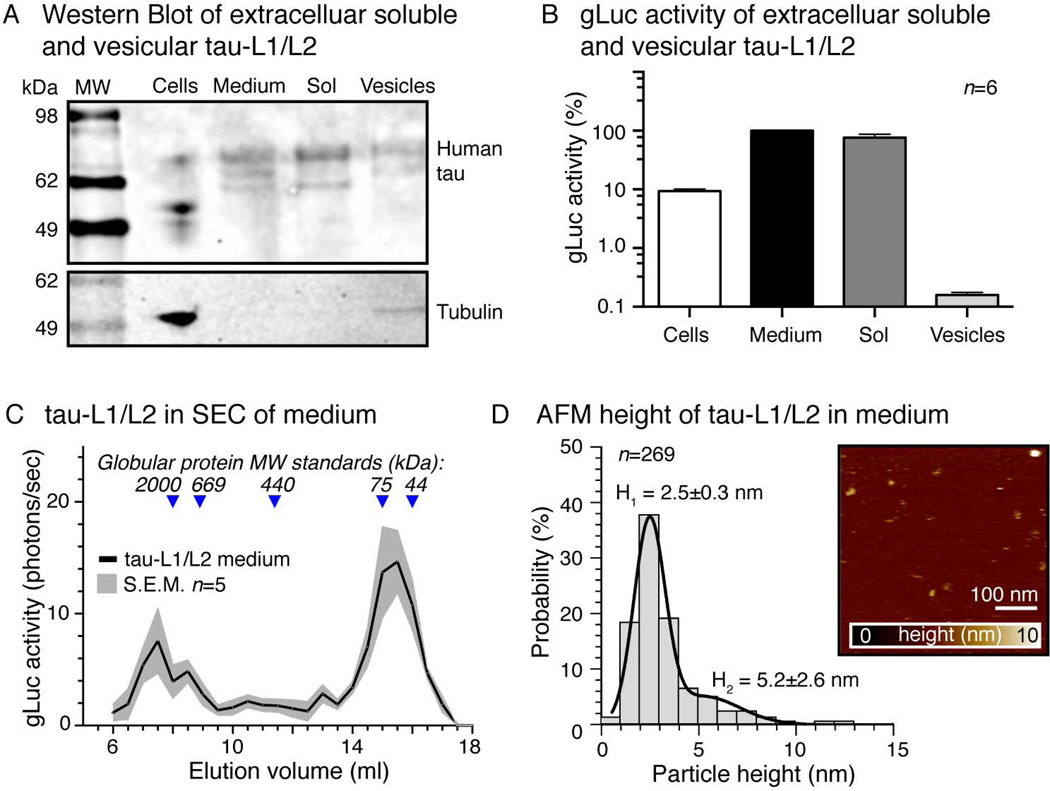
To test whether soluble or membrane-associated tau (for example associated with exosomes or microvesicles) is released into the culture medium, we collected culture medium of tau-L1/L2 expressing cells after 36h and separated the membrane-containing fraction by ultracentrifugation. Western Blot (Figure 3A) and gLuc assay (Figure 3B) of the soluble and the membrane fraction of tau-L1/L2 conditioned medium show only minor association of gLuc activity with secreted membranes. In fact, the residual gLuc signal in the membrane fraction could also reside from co-pelleted larger tau-L1/L2 aggregates. Cultured primary cortical neurons showed a similar enrichment of soluble extracellular tau-L1/L2 in the culture medium (Supplementary Figure 3A).
Figure 3. Released tau-L1/L2 is mostly soluble and oligomeric.
A) Western Blot for human tau in cells and medium from HEK tau-L1/L2 (40 hours expression). Human tau in the culture medium was further fractionated into soluble (Sol) and extracellular vesicle bound (Vesicles) tau by ultracentrifugation.
B) gLuc assay of cells and different medium fractions shown in (A) reveal that ~90% of tau-L1/L2 in the medium is soluble rather than vesicle/membrane-associated. Mean±SEM, n=2 experiments, 6 repeats.
C) Size exclusion chromatography (SEC) was performed on HEK tau-L1/L2 medium (40 hours) and the collected fractions (0.5 ml) were analyzed by gLuc assay to detect dimeric and oligomeric tau-L1/L2 but not monomeric tau. Most tau-L1/L2 appeared at a MW of truncated oligomeric forms of tau (elution vol. 14–16 ml), and a small amount existed as higher molecular weight oligomeric tau-L1/L2 (elution vol. 7–9 ml). Mean±SEM, n=5 experiments.
D) AFM topographies of human tau isolated from HEK tau-L1/L2 medium (40 h) by immuno-precipitation reveal particles sizes (= AFM heights) of mostly H1=2.5±0.3 nm and some of H2=5.2±2.6 nm. Mean±SD, n=269 particles.
