Abstract
Insecticides are by design toxic. They must be toxic to effectively kill target species of insects. Unfortunately, they also have off-target toxic effects that can harm other species, including humans. Developmental neurotoxicity is one of the most prominent off-target toxic risks of insecticides. Over the past seven decades several classes of insecticides have been developed, each with their own mechanisms of effect and toxic side effects. This review covers the developmental neurotoxicity of the succeeding generations of insecticides including organochlorines, organophosphates, pyrethroids, carbamates and neonicotinoids. The goal of new insecticide development is to more effectively kill target species with fewer toxic side effects on non-target species. From the experience with the developmental neurotoxicity caused by the generations of insecticides developed in the past advice is offered how to proceed with future insecticide development to decrease neurotoxic risk.
Keywords: Neurotoxicity, Development, Organochlorines, Organophosphates, Pyrethroids: Carbamates, Neonicotinoids
1. Introduction
Insecticides control insect attacks on crops, livestock and pets and prevent transmission of insect-borne diseases. However, insecticide exposures can also have adverse off-target effects including neurotoxicity. Over the past seven decades, a variety of insecticide classes have been introduced. The organochlorines (OCs), introduced in the market in the early 1940s, were the first insecticides active against a wide variety of pests, and effective for long periods of time (Casida and Quistad 1998). These were succeeded by organophosphates (OPs) and carbamates (CAs), pyrethroids (PIs), and most recently in the 1990s, by neonicotinoids (NEs) (Casida and Durkin 2013b). It is instructive to compare the risks posed by insecticides in all of these classes in the quest to develop safer strategies to maximize insecticidal actions while minimizing off-target toxic effects. One of the most prominent risks, and a source of intense regulatory activity, is developmental neurotoxicity (Grandjean and Landrigan 2014). This is the focus of the current review.
Each of the classes of insecticides was considered safe when introduced into the market, however, none is completely specific for insect pests. Indeed, insecticides primarily target the nervous system and similarities between the insect and human nervous systems often lead to cross-toxicity. Accordingly, experimental animal studies and epidemiological findings point to the health hazards associated with exposure to all of these classes of insecticides.
The developing nervous system is highly susceptible to the neurotoxicity of insecticides as it is for many types of environmental toxicants. This enhanced sensitivity occurs not only during prenatal development but also postnatally, extending into adolescence (Connors and others 2008). Impacts on the developing nervous system can have deleterious effects that last a lifetime, long after the end of exposure, because the toxicant causes malformations of the nervous system. This review will consider the targets and mechanisms of action of the main insecticide classes during brain development, highlighting morphological and neurochemical effects that culminate in behavioral dysfunction. Finally, we will suggest future directions for insecticide research that could lead to development of safer products.
For each class of insecticides, in vitro, experimental studies in animal models and epidemiological findings were included. Deleterious neural outcomes of developmental exposure are summarized in the main body of the review; tables [provided as supplementary material (SM)] also include studies that failed to find significant alterations. Dose effects for adverse neurodevelopmental effects in animal models are shown in figures plotted doses as proportions of the no-observed-adverse-effect level (NOAEL) as a common point of toxicodynamic potency and periods of exposure for representative insecticides on each class. For epidemiological findings, with few exceptions, we included only those studies in which exposure was quantified through measurement of parent compounds or metabolites in biological samples. Detailed information on insecticidal and off-target toxicity is provided as SM.
2. Organochlorines (OCs)
The Austrian chemist, Othmar Zeidler, first synthesized the prototypic OC DDT (dichlorodiphenyltrichloroethane) in 1874. However, more than 60 years later, it was the Swiss chemist, Paul Müller, who first demonstrated the effectiveness of DDT as an insecticide and, for that, he was awarded the 1948 Nobel Prize in Physiology or Medicine (Casida and Quistad 1998). Subsequently, other chlorinated compounds were identified as effective insecticides and, for decades, OCs dominated the market of insect control in both agriculture and home formulations. They were widely used over the span from 1940s, until they were largely restricted and banned in most countries during the 1970s and 1980s (Costa 2015), largely because of their persistence in the environment, bioaccumulation and emerging evidence of adverse effects on wildlife off-target species. Rachel Carson’s landmark book “Silent Spring”, published in 1962 (Carson 2002), played a major role in the decision to ban DDT and other OCs for agricultural uses in the US and, subsequently, in many other countries, and led to increased regulation of pesticides (for review: (Epstein 2014).
2.1. Developmental toxicity of OCs
Even though neurotoxicity was not among the most important reasons that led to regulation of OCs subsequent studies identified the developing brain as a sensitive target. Because of their lipophilicity OCs bioaccumulate in adipose tissue where they can remain for decades (Mrema and others 2013). When mobilized from adipocytes to the blood stream, OCs are readily secreted into breast milk (Mrema and others 2013; Shen and others 2007). OCs also can cross the placenta and the blood brain barrier and accumulate in the brain (Morrison 1971; Tebourbi and others 2006). OCs such as DDT are still permitted for use against mosquito-borne transmission of malaria in several countries due to their higher efficacy versus other insecticides (Bouwman and others 2011). Due to their persistence and continued use, developmental exposure to OCs still affects public health. Due to their lipophilicity and accumulation in the brain, neurotoxicity is a major concern.
In Subtopic 2.1.1 and in SM Table 1 we summarize data showing that OCs cause developmental behavioral and neurochemical disruption in experimental models. The mechanisms associated with neurotoxicity do not appear to be restricted to the blocking of sodium channels and γ-aminobutyric acid receptors that are involved in acute toxicity (see SM for a detailed description). As detailed below, these additional actions include altered neurotransmitter levels and endocrine disruption. Figure 1 further makes it clear that even very low levels of OC exposure during gestation and/or lactation of rodents cause deleterious neurobehavioral effects. Subsequently (Subtopic 2.1.2 and SM Table 2), parallel results from epidemiological studies that implicate early OCs exposure in adverse neurodevelopmental outcomes are presented.
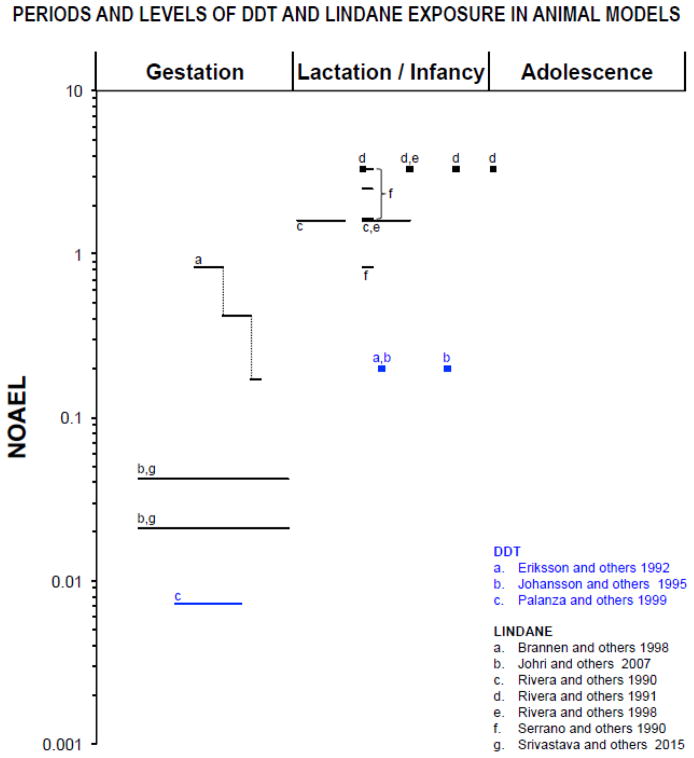
Figure 1.
Periods of exposure and NOAEL (no-observed-adverse-effect level) proportions in log scale based on animal model studies listed in table 1. Only doses and periods of exposure that elicited significant effects are reported. The NOAELs for neurotoxic effects of DDT (25 mg/kg; (ATSDR 2002), and of Lindane (6 mg/kg; (WHO 2004) were chosen based on acute, oral administration in adult rats. The symbol “■” represents single dose, “—” represents longer periods of exposure and “.....” represents dose change. Lactation/infancy extends from PN1 to PN27 (PN=postnatal day). Adolescence assumed to begin at PN28.
2.1.1. Evidence of OCs developmental neurotoxicity from in vitro and animal models
In vitro studies show that OCs affect neuronal differentiation, function and survival. At nanomolar concentrations, dieldrin, lindane and endosulfan inhibit voltage-gated calcium channels, decreasing the intracellular calcium concentration in pheochromocytoma (PC12) cells (Heusinkveld and others 2010; Heusinkveld and Westerink 2012; Meijer and others 2015). At higher (micromolar) concentrations, dieldrin decreases cell viability and cell proliferation in undifferentiated PC12 cells (Slotkin and others 2007a) and in mouse neuroprogenitor cells (Culbreth and others 2012). This OC decreases the number of dopaminergic and GABAergic cells, as well as the number and length of neurites in neonatal mesencephalic cells (Sanchez-Ramos and others 1998). Dieldrin and heptachlor evoke apoptosis in cell cultures, an event mediated by oxidative stress and mitochondrial damage (Culbreth and others 2012; Hong and others 2014; Slotkin and Seidler 2009a). In PC12 cells, dieldrin also produces a shift in differentiation fate in those cells that survive the initial oxidative damage (Slotkin and others 2007a). Cells which normally develop to express the cholinergic phenotype instead are directed toward the catecholaminergic phenotype, caused by shifts in several classes of neurotrophic factors (Slotkin and others 2010), as well as protein kinase C isoforms and their modulators (Slotkin and Seidler 2009b).
Rodent models have also shown that OC exposure induces neurobehavioral impairment. Pre and postnatal exposure to a mixture of OC insecticides that includes DDT, dieldrin and aldrin evokes robust reductions in gene expression for muscarinic cholinergic receptors (mAChR) and acetylcholinesterase that persists into adulthood in female rats (Gill and others 2013). Similarly, DDT exposure during the neonatal period evokes downregulation of muscarinic acetylcholine receptors (Eriksson and others 1992). Gestational exposure to lindane causes persistent increases in dopamine D2, serotonergic 5HT2 and GABA-A receptors in frontal cortex, cerebellum and striatum of rat offspring (Srivastava and others 2015). With postnatal exposure, lindane alters monoamine levels (Rivera and others 1998; Rivera and others 1991). Endosulfan exposure during the first three weeks of postnatal life also alters monoamine levels in brain regions such as hippocampus and brain stem (Lakshmana and Raju 1994). When exposure extends from gestation and throughout lactation, endosulfan increases glutamate, glutamine aspartate, taurine and GABA levels in the prefrontal cortex of infant and adolescent rats and decreases taurine and GABA levels when examined in adulthood (Cabaleiro and others 2008). Other effects include increased serotonin concentration and decreased serotonergic and dopaminergic turnover (Cabaleiro and others 2008). This OC is also able to elicit short and long-term alterations in norepinephrine, serotonin and dopamine content and turnover in the striatum (Lafuente and Pereiro 2013). In this region, further decreases in the dopamine transporter and tyrosine hydroxylase expression at adulthood suggest that endosulfan during gestation and lactation damages the nigrostriatal dopamine system and sensitizes dopaminergic neurons to a subsequent treatment with the dopaminergic neurotoxin MPTP (1- methyl -4- phenyl -1,2,3,6-tetrahydro pyridine) (Wilson and others 2014). Mice exposed to endosulfan during lactation also present depletion of striatal dopamine after re-exposure at adulthood (Jia and Misra 2007). Effects on the dopaminergic system were also shown in rodents exposed to other cyclodienes (Caudle and others 2005; Richardson and others 2006; Richardson and others 2008), which supports evidence that developmental exposure to this group of OCs is a risk factor for Parkinson’s disease (Hatcher and others 2008). Other effects include persistent increases in mRNA expression of cytochrome P450s and associated transcription factors as identified in several brain regions of rat offspring exposed to lindane during gestation (Srivastava and others 2015) As for postnatal exposure, lindane decreases myelination, which identifies glial cells as a target (Serrano and others 1990).
Behavioral dysfunction also results from early exposure to OCs. Heptachlor exposure from gestation to weaning increases locomotor activity at the same dose range that disrupts dopamine systems (Moser and others 2001). A similar effect was identified after gestational (Johri and others 2007) or postnatal exposure to lindane (Rivera and others 1998; Rivera and others 1990). Consistent with effects on acetylcholine synapses, cholinergic-dependent behaviors are particularly susceptible to disruption by OCs. Even a single exposure to DDT in the neonatal period leads to cognitive defects in adulthood, such as decreased habituation, a non-associative learning process (Eriksson and others 1992; Johansson and others 1995). Likewise, heptachlor exposure extending from gestation until adolescence, causes persistent deficits in spatial memory and learning (Moser and others 2001); endosulfan exposure during the first three postnatal weeks in rats causes deficits in operant learning and memory (Lakshmana and Raju 1994).
OCs have been identified as endocrine disruptors. They interfere with steroid signaling pathways either by binding to intracellular receptors or by inhibiting enzyme activities in steroidogenic pathways, actions which lead to decreased hormones production and increased levels of intermediate precursors (Mrema and others 2013). In addition, OCs such as DDT (and its metabolite DDE), dieldrin, endosulfan, and lindane alter thyroid hormone and thyroid-stimulating hormone (TSH) levels, adrenal corticosterone and response to adrenocorticotropic hormone (ACTH) in rodent cell lines (Zhang and others 2016), adrenal slice cultures (Lindhe and others 2001), rodent models (Liu and others 2011; Schantz and Widholm 2001; Yaglova and Yaglov 2014) and epidemiological studies (Freire and others 2012; Freire and others 2011). However, few studies actually studied the causative roles between OCs endocrine-disruption and brain development and behavioral outcomes. This issue was recently addressed in an elegant study from Briz and collaborators (Briz and others 2011) in which the effects of dieldrin, endosulfan, and lindane on two signaling pathways known to be involved in synaptic plasticity and synaptogenesis were investigated in primary cultures of cerebellar granule cells and cortical neurons: the mitogen-activated protein kinase (MAPK) and the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt). These authors demonstrated that these OCs exhibit endocrine-disrupting activity at micromolar concentrations and that interference on MAPK and PI3K/Akt pathways is mediated by OCs direct interaction with neuronal estrogen receptors. Considering the role of steroid hormones in brain development, OCs-mediated alterations in the physiological activation of these signaling pathways likely contribute to the cognitive and other behavioral deficits seen after developmental exposure (Parent and others 2011; Schantz and Widholm 2001).
2.1.2. Epidemiological studies on OCs neurodevelopmental adverse effects
Epidemiological studies assessed prenatal and/or postnatal exposure associations with neurobehavioral impairment (SM Table 2). As for OCs, most studies focus on DDT and its metabolite dichlorodiphenyl-dichloroethylene (DDE). However, evidence of deleterious outcomes of other compounds were also identified.
Findings from the North Carolina Breast Milk and Formula Project identified a positive association between DDE in breast milk and neonatal hyporeflexia (Rogan and others 1986). The Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) cohort study in California found prenatal levels of DDT and DDE to be associated with decreased mental and psychomotor development in 1 and 2-year old children from an agricultural community (Eskenazi and others 2006). In the Ribera d’Ebre cohort study, similar results were identified in 13-month old children from a village next to a factory that formerly produced DDT (Ribas-Fitó and others 2003) and, in a follow-up study, there were associations between cord serum levels of DDT at birth and decreased verbal, memory, quantitative, and perceptual performance skills at 4 years of age (Ribas-Fitó and others 2006). In a malaria-endemic region in Mexico, maternal DDE levels during gestation were associated with impaired motor development during the first year of life (Torres-Sánchez and others 2007) and, even though this association was no longer identified in children aged 1.5–2.5 years (Torres-Sánchez and others 2009), follow-ups at 4–5 years of age again indicated impaired quantitative, verbal, memory and general cognitive abilities (Torres-Sánchez and others 2013) as well as decreased spatial orientation performance (Osorio-Valencia and others 2015). Prenatal exposure to DDE was also associated with both inattention and hyperactivity Attention-Deficit Hyperactivity Disorder (ADHD) symptoms in children aged 7–11 years born to mothers residing near a contaminated harbor in Massachusetts, USA (Sagiv and others 2010). DDT levels in human breast milk were inversely correlated to the mental capacities in children aged 15 years in ten countries of three continents from the Programme for International Student Assessment (PISA) (Dörner and Plagemann 2002). DDE, but not DDT, exposure both pre- and postnatally during the first years of life may impair visual processing in pre-adolescents, as indicated from a study on children in Nunavik (Arctic Quebec, Canada), a region that presents high levels of several environmental contaminants, including OCs (Cartier and others 2014). This study was also the first to describe the impact of developmental OC exposure in sensory processing. Mirex levels at 4 years of age, were correlated with decreased working memory and impaired quantitative performance (Puertas and others 2010).
2.2. What did we learn with OCs and what is still to be learned?
Seventy years have passed since DDT was introduced to the market. The first lesson learned from OCs is that new generations of insecticides need to be less persistent in the environment and our bodies. Indeed, by the time the decisions to restrict and ban OCs took place, there were already major concerns regarding their persistence, consequent bioaccumulation, and effects on wildlife. Despite the decrease in use, the persistence of OC compounds results in low level exposure of people in virtually all countries contributed to a continuous effort of scientists to investigate the consequences of exposure and, in the following decades, developmental toxicity of mammals was identified as a sensitive adverse effect. Particularly disturbing is evidence that the mechanisms responsible for insect toxicity and off-target acute poisoning cannot explain the very distinct outcomes of developmental exposure, which indicates that these serve as poor predictors of liability. Thus, the bases established for OC regulation are not necessarily those that would serve to protect the fetus from adverse effects on brain development. This major theme will be even more critical in our assessment of the next insecticide generation, the OPs (see below).
While animal models and in vitro studies demonstrate that OC exposure during development is deleterious, for most OCs, there is still no systematic evaluation of developmental neurotoxic effects. As a result, while for some OCs (e.g. endosulfan, diedrin), there are extensive descriptions of neurotransmitter alterations without parallel behavioral studies, for others, either the investigation of neurotransmitter systems is limited (e.g heptachlor) or the behavioral consequences of exposure during particular developmental windows are missing (e.g. lindane, prenatal period). As for epidemiological findings, there is evidence that associate OCs with the later emergence of Parkinson’s disease, as well as decreased cognitive performance. Despite that, most studies focus on DDT and DDE, with scant work on the effects of other OCs. Accordingly, while there is evidence for developmental neurotoxicity or OC pesticides, there are still important gaps that prevent the complete assessment of the extent of the damage caused by exposure, and thus limit our ability to regulate human exposures based on these endpoints.
3. Organophosphates (OPs)
Organophosphorus compounds were first synthesized in the 1800s (Petroianu 2010), however, it was only in the late 1930s that the German chemist Gerhard Schrader, while working at Farbenfabriken Bayer, discovered the general chemical structure of anticholinesterase OP compounds, which lead to the synthesis of Baldan, the first commercialized OP insecticide [containing tetraethyl pyrophosphate (TEPP) as the active ingredient] (Casida and Quistad 1998; Klaassen and Casarett 2007). After World War II, the use of OPs increased at a rapid rate, with the development of synthetic insecticides that effectively killed insects with less acute toxicity than OP nerve gases. Since then, hundreds of OPs have been made and commercialized worldwide in a variety of formulations [e.g., malathion, parathion, diazinon, chlorpyrifos (CPF)]. An advantage of OPs when compared to OCs is the fact that OPs do not bioaccumulate. OPs were the first readily-biodegradable synthetic insecticides (Casida and Durkin 2013a). The progressive restriction or banning of OCs in the 1960s led to an increase in the use of OPs for both household and agricultural insect control.
OPs irreversibly inhibit acetylcholinesterase (AChE), the enzyme that catalyzes the breakdown of acetylcholine (ACh) to acetate and choline in synaptic clefts in both insects’ and off-target organisms’ nervous system (see SM for a detailed description). In humans and other mammals, when AChE inhibition exceeds 70–75%, acute poisoning results in a severe “cholinergic syndrome”, in which accumulation of ACh leads to peripheral signs such as increased sweating and salivation, bronchoconstriction, miosis, increased gastrointestinal motility and tremors; and central nervous system effects such as dizziness, mental confusion, and eventually, convulsions and death (Klaassen and Casarett 2007; Slotkin 2004a). Besides, OPs have been shown to have a variety of neurotoxic effects even at levels devoid of systemic toxicity, particularly with developmental exposure.
3.1. Developmental neurotoxicity of OPs
Concerns about OP exposure of pregnant women, infants and children emerged in the early 1990’s, when studies demonstrated that the residential use of CPF results in air and surface concentrations that can lead to exposure above the NOAEL (Fenske and others 1990; Gurunathan and others 1998). Animal studies of developmental OP exposure further indicated that the neurotoxicity of the widely used OP CPF in rodents is seen at substantially lower doses during development than in adulthood (e.g. (Pope and Chakraborti 1992; Pope and others 1991). These and other findings led to experimental designs that adopted low levels of exposure, and revealed deleterious effects of CPF and other OPs at doses that evoke little or no AChE inhibition. Indeed, developmental OP exposure has been associated with altered function of numerous proteins other than AChE and, as detailed below, these additional mechanisms provide the main route for disruption of brain development.
The developmental neurotoxicity of OPs, has been very extensively studied. Here we describe representative studies to provide an overview of mechanisms and outcomes of OP neurotoxicity. We focus on developmental effects of CPF, the most studied OP in animal models and in vitro studies (Subtopic 3.1.1 and SM Table 3) as well as information on developmental periods and levels of CPF exposure used in rodent models (Figure 2). Further, a comparative approach between CPF and other widely used OPs (parathion, diazinon and methyl parathion) are presented as SM. With a few exceptions, the studies cited below used OP doses that elicit no more than 25% inhibition of AChE, well below the threshold for overt signs of intoxication. Subsequently, results from human cohort studies that associate early OP exposures and adverse neurodevelopmental outcomes are presented (Subtopic 3.1.2 and SM Table 4).
