Abstract
Autism spectrum disorder (ASD) is a serious neurodevelopmental disorder that affects one in 45 children in the United States, with a similarly striking prevalence in countries around the world. However, mechanisms underlying its etiology and manifestations remain poorly understood. While ASD is diagnosed based on the presence and severity of impaired social communication and repetitive behavior, immune dysregulation and gastrointestinal issues are common co-morbidities. The microbiome is an integral part of human physiology; recent studies show that changes in the gut microbiota can modulate gastrointestinal physiology, immune function and even behavior. Links between particular bacteria from the indigenous gut microbiota and phenotypes relevant to ASD raise the important question of whether microbial dysbiosis plays a role in the development or presentation of ASD symptoms. Here we review reports of microbial dysbiosis in ASD. We further discuss potential effects of the microbiota on ASD-associated symptoms, drawing upon signaling mechanisms for reciprocal interactions between the microbiota, immunity, gut function and behavior. In addition, we discuss recent findings supporting a role for the microbiome as an interface between environmental and genetic risk factors that are associated with ASD. These studies highlight the integration of pathways across multiple body systems that together can impact brain and behavior and suggest that changes in the microbiome may contribute to symptoms of neurodevelopmental disease.
Keywords: microbiota, autism, inflammation, gastrointestinal tract, gut-brain-axis, neurodevelopment
Autism spectrum disorder and medical co-morbidities
ASD is a neurodevelopmental disorder that is characterized by impaired social communication and the presence of repetitive, or stereotyped, behaviors. In addition to the spectrum of behavioral abnormalities in ASD, several medical comorbidities are also observed in ASD individuals, ranging from seizures and anxiety to sleep deficiency and metabolic impairments (1–5). Brain changes in ASD include a reported 67% more neurons in the prefrontal cortex, more than 17% increase in brain weight, and abnormal cortical patterning. Further transcriptomic analysis of postmortem brains from human ASD individuals revealed altered expression of proteins that are important for functional synaptic activity in the prefrontal cortex and cerebellum (6–9). In addition, several brain imaging studies in living patients report correlations between abnormal frontal lobe connectivity, cortical morphology, amygdala activation and language control centers in ASD individuals compared to neurotypical controls (10–13).
The exact causes of ASD are unclear but are believed to involve a combination of genetic and environmental risk factors. It is estimated that the de novo mutations, common variants and short nucleotide polymorphisms identified across numerous ASD cases altogether account for approximately 50% of the disorder (14, 15). As such, many studies highlight the possibility for environmental risk factors and associated medical co-morbidities to contribute to core neurobehavioral symptoms of the disorder. Immune dysregulation and gastrointestinal disturbances are of particular interest in light of numerous studies reporting ASD-associated abnormalities in the peripheral, enteric and neuro-immune system. Postmortem brains of ASD patients show increased microglia and astroglia activation in the cerebellum and cerebral cortex, along with increased levels of proinflammatory cytokines in the cerebrospinal fluid and cortical regions of the brain (16). Moreover, there are ASD-associated genes that encode for features of the immune system, and mutations in those genes are linked with ASD phenotype, including loss of structural and functional connectivity in brain regions important for socio-communicative function (17, 18). Parallel studies reveal greater prevalence of gastrointestinal disorders and disturbances in ASD populations compared to controls (19, 20). Co-morbid gastrointestinal symptoms in subsets of ASD individuals include diarrhea/constipation, abdominal pain and gastric reflux. Deficient integrity of the gut epithelium and increased intestinal permeability are also reported (21).
These associations of ASD with greater prevalence of immune dysregulation and gastrointestinal issues motivate explorations of the ASD gut microbiome, which is emerging as a key regulator of intestinal physiology, neuroimmunity and host behavior. Many studies report dysbiosis of the gut microbiota in ASD individuals. Perhaps most intriguingly, gnotobiotic animal and probiotic studies demonstrate that microbiome changes can directly cause behavioral and neuropathological endophenotypes of human ASD. This avenue of research is critical for determining roles for microbiota dysbiosis and specific bacterial species that may contribute to and/or modify symptoms of ASD. In this review, we examine links between the microbiome and ASD symptoms, drawing upon data from animal experiments showing causal effects of the microbiome on immunity, brain and behavior. We further explore the notion that the microbiome plays an important role in mediating symptoms of ASD, and may be a key consideration for understanding immune and GI dysfunction in subsets of ASD individuals.
Gut microbiota on ASD-related endophenotypes in animal models
The microbiota plays an important role in regulating normal host physiology, metabolism, nutrition, and brain function. Since mammals are unable to synthesize many key nutrients, the gut microbiota assumes a primary role in digestion, synthesizing essential dietary vitamins and co-factors, such as vitamin B, riboflavin, thiamine and folate. In addition to roles for the microbiome in regulating digestion, GI physiology and immunity, increasing research reveals the ability of the gut microbiota to signal across the so-called “microbiota-gut-brain axis”. Raising animals in the absence of microbial colonization results in abnormalities in a variety of complex behaviors, pointing to the possibility that the microbiota modulates behavioral outcomes in animal models of neurodevelopmental and neurological disorders. Social communication deficits and the presence of stereotyped behaviors are hallmark diagnostic features of human ASD, and other behavioral abnormalities, such as anxiety, seizures, and hyperactivity are often co-morbid. Two independent studies demonstrate that germ-free mice exhibit decreased sociability or propensity to interact with a novel mouse versus non-social object, and reduced social preference to interact with an unfamiliar versus familiar mouse (22, 23). This is similarly seen in germ-free rats, which exhibit reduced social investigation of an unfamiliar partner (24). Germ-free mice also display differential gene expression, exon usage and RNA-editing in the amygdala, a key emotional center of the brain mediating responses to social stimuli (25). Interestingly, social behavioral abnormalities are impaired particularly in male mice, which parallels the male bias that is characteristic of ASD. Moreover, some of the social impairments are corrected by postnatal colonization of germ-free mice with a wildtype mouse gut microbiota at weaning, pointing to the ability to reverse abnormalities in social interactions (26). This is intriguing in light of reports that risperidone, an FDA-approved treatment for autism, does not correct social abnormalities in human ASD or mouse models of ASD (27, 28).
