Abstract
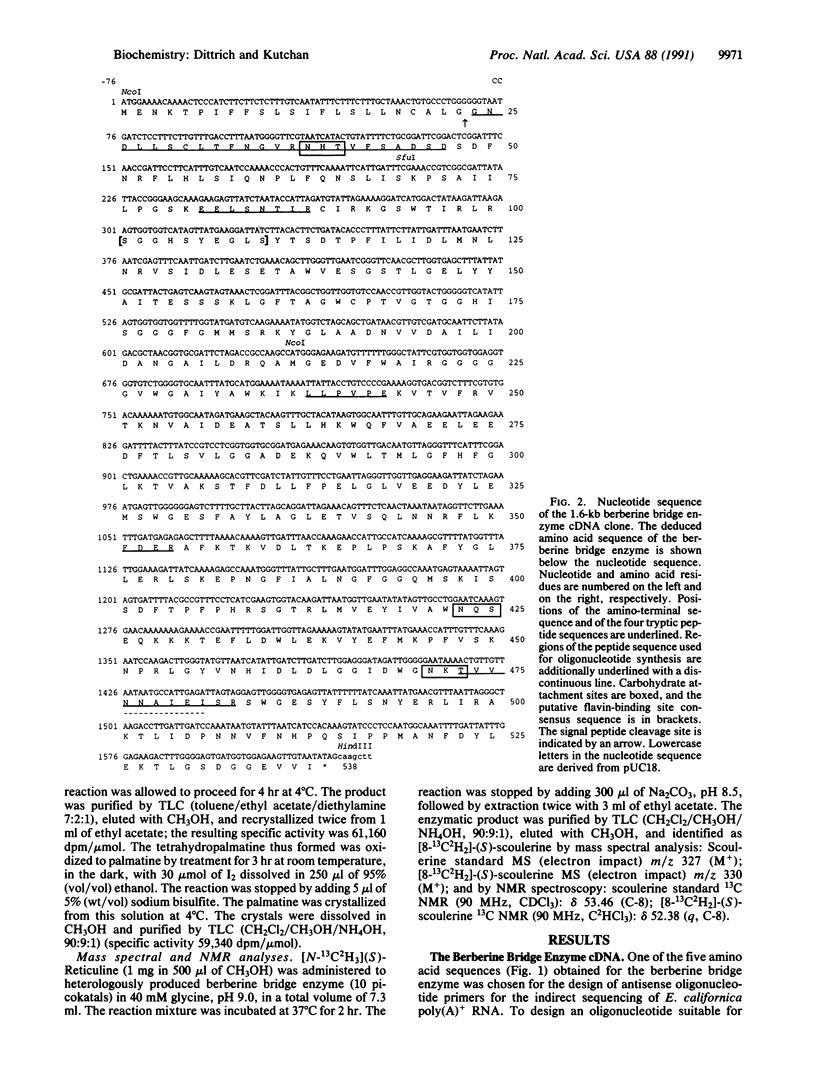
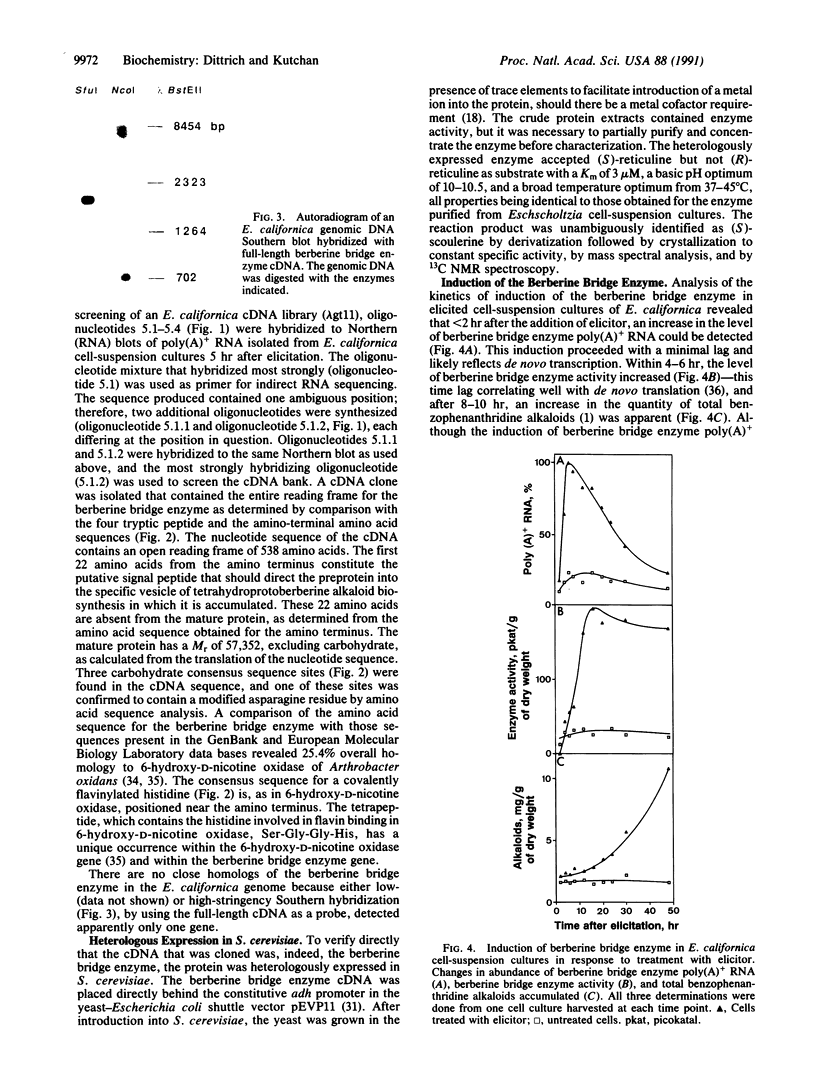
The berberine bridge enzyme [(S)-reticuline: oxygen oxidoreductase (methylene-bridge-forming), EC 1.5.3.9] is a vesicular plant enzyme that catalyzes the formation of the berberine bridgehead carbon of (S)-scoulerine from the N-methyl carbon of (S)-reticuline in a specific, unparalleled reaction along the biosynthetic pathway that leads to benzophenanthridine alkaloids. Cytotoxic benzophenanthridine alkaloids are accumulated in certain species of Papaveraceae and Fumariaceae in response to pathogenic attack and, therefore, function as phytoalexins. The berberine bridge enzyme has been purified to homogeneity from elicited cell-suspension cultures of Eschscholtzia californica, and partial amino acid sequences have been determined. A cDNA, isolated from a Agt11 cDNA bank of elicited E. californica cell-suspension cultures, coded for an open reading frame of 538 amino acids. The first 22 amino acids constitute the putative signal peptide. The mature protein has a Mr of 57,352, excluding carbohydrate. The berberine bridge enzyme was heterologously expressed in a catalytically active form in Saccharomyces cerevisiae. Southern hybridization with genomic DNA suggests that there is only one gene for the enzyme in the E. californica genome. Hybridized RNA blots from elicited E. californica cell-suspension cultures revealed a rapid and transient increase in poly(A)+ RNA levels that preceded both the increase in enzyme activity and the accumulation of benzophenanthridine alkaloids, emphasizing the integral role of the berberine bridge enzyme in the plant response to pathogens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandsch R., Bichler V. In vivo and in vitro expression of the 6-hydroxy-D-nicotine oxidase gene of Arthrobacter oxidans, cloned into Escherichia coli, as an enzymatically active, covalently flavinylated polypeptide. FEBS Lett. 1985 Nov 18;192(2):204–208. doi: 10.1016/0014-5793(85)80108-3. [DOI] [PubMed] [Google Scholar]
- Brandsch R., Hinkkanen A. E., Mauch L., Nagursky H., Decker K. 6-Hydroxy-D-nicotine oxidase of Arthrobacter oxidans. Gene structure of the flavoenzyme and its relationship to 6-hydroxy-L-nicotine oxidase. Eur J Biochem. 1987 Sep 1;167(2):315–320. doi: 10.1111/j.1432-1033.1987.tb13338.x. [DOI] [PubMed] [Google Scholar]