To characterize the composition and molecular weight of released tau-L1/L2 oligomers, we separated tau-L1/L2 conditioned medium by size exclusion chromatography (SEC) and detected the gLuc activity in each fraction (Figure 3C). In order to detect only oligomeric tau-L1/L2, we measured the gLuc activity in all SEC fractions, so that monomeric tau-L1 or tau-L2 was not detected due to the lack of gLuc complementation. One should keep in mind, that the SEC elution volume correlates inversely with protein size and that it does not necessarily reflect the actual molecular weight of a protein. The separation by SEC depends on the protein’s Stoke’s radius, which for intrinsically disordered proteins (IDP), such as tau, is not an absolute value and rather larger than for globular proteins; the flexible and often extended conformation of tau may impair the correlation with the globular MW standard proteins used for SEC column calibration, and the detected SEC sizes of tau-L1/L2 can only be taken to distinguish species with large differences in protein size. In case of tau-L1/L2, for example, higher order oligomers are expected to elute in small elution volumes, whereas dimers and oligomers of truncated tau species elute in larger elution volumes. Most gLuc activity was found in the lower molecular weight SEC fractions (elution volume ~15 ml), likely representing dimeric and smaller oligomeric truncated tau-L1/L2; truncated tau species were also present in culture medium after extended time of expression (~72h, Figure 2B). Only little tau-L1/L2 was detected in higher-order oligomeric species (elution volume ~7–9 ml). This distribution of tau-L1/L2 in the culture medium was comparable to SEC separation profile of recombinant tau preparations containing a mix of tau monomers, dimers, and higher-order oligomers (Supplementary Figure 3B,C), whereby shifts in the apparent MW may result from the actual MW difference between tau and tau-L1/L2 (recombinant tau ≈46 kDa; tau-L1/L2 ≈112 kDa) as well as from changes in protein conformation upon tau oligomerization or split-gLuc fusion. We conclude that most of the oligomeric tau released by cells consist of truncated tau species.
Slightly higher (non-significant) gLuc activity was detected when co-expressing one N-terminally and one C-terminally tagged tau (L1-tau + tau-L2) compared to two C-terminally tagged tau proteins (tau-L1 + tau-L2; Supplementary Figure 3D); no fibrillar tau aggregates were detected by Thioflavine-T assay in either case of tau monomer orientation in the cells or in the culture culture medium (Supplementary Figure 3E). This suggested that the oligomerization of tau in cells may have some preference towards an anti-parallel orientation of tau monomers – as previously suggested from in vitro studies (Wille et al. 1992, Rosenberg et al. 2008) – and that tau-L1/L2 activity originates from dimeric and oligomeric but not aggregated tau species. To further characterize the size of released tau oligomers, we affinity purified tau from tau-L1/L2 conditioned medium using magnetic beads coated with human tau specific antibody (Tau13) and imaged the extracted tau proteins by atomic force microscopy (AFM) in buffer solution (Figure 3D). We measured the height of the tau particles to estimate their differences in size. AFM revealed tau-L1/L2 particle heights of 2.5±0.3 nm and 5.2±2.6 nm, which may represent dimeric and oligomeric tau-L1/L2 species, respectively. For comparison, the AFM height of monomeric recombinant human tau was 1.0±0.7 nm (Supplementary Figure 3C), similar to what has been reported earlier for monomeric tau (Tepper et al. 2014, Kumar et al. 2014).
To examine if tau-L1/L2 oligomerization occurs before or after release into the medium, we mixed (1:1) conditioned medium of separately grown tau-L1 and tau-L2 cells and incubated it at 37°C. However, in the mix of tau-L1 and tau-L2, no gLuc activity could be detected during 72 hours, whereas medium of tau-L1/L2 cells retained its high gLuc actvity (Supplementary Figure 4A). Unfortunately, we were unable to test the reversibility of tau dimerization using the split-gLuc assay because the activity of pure gLuc, which was expressed as positive control for gLuc activity maintenance in tested dissociation conditions, decreased rapidly in conditions needed to dissociate tau dimers into monomers (Kim et al. 2015, Friedhoff et al. 1998b), namely ≥1mM DTT with or without elevated temperature (50–60 Celsius). This made it impossible to evaluate a successful dissociation and re-oligomerization using our assay (data not shown). In sum, our data suggest that “stable” tau oligomers are formed intracellularly, and that monomeric and oligomeric tau can be released by cells.