Modulation of the maternal environment is also of interest given the neurodevelopmental origins of ASD. Though there are numerous perinatal risk factors that influence maternal-fetal physiology including stress, infection, gestational diabetes, breast- versus formula-feeding, maternal age, antibiotic use and obesity, the changes in the gut microbiota can also be a relevant risk factor. A recent study by Buffington et al. showed that high fat diet induced-maternal obesity alters the offspring gut microbiome and causes social behavioral deficits that are linked to altered signaling in the mesolimbic reward system (29). Remarkably, transfer of the gut microbiota from control mice into offspring of high fat diet-fed mothers completely corrected the impairments in sociability and social novelty seen in the mice, demonstrating a key role for the gut microbiome in regulating mouse social behavior. Furthermore, treatment with the gut bacterium Lactobacillus reuteri alone sufficiently restores social behaviors, revealing specificity of social behavioral modulation in this model to a particular bacterial taxon. The beneficial effect of the microbiome in these studies was associated with its ability to promote hypothalamic levels of oxytocin and activation of neurons in the ventral tegmental area. This novel finding supports the promise of probiotic treatments for social behaviors. Importantly, however, we caution against use of L. reuteri for ASD until additional studies examine broader physiological effects of the bacterium on host biology and until such exploratory treatments are validated to be safe and effective in humans.
In addition to social interaction, there is some evidence that manipulation of the microbiome by probiotic treatment can modulate communicative and repetitive behavior in mice. In a mouse model of maternal immune activation, a principal environmental risk factor for autism, mice develop core behavioral features of ASD (impaired social communication and stereotyped behaviors), as well as several neuropathologies and co-morbid gastrointestinal and immunological symptoms relevant to the human disorder (30–32). Altering the postnatal gut microbiota by early life treatment with the human gut bacterium Bacteroides fragilis sufficiently ameliorated deficits in the frequency and quality of adult ultrasonic vocalizations and reduced stereotypic burying behavior exhibited by the ASD-like mice. While the mechanisms underlying the ability of the gut microbiota to modulate ASD-related behaviors are unclear, improvements in gastrointestinal integrity and alterations in serum metabolites could be involved. Consistent with a possible role for the microbiome in contributing to the symptoms of ASD, it would be interesting to examine the presence and severity of ASD-related behavioral and neuropathological abnormalities in ASD animal models raised on a germ-free background or depleted of gut microbes using treatment with broad spectrum antibiotics. Such studies would enable dissection of causal mechanisms linking the microbiome to core ASD behaviors and neuropathologies.
Anxiety-like behavior is also observed in subsets of individuals with ASD and is commonly recapitulated in animal models for ASD. The microbiome modulates anxiety-like behavior in mice, as germ-free mice exhibit increased locomotor activity and decreased anxietylike behavior in several tasks, including open field exploration, the elevated plus maze, light-dark box and platform step-down test (25, 33, 34). These behavioral changes are correlated with altered expression of genes involved in second messenger pathways and synaptic transmission, including PSD-95 and synaptophysin in the striatum (35). Moreover, these behavioral changes can be related to learning and memory deficits seen in both germ-free and antibiotic-treated mice (36, 37).
Germ-free animals also exhibit several abnormalities in brain gene expression and neurophysiology. For example, abnormal transcriptomic profiles are observed across the frontal cortex, striatum, amygdala and hippocampus (35), with altered expression of genes important for synaptic long-term potentiation, steroid hormone metabolism and neuronal transmission. Consistent with this, many studies report microbiome-mediated alterations in levels of brain-derived neurotrophic factor (BDNF) and synaptic proteins (23, 26, 33, 34, 36, 38). In addition, differences in serotonergic, dopaminergic, and glutamatergic signaling are observed in germ-free mice compared to conventionally-colonized controls (26, 34, 35, 39, 40). Germ free mice also display an exaggerated hypothalamic-pituitary-adrenal axis, with elevated corticosterone and adrenocorticotropic hormone levels in response to stress. Furthermore, germ-free mice also exhibit increased adult hippocampal neurogenesis compared to conventionally colonized controls (41). Interestingly, several of these effects are reversed upon colonization with a conventional gut microbiota, or even specific bacterial species (Table 1), suggesting that there are dynamic interactions across the microbiota-gut-brain axis that persists through adulthood.
Table 1.
Microbiota changes in ASD patients, mouse models with behavioral abnormalities, and links to immune and GI abnormalities.
| Subject | Behavior | Description | Microbiota | Immune | GI | Reference(s) |
|---|---|---|---|---|---|---|
|
children (43–84 mo) |
regressive- onset autism |
Broad-spectrum antibiotic use was linked to chronic diarrhea followed by loss of language, play, and social skills (n=11). |
X | X | (50) | |
| children | regressive- onset autism |
ASD (n=13) children all had GI symptoms (diarrehea and constipation) had more clostridial species, and significant amount of non-spore-forming anaerobes and microaerophilic bacteria compared to control (n=8). |
X | X | (42) | |
| children | autism | ASD children had elevated levels of Clostridium boltea, Clostridium group I and XI. |
X | (43) | ||
| mice | stress response |
GF mice have elevated stress response, reduced BDNF in cortex and hippocampus. GF colonization with Bifidobacterium infantis reversed stress response. |
X | (40) | ||
|
children (3–16 yo) |
autism | ASD patients (n=58) had taken antibiotics (34.5%), had GI complaints (91.4%), and were taking probiotics/prebiotics (53.4%). ASD patients had higher Clostridium clusters I and II compared to control (n=22). |
X | X | (44) | |
|
children (avg. 11–12 yo) |
regressive- autism (n=24) non- regressive autism (n=32) |
ASD patients (n=56) used significantly more antibiotics. |
X | (114) | ||
| mice | visceral hypersensitivity |
Lactobacillus paracasei NCC2461 normalized visceral sensitivity. |
X | (115) | ||
|
children (6.1 +/− 2.2 yo) |
autism | ASD children (n=15) had significantly higher use of oral antibiotics during first 12 mo of life. |
X | (116) | ||
| rats | depression like behavior |
Probiotic Bifidobacteria infantis treatment did not change behavior but decreased IFNγ, TNFα and IL-6 cytokines. |
X | X | (117) | |
| mice | anxiety-like behavior |
Colonic inflammation induced anxiety like behavior, decreased hippocampal BDNF mRNA, and increased circulating TNFα and IFNγ. Probiotic Bifidobacterium longum restored behavior and BDNF. |
X | X | X | (118) |
| rats | depression- like behavior |