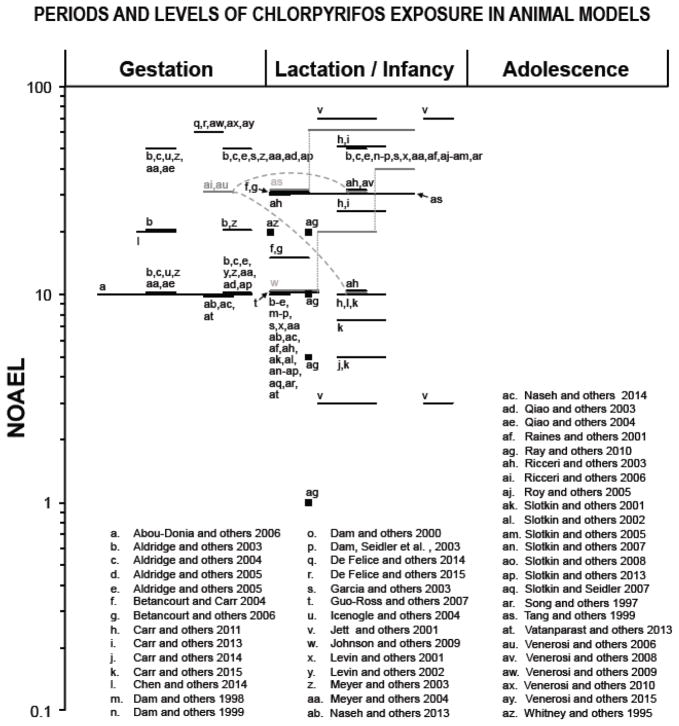
Figure 2.
Periods of exposure and percentages of the NOAEL (no-observed-adverse-effect level) proportions in log scale based on animal model studies listed in table 3. Only doses and periods of exposure that elicited significant effects are reported. For Chlorpyrifos, the NOAEL for cholinesterase inhibition in brain (0.1 mg/kg/day; (WHO 2009) was chosen based on subchronic, oral administration in adult rats. The symbol “■” represents single dose, “—” represents longer periods of exposure, “.....” represents dose change and “- - -” indicates a time interval between multiple exposures. Lactation/infancy extends from PN1 to PN27 (PN=postnatal day). Adolescence assumed to begin at PN28.
3.1.1. Evidence of CPF developmental neurotoxicity from in vitro and animal models
Studies in the 1990’s demonstrated that the maximum tolerated dose of CPF in neonatal rats is one sixth of that in adults, despite a faster recovery from AChE inhibition during early development (Lassiter and others 1998; Pope and Chakraborti 1992; Pope and others 1991; Song and others 1997). Factors behind the increased susceptibility to OP toxicity during development may include reduced detoxification capability during development (Hunter and others 1999; Moser and others 1998a). Also, there is differential sensitivity during development to several other biochemical and behavioral endpoints (e.g.(Moser and others 1998b; Zheng and others 2000) as described below.
Reducing the CPF exposure level in rats to the point where there is little or no AChE inhibition led to the identification of deleterious effects that, obviously, involve other underlying mechanisms. One of the first effects described was inhibition of DNA synthesis. A number of experiments indicated that the effects were indeed unrelated to anticholinesterase actions: (1) effects were evident before the formation of the majority of cholinergic synapses; (2) effects were equally present in brain regions with dense or sparse cholinergic innervation (Whitney and others 1995); and (3) effects were seen in models that prevent CPF metabolism into its oxon (the metabolite that inhibits AChE) (Song and others 1997; Song and others 1998; Whitney and others 1995). The conclusion of a non-cholinesterase mechanism for developmental neurotoxicity was confirmed is subsequent studies and in different models (Dam and others 1998; Garcia and others 2001; Jameson and others 2006). In the ensuing years, several other non-cholinesterase targets of CPF and other OP insecticides were identified.
CPF exposure in rodents throughout gestation and/or lactation periods using doses spanning the threshold for barely-detectable AChE inhibition, evokes both immediate and late-emergent morphological, neurochemical and behavioral effects (Abou-Donia and others 2006; Johnson and others 2009; Tang and others 1999; Venerosi and others 2015). However, because brain development is a dynamic process, the detailed outcomes vary as a function of the period in which exposure occurs. In this regard, a large number of studies in animal models have restricted prenatal CPF exposure to either mid-gestation, the developmental period spanning formation and closure of the neural tube, or to late-gestation, when the transition from replication to differentiation of major neuronal cell populations occurs. During postnatal life, studies have focused on phases of terminal neuronal differentiation and synaptogenesis that occur during lactation. As detailed below and in SM Table 3, this approach allowed the identification of separable windows of vulnerability to the effects of CPF; however, the main conclusion is that the brain is vulnerable to CPF over a very wide period of development, with the actual target shifting in accord with the stage at which exposure occurs.
Short-term CPF exposure restricted to mid-gestation in rodents results in altered cleavage plane orientation of apical neural progenitors (the neurogenesis onset cells located in the ventricular zone of the cerebral wall) (Chen and others 2014b), reduced and altered mitotic figures and mitotic layer, as well as an increased number of apoptotic cells in forebrain and hindbrain (Roy and others 1998). In addition, there are delayed-onset effects that emerge or are present in juvenile stages, adolescence or adulthood, notably, deficits in cholinergic activity and mAChR density, cell loss and deficits of neuritic projections (Qiao and others 2004); these are unrelated to the density of cholinergic innervation, as they are present in brain regions that are sparse in these projections, as well as in those that with dense cholinergic innervation. The cholinergic system is not the only neurotransmitter system affected. Serotonergic markers exhibit initial suppression, immediately followed by rebound elevation (Aldridge and others 2003), which is still evident at adulthood (Aldridge and others 2004). Behavioral studies show that mid-gestational CPF causes long-term deficits in adult working memory accompanied by decreased susceptibility to the amnestic effect of muscarinic cholinergic blockade indicating a decreased reliance on cholinergic circuits for memory function (Icenogle and others 2004). Both serotonin (5HT) and AChE are expressed prior to synaptogenesis and act as neurotropic factors early during brain development (Abreu-Villaça and others 2011; Nordquist and Oreland 2010; Wirth and others 2016). CPF at doses too low to inhibit AChE nevertheless interferes with its morphogenic activity (Yang and others 2008). Taken together with the effects of CPF on neuroprogenitor cells, cell survival and neurodifferentiation, indicate that CPF interferes with trophic functions that control early events in brain assembly.
Timing of exposure is critically important for the nature of the neurotoxic damage caused. When the window for CPF exposure is shifted to other developmental stages, the targets and consequences for adverse effects are likewise changed. Late gestational exposure in developing rats results in both immediate and long-lasting effects on adenylyl cyclase-mediated cell signaling cascades with sex-selective alterations (Aldridge and others 2003; Meyer and others 2004; Meyer and others 2003). These cascades are shared by several neurotransmitter systems, which suggests that the outcomes are closely associated with the widespread neurochemical consequences of CPF. Late-gestational exposure evokes immediate (Aldridge and others 2003) and long-term (Aldridge and others 2005b; Aldridge and others 2004) elevations of serotonergic biomarkers in rats, effects that are stronger than those identified after exposure at mid-gestation or during the postnatal period (Aldridge and others 2003; Aldridge and others 2004). By adolescence or adulthood, there are also deficits in presynaptic cholinergic activity that, distinct from mid-gestation exposure, are not accompanied by alterations in mAChR density (Qiao and others 2003), and thus involve different synaptic mechanisms from those of the earlier exposure paradigm. Nitric oxide synthase expression is transiently decreased in most cortical regions (Naseh and others 2013) whereas there are late-emergent increases in the hypothalamus (Naseh and Vatanparast 2014) and amygdala (Vatanparast and others 2013). Effects on neurotransmitter systems such as the cholinergic, serotonergic and nitrergic likely have pervasive consequences since they have established trophic roles in developmental events including neurogenesis, differentiation and synaptogenesis and also neuroplasticity (Abreu-Villaça and others 2011; Cossenza and others 2014; Okamoto and Lipton 2015; Wirth and others 2016). Most studies focus on effects on neurons, however, protein markers not only for developing axons but also for oligodendrocytes are enhanced shortly after exposure while, during adolescence, there are deficits (Garcia and others 2003), which indicates that glial cells are also impacted by CPF. Behavioral effects of late gestational CPF exposure include reduced motor behavior in neonates (Venerosi and others 2009), whereas in adolescents and adults, locomotor activity is increased (Levin and others 2002). Normal sex differences in locomotor activity are eliminated by late gestational CPF exposure (Levin and others 2002). Notably, the specific targeting of females extends to deficits in working and reference memory associated with cholinergic impairment. Ultrasonic calls, a behavior used as indicator of the emotional state of neonate rodents, is decreased during the lactation period (Venerosi and others 2009), while at adulthood, altered anxiety levels are still evident in females (Ricceri and others 2006; Venerosi and others 2010). In addition, at adulthood, rats fail to respond to selective serotonin re-uptake inhibitors in the forced swimming, a test used to measure depressive-like behavior (Venerosi and others 2010), which, together with the altered serotonergic transmission (Aldridge and others 2005b; Aldridge and others 2003; Aldridge and others 2004) poses this neurotransmitter system as an important target during this period of gestational development. The investigation of sex-specific behaviors demonstrates increased aggressive behavior in males (Ricceri and others 2006). However, females show increased ultrasonic vocalizations and investigation in a social task. Particularly, CPF exposure during late gestation abolishes sex differences by enhancing female offspring social investigation to the same level found in males (De Felice and others 2014). Overall, exposure during late gestation reaffirms the involvement of a variety of targets other than AChE in the neurotoxic effects induced by CPF and shows substantial differences in the effects when compared to early gestation. These include a higher sensitivity of the serotonergic system and adenylyl cyclase cascades, and the emergence of nitrergic alterations. Most effects are evident in both males and females; however, incipient sex-dependent effects on neurochemical markers and adenylyl cyclase were also identified. Particularly notable are sex-selective behavioral outcomes that suggest a greater sensitivity of females leading to the suppression of sex-dimorphic behavior through masculinization of female performance. While the mechanisms responsible for CPF sex-selective effects are not established, a recent study in children environmentally exposed to CPF during prenatal life failed to show expected sex differences in several cortical regions (Rauh and others 2012)- see Subtopic 3.1.2), which is both consistent with disruption of normal behavioral reported in animal models and supports that CPF interferes with differentiation of brain regions involved in sexual dimorphisms.
When CPF exposure is shifted even later, to postnatal stages, the most frequent approach has been to administer this insecticide during specific windows in the preweaning period. As for early-postnatal exposure, defined here as the 1st week of postnatal development in rats, several studies focused on gene transcription related to neuronal differentiation and survival. CPF affects a plethora of neurochemical targets evoking immediate transcriptional changes in genes involved in pathways for brain cell development (neural cell growth, development of glia and myelin, cell adhesion/migration, neural cell differentiation, neurothrophic factors, their receptors and signaling), cytotocicity (apoptosis, oxidative stress, excitotoxicity, mitochondrial dysfunction), cAMP-related cell signaling and development of neurotransmitter synthesis, storage and receptors for ACh, serotonin, norepinephrine and dopamine (Betancourt and others 2006; Ray and others 2010; Slotkin and Seidler 2007; Slotkin and others 2007b; 2008a). The effects are not restricted to the transcription level, as indicated by altered function of a wide variety of neural systems. The direct assessment of adenylyl cyclase signaling further allowed the identification of deficits in multiple components of the cascade a few days after exposure (Song and others 1997). At adulthood, there is a predominant elevation of adenylyl cyclase with emerging brain region and sex-selective changes (Meyer and others 2004) that are distinct from those identified with earlier exposures. As for neurotransmitter systems, mAChR density and choline acetyltransferase activity decrease after exposure (Betancourt and Carr 2004; Dam and others 1999; Guo-Ross and others 2007) and, despite the lack of immediate effects on cholinergic activity (Dam and others 1999), sex-selective effects emerge during adolescence and persist into adulthood (Slotkin and others 2001). Similar to gestational exposure, damage in neurotransmitter systems extends beyond cholinergic pathways. There are immediate and late-emergent effects on the serotonergic system, even though the effects are considerably smaller than those seen after gestational exposure (Aldridge and others 2005b; Aldridge and others 2003; Aldridge and others 2004; Raines and others 2001). Increases in norepinephrine and dopamine synaptic activity turnover are unrelated to the magnitude or temporal pattern of cholinesterase inhibition and most evident in brain regions with poor cholinergic innervation (Dam and others 1999). Neuronal nitric oxide synthase expression decreases in most cerebral cortical regions, in the hippocampus (Naseh and others 2013) and in the amygdala (Vatanparast and others 2013) while it increases in hypothalamus (Naseh and Vatanparast 2014) and the pattern and subregions affected is distinct than those after gestational exposure.
In association with these neurochemical alterations, early postnatal CPF evokes behavioral effects as well. There are immediate CPF-induced deficits in the development of coordination skills in females, while, later during development, decreases in locomotor activity and rearing are selective for males (Dam and others 2000). Long-term effects on cognitive behavior are identified in the radial-arm maze, where CPF-treated males exhibit an increase in errors while females reduce their error rates. These effects result in a reversal of the typical more accurate performance in visuo-spatial tasks exhibited by males (Levin and others 2001) and indicate that the period of development in which CPF exposure results in the disruption of normal sexual dimorphisms is not restricted to gestation, extending into the postnatal period. Interestingly, 5HT2 antagonism causes memory deficits only in CPF-treated animals, indicating an abnormal dependence on serotonergic systems (Aldridge and others 2005a). As for effects on serotonergic-related behaviors, anxiolysis and increased anhedonia are evident in adult males (Aldridge and others 2005a). These results clearly indicate that the high susceptibility to CPF extends into the postnatal period, however, while several targets of gestational exposure are also affected by exposure during postnatal life, the pattern of effects differ. Likewise, sex-selective effects are a hallmark, but the specific targeting of females identified during late gestation is no longer evident when exposure shifts to early postnatal development. Even though CPF-mediated sex-dependent effects suggest the role of hormonal factors and interference with differentiation of brain regions, a more thorough investigation of the underlying mechanisms should be performed. However, it also may be the case that developmental CPF exposure may affect brain regions that play different roles in behavioral functions of males and females apart from effects mediated via actions on sex hormones.
At late-postnatal stages of development (defined here to begin at the 2nd week of postnatal life), as demonstrated in younger animals, CPF decreases the activity and reactivity of the adenylyl cyclase signaling cascade (Song and others 1997). The deficits last into adulthood with strong sex-selectivity (Meyer and others 2004). There are immediate increases in catecholaminergic synaptic activity (Dam and others 1999), as well as widespread deficiencies in cholinergic synaptic function that persist into adulthood (Dam and others 1999; Slotkin and others 2001). Late postnatal CPF also inhibits the endocannabinoid metabolizing enzymes FAAH and MAGL and increases endocannabinoid levels in the brain (Carr and others 2013; Carr and others 2011; Carr and others 2014). Since the endocannabinoid signaling is involved in central nervous system functions such as modulation of mood, learning and memory, neurogenesis, cell differentiation, cell migration, neuronal specification and synaptogenesis (Anavi-Goffer and Mulder 2009; Younts and Castillo 2014), more studies investigating CPF-mediated interference in this system during this and other periods of development are warranted. Some deleterious effects are smaller in magnitude when compared to earlier periods of exposure. In this regard, serotonergic markers decrease but the effects are weak and tend to fade during the course of postnatal development (Aldridge and others 2005b; Aldridge and others 2003; Aldridge and others 2004; Raines and others 2001). In contrast, oxidative stress is more evident (Slotkin and others 2005) and, the analysis of the hippocampus indicates that this brain region is especially vulnerable to CPF-induced developmental neuro- and gliotoxicity (Garcia and others 2003; Roy and others 2005).
Behavioral alterations of late-postnatal CPF exposure include increased locomotor activity and reactivity to a novel environment in juveniles (Ricceri and others 2003), increased rearing and grooming (Dam and others 2000) but the effects are more modest than those observed after exposure at earlier ages. Decreased habituation (Levin and others 2001) and spatial memory/learning deficits (Jett and others 2001) have also been documented after late postnatal CPF exposure. Importantly, there are sex-selective effects of decreased responsiveness to the muscarinic antagonist scopolamine on radial-arm maze reference memory performance in females (Levin and others 2001). Increased aggressive behavior was seen in CPF-exposed males, increased maternal responsiveness toward pups in CPF-exposed virgin females and reduced anxiety levels and motivation to build the nest and defend it from a male intruder in lactating females (Carr and others 2015; Ricceri and others 2003; Ricceri and others 2006; Venerosi and others 2008). Even though there are significant effects of exposure at late-postnatal stages, the comparison between distinct periods of brain development indicates that several of them tend to reduce in magnitude and fade during the course of postnatal development, which is consistent with evidence of higher susceptibility to OPs in younger animals.
As detailed in SM Table 3, CPF developmental effects have also been investigated in complementary models such as cell cultures, chicks and zebrafish and, overall, these models both confirm several neurochemical and behavioral effects identified in the traditional rodent models and add information. The chick embryo model recapitulates the actions of early gestational CPF exposure in rats in showing CPF-induced deficits in cholinergic activity and decreased density of cortical serotonergic receptors. Since this model eliminates variables related to maternal physiology, the results indicate that these altered markers reflect direct CPF actions in the immature brain (Slotkin and others 2008b). Brain slices and embryonic cell cultures also have the advantage of eliminating the potential impact of the maternal-fetal unit and, neuronotypic and gliotypic cell lines further allow investigation during distinct stages of development, ranging from very immature phases to full differentiation (e.g. (Adigun and others 2010; Garcia and others 2001; Qiao and others 2001). In this regard, the use of these complementary models revealed neurochemical targets (e.g. (Slotkin and Seidler 2011; 2012) as well as mechanisms that underlie the effects of CPF exposure. As examples. CPF interference with AChE morphogenic activity plays a role in CPF inhibition of axonal growth (Yang and others 2008) and activation of MAP kinases induces apoptosis, as evidenced from cultures of neurons derived from embryonic and newborn rats (Caughlan and others 2004).
As for zebrafish, the number of studies in the field of developmental neurotoxicology has increased in the last years (Bailey and others 2013; Levin and Cerutti 2009). Exposure of zebrafish larvae to CPF decreases body length, inhibits hatchability and heart rate and results in a number of non-lethal developmental malformations. Early-life CPF exposure also alters the transcriptional levels of genes involved in early neurodevelopmental processes, indicative of neurotoxicity, induces oxidative stress and disturbs the innate immune system in larvae (Jin and others 2015). Other effects in larvae include decreased dopamine and serotonin levels and increased transmitter turnover (Eddins and others 2010), decreased swimming activity (Jin and others 2015; Levin and others 2004; Yen and others 2011) and decreased habituation of swimming over time (Levin and others 2004). Reduced anxiety-related behavior (thigmotaxis) by CPF is unrelated to changes in AChE activity (Richendrfer and Creton 2015; Richendrfer and others 2012). The assessment of adult fish indicates reduced dopamine levels, spatial learning impairments, increased swimming activity and increased sensorimotor response (startle response) (Eddins and others 2010; Levin and others 2003; Sledge and others 2011).
3.1.2. Epidemiological studies on OPs neurodevelopmental adverse effects
Most epidemiological studies described below and in SM Table 4 assessed OP exposure based on biological samples. The most frequent biomarkers of exposure are dialkyl phosphates (DAPs) that can be measured in urine, and represent the sum of diethylphosphates (DEP) and dimethylphosphates (DMP). DEPs include metabolites from ten OP insecticides and DMPs include metabolites of seventeen methyl OP insecticides (Burns and others 2013). Whole blood cholinesterase, erythrocyte AChE and plasma butyrylcholinesterase are also nonspecific markers of OP (and CA) exposure (Eskenazi and others 2004; Suarez-Lopez and others 2013). Even though DAPs and cholinesterases can not be traced back to the specific parent compound(s), these metabolites provide useful information about cumulative exposure to OPs as a class (Barr and others 2004). In this regard, AChE activity long recovery time provides a more stable assessment of past exposures when compared to other biomarkers. Other metabolites frequently measured in urine include 3,5,6-trichloro-2-pyridinol (TCPy), a specific biomarker of CPF exposure, malathion dicarboxylic acid (MDA), used to estimate malathion exposure, and 4-nitrophenol (PNP), a metabolite of parathion and methylparathion (Eskenazi and others 2004). Parent compounds such as CPF and diazinon are measured directly in blood (Barr and Angerer 2006; Whyatt and others 2004).
OP exposure has been most frequently assessed during gestation and the majority of studies provide evidence that early exposure to OP is a risk factor for poor neurodevelopment (SM Table 4). Findings from the Columbia Center for Children’s Environmental Health (CCCEH) cohort demonstrated an inverse relation between CPF cord blood levels and birth weight and length in minority communities in New York City (Whyatt and others 2005; Whyatt and others 2004), but see (Barr and others 2010). In this same cohort, children with the higher prenatal CPF levels were more likely to present psychomotor and mental development delays than children with lower levels of exposure, and their mothers reported more attention problems, AHDH, and pervasive developmental disorder symptoms at 3 years of age (Lovasi and others 2011; Rauh and others 2006). At 7 years of age, a significant relation between prenatal CPF levels and lower Full-Scale IQ and Working Memory was evident (Rauh and others 2011) and, recently, abnormalities in morphological measures of several cortical surface areas were associated with higher prenatal CPF exposure in children at 6–11 years of age (Rauh and others 2012). When TCPy, a CPF metabolite, was assessed in urine in pregnant women from Mexico city that were enrolled in the Early Life Exposures in Mexico to Environmental Toxicants (ELEMENT) study, there were trends toward increased ADHD in boys and increased attentional problems in girls at 6–11 years of age (Fortenberry and others 2014). However, the CHAMACOS study (Eskenazi and others 2004; Eskenazi and others 2007) did not find these sorts of associations. It is important to keep in mind that TCPy is not a specific marker of OP exposure; it is also found in fruits and vegetables. Therefore, its direct intake may have interfered with the results, which might explain the lack of significant effects in the latter studies (Eaton and others 2008).
As for malathion, even though most epidemiological studies have not found significant neurobehavioral effects of gestational exposure on early postnatal development (Engel and others 2007; Eskenazi and others 2004; Eskenazi and others 2007; Wolff and others 2007), Engel and collaborators did identify an association between increasing MDA levels and increased abnormal neonatal reflexes of children in the Mount Sinai Children’s Environmental Health Cohort (Engel, Berkowitz et al. 2007).