Internalization of oligomeric tau from the culture medium
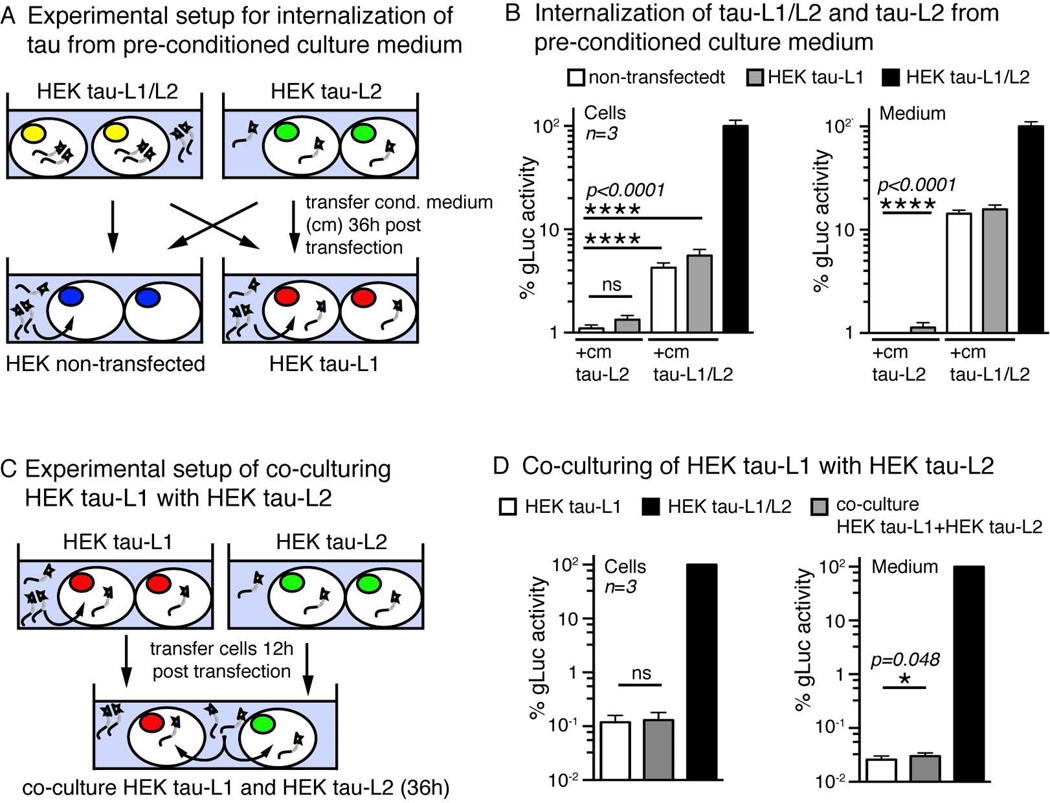
Using fluorescence techniques it has been suggested that extracellular oligomeric but not monomeric tau is taken up by cells (Wu et al. 2013, Takeda et al. 2015). We examined the uptake of oligomeric and monomeric tau by making use of the highly sensitive split-gLuc complementation assay. We incubated non-transfected naive cells and cells expressing only tau-L1 with pre-conditioned medium from tau-L1/L2 cells, or from tau-L2 cells for 36 hours (Figure 4A). Determining the intracellular gLuc activity, we found a significant amount of pre-oligomerized tau-L1/L2 to be taken up by non-transfected cells as well as by tau-L1 cells (Figure 4B). The uptake of oligomeric tau-L1/L2 (after 36 hours) was dose dependent and corresponded to ~12.4% of gLuc activity in the treatment medium (Supplementary Figure 3A). These data show that tau oligomers in the surrounding medium can be internalized by cells. When treating tau-L1 expressing cells with culture medium from tau-L2 expressing cells – likely containing mainly homo-oligomeric tau-L2/L2 with no gLuc activity and some monomeric tau-L2 - very little tau-L1/L2 formation occurred in the cells (non-significant) and in the medium (p<0.0001, Figure 4B). This indicated that tau-L1/L2 oligomerization from preformed tau oligomers is very inefficient, supporting the idea of a high stability of tau multimers.
Figure 4. Internalization by HEK cells and extracellular oligomerization of tau.
A) Experimental setup of treating HEK and HEK tau-L1 with pre-conditioned medium from either HEK tau-L2 or HEK tau-L1/L2 cells.
B) gLuc activity measured in cells and medium after treating cells as shown in (A). The intracellular (cells) and extracellular (medium) tau oligomerization (=gLuc activity) after treatment of HEK tau-L1 with tau-L2 medium was very inefficient, maybe due to the high stability of already formed intracellular tau-L1/L1 oligomers against re-arrangements and integration of tau-L2. Uptake of preformed tau-L1/L2 oligomers was efficient in both non-transfected and tau-L1 HEK cells, and treatment with tau-L1/L2 medium led to pronounced gLuc activity in the medium (after 36 h), suggesting a high stability of tau oligomers in culture conditions. Mean±SEM, n=3 experiments.
C) Experimental setup to monitor the exchange of tau between cells by co-culturing HEK tau-L1 with HEK tau-L2.
D) Tau-L1/L2 oligomerization after co-culturing of HEK tau-L1 with HEK tau-L2 for 36 hours was negligible in cells and minor in the medium. This suggested the rapid intracellular formation and release of stable tau-L1/L1 and tau-L2/L2 oligomers, which are rather resistant to re-arrangements and integration of tau internalized or encountered in the medium. Mean±SEM, n=3 experiments.