Probiotic Bifidobacterium infantis treatment in a maternal separation stress model normalized IL-6 levels, increased swim behavior and reduced immobility in forced swim test, and restored basal noradrenaline levels in brainstem. |
X | (119) | ||
| rats | visceral hypersensitivity |
Probiotic Bifidobacterium infantis 35624 reduces visceral pain. |
X | (120) | ||
|
children (2–13 yo) |
Impaired social, language, and verbal skills. Repetitive stereotypical behaviors. |
ASD patients (n=33) had varying GI symptoms. More severe autism had higher Desulfovibrio, Bacteroides vulgatus, and Bacteroidetes. Firmicutes was higher in control (n=15). |
X | X | (121) | |
| mice | motor activity and anxiety- like behavior |
GF mice have increased motor activity and decreased anxiety. Changes in PSD-95 and synaptophysin expression in striatum. |
X | (35) | ||
| mice | anxiety- and depression- related behaviors |
Probiotic L. rhamnosus treatment of mice in a stress model reduced stress and increased GABA receptor expression in prefrontal cortex. |
X | (122) | ||
| mice | anxiety-like behavior |
Chemical colitis mouse model treated with probiotic (Bifidobacterium longum) had normalized anxiety like behavior. |
X | X | X | (123) |
|
rats and adults (avg 42 yo) |
anxiety, depression, and stress |
Probiotic (Lactobacillus helveticus and Bifidobacterium longum) reduced anxiety-like behavior in rats and reduced psychological stress in patients. |
X | (124) | ||
|
children (onset 13.4–5.4 mo) |
autism | ASD patients with GI symptoms (n=15) had a decrease in disaccharidases and hexose transporters, they also had decreases in Bacteroidetes, increase in Firmicutes/Bacteroidetes ratio, and increase in Betaproteobacteria compared to patients with only GI (n = 7). |
X | X | (47) | |
| mice | stress-induced corticosterone, anxiety-, and depression- related behavior |
L. rhamnosus increased cortical GABA(B1b) receptor expression, decreased GABA(Aα2) expression in prefrontal cortex and amygdala, but increased in hippocampus. L. rhamnosus reduced stress, anxiety and depression behavior. |
X | (122) | ||
| mice | anxiety-like behavior |
Chronic colitis model has increased anxiety. Bifidobacterium longum normalized behavior, but no change in BDNF expression. |
X | X | (123) | |
| adults (avg 42 yo) | anxiety and depression |
Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 decreased hospital anxiety and depression. n=10 treated. |
X | (125) | ||
|
children (2–18 yo) |
autism, Asperger’s |
ASD children (n=58) had GI symptoms decreased fecal SCFAs, lower levels of Bifidobacterium and higher levels of Lactobacillus |
X | X | (19) | |
| children (avg 123 mo) | autism | ASD children (n=23) had elevated fecal SCFAs |
X | (126) | ||
| mice | ASD-like behaviors |
MIA mice have decreased GI barrier, increased IL-6, decreased cytokine/chemokine, and gut microbiota dysbiosis, and autism- related behaviors that were restored following colonization with B. fragilis. |
X | X | X | (32) |
| mice | social preference and repetitive behaviors |
GF mice had deficits in social avoidance, social novelty, social investigation. GF mice also had increased repetitive self- grooming. |
X | (22) | ||
| mice | social behavior |
Maternal high fat diet induced social deficits in offspring are restored following colonization with Lactobacillus reuteri |
X | X | (29) |
Potential roles for the microbiome in ASD
Alterations in the gut microbiota are observed in ASD individuals compared to neurotypical controls (Table 1). Fecal bacterial profiling reveals a higher abundance of bacteria in the genus Clostridium in ASD patients (42–44). ASD patients also exhibited decreased Bacteroidetes/Firmicutes ratio, increased Lactobacillus and Desulfovibrio species, which correlated with ASD severity (45). ASD severity was also linked to a reduction in SCFAs, including acetate, proprionate and butyrate (19), which are modulated by gut microbes. Bacterial genera important for carbohydrate-degradation and fermentation, including Prevotella, Coprococcus and Veilonellaceae, were decreased in ASD patients (46, 47). On the other hand, ASD patients were shown to have elevated abundance of Sutterella, which regulates mucosal metabolism and intestinal epithelial integrity (20, 48). Together, these studies suggest that ASD is associated with altered composition and function of the gut microbiota.
Despite these reports of microbial dysbiosis in ASD, there is little consensus on specific bacterial species that are similarly altered across separate studies. That is, no defined microbial signature has been identified for ASD, though many studies report microbiome differences within independent cohorts of ASD and controls (Table 1). Several factors could contribute to these discrepancies, including methodological variations and inherent heterogeneity of ASD cohorts based on symptom severity, co-morbid conditions, varied lifestyle and medical history. ASD-associated alterations in eating behavior and diet are likely to play a role, as the gut microbiota can be stably altered in response to dietary changes and exposures to xenobiotics (49).
Whether alterations in the microbiota may contribute to development of ASD is unknown. Interestingly, a small clinical study of vancomycin treatment in ASD children reported some improvements in ASD behaviors, which waned when antibiotic treatment was discontinued (50), suggesting that the microbiome may contribute actively to the severity of behavioral abnormalities in ASD. Particular case studies also link antibiotic treatment to improvements in ASD behaviors and co-morbid conditions (51). In addition, the antibiotics d-cycloserine and minocycline are promising in light of their ability to treat behavioral symptoms of ASD in clinical trials and animal models (52–54). While both antibiotics are used to treat infections, their neuroprotective effects are commonly attributed to their roles as partial NMDA receptor agonist and microglial activation inhibitor, respectively.
Links between the gut microbiome and ASD-associated GI abnormalities
GI symptoms are variably present in ASD individuals (Table 2), ranging from 9–90% in prevalence (55, 56). Although the precise incidence varies from study to study, there is a general consensus that GI problems are common in individuals with autism (57) and that they could potentiate behavioral issues (57). A large meta-analysis of autism cases versus controls from 1980 to 2012 reveals greater incidence of intestinal symptoms, such as diarrhea, constipation and abdominal pain, despite high methodological variability. Consistent with this, a multicenter study of over 14,000 ASD individuals reports a higher prevalence of inflammatory bowel disease and other bowel disorders in ASD patients compared to controls (4). Notably, in an examination of 960 children from the CHildhood Autism Risks from Genetics and Environment (CHARGE) study, frequency of abdominal pain, diarrhea, constipation or gaseousness was associated with greater social withdrawal, stereotypy, irritability and hyperactivity as measured by the Abberant Behaviors Checklist (ABC) (58). Autism severity was also strongly correlated to the presence of gastrointestinal symptoms as measured by the Autism Treatment Evaluation Checklist (ATEC) and GI severity index (6-GSI) (57).
Table 2.