- Bruce R. J., West C. A. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989 Nov;91(3):889–897. doi: 10.1104/pp.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Dzink J. L., Socransky S. S. Comparative in vitro activity of sanguinarine against oral microbial isolates. Antimicrob Agents Chemother. 1985 Apr;27(4):663–665. doi: 10.1128/aac.27.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerskorn C., Mewes W., Goretzki H., Lottspeich F. A new siliconized-glass fiber as support for protein-chemical analysis of electroblotted proteins. Eur J Biochem. 1988 Oct 1;176(3):509–519. doi: 10.1111/j.1432-1033.1988.tb14308.x. [DOI] [PubMed] [Google Scholar]
- Edmondson D. E., Ackrell B. A., Kearney E. B. Identification of neutral and anionic 8 alpha-substituted flavin semiquinones in flavoproteins by electron spin resonance spectroscopy. Arch Biochem Biophys. 1981 Apr 15;208(1):69–74. doi: 10.1016/0003-9861(81)90124-7. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kuhn D. N., Chappell J., Boudet A., Hahlbrock K. Induction of phenylalanine ammonia-lyase and 4-coumarate:CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1102–1106. doi: 10.1073/pnas.81.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutchan T. M. Expression of enzymatically active cloned strictosidine synthase from the higher plant Rauvolfia serpentina in Escherichia coli. FEBS Lett. 1989 Oct 23;257(1):127–130. doi: 10.1016/0014-5793(89)81802-2. [DOI] [PubMed] [Google Scholar]
- Kutchan T. M., Hampp N., Lottspeich F., Beyreuther K., Zenk M. H. The cDNA clone for strictosidine synthase from Rauvolfia serpentina. DNA sequence determination and expression in Escherichia coli. FEBS Lett. 1988 Sep 12;237(1-2):40–44. doi: 10.1016/0014-5793(88)80167-4. [DOI] [PubMed] [Google Scholar]
- Köhrer K., Kutchan T. M., Domdey H. Specific oligodeoxynucleotide probes obtained through RNA sequencing. DNA. 1989 Mar;8(2):143–147. doi: 10.1089/dna.1.1989.8.143. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink E., Böhm H. Conversion of reticuline into scoulerine by a cell free preparation from Macleaya microcarpa cell suspension cultures. FEBS Lett. 1975 Jan 1;49(3):396–399. doi: 10.1016/0014-5793(75)80794-0. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986 Apr 11;45(1):145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- Schumacher H. M., Rüffer M., Nagakura N., Zenk M. H. Partial Purification and Properties of (S)-Norlaudanosoline Synthase from Eschscholtzia tenuifolia Cell Cultures. Planta Med. 1983 Aug;48(8):212–220. doi: 10.1055/s-2007-969923. [DOI] [PubMed] [Google Scholar]