We further tested these results in a different experimental setup, in which uptake and release of tau from co-cultured cells expressing tau-L1 and cells expressing tau-L2 (Figure 4C) was examined. In this experiment, monomeric tau-L1 and tau-L2, and homo-oligomeric tau-L1/L1 and tau-L2/L2 are released into the medium. Intracellular tau-L1/L2 indicated the uptake of extracellular tau fused to the respective complementary part of gLuc, whereas tau-L1/L2 in the medium results from extracellular gLuc complementation. After 36h in co-culture, no intracellular tau-L1/L2 was detected, and only minor (p=0.048) tau oligomerization occurred in the co-culture medium (Figure 4D), again suggesting formation of stable tau oligomers with little to no exchange of tau monomers between oligomers. Taken together, these data are consistent with the hypothesis that tau dimerization and oligomerization occurs rapidly inside the cell, before the release into the medium. Furthermore, the inefficient exchange of tau monomers between oligomers, which would involve re-arrangements and dissociation of existing dimers and oligomers, indicates a high stability of intra- and extracellular tau oligomers.
Tau oligomerization is triggered by pro-aggregation agents
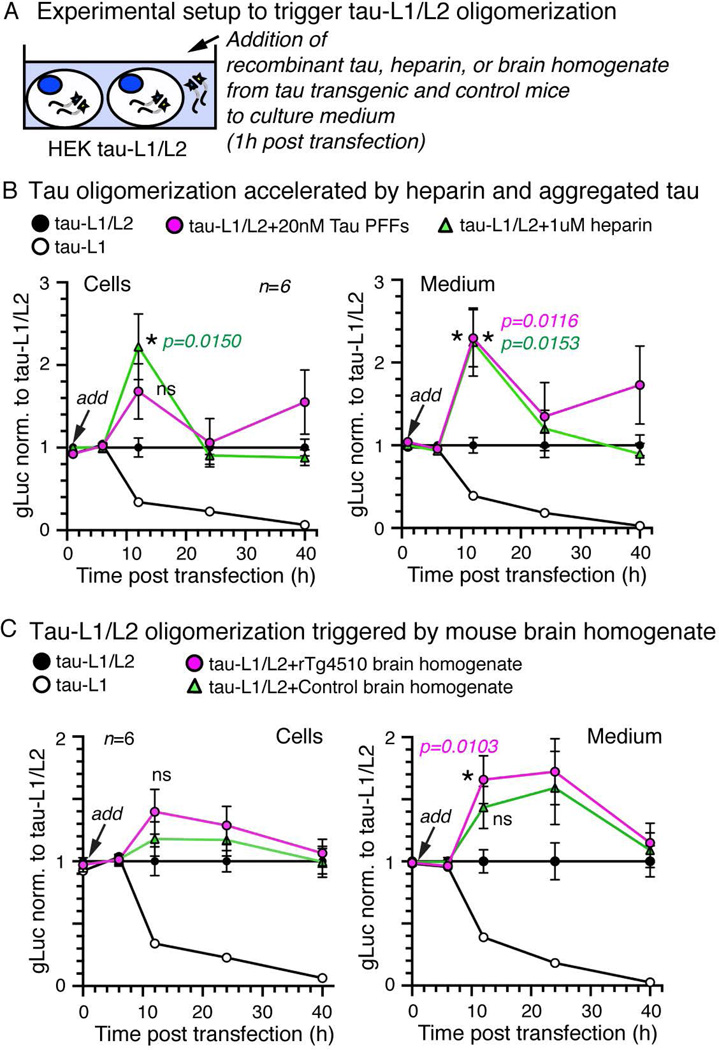
The formation of tau dimers appears to be the initial and rate-limiting step for tau aggregation in vitro (Schweers et al. 1995). The subsequent formation of fibrillar tau aggregates is enhanced in the presence of polyanions such as heparin (Friedhoff et al. 1998a, Barghorn & Mandelkow 2002) and can be seeded in the presence of preformed tau aggregates in vitro (Frost et al. 2009b) and in cells (Frost et al. 2009a). To test if tau-L1/L2 complementation results from similar biophysical interactions as tau aggregation, we triggered tau aggregation by adding heparin, pre-formed fibrils (PFFs) of recombinant full-length tau (Wegmann et al. 2010), and mouse brain lysate from rTg4510 mice (containing tauP301L aggregates, (Takeda et al. 2015)) and control mice to HEK tau-L1/L2 cells 1 hour after transfection (Figure 5A). Treatment of HEK tau-L1/L2 cells with pre-aggregated tau (PFFs, 20 nM) or heparin (1 µM) led to a significant transient increase at 12 hours post transfection (~1.6 to ~2.4-fold compared to non-treated HEK tau-L1/L2 cells) of both intra- and extracellular gLuc activity (Figure 5B). Treatment with brain homogenate from control and rTg4510 and mice (same amount of total protein and 60 ng/ml human tau in rTg4510 lysate) both modestly increased the intra- and extracellular tau-L1/L2 dimerization ~1.2 to 1.6-fold at 12 hours (Figure 5C); this effect was still detected at 24 hours post transfection. Interestingly, the content of control brain homogenate - spun at low velocity of 3000 g - appeared to have some dimerization activity in itself. These results indicate that the initial intracellular formation of tau-L1/L2 oligomers may occur faster in the presence of tau aggregation enhancers (heparin and pre-aggregated recombinant tau). After 40 hours, the gLuc activity in cells treated with heparin, aggregated tau, and brain homogenate was again similar to non-treated tau-L1/L2 cells, suggesting that the overall amount of tau-L1/L2 oligomers remained the same, but the oligomerization kinetics may be accelerated in presence of the different aggregation triggers.