GI abnormalities in ASD patients and links to microbiota and immune changes.
| Subject | Behavior | Description | Microbiota | Immune | GI | Reference(s) |
|---|---|---|---|---|---|---|
| children (4–16 yo) | infantile autism | 43% (n=9/21) of ASD children had abnormal intestinal permeability. |
X | (127) | ||
| children (2.6–16 yo) | social interaction, communication, interests |
Patients with Celiac disease (n=120) did not show autistic like behaviors. |
X | (128) | ||
| children (3.5–16.3 yo) | autism | Children with ASD (n=21) and bowel symptoms had increased basement membrane thickness, mucosal gamma delta cell density, CD8 (+) density and intraepithelial lymphocyte numbers compared to patients with only inflammatory bowel diseases. |
X | X | (129) | |
| children (avg 6.2 yo) | regressive autism | ASD children with GI symptoms (n=20/25) show autoantibody binding to epithelial cells and co- localize with complement proteins in the intestinal mucosa. |
X | X | (130) | |
| children (1–10 yo) | autism | ASD children with GI symptoms (diarrehea and constipation) (n=75) showed increased production of TNF-α/IL-12 upon stimulation with cow’s milk protein. |
X | X | (56) | |
| children (>1 yo) | autism | ASD children (n=3325) had elevated link with family members with gastrointestinal autoimmune diseases such as Celiac, Chron and ulcerative colitis |
X | X | (131) | |
|
children (avg 7.4 ± 5.1 yo) |
autism | 36.7% of ASD patients (n=33/90) had abnormal intestinal permeability and GI symptoms (constipation, diarrhea, and abdominal pain). |
X | (132) | ||
| children (3–10 yo) | autism | ASD children (n=12/23) with GI symptoms had elevated levels of Sutterella compared to control children (n=9/9) with GI symptoms. There was also IgG or IgM antibody reactivity to Sutterella wadsworthensis in ASD-GI children. |
X | X | X | (20) |
| human | autism | Higher rates of GI disorders in ASD patients, range 9- 91% of GI disorders in ASD children, abdominal pain is 2–41%, constipation is 6- 45%, and diarrhea is 3– 77%. |
X | (133) | ||
| children (>4 yo) | autism | ASD patients (n=88) had more impaired intestinal permeability and increased antibodies against food antigens. |
X | X | (134) | |
|
children (avg 7.8 ± 2.9 yo) |
autism | ASD patients (n=37) had higher levels of IgG antibody to gliadin and correlated to GI symptoms, but not associated with Celiac disease. |
X | X | (135) | |
| children (10–14 yo) | regressive, atypical autism |
No difference in small intestine permeability between ASD (n=103) and special needs (n=30) children. |
X | (136) |
Whether any changes in the microbiome are caused by GI symptoms or whether they contribute to the manifestation of GI symptoms in ASD is unclear. Gut microbes influence various aspects of gut physiology, including intestinal barrier integrity, epithelial cell regeneration, mucus production and gastrointestinal motility (59). Interestingly, the severity of GI symptoms in ASD has been associated with alterations in the gut microbiota in response to treatment with antibiotics, prebiotics, or probiotics (57). In light of the intricate interactions of the gut microbiome with the gut epithelium, (57) it would be interesting to examine if microbiome abnormalities in ASD are enriched in or even specific to ASD individuals with co-morbid gastrointestinal issues. In addition, investigations into whether particular microbiome changes are associated with specific ASD-associated dietary regimens, treatments and co-morbid medical symptoms would be of significant interest.
Links between the gut microbiome and ASD-associated immune dysregulation
The gut microbiota exhibits important bidirectional interactions with the immune system. Many facets of immunity are dysregulated in ASD (Table 3). Alterations in circulating and brain cytokines, chemokines and other inflammatory factors are frequently observed in ASD, as well as abnormal distributions and/or responsiveness of various leukocyte subtypes (16–18, 60). These particular ASD-related immune abnormalities are the subject of several recent reviews (17, 18, 61). Many of the immunophenotypes seen in ASD are consistent with elevated “proinflammatory” status, as indicated by an increase in cytokines and chemokines including IFN-γ, IL-β, IL-6, IL-12p40, TNF-α, MCP-1, TGFβ and CCL-2, as well as a hyperactive cellular immune responses (62–65). However, ASD patients demonstrate varying immune abnormalities including differential changes in their immune/cytokine profiles, as well as the degree of changes (66), making it difficult to pinpoint direct links between immune and microbiota alterations in ASD individuals. In addition, confounding factors such as patient-to-patient variability in diet, lifestyle, and genetics can also modify immune activity. Nevertheless, subsets ASD individuals exhibit aberrant immune activation. Many of the immunophenotypes observed involve factors and pathways that are known to be influenced by the gut microbiota, raising the question of whether ASD-associated microbial dysbiosis can contribute to the widespread immune dysregulation seen in ASD individuals.
Table 3.
Immune alterations in ASD patients and links to microbiota and GI abnormalities.
| Subject | Behavior | Description | Microbiota | Immune | GI | Reference(s) |
|---|---|---|---|---|---|---|
|
human (3–28 yo) |
autism | 46% (n=28/61) of ASD patients had family members with autoimmune disorders; immediate relatives with autoimmune disorders increased prevalence of autism diagnosis from 4 to 21%; autoimmune disorders include type 1 diabetes, rheumatoid arthritis, hypothyroidism, and system lupus erythematosus. |
X | (137) | ||
|
children (1–17 yo) |
autism, Asperger’s | LPS stimulated innate immune reaction that was stronger in ASD individuals (n=71), leading to elevated TNF-α, IL- 1β and IL-6 production. |
X | X | (63, 138) | |
| human (5–44 yo) | autism | Postmortem brain show increased microglia and astroglia activation. Brain and CSF showed increased proinflammatory cytokines. |
X | (16) | ||
|
children (5.9 ± 3.9 yo) |
autism | ASD (n=37) patients compared to control (n=29) had elevated sera IgG and IgM BDNF levels. |
X | (139) | ||
|
children (4–15 yo) |
autism, Asperger’s | ASD with GI (n= 18) compared to control (n=27) had enhanced pro- inflammatory cytokine profile, increased TNFα, INFγ, IL-4, and IL-5, decreased regulatory cytokine IL-10. |
X | X | (140) | |
|
children (42 ± 9.8 mo) |
autism, early onset, regressive |
ASD (n=116), contol (n= 96), developmental delays (n=32), ASD had decreased levels of IgG and IgM subclass. |
X | (141) | ||
| ASD mothers | autism | Maternal antibodies for fetal brain proteins were elevated in mothers of ASD children. ASD mothers (n=61), typical mothers (n=62), developmental delay mothers (n=40). |
X | (142) | ||
|
children (avg 3.47 yo) |
autism | ASD (n=114), contol (n= 96), developmental delays (n=31), ASD had increased levels of IgG4 subclass. |
X | (143) | ||
|
children (avg 3.2 yo) |
autism | ASD patients had elevated autoantibodies in plasma that were directed to cerebellar protein extracts. |
X | (144, 145) |
||
| children (>1 yo) | autism, Asperger | 3325 diagnosed children with ASD in Denmark had increased risk of ASD diagnosis when they had a family history of type 1 diabetes and rheumatoid arthritis. |
X | (131) | ||
| adult (18–44 yo) | severe autism | ASD patients (n=22) had elevated levels of serum endotoxin that were correlated with decreased VABX socialization scores and trend towards increase in proinflammatory cytokines IL-1β and IL-6, but not significant. |
X | X | (21) | |
|
children (median 3.6 yo) |
lethargy, stereotypy, hyperactivity, impaired communication/so cialization |
Elevated brain and CSF chemokine (MCP-1, RANTES, and eotaxin) in ASD patients (n=80) was associated with higher aberrant behavior and impaired learning and social skills. |
X | (146) | ||
|
children (median 3.4 yo) |
non-regressive and regressive autism |