Both cholinesterase activity and DAP levels are sensitive non-specific markers of exposure, and associations between prenatal exposure and deleterious outcomes were described. In both the Mount Sinai Cohort in New York City, USA (Engel and others 2007) and the CHAMACOS study in Salinas Valley, an agricultural community in California, USA (Young and others 2005), increasing prenatal DAP levels augmented the risk of abnormal reflexes in neonates. In the CHAMACOS, lower AChE activity in blood umbilical cord was furhter associated with shorter length of gestation and increased likelihood of preterm delivery (Eskenazi and others 2004) and, a recent study in China described a negative association between DAP gestational levels and neonatal passive and active tones and reflex (Zhang and others 2014). In the Mt. Sinai study, DAP levels were associated with decreased cognitive abilities in Black and Hispanic but not in White children at 1 and 2 years of age (Engel and others 2011). In follow ups from the CHAMACOS, prenatal exposure increased the likelihood of decreased cognitive performance and increased pervasive developmental disorder symptoms at 2 years (Eskenazi and others 2007) and the risk of ADHD at 5 years (Marks and others 2010). Decreased cognitive scores were consistently associated with DAP levels in CHAMACOS children at 7 years of age (Bouchard and others 2011), while poorer motor and visuospatial functions and impaired visuospatial memory were identified in Ecuadorian 6–8 years old children whose mothers were occupationally exposed to OP insecticides (Harari and others 2010). The Childhood Autism Risks from Genes and Environment (CHARGE) study, by linking commercial pesticide application data from the California Pesticide Use Report to maternal addresses during pregnancy, found a positive association between residential proximity to OPs exposure during the third trimester of gestation and autism spectrum disorders (Shelton and others 2014).
As for the effects of postnatal exposure (SM Table 4), there are fewer studies, and most of them used non-specific markers in child urine. In China, increasing DAP levels failed to increase the risk of poor motor and mental development at 2 years of age (Guodong and others 2012). Distinctively, in the CHAMACOS study, child DAPs levels at this same age were associated with increased risk of pervasive developmental disorder (consistent with Autistism Spectrum Disorders), but also with impaired congitive performance (Eskenazi and others 2007). Marks and collaborators (Marks and others 2010) described an association between child exposure and ADHD in CHAMACOS 5 year-old children and, later during development, this same association was identified in a representative sampling of the U.S. population, as part of the National Health and Nutrition Examination Survey (NHANES) (Bouchard and others 2010). While both in the CHAMACOS (Bouchard and others 2011) and in a study in Ecuador (Harari and others 2010) there were no consistent associations between DAP levels and cognitive performance at 6–8 years of age, higher child DAP levels increased the risk of poorer motor and cognitive performance in 7 year-old children (Sánchez Lizardi and others 2008) and lower AChE activity was associated with lower attention/executive functioning, and memory/learning in 4–9 year old boys (Suarez-Lopez and others 2013) living in agricultural/floricultural communities in the USA and Ecuador, respectively. In addition, occupational agricultural exposure in Egypt resulted in decreased AChE activity, impaired neurobehavioral performance, and more reports of neurological symptoms in male children between the ages of 9 and 18 years (Abdel Rasoul and others 2008).
Regarding other biomarkers, in Mississipi and Ohio in the USA, the investigation of the effects of illegal spraying of methyl parathion in residences demonstrated that the presence of PNP in the urine of household members increased the risk of short-term memory and attention deficits and increased the likelihood of parents reports of behavioral and motor skill problems in 2–12 years old children (Ruckart and others 2004). However, there was no association detected between cognitive performance and TCPy levels in 4–10 year-old children whose parents worked in agriculture in Costa Rica (Lu and others 2009).
Paraoxonase 1 (PON1) is a key enzyme in the metabolism of OPs catalyzing oxon detoxification. Polymorphisms in different PON1 gene regions determine the expression or activity of the enzyme and the rate of OPs biotransformation. The PON108 CC genotype is associated with higher PON1 levels, and the TT variant leads to lower levels of the enzyme. Having both reduced PON1 levels/activity and vulnerable genotypes may lead to higher toxicity, are candidate biomarkers of susceptibility to the effects of OPs pesticides, both in animals and in humans (Costa and others 2003; Muñoz-Quezada and others 2013). In addition, in fetuses and children, PON1 levels and activity are much lower than those in adults (Chen and others 2003; Furlong and others 2006; Huen and others 2010), which may contribute to the greater vulnerability to OP pesticide toxicity during development. Three cohorts have investigated the interference of PON1 in outcomes of OP exposure during development: the Mt. Sinai and the CHAMACOS in the USA and, recently, the Study of Asian Women and Offspring’s Development and Environmental Exposures (SAWASDEE), in Thailand. Their results indicate that PON1 is a useful tool in the investigation of associations between OP exposure and neurodevelopment.
In mothers from the urban Mt. Sinai cohort and from the agricultural SAWASDEE study that presented low PON1 activity DAP levels were associated with more abnormal neonatal reflexes (Engel and others 2007) and, at 6–9 years, with decreases in perceptual reasoning (Engel and others 2011), particularly among children of mothers with low PON1 activity or the genotype associated with slow OP metabolism. In the CHAMACOS, even though children at 2 years were more likely to display pervasive developmental disorder and poorer motor and mental development when they or their mothers had lower PON1 levels or activity, interactions between DAPs and PON1 failed to reach significance (Eskenazi and others 2010). Later during development, there were associations between both maternal and child PON1 levels and cognitive deficits in the children, however, maternal and child PON1 genotype weakly modified the relationship of maternal DAPs and cognition. For 7 year-old children of mothers with lowest PON1 levels, the inverse association between DAP levels and cognition reached significance for verbal comprehension (Eskenazi and others 2014).
3.2. What did we learn with OPs and what is still to be learned?
The big advantage of OPs when compared to OCs is their reduced persistence in the environment, is largely contravened by the obvious toxic nature of their classic mechanism of action – AChE inhibition – not only to target but also to off-target organisms, not to mention other more recently identified mechanisms. Indeed, there is strong evidence that deleterious outcomes of developmental OP exposure result from multiple mechanisms of action - all OPs inhibit AChE but have varying effects at lower doses below the threshold for AChE inhibition. The multiple non-cholinergic mechanisms of OP neurotoxicity brings up two major points. The first is that there is a clear disconnection between endpoints used for regulatory decisions when compared the mechanisms that disrupt mammalian development; this class of insecticides is an example of the poor demands of regulatory agencies before approval of potentially toxic substances for commercial purposes. The second is that compounds grouped in the same mode of action classification may have distinct biological targets relevant to human health, which further indicate the need to evaluate potentially toxic effects of less studied OP components in developing organisms. The fact that the low-dose neurobehavioral toxicity of OPs is often subtle in nature does not decrease its societal importance given that substantially more people are exposed to the low OP levels than the higher levels.
Currently, OPs compose the class of insecticides for which there is the most complete body of evidence concerning developmental neurotoxicity. The period of high sensitivity for OP neurotoxicity ranges from early gestation and into the postnatal period and, among OPs, CPF appears to be particularly problematic at the low dose exposure level that is widespread in the population. This class is an example of decades of complimentary efforts of researchers in epidemiology and basic sciences in revealing outcomes of exposure and reinforcing the need of stronger regulatory actions for the use of pesticides in susceptible subpopulations. Indeed, despite the dichotomy in points of view between regulatory agencies and academic researchers described above, this effort resulted in the ban of CPF for indoor use in several countries as well as restricted use of other OPs both in residences and agriculture. Overall, there is still need of cohort studies focusing on the investigation of short- and long-term effects of postnatal exposure, as well as the use of animal models to assess effects of other less studied OP components. However, with OPs, it became clear that fetuses and children are more vulnerable and should be particularly protected and that, in addition to the reduced persistency in the environment, safer insecticides must have greater selective toxicity for target insects.
4. Carbamates (CAs)
Derived from carbamic acid, the majority of CA insecticides are N-methyl carbamates (e.g. aldicarb, carbaryl, carbofuran, bendiocarb, methiocarb, methomyl, oxamyl, propoxur, and thiodicarb). CAs were introduced in the insecticide market in the 1960s in response to a search for insecticides with lower mammalian toxicity than OPs. Indeed, this class of pesticides is still used in agriculture as an alternative to OPs (Vale and Lotti 2015).
4.1. Developmental neurotoxicity of CAs
Similar to OPs, CAs primary mode of action in insects and off-target organisms is the inhibition of AChE, which results in accumulation of ACh at synapses in the nervous system and acute toxicity. However, while OPs bind irreversibly to AChE, inhibition elicited by CAs is rapidly reversible and, as a result, CAs intoxication syndrome tends to be less severe than that elicited by OPs poisoning. Despite that, CAs have been shown to evoke neurotoxic effects and, as for other classes of insecticides, exposure during development is more deleterious than at adulthood.
The comparison between rodents exposed to several CA insecticides at different postnatal ages shows that the younger the animal, the smaller the dose necessary to cause AChE inhibition (Moser 1999; Moser and others 2010; Moser and others 2015; Moser and others 2013) and to elicit cholinergic toxicity (Moser 1999). The increased sensitivity could be due to a reduced clearance and consequent increased brain CA levels in younger animals, as demonstrated to occur with carbaryl and 1-naphthol (Moser and others 2013) but see (Herr and others 2010). However, whether similar pharmacokinetic differences also occur with other components of the same class, and with prolonged periods of exposure is still poorly understood.
Compared to OPs, few studies describe behavioral or neurochemical outcomes of CA exposure in vitro and in animal models (SM Table 5 and Figure 3). However, effects of developmental exposure are diverse and alterations are identified even at adulthood. As described below, deleterious outcomes are largely unrelated to the level of acute toxicity; compounds highly toxic (e.g. carbofuran) or that present moderate (e.g. carbosulfan) or low (e.g. carbaryl) acute toxicity all cause neurobehavioral effects with developmental exposure. There is also evidence that developmental exposure to distinct components of the CA class elicits disparate effects, which, together with the transient inhibition of AChE, indicates that the mechanisms associated are not restricted to those involved in the acute toxicity.
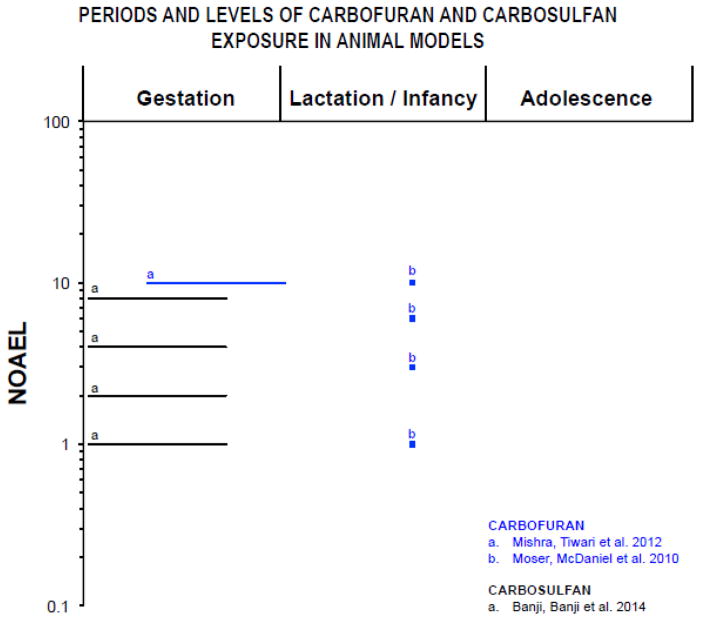
Figure 3.
Periods of exposure and percentages of the NOAEL (no-observed-adverse-effect level) proportions in log scale based on animal model studies listed in table 6. Only doses and periods of exposure that elicited significant effects are reported. For Carbofuran (0.1 mg/kg/day; (FAO 2002), and of Carbosulfan (0.5 mg/kg; (Wolterink and van Hoeven Arentzen 2003), the NOAEL for for cholinesterase inhibition in brain was chosen based on acute, oral administration in adult rats. The symbol “■” represents single dose and “—” represents longer periods of exposure. Lactation/infancy extends from PN1 to PN27 (PN=postnatal day). Adolescence assumed to begin at PN28.
4.1.1. Evidence of CAs developmental neurotoxicity from in vitro and animal models
In vitro studies show that carbaryl, at doses that do not cause cell death, inhibits outgrowth of axon-like processes in N2a neuroblastoma cells (Flaskos and others 1999). As for carbofuran, it evokes cytotoxicity and increases apoptotic cell death and nAChR downregulation in cultures of rats embryonic hippocampal and cortical cells (Kim and others 2004). Consistent with these findings, the analysis of the hippocampus of juvenile and adolescent rats exposed during gestation demonstrates neurodegeneration, decreased neurogenesis and long-term survival of progenitor cells, altered fate specification of new generated cells and deficits in hippocampal-dependent learning and memory processes (Mishra and others 2012). The only study available on gestational exposure to carbosulfan (Banji and others 2014) describes increased oxidative stress and damage to cerebellar Purkinje cells in rats. Early exposure to this CA also delays motor and sensorimotor development in lactating rats and increases anxiety in adolescents (Banji and others 2014).
The postnatal brain is less affected by CAs. Moser and collaborators demonstrate decreased motor activity in response to acute postnatal exposure of rats to several CAs (Moser 1999; Moser and others 2010; Moser and others 2015). A detailed investigation of neurobehavioral effects of aldicarb demonstrates decreased rearing and arousal after acute postnatal exposure but the effects are no longer present 24 hours after exposure and are only identified at doses close to the one that elicited cholinergic toxicity (Moser 1999).
In addition to the effects described above, there is evidence of CAs endocrine disrupting activity. In this regard, carbaryl was shown to inhibit progesterone biosynthesis in primary human granulosa-lutein cell (Cheng and others 2006), while, carbaryl and its metabolite 1-naphthol present thyroid receptor antagonist activity, as evidenced in HepG2 (human liver cancer cell line) transfected cells expressing β1- Thyroid hormone receptors (Sun and others 2008) Despite the established roles of thyroid and steroid hormones in brain development, whether endocrine-disrupting mechanisms of action contribute to the outcomes of CAs developmental exposure still needs to be investigated.
Carbaryl was also shown to interact with aryl hydrocarbon receptors (AhR). Even though this CA does not t easily into the structural characteristics that define typical AhR ligands, it is able to take a planar or close-to-planar conformation that is accessible for interaction with AhR (Casado and others 2006), as demonstrated in cultured mammalian cells and in yeast-based bioassays (Boronat and others 2007). The investigation of transcriptional pathways indicates that the peculiar structure of carbaryl modifies key surfaces of interaction with coactivators. Particularly, it was suggested that the AhR-carbaryl complex interacts with the cofactor AhR nuclear translocator (ARNT), but does not interact with coactivators (e.g. cAMP response element-binding protein) that are required for transcriptional activation (Boronat and others 2007). In cell types where ARNT provides for the missing interactions, the system behaves as an agonist, which results in transcriptional activation. In contrast, in cell lines where these interactions are missing, carbaryl acts as a competitive antagonist, precluding transcription activation (Boronat and others 2007). Considering that activation of AhR by xenobiotics constitutes an initial step leading to toxic effects such as endocrine disruption, reproductive toxicity and developmental defects, this may constitute a relevant mechanism of action of CAs developmental toxicity.
4.1.2. Epidemiological studies on CAs neurodevelopmental adverse effects
Epidemiological studies that investigated exposure to CA insecticides during development detected CAs or metabolites in 30% to 93% of samples from mothers/newborns of several countries (Barr and others 2010; Forde and others 2015; Ostrea and others 2009; Whyatt and others 2003) and in up to 11% of samples from children and adolescents (Needham and others 2005). While these and other studies found a significant exposure to CAs during development, we found few epidemiological studies describing developmental effects of this class of insecticide, and all of them focused on prenatal exposures (SM Table 6). In the Philippines, Ostrea and collaborators (Ostrea Jr and others 2012) identified a significant relationship between prenatal propoxur exposure and slower motor development for children at 2 years of age. Shelton and collaborators (Shelton and others 2014) used commercial pesticide application data from the California Pesticide Use Report to investigate whether residential proximity to agricultural pesticides during pregnancy was associated with autism spectrum disorders or developmental delay (delayed cognitive or adaptative development). They did not find a significant association between CA exposure and autism spectrum disorders, however, they did find an association between residential proximity to carbaryl and methomyl during gestation and neurodevelopmental delay in 2–5 years old children.
4.2. What did we learn with CAs and what is still to be learned?
The most toxic CA compounds to mammals, carbofuran and aldicarb, were banned in several countries (ANVISA 2012, 2015; EC 2003, 2007; EPA 2007), however, most other CAs are still extensively used. Accordingly, epidemiological studies demonstrate that CAs are frequently present in distinct populations not only during gestation but also during postnatal development.
There is evidence of increased sensitivity of developing organisms and, similar to OPs, the classic mechanism of action of CAs, responsible for insect toxicity and acute toxicity in off-target species, do not explain the effects of developmental exposure. In this regard, even though most experimental studies that describe adverse outcomes of developmental CA exposure in brain or behavior used doses not devoid of AChE inhibition, which makes it difficult to clearly define non-cholinesterase mechanisms of action, a number of experiments indicate that deleterious outcomes result from multiple mechanisms of action: (1) large variety of effects, (2) deleterious outcomes unrelated to the level of acute toxicity, (3) disparate effects evoked by distinct components of the class and, (4) identification of effects even long after the end of exposure and reversal of AChE inhibition.
Still, compared to other classes of insecticides, there is evidence for fewer deleterious effects during development. While this indicates that this class of insecticides is safer to off-target species than other classes, gaps in the literature such as the reduced number of studies of subchronic exposures in animal models and the lack of human cohort studies that investigate the associations between postnatal exposures and neurodevelopment, do not allow the scientific community to discard the possibility of other harmful consequences of CA exposure during development.
5. Pyrethroids (PIs)
PIs are synthetic analogs that are based structurally on the six naturally occurring esters (pyrethrins) present in the flower heads of the Chrysanthemum plant. Similar to these natural esters, synthetic PIs have high insecticidal potency and relatively low mammalian toxicity, with the advantage of being less susceptible to hydrolysis and photodegradation than the prototypic compounds (Clark and Symington 2012). In addition to their value in controlling agricultural pests, PIs are active ingredients of household insecticides and are also frequently used to control insect attacks on pets.
Based on structural differences and on toxic signs of acute exposure in rodents, PIs have been divided into types I and II (Soderlund 2012). Type I PIs (e.g. allethrin and permethrin) comprise a wide structural variety of compounds lacking a cyano moiety at the α-position and elicit an intoxication syndrome that includes general tremor and convulsive twitching. Distinctively, type II PIs (e.g. deltamethrin and cypermethrin) display the cyano moiety and elicit an intoxication syndrome that includes salivation and choreoathetosis (Soderlund 2012).
Both types of PIs slow the activation and inactivation of voltage-gated sodium channels leading to neuronal hyperstimulation in insects and off-target organisms. However, in the later, in addition to the sodium channels, each type of PI exhibit secondary targets also implicated in their acute neurotoxic actions (Soderlund 2012) (see SM for a detailed description).
5.1. Developmental neurotoxicity of PIs
PIs are rapidly metabolized, and therefore are unlikely to bioaccumulate. This, associated to their low mammalian toxicity and limited persistence in the environment make this class of insecticide safer than other classes such as OCs and OPs. Despite that, there is evidence of toxic effects even at low doses of exposure and of increased sensitivity of developing organisms. Age-related differences are largely attributed to toxicokinetic differences between juvenile and adult rodents, as, during the neonatal period, immature rodents have limited metabolic capacity (Anand and others 2006b; Cantalamessa 1993; Sheets and others 1994). As an example, deltamethrin, a type II PI, is detoxified by liver cytochrome P450s and carboxylesterases, and the capacity of these enzymes to metabolize this PI increases during postnatal development of rats (Anand and others 2006a). In accordance with these data, there is an inverse correlation between the severity of deltamethrin-induced neurotoxicity and age (Kim and others 2010).
Increased blood-brain barrier permeability was also reported as a result of early PI exposure (Gupta and others 1999a; Gupta and others 1999b; Maurya and others 2014; Sinha and others 2004; Sinha and others 2006). In addition, with the development of new methodological approaches, toxicodynamic factors began to be investigated. Voltage-gated sodium channel isoforms are developmentally regulated and differences in sensitivity to PIs between isoforms expressed in the developing and mature central nervous systems are suggested to play a role in the age-dependent toxicity of these compounds. To test this hypothesis, Meacham and collaborators (Meacham and others 2008), investigated the effects of several type II PIs on sodium currents in Xenopus laevis oocytes injected with different combinations of rat α (Nav1.2 or Nav1.3) and β (β1 or β3) subunits. There are significantly greater effects on Nav1.3/β3 channels, which are preferentially expressed in embryonic phases in rats, when compared to the Nav1.2/β1 channels, which are highly expressed in adults, suggesting that the predominance of more susceptible voltage-gated sodium channels during development may in part explain age-dependent outcomes (Meacham and others 2008).
In Subtopic 5.1.1 and in SM Tables 7 and 8 we summarize data that associate developmental exposure to types I and II PIs to neurochemical and behavioral outcomes in vitro and in animal models. Even though most studies do not investigate the mechanisms that underlie the effects, the variety of neurochemical and behavioral alterations suggests that the mechanisms include but are not restricted to those involved in the acute toxicity (see SM material for a detailed description). Figures 4 and 5 further make it clear that even very low levels of exposure during gestation and/or lactation of rodents cause deleterious effects. Subsequently, in Subtopic 5.1.2 and SM Table 9, evidence from epidemiological studies that implicate early PIs exposure in adverse neurodevelopmental outcomes are described. Since the classification of PIs into types I and II is widely used in the literature, it is also be adopted in this review. However, it should be noted that it is based on doses that cause overt neurotoxicity and, therefore, may not be the best fit for low-dose and developmental exposures.
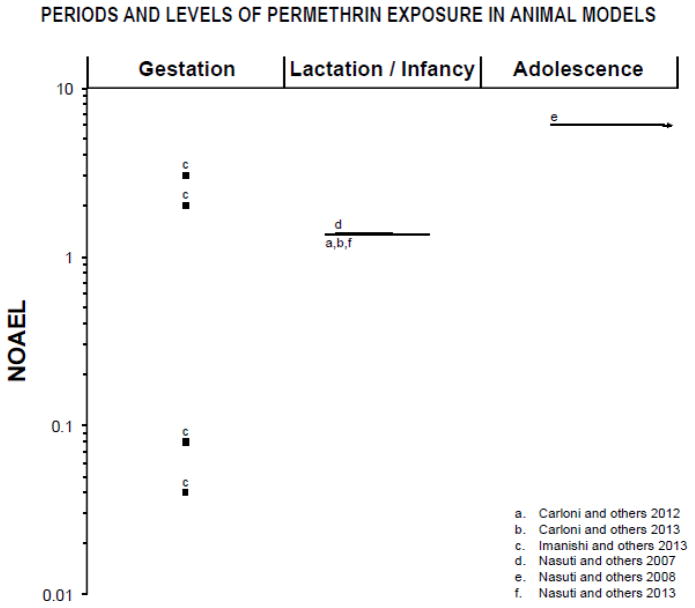
Figure 4.