Figure 5. Tau-L1/L2 dimerization is triggered by agents inducing tau aggregation.
A) Experimental setup of treating HEK tau-L1/L2 cells with aggregated recombinant tau (20 nM PFFs), heparin (1 µM), or mouse brain homogenates from tauP301L transgenic rTg4510 mice or control animals.
B) gLuc activity detected in cells and medium of HEK tau-L1/L2 after addition of human recombinant pre-aggregated tau (PFFs) or heparin, relative to untreated HEK tau-L1/L2. Both PFF and heparin treatment accelerate oligomers formation early after 12 hours. Mean±SEM, n=6 experiments.
C) Treatment of HEK tau-L1/L2 with rTg4510 and control brain homogenate both enhances oligomer formation after 12 and 24 hours compared to untreated HEK tau-L1/L2. After 40 hours, all tau-L1/L2 oligomers have formed and no more difference between treated and untreated HEK tau-L1/L2 could be detected. Mean±SEM, n=6 experiments.
We conclude from our results, that tau rapidly forms intracellular dimers and oligomers, which are stable against dissociation and are released into and taken up from the surrounding medium. However, if cellular tau oligomerization is triggered by pro-aggregation agents remains to be clarified.
INTERPRETATION
Although deposition of tau aggregates as insoluble NFTs resembles a major part of the pathology in AD and other tauopathies, soluble oligomeric tau species appear to be highly neurotoxic and may thus be - at least in part – involved in the pathological changes in these diseases.
Tau protein propagation from across neuronal networks has been shown in vivo (de Calignon et al. 2012, Liu et al. 2012, Wegmann et al. 2015) and in vitro (Takeda et al. 2015, Wu et al. 2013), but the nature of the released and uptake of tau species remains somewhat enigmatic. In order to further examine the formation, release, and uptake of soluble forms of tau, we have developed an assay, based on split-luciferase complementation, that reports the formation of dimers and oligomers and can detected tau oligomerization in an ultrasensitive range of ~10 pg/ml. Using this assay, we demonstrated that oligomeric tau species (i) are primarily generated intracellularly both in HEK cells and in neurons, (ii) are secreted as mostly non-membrane associated soluble forms of tau, (iii) are stable once formed, (iv) can be taken up by cells, and (v) are sensitive to aggregation catalysts that initiate paired helical filament formation in vitro.
Mixing of tau-L1 with tau-L2 in conditioned medium or co-culturing of tau-L1 with tau-L2 cells led to only minor reconstitution of luciferase activity, presumably because homo-oligomers of tau (e.g. tau-L1/L1) that are secreted into the media are relatively stable and do not dissociate and re-oligomerize as tau-L1/L2 hetero-oligomers that support enzyme activity. On the other hand, addition of preformed tau-L1/L2 complexes to cells with previously no luciferase activity led to robust intracellular luciferase activity. We hypothesize that preformed stable tau oligomers are readily taken up by cells, leading to intracellular luciferase activity after uptake of tau-L1/L2 hetero-oligomers and no activity after uptake of homo-oligomers (tau-L1/L1 or tau-L2/L2).
In our assay, the main tau species produced by cells appear to be dimers or smaller oligomers, as for example concluded from luciferase activity after SEC of tau-L1/L2 culture medium and by AFM. Based on the idea that small oligomers are likely building blocks of paired helical filaments (Wegmann et al. 2010), these tau oligomers could well be “on pathway” towards larger paired helical filament-like tau aggregates (Friedhoff et al. 1998b). This idea is to some extent supported by the observation that known pro-aggregation agents, such as heparin and preformed recombinant tau fibrils, appeared to enhance initial luciferase complementation, hence tau oligomerization. The addition of pre-aggregated tau (PFFs) and heparin accelerated tau oligomerization, possibly by favoring the multimerization of monomeric tau present in cells and in the culture medium, similar to what has been reported for heparin in in vitro tau aggregation experiments using recombinant tau (Paudel & Li 1999). Heparan sulfate proteoglycans (HSPGs) are normal constituents in the brain (Zhang et al. 2014) and their interference with extracellular tau uptake – but not oligomerization - has been shown previously (Holmes et al. 2013, Mirbaha et al. 2015). However, the acceleration of intracellular tau oligomerization by heparin may also be due to a secondary effect due to heparin cell toxicity (Linhardt 2004).
However, control brain lysate caused a similar increase of luciferase activity compared to Tg4510 lysate, indicating that treatment of cells with brain lysate in general has an effect on either protein production or tau oligomer formation, for example by changes in the PTMs of tau that favor its dimer and oligomer formation, or cause general cell stress.