ASD children (n=97) showed higher plasma levels of IL-6 and IL-12p40. |
X | (147) | ||
|
children (7–15 yo) |
high functioning autism |
Increased levels of serum IL-17 in male subjects with high functioning ASD (n=28). |
X | (64) | ||
|
children (5–17 yo) |
Regressive autism | ASD children (n=34) had decreased levels of plasma IL-23, but no changes in IL-17. |
X | (148, 149) |
||
|
children (24–60 mo) |
autism | Increased production of IL −17 and IL-13 in co-morbid autism (n=45) and asthma children (n=12). |
X | (150) |
i. The microbiome and neuroimmune abnormalities of ASD
Both elevated microglial activation and altered microglia to neuron spatial distribution patterns are seen in the cerebral cortex and cerebellum of postmortem ASD brains (16, 67–69) and surrogate markers of increased microglial activation are observed by PET imaging of living ASD individuals (70). Interestingly, Erny and colleagues demonstrate that the microbiome is required for proper development and function of adult brain microglia (71). Microglia from germ-free mice exhibit altered transcriptomes including downregulation of cell activation genes (eg. Mapk 8, Fcgr2β, and Hif1α), reduction of genes for type 1 IFN receptor signaling (eg. Jak3 and Stat1), and upregulation of microglia transcription and survival factors (eg. Sfpi1 and Csf1r), as compared to those isolated from conventionally-colonized controls. Microglia from germ-free mice also exhibit altered morphology, with longer processes and increased branching. Following exposure to bacterial or viral challenge, microglia from germ-free mice maintain altered morphology and reduced inflammatory responses compared to those from conventionally-colonized mice. Remarkably, re-colonization of adult gnotobiotic mice with a conventional gut microbiota or supplementation with SCFAs, the primary products of bacterial fermentation, sufficiently corrects these deficiencies in microglial activation (71). These findings suggest that indigenous gut microbes reversibly modulate microglial function, and further motivate the identification of specific bacterial species from the gut microbiota that confer these neuro-immunomodulatory effects.
ii. Peripheral immune regulation and the microbiome
Various systemic immune abnormalities observed in ASD may also be influenced by the microbiota. For example, specific bacterial species from the gut microbiota regulate differentiation of T lymphocyte subtypes. Colonization with segmented filamentous bacteria stimulates the accumulation of inflammatory IL-17-producing Th17 cells via the acute phase protein serum amyloid A, which predisposes to symptoms of autoimmune disease in animal models (72, 73). In contrast, both Bacteroides fragilis and a particular consortium of Clostridial species upregulate levels of IL-10-producing T regulatory cells. By this mechanism, B. fragilis and Clostridial consortium sufficiently correct symptoms of intestinal disease and multiple sclerosis in animal models (74, 75) and continue to be tested for clinical translation into patient populations. The interplay between the gut microbiome and immune system could be relevant to the immune dysregulation observed in ASD, where abnormal distributions and functions of various leukocyte subtypes are observed. For example, deficiencies in regulatory T cells and other T helper cell subtypes are reported in ASD individuals compared to controls (76). In addition, peripheral blood monocytes and macrophages from ASD individuals are hyperresponsive to stimulation as compared to those isolated from neurotypical controls, and the microbiota fundamentally regulates systemic myeloid development and differentiation (77, 78).
Overall, this research raises the fascinating question of whether microbial dysbiosis can contribute to the immune dysregulation seen in ASD, such as microglial activation and T regulatory cell deficits, and whether manipulations of the microbiota can ameliorate ASD-related immune abnormalities. While parallel studies of immune problems in ASD and effects of the microbiome on the immune system are revealing some converging pathways, additional preclinical studies are required to determine whether microbiome changes in ASD can sufficiently cause any of the immune abnormalities seen in the disorder. Moreover, it will be important to determine whether existing animal models for ASD, which display core behavioral and neuropathological symptoms of the disorder, also exhibit immune abnormalities and microbiome changes seen in ASD. Such associations have been reported in a few mouse models of ASD environmental risk factors (32, 79), but information for additional environmental and genetic models is currently lacking.
The microbiome as a potential mediator of risk factors in ASD
While there is evidence that ASD-associated microbial dysbiosis could modulate corresponding immune, gastrointestinal and even behavioral symptoms, whether microbiome alterations contribute to the etiopathogenesis of ASD is unclear. Idiopathic ASD is thought to be a result of a combination of several genetic and environmental factors that each contributes a fraction of disease risk. The strong concordance of ASD in monozygotic twins compared to dizygotic twins reveals an ASD heritability rate of about 50% (80, 81). Several genetic factors increase ASD risk, including short nucleotide polymorphisms (SNPs), copy number variants (CNVs) and de novo mutations in genes involved in synaptic transmission and neuronal activity (82–84). Interestingly, some ASD susceptibility genes encode components of the immune system (17). In addition, several environmental risk factors have been identified to increase risk for autism.
The microbiota is well positioned at the intersection between genes and environment, as its composition and function are dependent on genetic background and critically shaped by environmental factors, including age, infection, diet and xenobiotics. Moreover, early life changes in the microbiota can have lasting effects on health and disease. For example, several diet-induced host phenotypes are sufficiently mediated by changes in the gut microbiota (85–88). The microbiota also conveys lasting effects of infection to the host (89) and can regulate epigenetic modification of the host genome (90, 91). Interestingly, microbiota mediated epigenetic changes can determine host transcriptional profiles. For example, SCFA butyrate can act as an HDAC inhibitor. HDAC are involved in cell cycle progression, gene silencing, differentiation, and genotoxic responses (92).
Whether the microbiota mediates effects of genetic and/or environmental risk factors on the development of ASD symptoms is unclear. However, increasing evidence suggests that the microbiota is altered in response to etiological risk factors for ASD. Maternal infection is a primary environmental risk factor for ASD based on numerous epidemiological, clinical and animal studies (93–98). Modeling maternal immune activation in mice results in global changes in the composition of the adult offspring microbiome (99, 100). This microbial dysbiosis is correlated with lasting behavioral abnormalities, neuropathologies, immune dysfunction and deficient gastrointestinal integrity. Interestingly, altering the microbiome via postnatal treatment with the human commensal B. fragilis improved gastrointestinal physiology and performance in some tasks measuring core ASD-related behaviors. Similarly, modeling maternal exposure to valproic acid, an anticonvulsant drug that is associated with increased risk for ASD, (79, 101) rendered offspring with lasting changes in gut microbiota composition, as well as neuroinflammation, abnormal gastrointestinal physiology and ASD-related behavioral abnormalities (79). Whether exposures to ASD risk factors also result in microbiome alterations and whether there are any similarities across microbiota impairments across differing insults are important questions for future investigation.