Periods of exposure and percentages the NOAEL (no-observed-adverse-effect level) proportions in log scale based on animal model studies listed in table 8. Only doses and periods of exposure that elicited significant effects are reported. The NOAEL for neurotoxic effects of Permethrin (25 mg/kg/day; (ATSDR 2003) was chosen based on acute, oral administration in adult rats. The symbol “■” represents single dose and “—” represents longer periods of exposure. Lactation/infancy extends from PN1 to PN27 (PN=postnatal day). Adolescence assumed to begin at PN28.
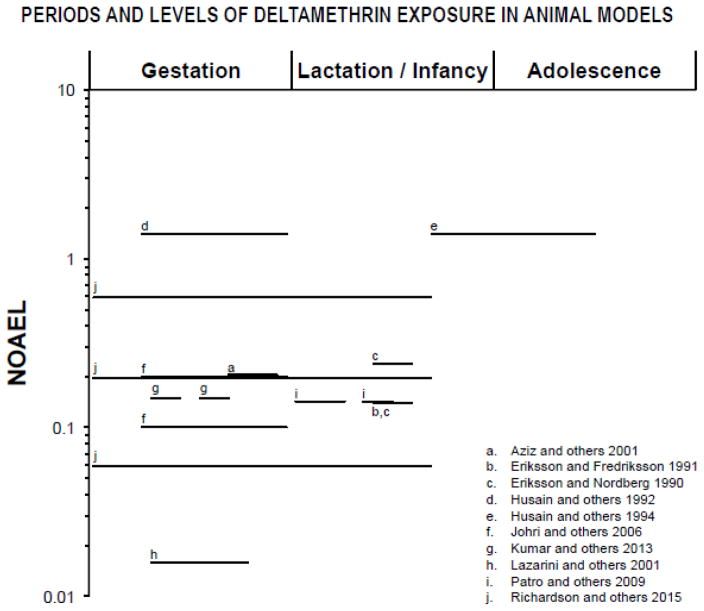
Figure 5.
Periods of exposure and percentages of the NOAEL (no-observed-adverse-effect level) proportions in log scale based on animal model studies listed in table 9. Only doses and periods of exposure that elicited significant effects are reported. The NOAEL for neurotoxic effects of Deltamethrin (5 mg/kg/day; (INCHEM 2000) was chosen based on acute, oral administration in adult rats. The symbol “■” represents single dose and “—” represents longer periods of exposure. Lactation/infancy extends from PN1 to PN27 (PN=postnatal day). Adolescence assumed to begin at PN28.
5.1.1. Evidence of PIs developmental neurotoxicity from in vitro and animal models
Developmental exposure to both types of pyrethroids has been associated to a wide range of behavioral, neurochemical and molecular effects. Permethrin, allethrin and bioalletrin are the most frequently studied type I PIs in vitro and in animal models (SM Table 7 and Figure 4). Gestational exposure to type I PIs alters brain vascular formation (Imanishi and others 2013), increases blood-brain barrier permeability (Sinha and others 2004), increases oxidative stress both shortly and long after the end of exposure (Sinha and others 2006), causes functional deficits in the cholinergic muscarinic system (Eriksson and Nordberg 1990), decreases monoamine levels and neocortical and hippocampal thicknesses during postnatal development (Imanishi and others 2013). These effects are accompanied by delayed physical and motor development, decreased locomotor activity, impaired motor coordination (Imanishi and others 2013; Syed and others 2015) and deficient learning and memory (Sinha and others 2006) even at adulthood.
Similarly, postnatal exposure increases blood-brain barrier permeability (Sinha and others 2004), causes oxidative stress (Gupta and others 1999b; Nasuti and others 2007; Sinha and others 2006) and impairs the cholinergic muscarinic, dopaminergic and noradrenergic systems (Ahlbom and others 1994; Carloni and others 2012; Carloni and others 2013; Eriksson and Nordberg 1990; Nasuti and others 2013; Nasuti and others 2007). However, several other effects were also identified. These include unbalance between pro- and anti-inflammatory responses and impairment of the serotonergic system, decreased nitric oxide, glutamate and calcium in several brain regions even several months after the end of exposure (Carloni and others 2012; Carloni and others 2013; Nasuti and others 2013). As for behavioral effects, both locomotor activity and rearing are increased in response to early postnatal exposure (Ahlbom and others 1994; Nasuti and others 2007; Talts and others 1998), whereas spatial working memory (Carloni and others 2012; Carloni and others 2013; Nasuti and others 2013), and conditioned avoidance responses are decreased (Sinha and others 2006). Behavioral effects are identified even long after the end of exposure, in adult and aged rats, which suggests that early life exposure to type I PIs interferes with brain-aging processes.
Learning and memory impairments evoked by type I PIs are likely associated with deficits in the glutamatergic system. In this regard, in addition to the decreased glutamate and calcium levels identified after postnatal exposures (Carloni and others 2012; Carloni and others 2013), in cultures of neonatal hippocampal neurons, the activity of glutamatergic neuronal networks and the spontaneous release of glutamate in synapses are decreased (Meyer and others 2008) and, recently, a NMDA receptor-mediated mechanism was suggested to play a role in PIs neurotoxic actions during development. Cao and collaborators (Cao and others 2014) demonstrated that, in primary cultures of neonatal cortical neurons, bifenthrin, at concentrations well below those that delay sodium channel inactivation and affect sodium current, alters spontaneous synchronized calcium oscillations that are essential for normal neuronal development (Dolmetsch and others 1998; Wayman and others 2006) and increases phosphorylated CREB and activity-dependent dendritic growth. These effects are partially suppressed by NMDA receptors antagonists (Cao and others 2014).
As for type II PIs (SM Table 8 and Figure 5), most studies focus on deltamethrin, which is widely used in agricultural and home formulations. However, neurotoxic effects of cyhalothrin, fenvalerate, cypermethrin and cyfluthrin are also identified. In vitro studies have provided valuable data on the effects and mechanisms of action of type II PIs. In cultures of hippocampal neurons, the activity of glutamatergic neuronal networks and the spontaneous release of glutamate in synapses decrease in response to deltamethrin (Meyer and others 2008). In cultures of neocortical neurons, c-fos and brain-derived neurotrophic factor are increased (Ihara and others 2012; Imamura and others 2006), as well as cell survival and neurite growth (Ihara and others 2012). Damage to astrocytes is reported; in vitro exposure to cypermethrin decreases cell viability, decreases the number of astrocytes processes and increases levels of proteins involved in astrocyte and neuron migration signaling (Maurya and others 2014).
Experimental studies in animal models demonstrate that gestational exposure to high doses of type II pyrethroids elicits severe effects such as increased resorptions and neonatal death, decreased brain regions weight and delayed physical and motor development in rodents (Husain and others 1992; Syed and others 2015). However, significant effects are also identified at low doses. In this regard, long-lasting increases in the expression of xenobiotic metabolizing P450 enzymes in brain (Johri and others 2006; Singh and others 2013), impairment of the dopaminergic (Lazarini and others 2001) and muscarinic cholinergic system (Aziz and others 2001) in striatum and hippocampus, respectively, and morphological alterations in cerebellum (Kumar and others 2013) are described. These effects are associated with a number of behavior alterations including decreased locomotor activity (Johri and others 2006; Singh and others 2013), impaired motor coordination (Kumar and others 2013), reduced exploratory behavior (Gomes and others 1991; Singh and others 2013), impaired working memory (Aziz and others 2001) and increased depressive-like behavior (Lazarini and others 2001).
Interestingly, the marked morphological alterations in the cerebellum, oxidative stress, and the central muscarinic cholinergic and dopaminergic systems alterations that result from prenatal exposure to type II PIs are also present in rodents exposed during postnatal life (Ansari and others 2012a; b; Eriksson and Fredriksson 1991; Eriksson and Nordberg 1990; Husain and others 1994; Nasuti and others 2007; Patro and others 2009; Singh and others 2012). Particularly, the dopaminergic system is an important target. Altered levels of dopamine and its metabolites and downregulation of dopamine receptors in the striatum, neurodegeneration in substantia nigra and increased oxidative stress, decreased locomotor activity and deficits in motor coordination during adolescence and at adulthood (Ansari and others 2012b; Nasuti and others 2007; Singh and others 2012) suggest an association between exposure to type II PIs and environmentally induced Parkinson’s disease. In addition, recently, Richardson and collaborators (Richardson and others 2015) demonstrated that male mice exposed to deltamethrin throughout gestation and lactation reproduce the core behavioral symptoms of ADHD, including hyperactivity, impulsive-like behavior, and deficits in working memory and attention and that these behavior effects are also associated to dopaminergic alterations such as decreased extracellular dopamine, increased dopamine transporter and dopamine-D1 receptor levels in striatum.
5.1.2. Epidemiological studies on PIs neurodevelopmental adverse effects
With increasing OP restrictions, exposure of pregnant women to PIs for residential pest control has increased (Williams and others 2008). However, few epidemiological studies investigated the effects of PI insecticide exposure at environmental levels during prenatal and childhood development, and data on neurodevelopmental effects are particularly sparse (SM Table 9). Out of five studies that evaluated the effects of prenatal PI exposure on children’s neurodevelopment, three identified evidence of deleterious outcomes. Xue and collaborators (Xue and others 2013) quantified 3-PBA, a metabolite of permethrin, cypermethrin, deltamethrin, allethrin, resmethrin, fenvalerate, cyhalothrin, fenpropathrin, and tralomethrin, in the urine of pregnant women and reported an inverse association between prenatal PI exposure and measures of motor function, social adaptation, and intelligence in Chinese infants at 1 year of age. As part of the CCCEH study, Horton and collaborators (Horton and others 2011) assessed permethrin in personal air samples collected during pregnancy. Since PIs are difficult to measure in air (Laskowski 2002), piperonyl butoxide, a synergist commonly formulated with pyrethroids was also quantified and used as an indicator of maternal PI insecticide use. They identified a negative association between piperonyl butoxide and neurodevelopment, as indicated by a four points lower score on the Bayley Mental Developmental Index in 3-year old children that were more highly exposed to piperonyl butoxide than those with lower exposures during pregnancy. Recent data from the CHARGE study linked commercial pesticide application data from the California Pesticide Use Report to the addresses during pregnancy. This ellegant study found a positive association between residential proximity to type II PIs preconception and during the third trimester of gestation and both autism spectrum disorders and developmental delay (delayed cognitive or adaptative development) (Shelton and others 2014).
As for the effects of exposure during childhood, four studies investigated the association between urinary levels of PI metabolites and cognitive outcomes. Using data extracted from the NHANES study, it was described that children with detectable levels of the metabolite 3-PBA were more than twice as likely to be diagnosed with ADHD (Richardson and others 2015). Wagner-Schuman and collaborators (Wagner-Schuman and others 2015) further described that the higher the 3-PBA levels, the higher the number of hyperactive-impulsive symptoms in boys. However, using a distinct cycle of NHANES, Quirós-Alcalá and collaborators (Quirós-Alcalá and others 2014) failed to find associations between the presence of PI metabolites in urine and parental report of both learning disability and ADHD. In the fourth study, data from the Canadian Health Measures Survey (CHMS) indicated an association between PI urinary metabolites in children between 6 and 11 years and high scores for total difficulties on the Strengths and Difficulties Questionnaire, suggestive of higher prevalence of clinical psychopathology disorders (Oulhote and Bouchard 2013).
5.2. What did we learn with PIs and what is still to be learned?
Altogether, epidemiological and experimental findings indicate that even though this class of insecticides is classically considered to be safer than other classes to the human health, there are concerning findings of developmental neurobehavioral toxicity. The findings further reinforce lessons learned with OP compounds; that the mechanism responsible for insect toxicity does not explain developmental neurotoxicity, that fetuses and children are particularly vulnerable, and that new developed insecticides must have higher selective toxicity to target organisms. In addition, since deleterious effects are identified long after exposure, even biotransformation into nontoxic metabolites and lack of significant bioaccumulation do not assure safety to off-target organisms.
Data obtained from in vitro and animal models of developmental exposure to both type I and type II PIs suggest that components highly used indoor and outdoor produce a spectrum of neurochemical and behavioral effects during development, with a window of vulnerability ranging from early gestation up to late postnatal period. Deltamethrin is the focus of several studies, however, other constituents of this class have received too little attention. This is concerning, because there are significant structural and functional differences between type I and type II PIs and even among components of each PI type that may be associated with dissimilar outcomes (Costa 2015; Soderlund 2012). Of note, despite a restricted number of epidemiological studies on the effects of PIs during development, most of them, which associate prenatal and postnatal exposures with deleterious neurodevelopmental outcomes, were published within the last five years, indicating an increasing interest in the subject.
6. Neonicotinoids (NEs)
Developed to replace OPs and CAs, NEs compose the newest and fastest growing class of insecticides introduced in the market. The seven NEs approved for commercial use worldwide: imidacloprid, thiacloprid, clothianidin, thiamethoxam, acetamiprid, nitenpyram, and dinotefuran, compose the most widely used class of insecticides worldwide (Jeschke and others 2011). NEs are broad spectrum insecticides, which contributed to their successful introduction in the market. They are used in urban pest control, in veterinary products and to pest control in fish farming (Simon-Delso and others 2015). NEs translocate to all parts of the plant thus making them toxic to herbivorous insects (Simon-Delso and others 2015). This also makes the entire plant a potential vector for transfer of the NEs to non-target species. Unlike surface insecticides, NEs cannot simply be washed off for safe food consumption. These compounds are also more persistent than OPs, CAs and PIs, and, these last characteristics, while make them safer for agricultural workers (due to lower frequency of applications and also seed/soil treatments rather than sprays), but may be associated with higher consumer risk (Simon-Delso and others 2015). NEs broad spectrum also leads to undesirable effects on non-target insects such as honeybees (Pisa and others 2015).
6.1. Developmental neurotoxicity of NEs
Nicotine, the prototypic nicotinic ligand, is an established neurotoxicant. NEs are similar to nicotine in their structure and act as agonists at the nAChRs of insects (Tomizawa and Casida 2005) (see SM for a detailed description). They were designed to act preferentially on insect rather than mammalian nicotinic receptors (Matsuda and others 2001; Sheets and others 2016). However, relatively few published studies investigated off-target toxic effects of NEs and most of these focused on imidacloprid, the first NE insecticide in the market, and on effects of adult exposure. NE metabolites, which were shown to have high affinity to mammalian nAChRs, comparable to or higher than nicotine (Tomizawa and Casida 2000), have also received little attention. The expression of nAChR subtypes and, as a result, the properties of the nAChRs, vary during prenatal and postnatal development of mammals (Abreu-Villaça and others 2011), which raises the possibility that the binding properties and responses of developing organisms to NEs change with age. Given the importance of nAChRs for mammalian brain development (Abreu-Villaça and others 2011), the scarcity of studies that have addressed the effects of early exposure to NEs and their metabolites is somewhat surprising, mainly because recent findings point to significant physiological and behavioral effects in animal models (see below and in SM Tables 10 and 11 and Figure 6).
Figure 6.
Periods of exposure and percentages of the NOAEL (no-observed-adverse-effect level) proportions in log scale based on animal model studies listed in table 10. Only doses and periods of exposure that elicited significant effects are reported. The NOAEL for neurotoxic effects of Imidacloprid (9 mg/kg; (EPA 2006) was chosen based on acute, oral administration in adult rats. As for Clothianidin, the NOAEL (60 mg/kg; (EPA 2003) was chosen based on subchronic, oral administration in adult rats. The symbol “■” represents single dose and “—” represents longer periods of exposure. Lactation/infancy extends from PN1 to PN27 (PN=postnatal day). Adolescence assumed to begin at PN28.
6.1.1. Evidence of NEs developmental neurotoxicity from in vitro and animal models
There have been studies that show that NEs have effects on mammalian nicotinic receptors. Application of 10 μM imidacloprid to brainstem slices from infant mice produces depolarizing inward currents, increasing the level of excitability of neurons that have nAChRs (Bal and others 2010). In primary cultures of cerebellar neurons from neonatal rats, imidacloprid and acetamiprid at concentrations as low as 1 μM increase physiological activity of neurons that express the mRNA of the α3, α4, and α7 nAChR subunits (Kimura-Kuroda and others 2012). In addition, cholinergic agonists significantly decrease the proportions of neurons that are excited by imidacloprid and acetamiprid, indicating the involvement of several nAChRs subunits in the excitatory response (Kimura-Kuroda and others 2012). In undifferentiated PC12 cells, imidacloprid has mixed agonist and antagonist effects in nAChRs (Nagata and others 1998). In rats, acute exposure during early gestation produces sensorimotor impairment and increased brain AChE activity, mAChR binding and glial fibrillary acidic protein (GFAP) expression in motor cortex and hippocampus in adolescent offspring (Abou-Donia and others 2008).
Fish models have also contributed to the literature that NEs have neurobehavioral effects on non-insect species. Recently, we (Crosby and others 2015) investigated in zebrafish the neurobehavioral effects of developmental exposure to imidacloprid, a prototypic neonicotinoid pesticide. Nicotine was also administered for comparison. Zebrafish were exposed during development via immersion in aqueous solutions containing 45 μM or 60 μM of imidacloprid or nicotine at the same concentrations (or vehicle control) from 4 hours to 5 days post fertilization. The functional effects of developmental exposure to both imidacloprid and nicotine were assessed in larvae using an activity assay and during adolescence and adulthood using a battery of neurobehavioral assays, including assessment of sensorimotor response and habituation in a tactile startle test, novel tank swimming, and shoaling behavior. In larvae, developmental imidacloprid exposure at both doses significantly decreases swimming activity. The 5D strain of zebrafish were more sensitive to both nicotine and imidacloprid than the AB* strain. In adolescent and adult fish, a history of developmental exposure to imidacloprid significantly decreased novel tank exploration and increased sensorimotor response to a startling tap stimulus. While developmental nicotine exposure to the same molar doses did not affect novel tank swimming, it did increase sensorimotor response to startle stimuli at the low dose. No effects of either compound were found on shoaling behavior or habituation to the startling stimulus. Early developmental exposure to imidacloprid has both early-life and persisting effects on neurobehavioral function in zebrafish.
Three studies investigated the effects of developmental exposure to clothianidin. Offspring from mouse dams exposed to doses ranging from 20 to 180 ppm throughout gestation and lactation (Tanaka 2012a) or from gestation until offspring adulthood (Tanaka 2012b) showed sex-selective effects during lactation, most evident at low and intermediate doses, suggesting impaired sensory and motor development (Tanaka 2012a; b). Clothianidin exposure from the second postnatal week until adulthood was not found to affect acquisition of learning/memory but it did impair memory consolidation in adult rats tested in the Morris water maze. However, no alterations were found in mRNA expression levels of related genes in the hippocampus (N-methyl D-aspartate 1, m1 AChR, synaptophysin, and growth associated protein 43) (Özdemir and others 2014).
6.1.2. NEs guideline studies
Due to the limited number of studies and to complement data on developmental toxicity of NEs, the Environmental Protection Agency (EPA) performed additional studies in which rats were exposed throughout gestation and lactation to imidacloprid, acetamiprid, thiacloprid, clothianidin, thiamethoxam or dinotefuran at three dose levels in accordance with established guidelines (EPA 1998). For each NE, the doses used were selected based on the following criteria: to induce evidence of toxicity in the dam or pups (high dose), less toxicity (intermediate dose) than the high dose and no evidence of toxicity (low dose) (Sheets and others 2016). Results from these studies were recently published (Sheets and others 2016) and will be briefly presented below. More detailed descriptions are provided as SM (SM text and SM Table 11).
Dinutefuran exposure fails to elicit detectable adverse effects in offspring (EPA 2009; Sheets and others 2016), however, all other NEs evoke deleterious outcomes during development. Imidacloprid at the high dose decreases locomotor activity in offspring at the end of lactation period. In offspring females, the caudate-putamen width is reduced during lactation and at adulthood, and the thickness of the corpus callosum is reduced during lactation (EPA 2006; Sheets and others 2016). Despite that, morphometric brain measurements were not performed in the intermediate and low dose-groups (EPA 2006). Exposure to the high dose of acetamiprid decreases the acoustic startle response (indicative of decreased habituation) by the end of the lactation period and at adulthood. There is a marginally significant increase in the number of errors in the Biel maze learning/memory test in male offspring by the end of the lactation period (EPA 2013; Sheets and others 2016). The intermediate and high doses of thiacloprid evoke signs of developmental delays such as late preputial separation and delayed vaginal opening. Males present reduced corpus callosum and striatum during lactation and reduced striatum and dentate gyrus at adulthood (EPA 2002; Sheets and others 2016). The higher and intermediate doses of clothianidin decrease motor activity and acoustic startle habituation responses during lactation. The cerebellum height is increased during lactation while the hippocampal gyrus thickness is increased during lactation and decreased in adult females exposed to the high dose (Sheets and others 2016). As for thiamethoxam, offspring exposed to the high dose presented reductions in morphometric measurements in several brain regions both during lactation and at adulthood. These effects explain the absolute brain weight reduction at both analyzed ages (Sheets and others 2016).
6.1.3. Epidemiological studies on NE’s neurodevelopmental adverse effects
NEs are present in a variety of fruits, vegetables, and in honey that are available in the market for human consumption (Chen and others 2014a). These compounds are present in food even in countries where their use was suspended for seed treatment, evidencing illicit use (Sabatino and others 2013). Despite that, up to now, data on human toxicity are limited. At adulthood, the data are almost entirely derived from descriptive studies of acute poisoning (e.g. (Lin and others 2013; Vinod and others 2015) and there is only one study on the effects of exposure during development. In this study (Table 12), data from the CHARGE case–control study was used to investigate whether household imidacloprid usage as a flea and tick treatment on household pets during pregnancy and first 3 years of infancy was associated with autism spectrum disorders. This pioneer study identified increased risk of autism spectrum disorders and pregnancy as the most sensitive period of exposure (Keil and others 2014). There are no epidemiological studies on other NEs and on other neurodevelopmental outcomes.