It is also possible that the oligomers detected in the complementation assay are prevented from further growth by the presence of the small split-luciferase tags (~10 kDa each), although similar extents and efficiencies of tau oligomers occurred regardless of whether the L1 and L2 tags were placed on the N- or C-terminal ends of tau.
Not much is known about the existence or role of tau oligomers in cells, neurons, or even the brain. The identification and characterization of tau oligomers has been brought forward by in vitro experiments using recombinant human tau produced in E. coli, showing that tau dimers are stabilized by a disulfide bond and appear to be an initial critical step for tau aggregation (Friedhoff et al. 1998b). Tau split-FP constructs expressed in cells (Kim et al. 2015) showed dimer formation, but the assay in itself is insufficient to study the dynamics of tau dimerization because of the irreversible complementation of FPs. Accordingly, this is one of the first studies reporting natural stable tau oligomers and their release from cells, even in the absence of higher order oligomers and aggregates. From our results it appears that the rapid formation of tau oligomers is a physiological process in the cell and may, thus, not have any particular toxic or disease related consequences. It has been reported that the overexpression of non-endogenous wild-type human tau in cultured murine neurons can cause synapse loss and mitochondria trafficking deficits in (Thies & Mandelkow 2007). Similarly, synaptic dysfunction in absence of tau aggregation and neurodegeneration has been seen in mice overexpressing wild-type human tau throughout the brain (Hoover et al. 2010). It seems as if the synaptotoxicity of soluble tau (monomers, dimers, and oligomers) maybe follow a different pathway than NFT neurotoxicity, but it remains to be clarified if the synaptotoxic effects of soluble tau species are due to general neuronal tau overload, if they involve tau monomers, dimers, and oligomers (or all of the above), and if they actually represent signs of early tau toxicity relevant in AD and tauopathies.
Recent studies showed that a lot of the tau detected in cerebrospinal fluid from AD patients has a molecular weight similar to dimeric and oligomeric of truncated tau, at least as determined by size exclusion (Takeda et al. 2016); similar low molecular weight tau derived from rTg4510 mouse brain lysates did not support the aggregation of TauRDP301S in a cell assay designed to sensitively detect tau aggregation (Takeda et al. 2015). These observations highlight the possibility that secreted stable oligomeric tau is a relevant physiological product. Our current study suggests that tau oligomers, even if present in small amounts, are readily secreted and taken up by cells and are fairly stable under in vitro physiological conditions. These tau species represent metabolic tau intermediates, which – under pathologic circumstances – can enter the tau aggregation pathway that leads to paired helical filaments. The assay we describe may help elucidate the role of physiological tau oligomers under different cellular conditions, and highlight the intracellular formation, release, and uptake of dimeric and small oligomeric tau as a new feature of tau biology.
Supplementary Material
Acknowledgments
We thank Daniel Mueller for discussion and providing access to his AFM instruments. There are no conflicts of interest from any of the authors. The work has been funded by Massachusetts General Hospital, the German research foundation (DFG, WE 5324/1-1) and the NIH.
Abbreviations
- AD
Alzheimer's disease
- FTD
frontotemporal dementia
- TauRD
repeat domain of full-length human 4R tau
- HMW
high molecular weight
- gLuc
Gaussia luciferase
- IDP
intrinsically disordered protein
- SEC
size exclusion chromatography
REFERENCES
- Barghorn S, Biernat J, Mandelkow E. Purification of recombinant tau protein and preparation of Alzheimer-paired helical filaments in vitro. Methods Mol Biol. 2005;299:35–51. doi: 10.1385/1-59259-874-9:035. [DOI] [PubMed] [Google Scholar]
- Barghorn S, Mandelkow E. Toward a unified scheme for the aggregation of tau into Alzheimer paired helical filaments. Biochemistry. 2002;41:14885–14896. doi: 10.1021/bi026469j. [DOI] [PubMed] [Google Scholar]
- Bibow S, Mukrasch MD, Chinnathambi S, Biernat J, Griesinger C, Mandelkow E, Zweckstetter M. The dynamic structure of filamentous tau. Angew Chem Int Ed Engl. 2011;50:11520–11524. doi: 10.1002/anie.201105493. [DOI] [PubMed] [Google Scholar]
- Daebel V, Chinnathambi S, Biernat J, et al. beta-Sheet core of tau paired helical filaments revealed by solid-state NMR. J Am Chem Soc. 2012;134:13982–13989. doi: 10.1021/ja305470p. [DOI] [PubMed] [Google Scholar]