Importantly, alterations in the maternal microbiome in response to environmental risk exposure or genetic risk transmission can be passed onto offspring at birth. The developing embryo is largely devoid of microbial colonization, and a preponderance of evidence suggests that mammals inherit their initial microbiome through the birthing process. Mode of birth, whether through natural birthing process or Caesarean section (C-section), drives the initial seeding of the infant microbiome, such that babies delivered via the vaginal canal can be discriminated from those delivered by C-section based on their microbiome (102–104). This has significant implications for developmental disorders, including ASD, where maternal or prenatal exposures to genetic or environmental risk are believed to contribute to disease etiopathogenesis. Animal models demonstrate that microbiome changes in response to maternal stress are passed onto offspring at birth, setting in motion microbial dysbiosis that persists into adulthood (105–107). Maternal-to-offspring transmission is also believed to cause the chronic microbiota abnormalities seen in adult offspring of immune-activated mothers (18). Consistent with this, some studies report that C-section is associated with elevated risk for ASD in the offspring (108, 109), though one reported no link between C-section and ASD symptoms (110). While recent studies show offspring delivered by C-section have reduced microbial diversity compared to those by vaginal birth (111–113), there is also evidence that early life microbiome is plastic and other exposures can shape the infant microbiota. Other confounding perinatal risk factors for ASD that appear with C-section but are unrelated to the microbiome include anesthesia applied during labor, preterm birth, maternal age, and oxytocin administration. Future studies will require careful consideration of study subjects and perinatal risk factors.
Future directions for investigating effects of the microbiome on ASD
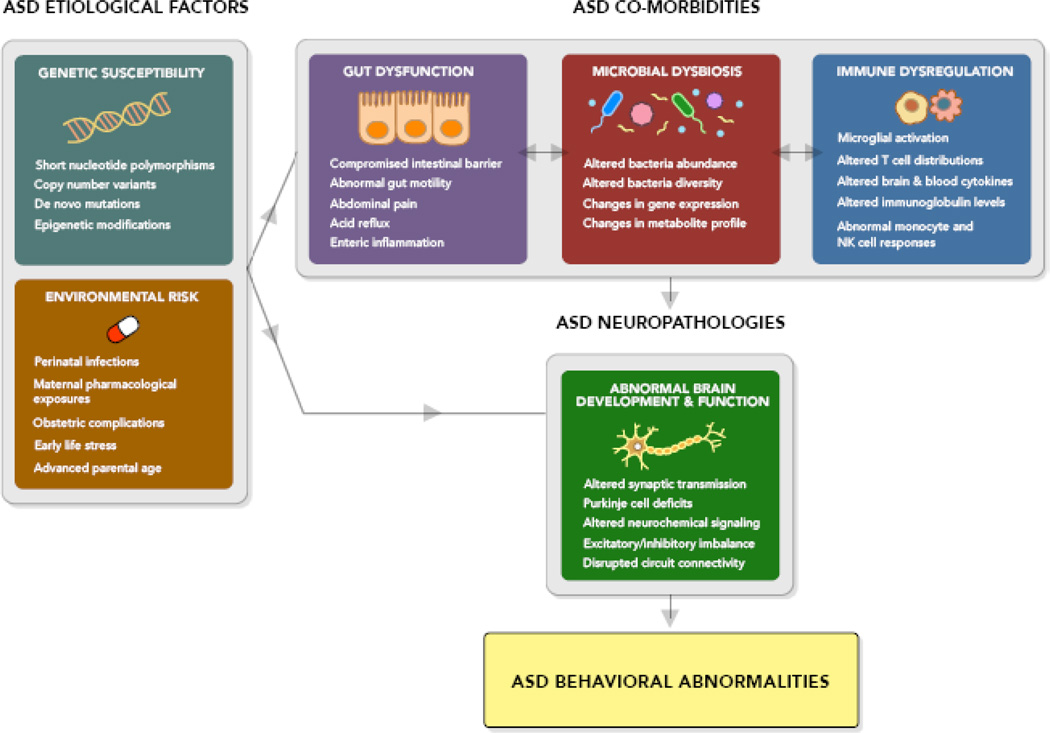
Emerging studies suggest the microbiota is an important regulator of gastrointestinal physiology, immune function and behavior (Figure 1). Abnormalities in each of these domains are reported in ASD, but additional characterization of co-morbid medical symptoms is required to clarify the nature, strength and reproducibility of specific associations. Evaluation of genetic background, medical history and ASD severity, among other variables, would provide insight into whether particular symptoms are enriched in specific subtypes of ASD and would further drive hypotheses regarding possible contributions of microbial dysbiosis, gastrointestinal dysfunction and/or immune dysregulation to the development or persistence of ASD behaviors. Similar efforts to characterize co-morbid microbiota, GI and immune symptoms across new and existing animal models for ASD are needed, with an emphasis on identifying converging phenotypic signatures across models of different genetic and environmental ASD risk factors. Further experiments are required to determine whether microbiome, gastrointestinal and/or immune abnormalities can sufficiently cause primary behavioral features of ASD. Such investigations should begin with studies using gnotobiotic or xenobiotic animals to identify peripheral targets and specific brain changes for development of novel ASD therapeutics. Of particular relevance to ASD-related microbial dysbiosis studies, it would be important to determine whether fecal transplant of ASD microbiota into animals is sufficient to cause behavioral impairments, neuropathologies and medical co-morbidities seen in ASD. Moreover, well-controlled studies on the efficacy of fecal microbiota transplant in ASD patients would provide much-needed guidance for the ASD community.
Figure 1. Model for Roles of the Microbiome in Autism Spectrum Disorder.
The microbiota is shaped by host genetics and environmental exposures. Select genetic and environmental risk factors for ASD could directly cause changes in the indigenous microbiota. Alternatively, the microbiota could be indirectly influenced by other medical co-morbidities associated with ASD, including gastrointestinal issues and immune dysfunction. The microbiota exhibits reciprocal interactions with the gastrointestinal tract, immune system, brain and behavior and abnormalities in any one component of this integrated system could the others. In particular, dysbiosis of the intestinal microbiota, in addition to immune and gastrointestinal symptoms seen in ASD, can influence neurodevelopment, neural activity and the manifestation of abnormal behaviors characteristic to ASD.