6.2. What did we learn with NEs and what is still to be learned?
Among the major classes of insecticides, the NE is the least studied, which in part reflects the fact that it was the most recent one introduced to the market. However, either the assurance of NEs reduced harm to the developing brain or the identification of significant damage caused by exposure will only be determined with further studies. The current set of in vitro studies suggests that even low doses of imidacloprid and acetamiprid induce nAChR-mediated excitation of neurons, however the translational potential of these results still needs to be verified. NEs were designed to be specific to insects, however developmental neurotoxicity is seen in vertebrates. Particularly worrisome is the fact that NEs are incorporated into the physiology of the plants rather than being on the surface, which presents a problem as they cannot be cleaned off before consumption like surface insecticides. Other important under-investigated issues include the developmental effects of NE metabolites in off-target organisms, and the potential differential sensitivity of fetal/early postnatal off-target nAChRs. In parallel, epidemiological studies are warranted.
7. How to improve safety
Insecticide safety should be determined before introduction to the market, however, frequently, the sequence of events is reversed so that after years of use, evidence of toxic effects identified by the scientific community lead regulatory agencies to review safe doses, restrict use and even ban products. The review of the registration status of chemicals already in use is indispensable for identifying and correcting errors that have been made. However, a better practice is to identify risks before a pesticide is registered. In this regard, it is vital that the studies conducted in support of registration of pesticides be made public so that the broader scientific community and society at large can independently evaluate the studies and their conclusions. Knowledge about the risk of these chemicals cannot reasonably be kept private when the chemicals themselves are commercialized to be so broadly cast into the public environment. As discussed below, improving the identification of toxic outcomes may be challenging but it is also strategic and prophylactic.
7.1. Identifying toxic outcomes
The restriction or ban of an insecticide theoretically implies the development of less harmful products to replace it. However, currently, the safety of new products is not assured before its introduction in the market. As discussed in other reviews (Colborn 2006; Slotkin 2004b), this occurs in part due to outdated standardized testing of developmental neurotoxicity required by regulatory agencies. As a result, frequently, more sensitive techniques and/or experimental designs used by the academic scientific community allows the identification of outcomes distinct than those assessed by following established guidelines. This indicates that current guidelines must be reviewed. In addition, regulatory agencies should consistently consult independent studies from the scientific community during the process of determining the toxicity of a chemical. The confidential nature of preapproval toxicity testing protects the commercial interests of the companies producing the new products, but does nothing to protect the wellbeing of the people exposed to the product once it is released into the environment upon approval. The insecticides themselves become broadcast in the environment, while information about the pre-approval toxicity testing often remains restricted. A spectrum of complementary models from in vitro cell based assays through non-mammalian models such as c. Elegans and zebrafish through classic rodent models as well as epidemiologic and clinical studies can provide a more efficient way to determine neurotoxic risks posed by insecticides. Opening pre-approval testing to a broader array of researchers could help avoid discovering health risks only after they have occurred in the human population.
7.2. Challenges
The scientific literature thus far has focused mainly on single chemical exposures. An important challenge associated with the use of chemical substances is the combined or sequential exposure (Boobis and others 2008). Insecticides with both similar and distinct modes of action are frequently used in the same crop (USDA 2014) and are concurrently identified in biological samples (e.g. (Barr and others 2010; Forde and others 2015; Reiler and others 2015). Despite that, there is still a restricted number of studies that investigate the effects and mechanisms associated with dual exposures.
As examples, three studies described the impact of an early exposure to PIs on the susceptibility to a subsequent exposure. Mice exposed to bioallethrin during early postnatal life and again at adulthood present a more potent increase in locomotor response and decreased relearning in the Morris water maze (Talts and others 1998). Rats exposed to cypermethrin during postnatal development and re-exposed at adulthood present decreased locomotor activity and motor coordination and those are accompanied by deficits in the dopaminergic system (Singh and others 2012). Prenatal exposure to cypermethrin followed by re-exposure at adulthood elicits a more pronounced expression of xenobiotic metabolizing P450 in brain (Singh and others 2013), which may have toxic consequences, as these enzymes activity are shown to affect both dopaminergic and serotonergic systems (Ingelman-Sundberg and others 2014; Shahabi and others 2008).
Insecticides may also interact with other agents. In this regard, the impact of developmental exposure to CPF and dexamethasone, a glucocorticoids routinely administered in preterm labor was recently investigated. Dexamethasone given in late gestation of rats augments the adverse effects of neonatal CPF, notably, by worsening deficits in indices of presynaptic and postsynaptic cholinergic activities in adolescent and adult offspring, preferably in females, and including effects that are only present with the dual exposure (Slotkin and others 2013). There are also more robust effects on locomotor hyperactivity, novelty suppressed feeding and object exploration tests and reversal of sex differences in both locomotor performance and object exploration levels with the combined exposure (Levin and others 2014).
With the increasing number of synthetic substances produced each year, environmental exposure is frequently inescapable and, the possibility of additive or interactive effects should be more completely investigated. The aforementioned results, as well as findings from other studies (e.g. (Slotkin and Seidler 2015; Slotkin and others 2015) indicate that cumulative developmental exposure to toxicants may lead to poorer health outcomes and calls attention to the need of the careful examination of populations exposed.
7.3. Looking for alternatives
The increasing worldwide popularity of organic foods, produced by methods that restrict or prohibit the use of synthetic products indicates a trend toward a reduction in insecticides use. Despite that, insecticides still rule the market and most of insecticide classes target the nervous system. Since this is a very effective way to kill insects, it is likely that neurotoxicity will remain an important action for new insecticides. To decrease cross-toxicity between insects and vertebrates the development of newer classes of insecticides has attempted to target biological processes that differ between insects and vertebrates. Neonicotinoid insecticides were developed with the aim to target nicotinic receptors of insects but not vertebrates. However, there seems to still be carryover effects affecting vertebrate brain development.
Insecticides not affecting neural activity are coming into use. In this regard, compounds that disrupt insects’ energy production (respiration targets) or alter growth and development can be highly effective with the possibility of superior selectivity to insects (Sparks and Nauen 2015). Another interesting option is the genetic modification of crops to express endotoxins such as the Cry protein (δ-endotoxin) produced by the Bacillus thuringiensis, which is toxic to a wide variety of insects (Rubio-Infante and Moreno-Fierros 2016). Commercialization of transgenic crops expressing Cry toxins has increased dramatically since the late 1990’s and this biological insecticide at this point appears to have lower toxicity in vertebrates (Rubio-Infante and Moreno-Fierros 2016).
Insecticides provide substantial benefits to the human population by controlling insect pests that carry disease or destroy crops. However, chemicals that kill insects have also been shown to damage human health. This has been seen through the numerous generations of insecticides introduced over the decades. Our experience with the mechanisms of efficacy for killing insects and mechanisms of risk for harming humans should provide ways to develop new methods for controlling insect pests that are effective with lessened risk.
Supplementary Material
Highlights.
Developmental neurotoxicity is one of the most prominent off-target toxic risks of insecticides.
Several classes of insecticides have been developed, each with neurotoxic side effects.
Organochlorines, organophosphates, pyrethroids, carbamates and neonicotinoids each produce developmental neurotoxicity.
The goal of new insecticide development is to effectively kill target species with less risk of developmental neurotoxicity.
Acknowledgments
This work was supported by a fellowship from Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES-Brazil) [grant number BEX 2037/15-7 to YA-V] and the Duke University Superfund Center (ES010356). The authors are thankful to Dr. Alex C. Manhães for his help with figure formatting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel Rasoul GM, Abou Salem ME, Mechael AA, Hendy OM, Rohlman DS, Ismail AA. Effects of occupational pesticide exposure on children applying pesticides. NeuroToxicology. 2008;29:833–838. doi: 10.1016/j.neuro.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB, Goldstein LB, Bullman S, Tu T, Khan WA, Dechkovskaia AM, Abdel-Rahman AA. Imidacloprid Induces Neurobehavioral Deficits and Increases Expression of Glial Fibrillary Acidic Protein in the Motor Cortex and Hippocampus in Offspring Rats Following in Utero Exposure. Journal of Toxicology and Environmental Health, Part A. 2008;71:119–130. doi: 10.1080/15287390701613140. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB, Khan WA, Dechkovskaia AM, Goldstein LB, Bullman SL, Abdel-Rahman A. In utero exposure to nicotine and chlorpyrifos alone, and in combination produces persistent sensorimotor deficits and Purkinje neuron loss in the cerebellum of adult offspring rats. Archives of Toxicology. 2006;80:620–631. doi: 10.1007/s00204-006-0077-1. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y, Filgueiras CC, Manhães AC. Developmental aspects of the cholinergic system. Behavioural Brain Research. 2011;221:367–378. doi: 10.1016/j.bbr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- Adigun AA, Ryde IT, Seidler FJ, Slotkin TA. Organophosphate Exposure During a Critical Developmental Stage Reprograms Adenylyl Cyclase Signaling in PC12 Cells. Brain research. 2010;1329:36–44. doi: 10.1016/j.brainres.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlbom J, Fredriksson A, Eriksson P. Neonatal exposure to a type-I pyrethroid (bioallethrin) induces dose—response changes in brain muscarinic receptors and behaviour in neonatal and adult mice. Brain Research. 1994;645:318–324. doi: 10.1016/0006-8993(94)91666-7. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental Exposure of Rats to Chlorpyrifos Leads to Behavioral Alterations in Adulthood, Involving Serotonergic Mechanisms and Resembling Animal Models of Depression. Environmental Health Perspectives. 2005a;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in Central Nervous System Serotonergic and Dopaminergic Synaptic Activity in Adulthood after Prenatal or Neonatal Chlorpyrifos Exposure. Environmental Health Perspectives. 2005b;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environmental Health Perspectives. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environmental Health Perspectives. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand SS, Bruckner JV, Haines WT, Muralidhara S, Fisher JW, Padilla S. Characterization of deltamethrin metabolism by rat plasma and liver microsomes. Toxicology and Applied Pharmacology. 2006a;212:156–166. doi: 10.1016/j.taap.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Anand SS, Kim KB, Padilla S, Muralidhara S, Kim HJ, Fisher JW, Bruckner JV. ONTOGENY OF HEPATIC AND PLASMA METABOLISM OF DELTAMETHRIN IN VITRO: ROLE IN AGE-DEPENDENT ACUTE NEUROTOXICITY. Drug Metabolism and Disposition. 2006b;34:389–397. doi: 10.1124/dmd.105.007807. [DOI] [PubMed] [Google Scholar]
- Anavi-Goffer S, Mulder J. The Polarised Life of the Endocannabinoid System in CNS Development. ChemBioChem. 2009;10:1591–1598. doi: 10.1002/cbic.200800827. [DOI] [PubMed] [Google Scholar]
- Ansari RW, Shukla RK, Yadav RS, Seth K, Pant AB, Singh D, Agrawal AK, Islam F, Khanna VK. Cholinergic Dysfunctions and Enhanced Oxidative Stress in the Neurobehavioral Toxicity of Lambda-Cyhalothrin in Developing Rats. Neurotoxicity Research. 2012a;22:292–309. doi: 10.1007/s12640-012-9313-z. [DOI] [PubMed] [Google Scholar]
- Ansari RW, Shukla RK, Yadav RS, Seth K, Pant AB, Singh D, Agrawal AK, Islam F, Khanna VK. Involvement of dopaminergic and serotonergic systems in the neurobehavioral toxicity of lambda-cyhalothrin in developing rats. Toxicology Letters. 2012b;211:1–9. doi: 10.1016/j.toxlet.2012.02.012. [DOI] [PubMed] [Google Scholar]
- ANVISA, A.N.d.V.S. Agrotóxico utilizado como chumbinho é retirado do mercado brasileiro. 2012 http://portal.anvisa.gov.br/wps/content/anvisa+portal/anvisa/sala+de+imprensa/menu+-+noticias+anos/2012+noticias/agrotoxico+utilizado+como+chumbinho+e+retirado+do+mercado+brasileirohttp://portal.anvisa.gov.br/wps/wcm/connect/5c720f80474591b499c8dd3fbc4c6735/aldicarbe.pdf?MOD=AJPERES.
- ANVISA, A.N.d.V.S. Nota técnica de reavaliação do ingrediente ativo de agrotóxico carbofurano. 2015 http://portal.anvisa.gov.br/wps/wcm/connect/b46868804b0552fabc7ebe0bd53a8764/CP+114-2015+-+Nota+tecnica.pdf?MOD=AJPERES.
- ATSDR; Registry A.f.T.S.a.D, editor. Toxicological Profile for DDT, DDE, and DDD. 2002 http://www.atsdr.cdc.gov/ToxProfiles/tp35-c3.pdf. [PubMed]
- ATSDR; Registry A.f.T.S.a.D, editor. Toxicological profile for pyrethrins and pyrethroids. 2003 http://www.atsdr.cdc.gov/ToxProfiles/tp155.pdf. [PubMed]
- Aziz MH, Agrawal AK, Adhami VM, Shukla Y, Seth PK. Neurodevelopmental consequences of gestational exposure (GD14–GD20) to low dose deltamethrin in rats. Neuroscience Letters. 2001;300:161–165. doi: 10.1016/s0304-3940(01)01543-9. [DOI] [PubMed] [Google Scholar]
- Bailey J, Oliveri A, Levin ED. Zebrafish Model Systems for Developmental Neurobehavioral Toxicology. Birth defects research Part C, Embryo today: reviews. 2013;99:14–23. doi: 10.1002/bdrc.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal R, Erdogan S, Theophilidis G, Baydas G, Naziroglu M. Assessing the effects of the neonicotinoid insecticide imidacloprid in the cholinergic synapses of the stellate cells of the mouse cochlear nucleus using whole-cell patch-clamp recording. NeuroToxicology. 2010;31:113–120. doi: 10.1016/j.neuro.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Banji D, Banji OJF, Ragini M, Annamalai AR. Carbosulfan exposure during embryonic period can cause developmental disability in rats. Environmental Toxicology and Pharmacology. 2014;38:230–238. doi: 10.1016/j.etap.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Barr DB, Ananth CV, Yan X, Lashley S, Smulian JC, Ledoux TA, Hore P, Robson MG. Pesticide concentrations in maternal and umbilical cord sera and their relation to birth outcomes in a population of pregnant women and newborns in New Jersey. Science of The Total Environment. 2010;408:790–795. doi: 10.1016/j.scitotenv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Barr DB, Angerer J. Potential Uses of Biomonitoring Data: A Case Study Using the Organophosphorus Pesticides Chlorpyrifos and Malathion. Environmental Health Perspectives. 2006;114:1763–1769. doi: 10.1289/ehp.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Bravo R, Weerasekera G, Caltabiano LM, Whitehead RD, Olsson AO, Caudill SP, Schober SE, Pirkle JL, Sampson EJ, Jackson RJ, Needham LL. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environmental Health Perspectives. 2004;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt AM, Burgess SC, Carr RL. Effect of Developmental Exposure to Chlorpyrifos on the Expression of Neurotrophin Growth Factors and Cell-Specific Markers in Neonatal Rat Brain. Toxicological Sciences. 2006;92:500–506. doi: 10.1093/toxsci/kfl004. [DOI] [PubMed] [Google Scholar]
- Betancourt AM, Carr RL. The Effect of Chlorpyrifos and Chlorpyrifos-Oxon on Brain Cholinesterase, Muscarinic Receptor Binding, and Neurotrophin Levels in Rats Following Early Postnatal Exposure. Toxicological Sciences. 2004;77:63–71. doi: 10.1093/toxsci/kfh003. [DOI] [PubMed] [Google Scholar]
- Boobis AR, Ossendorp BC, Banasiak U, Hamey PY, Sebestyen I, Moretto A. Cumulative risk assessment of pesticide residues in food. Toxicology Letters. 2008;180:137–150. doi: 10.1016/j.toxlet.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Boronat S, Casado S, Navas JM, Piña B. Modulation of aryl hydrocarbon receptor transactivation by carbaryl, a nonconventional ligand. FEBS Journal. 2007;274:3327–3339. doi: 10.1111/j.1742-4658.2007.05867.x. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. ATTENTION DEFICIT/HYPERACTIVITY DISORDER AND URINARY METABOLITES OF ORGANOPHOSPHATE PESTICIDES IN U.S. CHILDREN 8–15 YEARS. Pediatrics. 2010;125:e1270–e1277. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB, Eskenazi B. Prenatal Exposure to Organophosphate Pesticides and IQ in 7-Year-Old Children. Environmental Health Perspectives. 2011;119:1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwman H, van den Berg H, Kylin H. DDT and Malaria Prevention: Addressing the Paradox. Environmental Health Perspectives. 2011;119:744–747. doi: 10.1289/ehp.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz V, Molina-Molina JM, Sánchez-Redondo S, Fernández MF, Grimalt JO, Olea N, Rodríguez-Farré E, Suñol C. Differential Estrogenic Effects of the Persistent Organochlorine Pesticides Dieldrin, Endosulfan, and Lindane in Primary Neuronal Cultures. Toxicological Sciences. 2011;120:413–427. doi: 10.1093/toxsci/kfr019. [DOI] [PubMed] [Google Scholar]
- Burns CJ, McIntosh LJ, Mink PJ, Jurek AM, Li AA. Pesticide Exposure and Neurodevelopmental Outcomes: Review of the Epidemiologic and Animal Studies. Journal of Toxicology and Environmental Health Part B, Critical Reviews. 2013;16:127–283. doi: 10.1080/10937404.2013.783383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaleiro T, Caride A, Romero A, Lafuente A. Effects of in utero and lactational exposure to endosulfan in prefrontal cortex of male rats. Toxicology Letters. 2008;176:58–67. doi: 10.1016/j.toxlet.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Cantalamessa F. Acute toxicity of two pyrethroids, permethrin, and cypermethrin in neonatal and adult rats. Archives of Toxicology. 1993;67:510–513. doi: 10.1007/BF01969923. [DOI] [PubMed] [Google Scholar]
- Cao Z, Cui Y, Nguyen HM, Jenkins DP, Wulff H, Pessah IN. Nanomolar Bifenthrin Alters Synchronous Ca(2+) Oscillations and Cortical Neuron Development Independent of Sodium Channel Activity. Molecular Pharmacology. 2014;85:630–639. doi: 10.1124/mol.113.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carloni M, Nasuti C, Fedeli D, Montani M, Amici A, Vadhana MSD, Gabbianelli R. The impact of early life permethrin exposure on development of neurodegeneration in adulthood. Experimental Gerontology. 2012;47:60–66. doi: 10.1016/j.exger.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Carloni M, Nasuti C, Fedeli D, Montani M, Vadhana MSD, Amici A, Gabbianelli R. Early life permethrin exposure induces long-term brain changes in Nurr1, NF-kB and Nrf-2. Brain Research. 2013;1515:19–28. doi: 10.1016/j.brainres.2013.03.048. [DOI] [PubMed] [Google Scholar]
- Carr RL, Adams AL, Kepler DR, Ward AB, Ross MK. Induction of Endocannabinoid Levels in Juvenile Rat Brain Following Developmental Chlorpyrifos Exposure. Toxicological Sciences. 2013;135:193–201. doi: 10.1093/toxsci/kft126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Armstrong NH, Buchanan AT, Eells JB, Mohammed AN, Ross MK, Nail CA. Decreased anxiety in juvenile rats following exposure to low levels of chlorpyrifos during development. NeuroToxicology. 2015 doi: 10.1016/j.neuro.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Borazjani A, Ross MK. Effect of Developmental Chlorpyrifos Exposure, on Endocannabinoid Metabolizing Enzymes, in the Brain of Juvenile Rats. Toxicological Sciences. 2011;122:112–120. doi: 10.1093/toxsci/kfr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Graves CA, Mangum LC, Nail CA, Ross MK. Low Level Chlorpyrifos Exposure Increases Anandamide Accumulation in Juvenile Rat Brain in the Absence of Brain Cholinesterase Inhibition. Neurotoxicology. 2014;43:82–89. doi: 10.1016/j.neuro.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson R. Silent spring. Vol. 2002 Boston: Houghton Mifflin; 2002. [Google Scholar]
- Cartier C, Muckle G, Jacobson SW, Jacobson JL, Dewailly É, Ayotte P, Chevrier C, Saint-Amour D. Prenatal and 5-year p, p′-DDE exposures are associated with altered sensory processing in school-aged children in Nunavik: A visual evoked potential study. NeuroToxicology. 2014;44:8–16. doi: 10.1016/j.neuro.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Casado S, Alonso M, Herradón B, Tarazona JV, Navas JMA. Activation of the aryl hydrocarbon receptor by carbaryl: Computational evidence of the ability of carbaryl to assume a planar conformation. Environmental Toxicology and Chemistry. 2006;25:3141–3147. doi: 10.1897/06-131r.1. [DOI] [PubMed] [Google Scholar]
- Casida JE, Durkin KA. Anticholinesterase insecticide retrospective. Chemico-Biological Interactions. 2013a;203:221–225. doi: 10.1016/j.cbi.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE, Durkin KA. Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects. Annual Review of Entomology. 2013b;58:99–117. doi: 10.1146/annurev-ento-120811-153645. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Golden Age of Insecticide Research: Past, Present, or Future? Annual Review of Entomology. 1998;43:1–16. doi: 10.1146/annurev.ento.43.1.1. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang M, Miller GW. Perinatal Heptachlor Exposure Increases Expression of Presynaptic Dopaminergic Markers in Mouse Striatum. NeuroToxicology. 2005;26:721–728. doi: 10.1016/j.neuro.2004.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughlan A, Newhouse K, Namgung U, Xia Z. Chlorpyrifos Induces Apoptosis in Rat Cortical Neurons that is Regulated by a Balance Between p38 and ERK/JNK MAP Kinases. Toxicological Sciences. 2004;78:125–134. doi: 10.1093/toxsci/kfh038. [DOI] [PubMed] [Google Scholar]
- Chen J, Kumar M, Chan W, Berkowitz G, Wetmur JG. Increased influence of genetic variation on PON1 activity in neonates. Environmental Health Perspectives. 2003;111:1403–1409. doi: 10.1289/ehp.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Tao L, McLean J, Lu C. Quantitative Analysis of Neonicotinoid Insecticide Residues in Foods: Implication for Dietary Exposures. Journal of Agricultural and Food Chemistry. 2014a;62:6082–6090. doi: 10.1021/jf501397m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XP, Chen WF, Wang DW. Prenatal Organophosphates Exposure Alternates the Cleavage Plane Orientation of Apical Neural Progenitor in Developing Neocortex. PLoS ONE. 2014b;9:e95343. doi: 10.1371/journal.pone.0095343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Chen J, Qiu Y, Hong X, Xia Y, Feng T, Liu J, Song L, Zhang Z, Wang X. Carbaryl inhibits basal and FSH-induced progesterone biosynthesis of primary human granulosa-lutein cells. Toxicology. 2006;220:37–45. doi: 10.1016/j.tox.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Clark JM, Symington S. Advances in the Mode of Action of Pyrethroids. In: Matsuo N, Mori T, editors. Pyrethroids. Springer; Berlin Heidelberg: 2012. [DOI] [PubMed] [Google Scholar]
- Colborn T. A Case for Revisiting the Safety of Pesticides: A Closer Look at Neurodevelopment. Environmental Health Perspectives. 2006;114:10–17. doi: 10.1289/ehp.7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors SL, Levitt P, Matthews SG, Slotkin TA, Johnston MV, Kinney HC, Johnson WG, Dailey RM, Zimmerman AW. Fetal Mechanisms in Neurodevelopmental Disorders. Pediatric Neurology. 2008;38:163–176. doi: 10.1016/j.pediatrneurol.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Cossenza M, Socodato R, Portugal CC, Domith ICL, Gladulich LFH, Encarnação TG, Calaza KC, Mendonça HR, Campello-Costa P, Paes-de-Carvalho R. Chapter Five - Nitric Oxide in the Nervous System: Biochemical, Developmental, and Neurobiological Aspects. In: Gerald L, editor. Vitamins & Hormones. Academic Press; 2014. [DOI] [PubMed] [Google Scholar]
- Costa LG. Chapter 9 - The neurotoxicity of organochlorine and pyrethroid pesticides. In: Marcello L, Margit LB, editors. Handbook of Clinical Neurology. Elsevier; 2015. [DOI] [PubMed] [Google Scholar]
- Costa LG, Richter RJ, Li WF, Cole T, Guizzetti M, Furlong CE. Paraoxonase (PON 1) as a biomarker of susceptibility for organophosphate toxicity. Biomarkers. 2003;8:1–12. doi: 10.1080/13547500210148315. [DOI] [PubMed] [Google Scholar]
- Crosby EB, Bailey JM, Oliveri AN, Levin ED. Neurobehavioral impairments caused by developmental imidacloprid exposure in zebrafish. Neurotoxicology and Teratology. 2015;49:81–90. doi: 10.1016/j.ntt.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbreth ME, Harrill JA, Freudenrich TM, Mundy WR, Shafer TJ. Comparison of chemical-induced changes in proliferation and apoptosis in human and mouse neuroprogenitor cells. NeuroToxicology. 2012;33:1499–1510. doi: 10.1016/j.neuro.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Dam K, Garcia SJ, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways. Developmental Brain Research. 1999;116:9–20. doi: 10.1016/s0165-3806(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: delayed targeting of DNA synthesis after repeated administration. Developmental Brain Research. 1998;108:39–45. doi: 10.1016/s0165-3806(98)00028-5. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Developmental Brain Research. 2000;121:179–187. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- De Felice A, Venerosi A, Ricceri L, Sabbioni M, Scattoni ML, Chiarotti F, Calamandrei G. Sex-dimorphic effects of gestational exposure to the organophosphate insecticide chlorpyrifos on social investigation in mice. Neurotoxicology and Teratology. 2014;46:32–39. doi: 10.1016/j.ntt.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- Dörner G, Plagemann A. DDT in human milk and mental capacities in children at school age: an additional view on PISA 2000. Neuro Endocrinology Letters. 2002;23:427–431. [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Coyle J, McKhann G, Mobley WC, Nadel L, Neubert D, Schulte-Hermann R, Spencer PS. Review of the Toxicology of Chlorpyrifos With an Emphasis on Human Exposure and Neurodevelopment. Critical Reviews in Toxicology. 2008;38:1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- EC; Commission E, editor. Off J Eur Union. 2003. Council Decision of 18 March 2003 concerning the non-inclusion of aldicarb in Annex I to Council Directive 91/414/EEC and the withdrawal of authorisations for plant protection products containing this active substance. [Google Scholar]
- EC; Commission E, editor. Off J Eur Union. 2007. Commission Decision of 13 June 2007 concerning the non-inclusion of carbofuran in Annex I to Council Directive 91/414/EEC and the withdrawal of authorizations for plant protection products containing that substance. [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: Comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicology and teratology. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, Wetmur JG, Wolff MS. Prenatal Organophosphate Metabolite and Organochlorine Levels and Performance on the Brazelton Neonatal Behavioral Assessment Scale in a Multiethnic Pregnancy Cohort. American Journal of Epidemiology. 2007;165:1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, Wolff MS. Prenatal Exposure to Organophosphates, Paraoxonase 1, and Cognitive Development in Childhood. Environmental Health Perspectives. 2011;119:1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA; Agency E.P, editor. Health effects test guidelines; OPPTS 870.6300; Developmental neurotoxicity study. 1998 http://www.regulations.gov/-!documentDetail;D=EPA-HQ-OPPT-2009-0156-0042.