- de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires-Jones TL, Hyman BT. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suarez-Calvet M, et al. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer U, Newman AJ, Luth ES, Bartels T, Selkoe D. In vivo cross-linking reveals principally oligomeric forms of alpha-synuclein and beta-synuclein in neurons and non-neural cells. J Biol Chem. 2013;288:6371–6385. doi: 10.1074/jbc.M112.403311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedhoff P, Schneider A, Mandelkow EM, Mandelkow E. Rapid assembly of Alzheimer-like paired helical filaments from microtubule-associated protein tau monitored by fluorescence in solution. Biochemistry. 1998a;37:10223–10230. doi: 10.1021/bi980537d. [DOI] [PubMed] [Google Scholar]
- Friedhoff P, von Bergen M, Mandelkow EM, Davies P, Mandelkow E. A nucleated assembly mechanism of Alzheimer paired helical filaments. Proc Natl Acad Sci U S A. 1998b;95:15712–15717. doi: 10.1073/pnas.95.26.15712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009a;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Ollesch J, Wille H, Diamond MI. Conformational diversity of wild-type Tau fibrils specified by templated conformation change. J Biol Chem. 2009b;284:3546–3551. doi: 10.1074/jbc.M805627200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigant B, Landrieu I, Fauquant C, Barbier P, Huvent I, Wieruszeski JM, Knossow M, Lippens G. Mechanism of Tau-promoted microtubule assembly as probed by NMR spectroscopy. J Am Chem Soc. 2014;136:12615–12623. doi: 10.1021/ja504864m. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Adams KW, Fan Z, McLean PJ, Hyman BT. Characterization of oligomer formation of amyloid-beta peptide using a split-luciferase complementation assay. J Biol Chem. 2011;286:27081–27091. doi: 10.1074/jbc.M111.257378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes BB, DeVos SL, Kfoury N, et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci U S A. 2013;110:E3138–E3147. doi: 10.1073/pnas.1301440110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover BR, Reed MN, Su J, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeganathan S, von Bergen M, Mandelkow EM, Mandelkow E. The natively unfolded character of tau and its aggregation to Alzheimer-like paired helical filaments. Biochemistry. 2008;47:10526–10539. doi: 10.1021/bi800783d. [DOI] [PubMed] [Google Scholar]
- Johnson GV, Seubert P, Cox TM, Motter R, Brown JP, Galasko D. The tau protein in human cerebrospinal fluid in Alzheimer's disease consists of proteolytically derived fragments. J Neurochem. 1997;68:430–433. doi: 10.1046/j.1471-4159.1997.68010430.x. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Kalia SK, Chau H, Lozano AM, Hyman BT, McLean PJ. Ubiquitinylation of alpha-synuclein by carboxyl terminus Hsp70-interacting protein (CHIP) is regulated by Bcl-2-associated athanogene 5 (BAG5) PLoS One. 2011;6:e14695. doi: 10.1371/journal.pone.0014695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Lim S, Haque MM, et al. Identification of disulfide cross-linked tau dimer responsible for tau propagation. Sci Rep. 2015;5:15231. doi: 10.1038/srep15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Tepper K, Kaniyappan S, Biernat J, Wegmann S, Mandelkow EM, Muller DJ, Mandelkow E. Stages and Conformations of the Tau Repeat Domain during Aggregation and Its Effect on Neuronal Toxicity. J Biol Chem. 2014;289:20318–20332. doi: 10.1074/jbc.M114.554725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Kiritoshi T, Neugebauer V, Jackson GR, Kayed R. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci Rep. 2012;2:700. doi: 10.1038/srep00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall G, Cole RD. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984;259:5301–5305. [PubMed] [Google Scholar]
- Linhardt RJ. Heparin-induced cancer cell death. Chem Biol. 2004;11:420–422. doi: 10.1016/j.chembiol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbaha H, Holmes BB, Sanders DW, Bieschke J, Diamond MI. Tau Trimers Are the Minimal Propagation Unit Spontaneously Internalized to Seed Intracellular Aggregation. J Biol Chem. 2015;290:14893–14903. doi: 10.1074/jbc.M115.652693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukrasch MD, Markwick P, Biernat J, Bergen M, Bernado P, Griesinger C, Mandelkow E, Zweckstetter M, Blackledge M. Highly populated turn conformations in natively unfolded tau protein identified from residual dipolar couplings and molecular simulation. J Am Chem Soc. 2007;129:5235–5243. doi: 10.1021/ja0690159. [DOI] [PubMed] [Google Scholar]
- Paudel HK, Li W. Heparin-induced conformational change in microtubule-associated protein Tau as detected by chemical cross-linking and phosphopeptide mapping. J Biol Chem. 1999;274:8029–8038. doi: 10.1074/jbc.274.12.8029. [DOI] [PubMed] [Google Scholar]
- Pooler AM, Phillips EC, Lau DH, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013;14:389–394. doi: 10.1038/embor.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss U, Biernat J, Mandelkow EM, Mandelkow E. The 'jaws' model of tau-microtubule interaction examined in CHO cells. J Cell Sci. 1997;110(Pt 6):789–800. doi: 10.1242/jcs.110.6.789. [DOI] [PubMed] [Google Scholar]