Acknowledgments
The authors are supported by funding from UCLA’s Department of Integrative Biology & Physiology, NIH Director’s Early Independence Award (5DP5OD017924) and Alfred P. Sloan Foundation’s Fellowship in Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors have no biomedical financial interests or potential conflicts of interest.
References
- 1.Bercum FM, Rodgers KM, Benison AM, Smith ZZ, Taylor J, Kornreich E, et al. Maternal Stress Combined with Terbutaline Leads to Comorbid Autistic-Like Behavior and Epilepsy in a Rat Model. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:15894–15902. doi: 10.1523/JNEUROSCI.2803-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antshel KM, Zhang-James Y, Wagner KE, Ledesma A, Faraone SV. An update on the comorbidity of ADHD and ASD: a focus on clinical management. Expert review of neurotherapeutics. 2016;16:279–293. doi: 10.1586/14737175.2016.1146591. [DOI] [PubMed] [Google Scholar]
- 3.Becker KG. Autism, asthma, inflammation, and the hygiene hypothesis. Medical hypotheses. 2007;69:731–740. doi: 10.1016/j.mehy.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohane IS, McMurry A, Weber G, MacFadden D, Rappaport L, Kunkel L, et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PloS one. 2012;7:e33224. doi: 10.1371/journal.pone.0033224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatric research. 2011;69:26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, et al. Neuron number and size in prefrontal cortex of children with autism. Jama. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 7.Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow ML, Pramparo T, Winn ME, Barnes CC, Li HR, Weiss L, et al. Age-dependent brain gene expression and copy number anomalies in autism suggest distinct pathological processes at young versus mature ages. PLoS genetics. 2012;8:e1002592. doi: 10.1371/journal.pgen.1002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broek JA, Guest PC, Rahmoune H, Bahn S. Proteomic analysis of post mortem brain tissue from autism patients: evidence for opposite changes in prefrontal cortex and cerebellum in synaptic connectivity-related proteins. Molecular autism. 2014;5:41. doi: 10.1186/2040-2392-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catani M, Dell’Acqua F, Budisavljevic S, Howells H, Thiebaut de Schotten M, Froudist-Walsh S, et al. Frontal networks in adults with autism spectrum disorder. Brain : a journal of neurology. 2016;139:616–630. doi: 10.1093/brain/awv351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang DY, Beam D, Pelphrey KA, Abdullahi S, Jou RJ. Cortical morphological markers in children with autism: a structural magnetic resonance imaging study of thickness, area, volume, and gyrification. Molecular autism. 2016;7:11. doi: 10.1186/s13229-016-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrington JD, Miller J, Pandey J, Schultz RT. Anxiety And Social Deficits Have Distinct Relationships with Amygdala Function in Autism Spectrum Disorder. Social cognitive and affective neuroscience. 2016 doi: 10.1093/scan/nsw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha S, Sohn IJ, Kim N, Sim HJ, Cheon KA. Characteristics of Brains in Autism Spectrum Disorder: Structure, Function and Connectivity across the Lifespan. Experimental neurobiology. 2015;24:273–284. doi: 10.5607/en.2015.24.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, et al. Most genetic risk for autism resides with common variation. Nature genetics. 2014;46:881–885. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iossifov I, Levy D, Allen J, Ye K, Ronemus M, Lee YH, et al. Low load for disruptive mutations in autism genes and their biased transmission. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E5600–E5607. doi: 10.1073/pnas.1516376112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of neurology. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 17.Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nature reviews Neuroscience. 2015;16:469–486. doi: 10.1038/nrn3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsiao EY. Immune dysregulation in autism spectrum disorder. International review of neurobiology. 2013;113:269–302. doi: 10.1016/B978-0-12-418700-9.00009-5. [DOI] [PubMed] [Google Scholar]
- 19.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC gastroenterology. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. mBio. 2012:3. doi: 10.1128/mBio.00261-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emanuele E, Orsi P, Boso M, Broglia D, Brondino N, Barale F, et al. Low-grade endotoxemia in patients with severe autism. Neuroscience letters. 2010;471:162–165. doi: 10.1016/j.neulet.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 22.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arentsen T, Raith H, Qian Y, Forssberg H, Heijtz Diaz R. Host microbiota modulates development of social preference in mice. Microbial ecology in health and disease. 2015;26:29719. doi: 10.3402/mehd.v26.29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Dauge V, et al. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–217. doi: 10.1016/j.psyneuen.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Stilling RM, Ryan FJ, Hoban AE, Shanahan F, Clarke G, Claesson MJ, et al. Microbes & neurodevelopment--Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav Immun. 2015;50:209–220. doi: 10.1016/j.bbi.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 27.Bonini SA, Mastinu A, Maccarinelli G, Mitola S, Premoli M, La Rosa LR, et al. Cortical Structure Alterations and Social Behavior Impairment in p50-Deficient Mice. Cerebral cortex. 2016 doi: 10.1093/cercor/bhw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Accordino RE, Kidd C, Politte LC, Henry CA, McDougle CJ. Psychopharmacological interventions in autism spectrum disorder. Expert opinion on pharmacotherapy. 2016:1–16. doi: 10.1517/14656566.2016.1154536. [DOI] [PubMed] [Google Scholar]
- 29.Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell. 2016;165:1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coiro P, Padmashri R, Suresh A, Spartz E, Pendyala G, Chou S, et al. Impaired synaptic development in a maternal immune activation mouse model of neurodevelopmental disorders. Brain Behav Immun. 2015;50:249–258. doi: 10.1016/j.bbi.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, et al. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23:255–264. e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 35.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frohlich EE, Farzi A, Mayerhofer R, Reichmann F, Jacan A, Wagner B, et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gareau MG. Microbiota-gut-brain axis and cognitive function. Advances in experimental medicine and biology. 2014;817:357–371. doi: 10.1007/978-1-4939-0897-4_16. [DOI] [PubMed] [Google Scholar]
- 38.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. 609, e591–e593. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 39.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural brain research. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 40.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. The Journal of physiology. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O’Leary OF. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol Psychiatry. 2015;78:e7–e9. doi: 10.1016/j.biopsych.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 42.Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, et al. Gastrointestinal microflora studies in late-onset autism. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;35:S6–S16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- 43.Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Applied and environmental microbiology. 2004;70:6459–6465. doi: 10.1128/AEM.70.11.6459-6465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. Journal of medical microbiology. 2005;54:987–991. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- 45.Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiology & behavior. 2015;138:179–187. doi: 10.1016/j.physbeh.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 46.Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PloS one. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PloS one. 2011;6:e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Molecular autism. 2013;4:42. doi: 10.1186/2040-2392-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taguer M, Maurice CF. The complex interplay of diet, xenobiotics, and microbial metabolism in the gut: Implications for clinical outcomes. Clinical pharmacology and therapeutics. 2016 doi: 10.1002/cpt.366. [DOI] [PubMed] [Google Scholar]