- EPA; Agency E.P, editor. Data evaluation record for YRC2894 (Thiacloprid): developmental neurotoxicity study – rat. 2002 https://www.federalregister.gov/articles/2003/09/26/03-24371/thiacloprid-pesticide-tolerances.
- EPA; Environmental Protection Agency O.o.P. Pesticides and Toxic Substances (7501C), editor Pesticide Fact Sheet: Clothianidin. 2003 https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-044309_30-May-03.pdf.
- EPA; California Environmental Protection Agency D.o.P.R, editor. Imidacloprid: Risk characterization document, dietary and drinking water exposure. 2006 http://www.cdpr.ca.gov/docs/risk/rcd/imidacloprid.pdf.
- EPA; Agency E.P, editor. Reregistration Eligibility Decision for Carbofuran. 2007 http://archive.epa.gov/pesticides/reregistration/web/pdf/carbofuran_red.pdf.
- EPA; Agency E.P, editor. Dinotefuran; Pesticide Tolerances. 2009 https://www.federalregister.gov/articles/2009/12/18/E9-30131/dinotefuran-pesticide-tolerances.
- EPA. Acetamiprid: pesticide tolerances. 2013 http://www.federalregister.com/Browse/Document/usa/na/fr/2013/6/19/2013-14653.
- Epstein L. Fifty Years Since Silent Spring. Annual Review of Phytopathology. 2014;52:377–402. doi: 10.1146/annurev-phyto-102313-045900. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Ahlbom J, Fredriksson A. Exposure to DDT during a defined period in neonatal life induces permanent changes in brain muscarinic receptors and behaviour in adult mice. Brain Research. 1992;582:277–281. doi: 10.1016/0006-8993(92)90144-x. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Fredriksson A. Neurotoxic effects of two different pyrethroids, bioallethrin and deltamethrin, on immature and adult mice: Changes in behavioral and muscarinic receptor variables. Toxicology and Applied Pharmacology. 1991;108:78–85. doi: 10.1016/0041-008x(91)90270-o. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Nordberg A. Effects of two pyrethroids, bioallethrin and deltamethrin, on subpopulations of muscarinic and nicotinic receptors in the neonatal mouse brain. Toxicology and Applied Pharmacology. 1990;102:456–463. doi: 10.1016/0041-008x(90)90041-r. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, Furlong CE, Holland NT. Association of in Utero Organophosphate Pesticide Exposure and Fetal Growth and Length of Gestation in an Agricultural Population. Environmental Health Perspectives. 2004;112:1116–1124. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Huen K, Marks A, Harley KG, Bradman A, Barr DB, Holland N. PON1 and Neurodevelopment in Children from the CHAMACOS Study Exposed to Organophosphate Pesticides in Utero. Environmental Health Perspectives. 2010;118:1775–1781. doi: 10.1289/ehp.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Kogut K, Huen K, Harley KG, Bouchard M, Bradman A, Boyd-Barr D, Johnson C, Holland N. Organophosphate pesticide exposure, PON1, and neurodevelopment in school-age children from the CHAMACOS study. Environmental research. 2014;134:149–157. doi: 10.1016/j.envres.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Fenster L, Johnson C, Barr DB, Jewell NP. In Utero Exposure to Dichlorodiphenyltrichloroethane (DDT) and Dichlorodiphenyldichloroethylene (DDE) and Neurodevelopment Among Young Mexican American Children. Pediatrics. 2006;118:233–241. doi: 10.1542/peds.2005-3117. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate Pesticide Exposure and Neurodevelopment in Young Mexican-American Children. Environmental Health Perspectives. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO; Nations F.a.A.O.o.t.U, editor. Carbofuran (096) Toxicology. 2002 http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report08/Carbofuran.pdf.
- Fenske RA, Black KG, Elkner KP, Lee CL, Methner MM, Soto R. Potential exposure and health risks of infants following indoor residential pesticide applications. American Journal of Public Health. 1990;80:689–693. doi: 10.2105/ajph.80.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaskos J, Fowler MJ, Teurtrie C, Hargreaves AJ. The effects of carbaryl and trichlorphon on differentiating mouse N2a neuroblastoma cells. Toxicology Letters. 1999;110:79–84. doi: 10.1016/s0378-4274(99)00142-3. [DOI] [PubMed] [Google Scholar]
- Forde MS, Robertson L, Laouan Sidi EA, Cote S, Gaudreau E, Drescher O, Ayotte P. Evaluation of exposure to organophosphate, carbamate, phenoxy acid, and chlorophenol pesticides in pregnant women from 10 Caribbean countries. Environmental Science: Processes & Impacts. 2015;17:1661–1671. doi: 10.1039/c5em00247h. [DOI] [PubMed] [Google Scholar]
- Fortenberry GZ, Meeker JD, Sánchez BN, Barr DB, Panuwet P, Bellinger D, Schnaas L, Solano-González M, Ettinger AS, Hernandez-Avila M, Hu H, Tellez-Rojo MM. Urinary 3, 5, 6-trichloro-2-pyridinol (TCPY) in pregnant women from Mexico City: distribution, temporal variability, and relationship with child attention and hyperactivity. International journal of hygiene and environmental health. 2014;217:405–412. doi: 10.1016/j.ijheh.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C, Koifman RJ, Sarcinelli P, Rosa AC, Clapauch R, Koifman S. Long term exposure to organochlorine pesticides and thyroid function in children from Cidade dos Meninos, Rio de Janeiro, Brazil. Environmental Research. 2012;117:68–74. doi: 10.1016/j.envres.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Freire C, Lopez-Espinosa MJ, Fernández M, Molina-Molina JM, Prada R, Olea N. Prenatal exposure to organochlorine pesticides and TSH status in newborns from Southern Spain. Science of The Total Environment. 2011;409:3281–3287. doi: 10.1016/j.scitotenv.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Furlong CE, Holland N, Richter RJ, Bradman A, Ho A, Eskenazi B. PON1 status of farmworker mothers and children as a predictor of organophosphate sensitivity. Pharmacogenetics and Genomics. 2006;16:183–190. doi: 10.1097/01.fpc.0000189796.21770.d3. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Crumpton TL, Slotkin TA. Does the developmental neurotoxicity of chlorpyrifos involve glial targets? Macromolecule synthesis, adenylyl cyclase signaling, nuclear transcription factors, and formation of reactive oxygen in C6 glioma cells. Brain Research. 2001;891:54–68. doi: 10.1016/s0006-8993(00)03189-9. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Slotkin TA. Developmental neurotoxicity elicited by prenatal or postnatal chlorpyrifos exposure: effects on neurospecific proteins indicate changing vulnerabilities. Environmental Health Perspectives. 2003;111:297–303. doi: 10.1289/ehp.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Bowers WJ, Nakai JS, Yagminas A, Mueller R, Pulido O. Effects of Environmentally Relevant Mixtures of Persistent Organic Pollutants on the Developmental Neurobiology in Rats. Toxicologic Pathology. 2013;41:38–47. doi: 10.1177/0192623312451370. [DOI] [PubMed] [Google Scholar]
- Gomes MdS, Bernardi MM, Spinosa HdS. Pyrethroid insecticides and pregnancy: effect on physical and behavioral development of rats. Vet Hum Toxicol. 1991;33:315–317. [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. The Lancet Neurology. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo-Ross SX, Chambers JE, Meek EC, Carr RL. Altered Muscarinic Acetylcholine Receptor Subtype Binding in Neonatal Rat Brain following Exposure to Chlorpyrifos or Methyl Parathion. Toxicological Sciences. 2007;100:118–127. doi: 10.1093/toxsci/kfm195. [DOI] [PubMed] [Google Scholar]
- Guodong D, Pei W, Ying T, Jun Z, Yu G, Xiaojin W, Rong S, Guoquan W, Xiaoming S. Organophosphate Pesticide Exposure and Neurodevelopment in Young Shanghai Children. Environmental Science & Technology. 2012;46:2911–2917. doi: 10.1021/es202583d. [DOI] [PubMed] [Google Scholar]
- Gupta A, Agarwal R, Shukla GS. Functional impairment of blood-brain barrier following pesticide exposure during early development in rats. Human & Experimental Toxicology. 1999a;18:174–179. doi: 10.1177/096032719901800307. [DOI] [PubMed] [Google Scholar]
- Gupta A, Nigam D, Gupta A, Shukla GS, Agarwal AK. Effect of pyrethroid-based liquid mosquito repellent inhalation on the blood–brain barrier function and oxidative damage in selected organs of developing rats. Journal of Applied Toxicology. 1999b;19:67–72. doi: 10.1002/(sici)1099-1263(199901/02)19:1<67::aid-jat540>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Gurunathan S, Robson M, Freeman N, Buckley B, Roy A, Meyer R, Bukowski J, Lioy PJ. Accumulation of chlorpyrifos on residential surfaces and toys accessible to children. Environmental Health Perspectives. 1998;106:9–16. doi: 10.1289/ehp.981069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari R, Julvez J, Murata K, Barr D, Bellinger DC, Debes F, Grandjean P. Neurobehavioral Deficits and Increased Blood Pressure in School-Age Children Prenatally Exposed to Pesticides. Environmental Health Perspectives. 2010;118:890–896. doi: 10.1289/ehp.0901582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher JM, Pennell KD, Miller GW. Parkinson’s disease and pesticides: a toxicological perspective. Trends in Pharmacological Sciences. 2008;29:322–329. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr DW, Mwanza JC, Lyke DF, Graff JE, Moser VC, Padilla S. Relationship between brain and plasma carbaryl levels and cholinesterase inhibition. Toxicology. 2010;276:172–183. doi: 10.1016/j.tox.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Heusinkveld HJ, Thomas GO, Lamot I, van den Berg M, Kroese ABA, Westerink RHS. Dual actions of lindane (γ-hexachlorocyclohexane) on calcium homeostasis and exocytosis in rat PC12 cells. Toxicology and Applied Pharmacology. 2010;248:12–19. doi: 10.1016/j.taap.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Heusinkveld HJ, Westerink RHS. Organochlorine Insecticides Lindane and Dieldrin and Their Binary Mixture Disturb Calcium Homeostasis in Dopaminergic PC12 Cells. Environmental Science & Technology. 2012;46:1842–1848. doi: 10.1021/es203303r. [DOI] [PubMed] [Google Scholar]
- Hong S, Hwang J, Kim JY, Shin KS, Kang SJ. Heptachlor induced nigral dopaminergic neuronal loss and Parkinsonism-like movement deficits in mice. Exp Mol Med. 2014;46:e80. doi: 10.1038/emm.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MK, Rundle A, Camann DE, Boyd Barr D, Rauh VA, Whyatt RM. Impact of Prenatal Exposure to Piperonyl Butoxide and Permethrin on 36-Month Neurodevelopment. Pediatrics. 2011;127:e699–e706. doi: 10.1542/peds.2010-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Harley K, Bradman A, Eskenazi B, Holland N. Longitudinal Changes in PON1 Enzymatic Activities in Mexican-American Mothers and Children with Different Genotypes and Haplotypes. Toxicology and applied pharmacology. 2010;244:181–189. doi: 10.1016/j.taap.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DL, Lassiter TL, Padilla S. Gestational Exposure to Chlorpyrifos: Comparative Distribution of Trichloropyridinol in the Fetus and Dam. Toxicology and Applied Pharmacology. 1999;158:16–23. doi: 10.1006/taap.1999.8689. [DOI] [PubMed] [Google Scholar]
- Husain R, Malaviya M, Seth PK, Husain R. Differential responses of regional brain polyamines following in utero exposure to synthetic pyrethroid insecticides: a preliminary report. Bull Environ Contam Toxicol. 1992;49:402–409. doi: 10.1007/BF01239644. [DOI] [PubMed] [Google Scholar]
- Husain R, Malaviya M, Seth PK, Husain R. Effect of Deltamethrin on Regional Brain Polyamines and Behaviour in Young Rats. Pharmacology & Toxicology. 1994;74:211–215. doi: 10.1111/j.1600-0773.1994.tb01100.x. [DOI] [PubMed] [Google Scholar]
- Icenogle LM, Christopher NC, Blackwelder WP, Caldwell DP, Qiao D, Seidler FJ, Slotkin TA, Levin ED. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicology and Teratology. 2004;26:95–101. doi: 10.1016/j.ntt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Ihara D, Fukuchi M, Honma D, Takasaki I, Ishikawa M, Tabuchi A, Tsuda M. Deltamethrin, a type II pyrethroid insecticide, has neurotrophic effects on neurons with continuous activation of the Bdnf promoter. Neuropharmacology. 2012;62:1091–1098. doi: 10.1016/j.neuropharm.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Imamura L, Yasuda M, Kuramitsu K, Hara D, Tabuchi A, Tsuda M. Deltamethrin, a Pyrethroid Insecticide, Is a Potent Inducer for the Activity-Dependent Gene Expression of Brain-Derived Neurotrophic Factor in Neurons. Journal of Pharmacology and Experimental Therapeutics. 2006;316:136–143. doi: 10.1124/jpet.105.092478. [DOI] [PubMed] [Google Scholar]
- Imanishi S, Okura M, Zaha H, Yamamoto T, Akanuma H, Nagano R, Shiraishi H, Fujimaki H, Sone H. Prenatal exposure to permethrin influences vascular development of fetal brain and adult behavior in mice offspring. Environmental Toxicology. 2013;28:617–629. doi: 10.1002/tox.20758. [DOI] [PubMed] [Google Scholar]
- INCHEM; Cancer I.A.f.R.o, editor. Pesticide residues in food 2000: DELTAMETHRIN. 2000 http://www.inchem.org/documents/jmpr/jmpmono/v00pr04.htm-_00042261.