- Remy I, Michnick SW. A highly sensitive protein-protein interaction assay based on Gaussia luciferase. Nat Methods. 2006;3:977–979. doi: 10.1038/nmeth979. [DOI] [PubMed] [Google Scholar]
- Rosenberg KJ, Ross JL, Feinstein HE, Feinstein SC, Israelachvili J. Complementary dimerization of microtubule-associated tau protein: Implications for microtubule bundling and tau-mediated pathogenesis. Proc Natl Acad Sci U S A. 2008;105:7445–7450. doi: 10.1073/pnas.0802036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SantaCruz K, Lewis J, Spires T, Paulson, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweers O, Mandelkow EM, Biernat J, Mandelkow E. Oxidation of cysteine-322 in the repeat domain of microtubule-associated protein tau controls the in vitro assembly of paired helical filaments. Proc Natl Acad Sci U S A. 1995;92:8463–8467. doi: 10.1073/pnas.92.18.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillen A, Leroy A, Wieruszeski JM, Loyens A, Beauvillain JC, Buee L, Landrieu I, Lippens G. Regions of tau implicated in the paired helical fragment core as defined by NMR. Chembiochem. 2005;6:1849–1856. doi: 10.1002/cbic.200400452. [DOI] [PubMed] [Google Scholar]
- Takeda S, Commins C, DeVos SL, et al. Seed-competent high-molecular-weight tau species accumulates in the cerebrospinal fluid of Alzheimer's disease mouse model and human patients. Ann Neurol. 2016 doi: 10.1002/ana.24716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Wegmann S, Cho H, et al. Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer's disease brain. Nat Commun. 2015;6:8490. doi: 10.1038/ncomms9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper K, Biernat J, Kumar S, et al. Oligomer formation of tau protein hyperphosphorylated in cells. J Biol Chem. 2014;289:34389–34407. doi: 10.1074/jbc.M114.611368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies E, Mandelkow EM. Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/Par-1. J Neurosci. 2007;27:2896–2907. doi: 10.1523/JNEUROSCI.4674-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinczek B, Ebneth A, Mandelkow EM, Mandelkow E. Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J Cell Sci. 1999;112(Pt 14):2355–2367. doi: 10.1242/jcs.112.14.2355. [DOI] [PubMed] [Google Scholar]
- von Bergen M, Barghorn S, Biernat J, Mandelkow EM, Mandelkow E. Tau aggregation is driven by a transition from random coil to beta sheet structure. Biochim Biophys Acta. 2005;1739:158–166. doi: 10.1016/j.bbadis.2004.09.010. [DOI] [PubMed] [Google Scholar]
- von Bergen M, Barghorn S, Muller SA, Pickhardt M, Biernat J, Mandelkow EM, Davies P, Aebi U, Mandelkow E. The core of tau-paired helical filaments studied by scanning transmission electron microscopy and limited proteolysis. Biochemistry. 2006;45:6446–6457. doi: 10.1021/bi052530j. [DOI] [PubMed] [Google Scholar]
- Wegmann S, Jung YJ, Chinnathambi S, Mandelkow EM, Mandelkow E, Muller DJ. Human Tau isoforms assemble into ribbon-like fibrils that display polymorphic structure and stability. J Biol Chem. 2010;285:27302–27313. doi: 10.1074/jbc.M110.145318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann S, Maury EA, Kirk MJ, et al. Removing endogenous tau does not prevent tau propagation yet reduces its neurotoxicity. EMBO J. 2015;34:3028–3041. doi: 10.15252/embj.201592748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann S, Medalsy ID, Mandelkow E, Muller DJ. The fuzzy coat of pathological human Tau fibrils is a two-layered polyelectrolyte brush. Proc Natl Acad Sci U S A. 2013;110:E313–E321. doi: 10.1073/pnas.1212100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille H, Drewes G, Biernat J, Mandelkow EM, Mandelkow E. Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J Cell Biol. 1992;118:573–584. doi: 10.1083/jcb.118.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Hudry E, Hashimoto T, Uemura K, Fan ZY, Berezovska O, Grosskreutz CL, Bacskai BJ, Hyman BT. Distinct dendritic spine and nuclear phases of calcineurin activation after exposure to amyloid-beta revealed by a novel fluorescence resonance energy transfer assay. J Neurosci. 2012;32:5298–5309. doi: 10.1523/JNEUROSCI.0227-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JW, Herman M, Liu L, et al. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem. 2013;288:1856–1870. doi: 10.1074/jbc.M112.394528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Cirrito JR, Stewart FR, et al. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci. 2011;31:13110–13117. doi: 10.1523/JNEUROSCI.2569-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori H, Khatoon S, Grundke-Iqbal I, Blennow K, Ewers M, Hampel H, Iqbal K. Tau in cerebrospinal fluid: a sensitive sandwich enzyme-linked immunosorbent assay using tyramide signal amplification. Neurosci Lett. 2007;418:186–189. doi: 10.1016/j.neulet.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GL, Zhang X, Wang XM, Li JP. Towards understanding the roles of heparan sulfate proteoglycans in Alzheimer's disease. Biomed Res Int. 2014;2014:516028. doi: 10.1155/2014/516028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.