- Ingelman-Sundberg M, Persson A, Jukic MM. Polymorphic expression of CYP2C19 and CYP2D6 in the developing and adult human brain causing variability in cognition, risk for depression and suicide: the search for the endogenous substrates. Pharmacogenomics. 2014;15:1841–1844. doi: 10.2217/pgs.14.151. [DOI] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos Affects Phenotypic Outcomes in a Model of Mammalian Neurodevelopment: Critical Stages Targeting Differentiation in PC12 Cells. Environmental Health Perspectives. 2006;114:667–672. doi: 10.1289/ehp.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke P, Nauen R, Schindler M, Elbert A. Overview of the Status and Global Strategy for Neonicotinoids. Journal of Agricultural and Food Chemistry. 2011;59:2897–2908. doi: 10.1021/jf101303g. [DOI] [PubMed] [Google Scholar]
- Jett DA, Navoa RV, Beckles RA, McLemore GL. Cognitive Function and Cholinergic Neurochemistry in Weanling Rats Exposed to Chlorpyrifos. Toxicology and Applied Pharmacology. 2001;174:89–98. doi: 10.1006/taap.2001.9198. [DOI] [PubMed] [Google Scholar]
- Jia Z, Misra HP. Developmental exposure to pesticides zineb and/or endosulfan renders the nigrostriatal dopamine system more susceptible to these environmental chemicals later in life. NeuroToxicology. 2007;28:727–735. doi: 10.1016/j.neuro.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Jin Y, Liu Z, Peng T, Fu Z. The toxicity of chlorpyrifos on the early life stage of zebrafish: A survey on the endpoints at development, locomotor behavior, oxidative stress and immunotoxicity. Fish & Shellfish Immunology. 2015;43:405–414. doi: 10.1016/j.fsi.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Johansson U, Fredriksson A, Eriksson P. Bioallethrin causes permanent changes in behavioural and muscarinic acetylcholine receptor variables in adult mice exposed neonatally to DDT. European Journal of Pharmacology: Environmental Toxicology and Pharmacology. 1995;293:159–166. doi: 10.1016/0926-6917(95)00012-7. [DOI] [PubMed] [Google Scholar]
- Johnson FO, Chambers JE, Nail CA, Givaruangsawat S, Carr RL. Developmental Chlorpyrifos and Methyl Parathion Exposure Alters Radial-Arm Maze Performance in Juvenile and Adult Rats. Toxicological Sciences. 2009;109:132–142. doi: 10.1093/toxsci/kfp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri A, Yadav S, Dhawan A, Parmar D. Overexpression of cerebral and hepatic cytochrome P450s alters behavioral activity of rat offspring following prenatal exposure to lindane. Toxicology and Applied Pharmacology. 2007;225:278–292. doi: 10.1016/j.taap.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Johri A, Yadav S, Singh RL, Dhawan A, Ali M, Parmar D. Long lasting effects of prenatal exposure to deltamethrin on cerebral and hepatic cytochrome P450s and behavioral activity in rat offspring. European Journal of Pharmacology. 2006;544:58–68. doi: 10.1016/j.ejphar.2006.06.042. [DOI] [PubMed] [Google Scholar]
- Keil AP, Daniels JL, Hertz-Picciotto I. Autism spectrum disorder, flea and tick medication, and adjustments for exposure misclassification: the CHARGE (CHildhood Autism Risks from Genetics and Environment) case–control study. Environmental Health. 2014;13:3–3. doi: 10.1186/1476-069X-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KB, Anand SS, Kim HJ, White CA, Fisher JW, Tornero-Velez R, Bruckner JV. Age, Dose, and Time-Dependency of Plasma and Tissue Distribution of Deltamethrin in Immature Rats. Toxicological Sciences. 2010;115:354–368. doi: 10.1093/toxsci/kfq074. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim JE, Ko BH, Moon IS. Carbofuran Induces Apoptosis of Rat Cortical Neurons and Down-Regulates Surface a7 Subunit of Acetylcholine Receptors. Mol Cells. 2004;17:242–247. [PubMed] [Google Scholar]
- Kimura-Kuroda J, Komuta Y, Kuroda Y, Hayashi M, Kawano H. Nicotine-Like Effects of the Neonicotinoid Insecticides Acetamiprid and Imidacloprid on Cerebellar Neurons from Neonatal Rats. PLoS ONE. 2012;7:e32432. doi: 10.1371/journal.pone.0032432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, Casarett LJ. Casarett and Doull’s Toxicology: The Basic Science of Poisons. Blacklick, OH, USA: McGraw-Hill Professional Publishing; 2007. [Google Scholar]
- Kumar K, Patro N, Patro I. Impaired Structural and Functional Development of Cerebellum Following Gestational Exposure of Deltamethrin in Rats: Role of Reelin. Cellular and Molecular Neurobiology. 2013;33:731–746. doi: 10.1007/s10571-013-9942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente A, Pereiro N. Neurotoxic effects induced by endosulfan exposure during pregnancy and lactation in female and male rat striatum. Toxicology. 2013;311:35–40. doi: 10.1016/j.tox.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Lakshmana MK, Raju TR. Endosulfan induces small but significant changes in the levels of noradrenaline, dopamine and serotonin in the developing rat brain and deficits in the operant learning performance. Toxicology. 1994;91:139–150. doi: 10.1016/0300-483x(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Laskowski D. Physical and Chemical Properties of Pyrethroids. In: Ware G, editor. Reviews of Environmental Contamination and Toxicology. Springer; New York: 2002. [DOI] [PubMed] [Google Scholar]
- Lassiter TL, Padilla S, Mortensen SR, Chanda SM, Moser VC, Barone S., Jr Gestational Exposure to Chlorpyrifos: Apparent Protection of the Fetus? Toxicology and Applied Pharmacology. 1998;152:56–65. doi: 10.1006/taap.1998.8514. [DOI] [PubMed] [Google Scholar]
- Lazarini CA, Florio JC, Lemonica IP, Bernardi MM. Effects of prenatal exposure to deltamethrin on forced swimming behavior, motor activity, and striatal dopamine levels in male and female rats. Neurotoxicology and Teratology. 2001;23:665–673. doi: 10.1016/s0892-0362(01)00170-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicology and Teratology. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Nakajima A, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Developmental Brain Research. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Cauley M, Johnson JE, Cooper EM, Stapleton HM, Ferguson PL, Seidler FJ, Slotkin TA. Prenatal Dexamethasone Augments the Neurobehavioral Teratology of Chlorpyrifos: Significance for Maternal Stress and Preterm Labor. Neurotoxicology and teratology. 2014;41:35–42. doi: 10.1016/j.ntt.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Cerutti DT. Behavioral Neuroscience of Zebrafish. In: Buccafusco J, editor. Methods of Behavior Analysis in Neuroscience. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. [PubMed] [Google Scholar]
- Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicology and Teratology. 2003;25:51–57. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Swain HA, Donerly S, Linney E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicology and Teratology. 2004;26:719–723. doi: 10.1016/j.ntt.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Lin PC, Lin HJ, Liao YY, Guo HR, Chen KT. Acute Poisoning with Neonicotinoid Insecticides: A Case Report and Literature Review. Basic & Clinical Pharmacology & Toxicology. 2013;112:282–286. doi: 10.1111/bcpt.12027. [DOI] [PubMed] [Google Scholar]
- Lindhe O, Lund BO, Bergman A, Brandt I. Irreversible binding and adrenocorticolytic activity of the DDT metabolite 3-methylsulfonyl-DDE examined in tissue-slice culture. Environmental Health Perspectives. 2001;109:105–110. doi: 10.1289/ehp.109-1240628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Shi Y, Li H, Wang Y, Yang K. p,p′-DDE Disturbs the Homeostasis of Thyroid Hormones via Thyroid Hormone Receptors, Transthyretin, and Hepatic Enzymes. Horm Metab Res. 2011;43:391–396. doi: 10.1055/s-0031-1277135. [DOI] [PubMed] [Google Scholar]
- Lovasi GS, Quinn JW, Rauh VA, Perera FP, Andrews HF, Garfinkel R, Hoepner L, Whyatt R, Rundle A. Chlorpyrifos Exposure and Urban Residential Environment Characteristics as Determinants of Early Childhood Neurodevelopment. American journal of public health. 2011;101:63–70. doi: 10.2105/AJPH.2009.168419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Essig C, Root C, Rohlman DS, McDonald T, Sulzbacher S. Assessing the association between pesticide exposure and cognitive development in rural Costa Rican children living in organic and conventional coffee farms. Int J Adolesc Med Health. 2009;21:609–621. doi: 10.1515/ijamh.2009.21.4.609. [DOI] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, Eskenazi B. Organophosphate Pesticide Exposure and Attention in Young Mexican-American Children: The CHAMACOS Study. Environmental Health Perspectives. 2010;118:1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends in Pharmacological Sciences. 2001;22:573–580. doi: 10.1016/s0165-6147(00)01820-4. [DOI] [PubMed] [Google Scholar]
- Maurya SK, Mishra J, Tripathi VK, Sharma R, Siddiqui MH. Cypermethrin induces astrocyte damage: Role of aberrant Ca2+, ROS, JNK, P38, matrix metalloproteinase 2 and migration related reelin protein. Pesticide Biochemistry and Physiology. 2014;111:51–59. doi: 10.1016/j.pestbp.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Meacham CA, Brodfuehrer PD, Watkins JA, Shafer TJ. Developmentally-regulated sodium channel subunits are differentially sensitive to α-cyano containing pyrethroids. Toxicology and Applied Pharmacology. 2008;231:273–281. doi: 10.1016/j.taap.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Meijer M, Brandsema JAR, Nieuwenhuis D, Wijnolts FMJ, Dingemans MML, Westerink RHS. Inhibition of Voltage-Gated Calcium Channels After Subchronic and Repeated Exposure of PC12 Cells to Different Classes of Insecticides. Toxicological Sciences. 2015;147:607–617. doi: 10.1093/toxsci/kfv154. [DOI] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Aldridge JE, Tate CA, Cousins MM, Slotkin TA. Critical periods for chlorpyrifos-induced developmental neurotoxicity: alterations in adenylyl cyclase signaling in adult rat brain regions after gestational or neonatal exposure. Environmental Health Perspectives. 2004;112:295–301. doi: 10.1289/ehp.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Cousins MM, Slotkin TA. Developmental neurotoxicity elicited by gestational exposure to chlorpyrifos: when is adenylyl cyclase a target? Environmental Health Perspectives. 2003;111:1871–1876. doi: 10.1289/ehp.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DA, Carter JM, Johnstone AFM, Shafer TJ. Pyrethroid modulation of spontaneous neuronal excitability and neurotransmission in hippocampal neurons in culture. NeuroToxicology. 2008;29:213–225. doi: 10.1016/j.neuro.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Mishra D, Tiwari SK, Agarwal S, Sharma VP, Chaturvedi RK. Prenatal Carbofuran Exposure Inhibits Hippocampal Neurogenesis and Causes Learning and Memory Deficits in Offspring. Toxicological Sciences. 2012;127:84–100. doi: 10.1093/toxsci/kfs004. [DOI] [PubMed] [Google Scholar]
- Morrison G. Penetration of the blood-brain-cerebral spinal fluid barrier by DDT. Bull Environ Contam Toxicol. 1971;6:48–54. doi: 10.1007/BF01559073. [DOI] [PubMed] [Google Scholar]
- Moser VC. Comparison of Aldicarb and Methamidophos Neurotoxicity at Different Ages in the Rat: Behavioral and Biochemical Parameters. Toxicology and Applied Pharmacology. 1999;157:94–106. doi: 10.1006/taap.1999.8675. [DOI] [PubMed] [Google Scholar]
- Moser VC, Chanda SM, Mortensen SR, Padilla S. Age- and Gender-Related Differences in Sensitivity to Chlorpyrifos in the Rat Reflect Developmental Profiles of Esterase Activities. Toxicological Sciences. 1998a;46:211–222. doi: 10.1006/toxs.1998.2526. [DOI] [PubMed] [Google Scholar]
- Moser VC, McDaniel KL, Phillips PM, Lowit AB. Time-Course, Dose-Response, and Age Comparative Sensitivity of N-Methyl Carbamates in Rats. Toxicological Sciences. 2010;114:113–123. doi: 10.1093/toxsci/kfp286. [DOI] [PubMed] [Google Scholar]
- Moser VC, Padilla S, Hunter DL, Marshall RS, McDaniel KL, Phillips PM. Age- and Gender-Related Differences in the Time Course of Behavioral and Biochemical Effects Produced by Oral Chlorpyrifos in Rats. Toxicology and Applied Pharmacology. 1998b;149:107–119. doi: 10.1006/taap.1997.8354. [DOI] [PubMed] [Google Scholar]
- Moser VC, Phillips PM, McDaniel KL. Assessment of biochemical and behavioral effects of carbaryl and methomyl in Brown-Norway rats from preweaning to senescence. Toxicology. 2015;331:1–13. doi: 10.1016/j.tox.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Moser VC, Phillips PM, McDaniel KL, Zehr RD, MacMillan DK, MacPhail RC. Carbaryl and 1-Naphthol Tissue Levels and Related Cholinesterase Inhibition in Male Brown Norway Rats from Preweaning to Senescence. Journal of Toxicology and Environmental Health, Part A. 2013;76:1151–1167. doi: 10.1080/15287394.2013.844751. [DOI] [PubMed] [Google Scholar]
- Moser VC, Shafer TJ, Ward TR, Meacham CA, Harris MW, Chapin RE. Neurotoxicological Outcomes of Perinatal Heptachlor Exposure in the Rat. Toxicological Sciences. 2001;60:315–326. doi: 10.1093/toxsci/60.2.315. [DOI] [PubMed] [Google Scholar]
- Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology. 2013;307:74–88. doi: 10.1016/j.tox.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Muñoz-Quezada MT, Lucero BA, Barr DB, Steenland K, Levy K, Ryan PB, Iglesias V, Alvarado S, Concha C, Rojas E, Vega C. Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: A systematic review. Neurotoxicology. 2013;39:158–168. doi: 10.1016/j.neuro.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Song JH, Shono T, Narahashi T. Modulation of the Neuronal Nicotinic Acetylcholine Receptor-Channel by the Nitromethylene Heterocycle Imidacloprid. Journal of Pharmacology and Experimental Therapeutics. 1998;285:731–738. [PubMed] [Google Scholar]
- Naseh M, Vatanparast J. Enhanced expression of hypothalamic nitric oxide synthase in rats developmentally exposed to organophosphates. Brain Research. 2014;1579:10–19. doi: 10.1016/j.brainres.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Naseh M, Vatanparast J, Baniasadi M, Hamidi GA. Alterations in nitric oxide synthase-expressing neurons in the forebrain regions of rats after developmental exposure to organophosphates. Neurotoxicology and Teratology. 2013;37:23–32. doi: 10.1016/j.ntt.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Nasuti C, Carloni M, Fedeli D, Gabbianelli R, Di Stefano A, Laura Serafina C, Silva I, Domingues V, Ciccocioppo R. Effects of early life permethrin exposure on spatial working memory and on monoamine levels in different brain areas of pre-senescent rats. Toxicology. 2013;303:162–168. doi: 10.1016/j.tox.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Nasuti C, Gabbianelli R, Falcioni ML, Di Stefano A, Sozio P, Cantalamessa F. Dopaminergic system modulation, behavioral changes, and oxidative stress after neonatal administration of pyrethroids. Toxicology. 2007;229:194–205. doi: 10.1016/j.tox.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Needham LL, Barr DB, Caudill SP, Pirkle JL, Turner WE, Osterloh J, Jones RL, Sampson EJ. Concentrations of Environmental Chemicals Associated with Neurodevelopmental Effects in U.S. Population. NeuroToxicology. 2005;26:531–545. doi: 10.1016/j.neuro.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Nordquist N, Oreland L. Serotonin, genetic variability, behaviour, and psychiatric disorders - a review. Upsala Journal of Medical Sciences. 2010;115:2–10. doi: 10.3109/03009730903573246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S-i, Lipton SA. S-Nitrosylation in neurogenesis and neuronal development. Biochimica et Biophysica Acta (BBA) - General Subjects. 2015;1850:1588–1593. doi: 10.1016/j.bbagen.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio-Valencia E, Torres-Sánchez L, López-Carrillo L, Cebrián ME, Rothenberg SJ, Hernández Chavez MdC, Schnaas L. Prenatal p,p′-DDE exposure and establishment of lateralization and spatial orientation in Mexican preschooler. Neurotoxicology. 2015;47:1–7. doi: 10.1016/j.neuro.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrea EM, Bielawski DM, Posecion NC, Corrion M, Villanueva-Uy E, Bernardo RC, Jin Y, Janisse JJ, Ager JW. Combined analysis of prenatal (maternal hair and blood) and neonatal (infant hair, cord blood and meconium) matrices to detect fetal exposure to environmental pesticides. Environmental research. 2009;109:116–122. doi: 10.1016/j.envres.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrea EM, Jr, Reyes A, Villanueva-Uy E, Pacifico R, Benitez B, Ramos E, Bernardo RC, Bielawski DM, Delaney-Black V, Chiodo L, Janisse JJ, Ager JW. Fetal exposure to propoxur and abnormal child neurodevelopment at 2 years of age. NeuroToxicology. 2012;33:669–675. doi: 10.1016/j.neuro.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Bouchard MF. Urinary Metabolites of Organophosphate and Pyrethroid Pesticides and Behavioral Problems in Canadian Children. Environmental Health Perspectives. 2013;121:1378–1384. doi: 10.1289/ehp.1306667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdemir HH, Kara M, Yumrutas O, Uckardes F, Eraslan E, Demir CF, Bal R. Determination of the effects on learning and memory performance and related gene expressions of clothianidin in rat models. Cognitive Neurodynamics. 2014;8:411–416. doi: 10.1007/s11571-014-9293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent AS, Naveau E, Gerard A, Bourguignon JP, Westbrook GL. Early developmental actions of endocrine disruptors on the hypothalamus, hippocampus and cerebral cortex. Journal of toxicology and environmental health Part B, Critical reviews. 2011;14:328–345. doi: 10.1080/10937404.2011.578556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro N, Shrivastava M, Tripathi S, Patro IK. S100β upregulation: A possible mechanism of deltamethrin toxicity and motor coordination deficits. Neurotoxicology and Teratology. 2009;31:169–176. doi: 10.1016/j.ntt.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Petroianu GA. History of organophosphate synthesis: the very early days. Pharmazie. 2010;65:306–311. [PubMed] [Google Scholar]
- Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, Kreutzweiser DP, Krupke C, Liess M, McField M, Morrissey CA, Noome DA, Settele J, Simon-Delso N, Stark JD, Van der Sluijs JP, Van Dyck H, Wiemers M. Effects of neonicotinoids and fipronil on non-target invertebrates. Environmental Science and Pollution Research. 2015;22:68–102. doi: 10.1007/s11356-014-3471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CN, Chakraborti TK. Dose-related inhibition of brain and plasma cholinesterase in neonatal and adult rats following sublethal organophosphate exposures. Toxicology. 1992;73:35–43. doi: 10.1016/0300-483x(92)90168-e. [DOI] [PubMed] [Google Scholar]
- Pope CN, Chakraborti TK, Chapman ML, Farrar JD, Arthun D. Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothioate insecticides. Toxicology. 1991;68:51–61. doi: 10.1016/0300-483x(91)90061-5. [DOI] [PubMed] [Google Scholar]
- Puertas R, Lopez-Espinosa MJ, Cruz F, Ramos R, Freire C, Pérez-García M, Abril A, Julvez J, Salvatierra M, Campoy C, Olea N. Prenatal exposure to mirex impairs neurodevelopment at age of 4 years. NeuroToxicology. 2010;31:154–160. doi: 10.1016/j.neuro.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Abreu-Villaça Y, Tate CA, Cousins MM, Slotkin TA. Chlorpyrifos exposure during neurulation: cholinergic synaptic dysfunction and cellular alterations in brain regions at adolescence and adulthood. Developmental Brain Research. 2004;148:43–52. doi: 10.1016/j.devbrainres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos modeled in vitro: comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environmental Health Perspectives. 2001;109:909–913. doi: 10.1289/ehp.01109909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environmental Health Perspectives. 2003;111:536–544. doi: 10.1289/ehp.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirós-Alcalá L, Mehta S, Eskenazi B. Pyrethroid Pesticide Exposure and Parental Report of Learning Disability and Attention Deficit/Hyperactivity Disorder in U.S. Children: NHANES 1999–2002. Environmental Health Perspectives. 2014;122:1336–1342. doi: 10.1289/ehp.1308031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines KW, Seidler FJ, Slotkin TA. Alterations in serotonin transporter expression in brain regions of rats exposed neonatally to chlorpyrifos. Developmental Brain Research. 2001;130:65–72. doi: 10.1016/s0165-3806(01)00211-5. [DOI] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. Seven-Year Neurodevelopmental Scores and Prenatal Exposure to Chlorpyrifos, a Common Agricultural Pesticide. Environmental Health Perspectives. 2011;119:1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. Impact of Prenatal Chlorpyrifos Exposure on Neurodevelopment in the First 3 Years of Life Among Inner-City Children. Pediatrics. 2006;118:e1845–e1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Liu J, Barr DB, Slotkin TA, Peterson BS. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7871–7876. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Liu J, Ayoubi P, Pope C. DOSE-RELATED GENE EXPRESSION CHANGES IN FOREBRAIN FOLLOWING ACUTE, LOW-LEVEL CHLORPYRIFOS EXPOSURE IN NEONATAL RATS. Toxicology and applied pharmacology. 2010;248:144–155. doi: 10.1016/j.taap.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiler E, Jørs E, Bælum J, Huici O, Alvarez Caero MM, Cedergreen N. The influence of tomato processing on residues of organochlorine and organophosphate insecticides and their associated dietary risk. Science of The Total Environment. 2015;527–528:262–269. doi: 10.1016/j.scitotenv.2015.04.081. [DOI] [PubMed] [Google Scholar]
- Ribas-Fitó N, Cardo E, Sala M, Eulàlia de Muga M, Mazón C, Verdú A, Kogevinas M, Grimalt JO, Sunyer J. Breastfeeding, Exposure to Organochlorine Compounds, and Neurodevelopment in Infants. Pediatrics. 2003;111:e580–e585. doi: 10.1542/peds.111.5.e580. [DOI] [PubMed] [Google Scholar]
- Ribas-Fitó N, Torrent M, Carrizo D, Muñoz-Ortiz L, Júlvez J, Grimalt JO, Sunyer J. In Utero Exposure to Background Concentrations of DDT and Cognitive Functioning among Preschoolers. American Journal of Epidemiology. 2006;164:955–962. doi: 10.1093/aje/kwj299. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Markina N, Valanzano A, Fortuna S, Cometa MF, Meneguz A, Calamandrei G. Developmental exposure to chlorpyrifos alters reactivity to environmental and social cues in adolescent mice. Toxicology and Applied Pharmacology. 2003;191:189–201. doi: 10.1016/s0041-008x(03)00229-1. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Venerosi A, Capone F, Cometa MF, Lorenzini P, Fortuna S, Calamandrei G. Developmental Neurotoxicity of Organophosphorous Pesticides: Fetal and Neonatal Exposure to Chlorpyrifos Alters Sex-Specific Behaviors at Adulthood in Mice. Toxicological Sciences. 2006;93:105–113. doi: 10.1093/toxsci/kfl032. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. The FASEB Journal. 2006;20:1695–1697. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang MZ, Dean ED, Pennell KD, Miller GW. Developmental heptachlor exposure increases susceptibility of dopamine neurons to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)in a gender-specific manner. NeuroToxicology. 2008;29:855–863. doi: 10.1016/j.neuro.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Taylor MM, Shalat SL, Guillot TS, Caudle WM, Hossain MM, Mathews TA, Jones SR, Cory-Slechta DA, Miller GW. Developmental pesticide exposure reproduces features of attention deficit hyperactivity disorder. The FASEB Journal. 2015;29:1960–1972. doi: 10.1096/fj.14-260901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richendrfer H, Creton R. Chlorpyrifos and malathion have opposite effects on behaviors and brain size that are not correlated to changes in AChE activity. NeuroToxicology. 2015;49:50–58. doi: 10.1016/j.neuro.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richendrfer H, Pelkowski SD, Colwill RM, Créton R. Developmental sub-chronic exposure to chlorpyrifos reduces anxiety-related behavior in zebrafish larvae. Neurotoxicology and teratology. 2012;34:458–465. doi: 10.1016/j.ntt.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera S, Rosa R, Martínez E, Suñol C, Serrano MT, Vendrell M, Rodríguez-Farré E, Sanfeliu C. Behavioral and Monoaminergic Changes After Lindane Exposure in Developing Rats. Neurotoxicology and Teratology. 1998;20:155–160. doi: 10.1016/s0892-0362(97)00079-2. [DOI] [PubMed] [Google Scholar]
- Rivera S, Sanfeliu C, Rodríguez-Farré E. Behavioral changes induced in developing rats by an early postnatal exposure to lindane. Neurotoxicology and Teratology. 1990;12:591–595. doi: 10.1016/0892-0362(90)90067-m. [DOI] [PubMed] [Google Scholar]
- Rivera S, Sanfeliu C, Suñol C, Rodríguez-Farré E. Regional effects on the cerebral concentration of noradrenaline, serotonin and dopamine in suckling rats after a single dose of lindane. Toxicology. 1991;69:43–54. doi: 10.1016/0300-483x(91)90152-q. [DOI] [PubMed] [Google Scholar]
- Rogan WJ, Gladen BC, McKinney JD, Carreras N, Hardy P, Thullen J, Tinglestad J, Tully M. Neonatal effects of transplacental exposure to PCBs and DDE. The Journal of Pediatrics. 1986;109:335–341. doi: 10.1016/s0022-3476(86)80397-3. [DOI] [PubMed] [Google Scholar]
- Roy TS, Andrews JE, Seidler FJ, Slotkin TA. Chlorpyrifos elicits mitotic abnormalities and apoptosis in neuroepithelium of cultured rat embryos. Teratology. 1998;58:62–68. doi: 10.1002/(SICI)1096-9926(199808)58:2<62::AID-TERA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Roy TS, Sharma V, Seidler FJ, Slotkin TA. Quantitative morphological assessment reveals neuronal and glial deficits in hippocampus after a brief subtoxic exposure to chlorpyrifos in neonatal rats. Developmental Brain Research. 2005;155:71–80. doi: 10.1016/j.devbrainres.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Rubio-Infante N, Moreno-Fierros L. An overview of the safety and biological effects of Bacillus thuringiensis Cry toxins in mammals. Journal of Applied Toxicology. 2016;36:630–648. doi: 10.1002/jat.3252. [DOI] [PubMed] [Google Scholar]
- Ruckart PZ, Kakolewski K, Bove FJ, Kaye WE. Long-term neurobehavioral health effects of methyl parathion exposure in children in Mississippi and Ohio. Environmental Health Perspectives. 2004;112:46–51. doi: 10.1289/ehp.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino L, Scordino M, Pantò V, Chiappara E, Traulo P, Gagliano G. Survey of neonicotinoids and fipronil in corn seeds for agriculture. Food Additives & Contaminants: Part B. 2013;6:11–16. doi: 10.1080/19393210.2012.717969. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA. Prenatal Organochlorine Exposure and Behaviors Associated With Attention Deficit Hyperactivity Disorder in School-Aged Children. American Journal of Epidemiology. 2010;171:593–601. doi: 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Lizardi P, O’Rourke MK, Morris RJ. The Effects of Organophosphate Pesticide Exposure on Hispanic Children’s Cognitive and Behavioral Functioning. Journal of Pediatric Psychology. 2008;33:91–101. doi: 10.1093/jpepsy/jsm047. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Facca A, Basit A, Song S. Toxicity of Dieldrin for Dopaminergic Neurons in Mesencephalic Cultures. Experimental Neurology. 1998;150:263–271. doi: 10.1006/exnr.1997.6770. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ. Cognitive effects of endocrine-disrupting chemicals in animals. Environmental Health Perspectives. 2001;109:1197–1206. doi: 10.1289/ehp.011091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano MT, Vendrell M, Rivera S, Serratosa J, Rodríguez-Farré E. Effect of lindane on the myelination process in the rat. Neurotoxicology and Teratology. 1990;12:577–583. doi: 10.1016/0892-0362(90)90065-k. [DOI] [PubMed] [Google Scholar]
- Shahabi HN, Andersson DR, Nissbrandt H. Cytochrome P450 2E1 in the substantia nigra: Relevance for dopaminergic neurotransmission and free radical production. Synapse. 2008;62:379–388. doi: 10.1002/syn.20505. [DOI] [PubMed] [Google Scholar]
- Sheets LP, Doherty JD, Law MW, Reiter LW, Crofton KM. Age-Dependent Differences in the Susceptibility of Rats to Deltamethrin. Toxicology and Applied Pharmacology. 1994;126:186–190. doi: 10.1006/taap.1994.1106. [DOI] [PubMed] [Google Scholar]
- Sheets LP, Lib AA, Minnemac DJ, Collierd RH, Creeke MR, Pefferc RC. A critical review of neonicotinoid insecticides for developmental neurotoxicity. Critical Reviews in Toxicology. 2016;46:153–190. doi: 10.3109/10408444.2015.1090948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, Hansen RL, Hertz-Picciotto I. Neurodevelopmental Disorders and Prenatal Residential Proximity to Agricultural Pesticides: The CHARGE Study. Environmental Health Perspectives. 2014;122:1103–1109. doi: 10.1289/ehp.1307044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Main KM, Virtanen HE, Damggard IN, Haavisto AM, Kaleva M, Boisen KA, Schmidt IM, Chellakooty M, Skakkebaek NE, Toppari J, Schramm KW. From mother to child: Investigation of prenatal and postnatal exposure to persistent bioaccumulating toxicants using breast milk and placenta biomonitoring. Chemosphere. 2007;67:S256–S262. doi: 10.1016/j.chemosphere.2006.05.106. [DOI] [PubMed] [Google Scholar]
- Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke CH, Liess M, Long E, McField M, Mineau P, Mitchell EAD, Morrissey CA, Noome DA, Pisa L, Settele J, Stark JD, Tapparo A, Van Dyck H, Van Praagh J, Van der Sluijs JP, Whitehorn PR, Wiemers M. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environmental Science and Pollution Research International. 2015;22:5–34. doi: 10.1007/s11356-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Yadav S, Srivastava V, Kumar R, Singh D, Sethumadhavan R, Parmar D. Imprinting of Cerebral and Hepatic Cytochrome P450s in Rat Offsprings Exposed Prenatally to Low Doses of Cypermethrin. Molecular Neurobiology. 2013;48:128–140. doi: 10.1007/s12035-013-8419-5. [DOI] [PubMed] [Google Scholar]
- Singh AK, Tiwari MN, Upadhyay G, Patel DK, Singh D, Prakash O, Singh MP. Long term exposure to cypermethrin induces nigrostriatal dopaminergic neurodegeneration in adult rats: postnatal exposure enhances the susceptibility during adulthood. Neurobiology of Aging. 2012;33:404–415. doi: 10.1016/j.neurobiolaging.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Sinha C, Agrawal AK, Islam F, Seth K, Chaturvedi RK, Shukla S, Seth PK. Mosquito repellent (pyrethroid-based) induced dysfunction of blood–brain barrier permeability in developing brain. International Journal of Developmental Neuroscience. 2004;22:31–37. doi: 10.1016/j.ijdevneu.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Sinha C, Seth K, Islam F, Chaturvedi RK, Shukla S, Mathur N, Srivastava N, Agrawal AK. Behavioral and neurochemical effects induced by pyrethroid-based mosquito repellent exposure in rat offsprings during prenatal and early postnatal period. Neurotoxicology and Teratology. 2006;28:472–481. doi: 10.1016/j.ntt.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Sledge D, Yen J, Morton T, Dishaw L, Petro A, Donerly S, Linney E, Levin ED. Critical Duration of Exposure for Developmental Chlorpyrifos-Induced Neurobehavioral Toxicity. Neurotoxicology and teratology. 2011;33:742–751. doi: 10.1016/j.ntt.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicology and Applied Pharmacology. 2004a;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Guidelines for Developmental Neurotoxicity and Their Impact on Organophosphate Pesticides: A Personal View from an Academic Perspective. NeuroToxicology. 2004b;25:631–640. doi: 10.1016/S0161-813X(03)00050-0. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Infante A, Seidler FJ. Prenatal Dexamethasone Augments the Sex-Selective Developmental Neurotoxicity of Chlorpyrifos: Implications for Vulnerability after Pharmacotherapy for Preterm Labor. Neurotoxicology and teratology. 2013;37:1–12. doi: 10.1016/j.ntt.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Cousins MM, Tate CA, Seidler FJ. Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Research. 2001;902:229–243. doi: 10.1016/s0006-8993(01)02387-3. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Tate CA, Seidler FJ. Screening for Developmental Neurotoxicity Using PC12 Cells: Comparisons of Organophosphates with a Carbamate, an Organochlorine, and Divalent Nickel. Environmental Health Perspectives. 2007a;115:93–101. doi: 10.1289/ehp.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Oliver CA, Seidler FJ. Critical periods for the role of oxidative stress in the developmental neurotoxicity of chlorpyrifos and terbutaline, alone or in combination. Developmental Brain Research. 2005;157:172–180. doi: 10.1016/j.devbrainres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Comparative Developmental Neurotoxicity of Organophosphates In Vivo: Transcriptional Responses of Pathways for Brain Cell Development, Cell Signaling, Cytotoxicity and Neurotransmitter Systems. Brain research bulletin. 2007;72:232–274. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Oxidative and Excitatory Mechanisms of Developmental Neurotoxicity: Transcriptional Profiles for Chlorpyrifos, Diazinon, Dieldrin, and Divalent Nickel in PC12 Cells. Environmental Health Perspectives. 2009a;117:587–596. doi: 10.1289/ehp.0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Protein kinase C is a target for diverse developmental neurotoxicants: Transcriptional responses to chlorpyrifos, diazinon, dieldrin and divalent nickel in PC12 cells. Brain Research. 2009b;1263:23–32. doi: 10.1016/j.brainres.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Developmental exposure to organophosphates triggers transcriptional changes in genes associated with Parkinson’s disease in vitro and in vivo. Brain Research Bulletin. 2011;86:340–347. doi: 10.1016/j.brainresbull.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. DEVELOPMENTAL NEUROTOXICITY OF ORGANOPHOSPHATES TARGETS CELL CYCLE AND APOPTOSIS, REVEALED BY TRANSCRIPTIONAL PROFILES IN VIVO AND IN VITRO. Neurotoxicology and Teratology. 2012;34:232–241. doi: 10.1016/j.ntt.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Prenatal Nicotine Alters the Developmental Neurotoxicity of Postnatal Chlorpyrifos Directed Toward Cholinergic Systems: Better, Worse, or Just “Different? ” Brain research bulletin. 2015;0:54–67. doi: 10.1016/j.brainresbull.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. Exposure to Organophosphates Reduces the Expression of Neurotrophic Factors in Neonatal Rat Brain Regions: Similarities and Differences in the Effects of Chlorpyrifos and Diazinon on the Fibroblast Growth Factor Superfamily. Environmental Health Perspectives. 2007b;115:909–916. doi: 10.1289/ehp.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. TARGETING OF NEUROTROPHIC FACTORS, THEIR RECEPTORS, AND SIGNALING PATHWAYS IN THE DEVELOPMENTAL NEUROTOXICITY OF ORGANOPHOSPHATES IN VIVO AND IN VITRO. Brain research bulletin. 2008a;76:424–438. doi: 10.1016/j.brainresbull.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. UNRELATED DEVELOPMENTAL NEUROTOXICANTS ELICIT SIMILAR TRANSCRIPTIONAL PROFILES FOR EFFECTS ON NEUROTROPHIC FACTORS AND THEIR RECEPTORS IN AN IN VITRO MODEL. Neurotoxicology and teratology. 2010;32:42. doi: 10.1016/j.ntt.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Ryde IT, Yanai J. DEVELOPMENTAL NEUROTOXIC EFFECTS OF CHLORPYRIFOS ON ACETYLCHOLINE AND SEROTONIN PATHWAYS IN AN AVIAN MODEL. Neurotoxicology and teratology. 2008b;30:433–439. doi: 10.1016/j.ntt.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S, Levin ED, Seidler FJ. Prenatal Nicotine Changes the Response to Postnatal Chlorpyrifos: Interactions Targeting Serotonergic Synaptic Function and Cognition. Brain research bulletin. 2015;111:84–96. doi: 10.1016/j.brainresbull.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM. Molecular Mechanisms of Pyrethroid Insecticide Neurotoxicity: Recent Advances. Archives of Toxicology. 2012;86:165–181. doi: 10.1007/s00204-011-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular Mechanisms for Developmental Toxicity of Chlorpyrifos: Targeting the Adenylyl Cyclase Signaling Cascade. Toxicology and Applied Pharmacology. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the Developmental Neurotoxicity of Chlorpyrifosin Vitro:Macromolecule Synthesis in PC12 Cells. Toxicology and Applied Pharmacology. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- Sparks TC, Nauen R. IRAC: Mode of action classification and insecticide resistance management. Pesticide Biochemistry and Physiology. 2015;121:122–128. doi: 10.1016/j.pestbp.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Singh A, Shukla RK, Khanna VK, Parmar D. Effect of prenatal exposure of lindane on alterations in the expression of cerebral cytochrome P450s and neurotransmitter receptors in brain regions. Food and Chemical Toxicology. 2015;77:74–81. doi: 10.1016/j.fct.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Himes JH, Jacobs DR, Alexander BH, Gunnar MR. Acetylcholinesterase Activity and Neurodevelopment in Boys and Girls. Pediatrics. 2013;132:e1649–e1658. doi: 10.1542/peds.2013-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Shen OX, Xu XL, Song L, Wang XR. Carbaryl, 1-naphthol and 2-naphthol inhibit the beta-1 thyroid hormone receptor-mediated transcription in vitro. Toxicology. 2008;249:238–242. doi: 10.1016/j.tox.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Syed F, John PJ, Soni I. Neurodevelopmental consequences of gestational and lactational exposure to pyrethroids in rats. Environmental Toxicology. 2015 doi: 10.1002/tox.22178. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Talts U, Fredriksson A, Eriksson P. Changes in behavior and muscarinic receptor density after neonatal and adult exposure to bioallethrin. Neurobiology of Aging. 1998;19:545–552. doi: 10.1016/s0197-4580(98)00093-1. [DOI] [PubMed] [Google Scholar]
- Tanaka T. Effects of maternal clothianidin exposure on behavioral development in F1 generation mice. Toxicology and Industrial Health. 2012a;28:697–707. doi: 10.1177/0748233711422726. [DOI] [PubMed] [Google Scholar]
- Tanaka T. Reproductive and Neurobehavioral Effects of Clothianidin Administered to Mice in the Diet. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2012b;95:151–159. doi: 10.1002/bdrb.20349. [DOI] [PubMed] [Google Scholar]
- Tang J, Carr RL, Chambers JE. Changes in rat brain cholinesterase activity and muscarinic receptor density during and after repeated oral exposure to chlorpyrifos in early postnatal development. Toxicological Sciences. 1999;51:265–272. doi: 10.1093/toxsci/51.2.265. [DOI] [PubMed] [Google Scholar]
- Tebourbi O, Driss MR, Sakly M, Rhouma KBEN. Metabolism of DDT in Different Tissues of Young Rats. Journal of Environmental Science and Health, Part B. 2006;41:167–176. doi: 10.1080/03601230500364674. [DOI] [PubMed] [Google Scholar]
- Tomizawa M, Casida JE. Imidacloprid, Thiacloprid, and Their Imine Derivatives Up-Regulate the α4β2 Nicotinic Acetylcholine Receptor in M10 Cells. Toxicology and Applied Pharmacology. 2000;169:114–120. doi: 10.1006/taap.2000.9057. [DOI] [PubMed] [Google Scholar]
- Tomizawa M, Casida JE. NEONICOTINOID INSECTICIDE TOXICOLOGY: Mechanisms of Selective Action. Annual Review of Pharmacology and Toxicology. 2005;45:247–268. doi: 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- Torres-Sánchez L, Rothenberg SJ, Schnaas L, Cebrián ME, Osorio E, del Carmen Hernández M, García-Hernández RM, del Rio-Garcia C, Wolff MS, López-Carrillo L. In Utero p,p′-DDE Exposure and Infant Neurodevelopment: A Perinatal Cohort in Mexico. Environmental Health Perspectives. 2007;115:435–439. doi: 10.1289/ehp.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Sánchez L, Schnaas L, Cebrián ME, Hernández MdC, Valencia EO, Hernández RMG, López-Carrillo L. Prenatal Dichlorodiphenyldichloroethylene (DDE) exposure and neurodevelopment: A follow-up from 12 to 30 months of age. Neurotoxicology. 2009;30:1162–1165. doi: 10.1016/j.neuro.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Sánchez L, Schnaas L, Rothenberg SJ, Cebrián ME, Osorio-Valencia E, Hernández MdC, García-Hernández RM, López-Carrillo L. Prenatal p,p′-DDE Exposure and Neurodevelopment among Children 3. 5–5 Years of Age. Environmental Health Perspectives. 2013;121:263–268. doi: 10.1289/ehp.1205034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA, U.S.D.o.A.R.I.C. A national umbrella site for the regional IMP centers. 2014 http://www.ipmcenters.org/CropProfiles/cropprofiles.cfm?cipmregion=national.
- Vale A, Lotti M. Chapter 10 - Organophosphorus and carbamate insecticide poisoning. In: Marcello L, Margit LB, editors. Handbook of Clinical Neurology. Elsevier; 2015. [DOI] [PubMed] [Google Scholar]
- Vatanparast J, Naseh M, Baniasadi M, Haghdoost-Yazdi H. Developmental exposure to chlorpyrifos and diazinon differentially affect passive avoidance performance and nitric oxide synthase-containing neurons in the basolateral complex of the amygdala. Brain Research. 2013;1494:17–27. doi: 10.1016/j.brainres.2012.11.049. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Cutuli D, Colonnello V, Cardona D, Ricceri L, Calamandrei G. Neonatal exposure to chlorpyrifos affects maternal responses and maternal aggression of female mice in adulthood. Neurotoxicology and Teratology. 2008;30:468–474. doi: 10.1016/j.ntt.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Ricceri L, Rungi A, Sanghez V, Calamandrei G. Gestational exposure to the organophosphate chlorpyrifos alters social–emotional behaviour and impairs responsiveness to the serotonin transporter inhibitor fluvoxamine in mice. Psychopharmacology. 2010;208:99–107. doi: 10.1007/s00213-009-1713-2. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Ricceri L, Scattoni ML, Calamandrei G. Prenatal chlorpyrifos exposure alters motor behavior and ultrasonic vocalization in cd-1 mouse pups. Environmental Health. 2009;8:12–12. doi: 10.1186/1476-069X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venerosi A, Tait S, Stecca L, Chiarotti F, De Felice A, Cometa MF, Volpe MT, Calamandrei G, Ricceri L. Effects of maternal chlorpyrifos diet on social investigation and brain neuroendocrine markers in the offspring – a mouse study. Environmental Health. 2015;14:32. doi: 10.1186/s12940-015-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KV, Srikant S, Thiruvikramaprakash G, Dutta TK. A fatal case of thiacloprid poisoning. The American Journal of Emergency Medicine. 2015;33:310.e315–310.e316. doi: 10.1016/j.ajem.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Wagner-Schuman M, Richardson JR, Auinger P, Braun JM, Lanphear BP, Epstein JN, Yolton K, Froehlich TE. Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of U.S. children. Environmental Health. 2015;14:44. doi: 10.1186/s12940-015-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-Dependent Dendritic Arborization Mediated by CaM-Kinase I Activation and Enhanced CREB-Dependent Transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Whitney KD, Seidler FJ, Slotkin TA. Developmental Neurotoxicity of Chlorpyrifos: Cellular Mechanisms. Toxicology and Applied Pharmacology. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- WHO; Organization W.H, editor. Lindane in Drinking-water: Background document for development of WHO Guidelines for Drinking-water Quality. WHO; 2004. http://www.who.int/water_sanitation_health/dwq/chemicals/lindane.pdf. [Google Scholar]
- WHO specifications and evaluations for public health pesticides. Chlorpyrifos. 2009 http://www.who.int/whopes/quality/Chlorpyrifos_WHO_specs_eval_Mar_2009.pdf.
- Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, Hoepner LA, Garfinkel R, Hazi Y, Reyes A, Ramirez J, Cosme Y, Perera FP. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environmental Health Perspectives. 2003;111:749–756. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Camann D, Perera FP, Rauh VA, Tang D, Kinney PL, Garfinkel R, Andrews H, Hoepner L, Barr DB. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicology and Applied Pharmacology. 2005;206:246–254. doi: 10.1016/j.taap.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, Hoepner LA, Diaz D, Dietrich J, Reyes A, Tang D, Kinney PL, Perera FP. Prenatal Insecticide Exposures and Birth Weight and Length among an Urban Minority Cohort. Environmental Health Perspectives. 2004;112:1125–1132. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MK, Rundle A, Holmes D, Reyes M, Hoepner LA, Barr DB, Camann DE, Perera FP, Whyatt RM. Changes in Pest Infestation Levels, Self-Reported Pesticide Use, and Permethrin Exposure during Pregnancy after the 2000–2001 U.S. Environmental Protection Agency Restriction of Organophosphates. Environmental Health Perspectives. 2008;116:1681–1688. doi: 10.1289/ehp.11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WW, Shapiro LP, Bradner JM, Caudle WM. Developmental exposure to the organochlorine insecticide endosulfan damages the nigrostriatal dopamine system in male offspring. NeuroToxicology. 2014;44:279–287. doi: 10.1016/j.neuro.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth A, Holst K, Ponimaskin E. How serotonin receptors regulate morphogenic signalling in neurons. Progress in Neurobiology. 2016 doi: 10.1016/j.pneurobio.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel S, Berkowitz G, Teitelbaum S, Siskind J, Barr DB, Wetmur J. Prenatal Pesticide and PCB Exposures and Birth Outcomes. Pediatr Res. 2007;61:243–250. doi: 10.1203/pdr.0b013e31802d77f0. [DOI] [PubMed] [Google Scholar]
- Wolterink G, van Hoeven Arentzen PH. Pesticide residues in food - 2003 - Joint FAO/WHO Meeting on Pesticide Residues: CARBOSULFAN. 2003 http://www.inchem.org/documents/jmpr/jmpmono/v2003pr02.htm.
- Xue Z, Li X, Su Q, Xu L, Zhang P, Kong Z, Xu J, Teng J. Effect of Synthetic Pyrethroid Pesticide Exposure During Pregnancy on the Growth and Development of Infants. Asia-Pacific Journal of Public Health. 2013;25:72S–79S. doi: 10.1177/1010539513496267. [DOI] [PubMed] [Google Scholar]
- Yaglova NV, Yaglov VV. Changes in Thyroid Status of Rats after Prolonged Exposure to Low Dose Dichlorodiphenyltrichloroethane. Bulletin of Experimental Biology and Medicine. 2014;156:760–762. doi: 10.1007/s10517-014-2443-y. [DOI] [PubMed] [Google Scholar]
- Yang D, Howard A, Bruun D, Ajua-Alemanj M, Pickart C, Lein PJ. Chlorpyrifos and Chlorpyrifos-Oxon Inhibit Axonal Growth by Interfering with the Morphogenic Activity of Acetylcholinesterase. Toxicology and applied pharmacology. 2008;228:32–41. doi: 10.1016/j.taap.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J, Donerly S, Levin ED, Linney EA. Differential acetylcholinesterase inhibition of chlorpyrifos, diazinon and parathion in larval zebrafish. Neurotoxicology and teratology. 2011;33:735–741. doi: 10.1016/j.ntt.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JG, Eskenazi B, Gladstone EA, Bradman A, Pedersen L, Johnson C, Barr DB, Furlong CE, Holland NT. Association Between In Utero Organophosphate Pesticide Exposure and Abnormal Reflexes in Neonates. NeuroToxicology. 2005;26:199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Younts TJ, Castillo PE. Endogenous cannabinoid signaling at inhibitory interneurons. Current opinion in neurobiology. 2014;0:42–50. doi: 10.1016/j.conb.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang J, Liu R, Gan J, Liu J, Liu W. Endocrine-Disrupting Effects of Pesticides through Interference with Human Glucocorticoid Receptor. Environmental Science & Technology. 2016;50:435–443. doi: 10.1021/acs.est.5b03731. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Han S, Liang D, Shi X, Wang F, Liu W, Zhang L, Chen L, Gu Y, Tian Y. Prenatal Exposure to Organophosphate Pesticides and Neurobehavioral Development of Neonates: A Birth Cohort Study in Shenyang, China. PLoS ONE. 2014;9:e88491. doi: 10.1371/journal.pone.0088491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Olivier K, Won YK, Pope CN. Comparative Cholinergic Neurotoxicity of Oral Chlorpyrifos Exposures in Preweanling and Adult Rats. Toxicological Sciences. 2000;55:124–132. doi: 10.1093/toxsci/55.1.124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.