- 50.Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Vaisanen ML, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. Journal of child neurology. 2000;15:429–435. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- 51.Ramirez PL, Barnhill K, Gutierrez A, Schutte C, Hewitson L. Improvements in Behavioral Symptoms following Antibiotic Therapy in a 14-Year-Old Male with Autism. Case reports in psychiatry. 2013;2013:239034. doi: 10.1155/2013/239034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wellmann KA, Varlinskaya EI, Mooney SM. D-Cycloserine ameliorates social alterations that result from prenatal exposure to valproic acid. Brain research bulletin. 2014;108:1–9. doi: 10.1016/j.brainresbull.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urbano M, Okwara L, Manser P, Hartmann K, Herndon A, Deutsch SI. A trial of D-cycloserine to treat stereotypies in older adolescents and young adults with autism spectrum disorder. Clinical neuropharmacology. 2014;37:69–72. doi: 10.1097/WNF.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar H, Sharma B. Minocycline ameliorates prenatal valproic acid induced autistic behaviour, biochemistry and blood brain barrier impairments in rats. Brain research. 2016;1630:83–97. doi: 10.1016/j.brainres.2015.10.052. [DOI] [PubMed] [Google Scholar]
- 55.Buie T, Campbell DB, Fuchs GJ, 3rd, Furuta GT, Levy J, Vandewater J, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Suppl 1):S1–S18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- 56.Jyonouchi H, Geng L, Ruby A, Reddy C, Zimmerman-Bier B. Evaluation of an association between gastrointestinal symptoms and cytokine production against common dietary proteins in children with autism spectrum disorders. The Journal of pediatrics. 2005;146:605–610. doi: 10.1016/j.jpeds.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 57.McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics. 2014;133:872–883. doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- 58.Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. Journal of autism and developmental disorders. 2014;44:1117–1127. doi: 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. The Biochemical journal. 2009;420:211–219. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- 60.Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 61.Careaga M, Ashwood P. Autism spectrum disorders: from immunity to behavior. Methods in molecular biology. 2012;934:219–240. doi: 10.1007/978-1-62703-071-7_12. [DOI] [PubMed] [Google Scholar]
- 62.El-Ansary A, Al-Ayadhi L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. Journal of neuroinflammation. 2014;11:189. doi: 10.1186/s12974-014-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. Journal of neuroimmunology. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, et al. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PloS one. 2011;6:e20470. doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu N, Li X, Zhong Y. Inflammatory cytokines: potential biomarkers of immunologic dysfunction in autism spectrum disorders. Mediators of inflammation. 2015;2015:531518. doi: 10.1155/2015/531518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry. 2015;20:440–446. doi: 10.1038/mp.2014.59. [DOI] [PubMed] [Google Scholar]
- 67.Morgan JT, Chana G, Abramson I, Semendeferi K, Courchesne E, Everall IP. Abnormal microglial-neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain research. 2012;1456:72–81. doi: 10.1016/j.brainres.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 68.Tetreault NA, Hakeem AY, Jiang S, Williams BA, Allman E, Wold BJ, et al. Microglia in the cerebral cortex in autism. Journal of autism and developmental disorders. 2012;42:2569–2584. doi: 10.1007/s10803-012-1513-0. [DOI] [PubMed] [Google Scholar]
- 69.Edmonson C, Ziats MN, Rennert OM. Altered glial marker expression in autistic post-mortem prefrontal cortex and cerebellum. Molecular autism. 2014;5:3. doi: 10.1186/2040-2392-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, et al. Microglial activation in young adults with autism spectrum disorder. JAMA psychiatry. 2013;70:49–58. doi: 10.1001/jamapsychiatry.2013.272. [DOI] [PubMed] [Google Scholar]
- 71.Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nature neuroscience. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. Journal of immunology. 2010;185:4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- 76.Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, et al. Elevated immune response in the brain of autistic patients. Journal of neuroimmunology. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grigorenko EL, Han SS, Yrigollen CM, Leng L, Mizue Y, Anderson GM, et al. Macrophage migration inhibitory factor and autism spectrum disorders. Pediatrics. 2008;122:e438–e445. doi: 10.1542/peds.2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Theije CG, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, et al. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun. 2014;37:197–206. doi: 10.1016/j.bbi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 80.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. Jama. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Colvert E, Tick B, McEwen F, Stewart C, Curran SR, Woodhouse E, et al. Heritability of Autism Spectrum Disorder in a UK Population-Based Twin Sample. JAMA psychiatry. 2015;72:415–423. doi: 10.1001/jamapsychiatry.2014.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Micheau J, Vimeney A, Normand E, Mulle C, Riedel G. Impaired hippocampus-dependent spatial flexibility and sociability represent autism-like phenotypes in GluK2 mice. Hippocampus. 2014;24:1059–1069. doi: 10.1002/hipo.22290. [DOI] [PubMed] [Google Scholar]
- 83.Aller MI, Pecoraro V, Paternain AV, Canals S, Lerma J. Increased Dosage of High-Affinity Kainate Receptor Gene grik4 Alters Synaptic Transmission and Reproduces Autism Spectrum Disorders Features. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:13619–13628. doi: 10.1523/JNEUROSCI.2217-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rendall AR, Truong DT, Fitch RH. Learning delays in a mouse model of Autism Spectrum Disorder. Behavioural brain research. 2016;303:201–207. doi: 10.1016/j.bbr.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature medicine. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548–554. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 88.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell host & microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, et al. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infection and immunity. 2012;80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumar H, Lund R, Laiho A, Lundelin K, Ley RE, Isolauri E, et al. Gut microbiota as an epigenetic regulator: pilot study based on whole-genome methylation analysis. mBio. 2014:5. doi: 10.1128/mBio.02113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cortese R, Lu L, Yu Y, Ruden D, Claud EC. Epigenome-Microbiome crosstalk: A potential new paradigm influencing neonatal susceptibility to disease. Epigenetics. 2016 doi: 10.1080/15592294.2016.1155011. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. The Journal of nutritional biochemistry. 2008;19:587–593. doi: 10.1016/j.jnutbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 93.Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun. 2012;26:623–634. doi: 10.1016/j.bbi.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Le Belle JE, Sperry J, Ngo A, Ghochani Y, Laks DR, Lopez-Aranda M, et al. Maternal inflammation contributes to brain overgrowth and autism-associated behaviors through altered redox signaling in stem and progenitor cells. Stem cell reports. 2014;3:725–734. doi: 10.1016/j.stemcr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee BK, Magnusson C, Gardner RM, Blomstrom A, Newschaffer CJ, Burstyn I, et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun. 2015;44:100–105. doi: 10.1016/j.bbi.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. Journal of autism and developmental disorders. 2013;43:25–33. doi: 10.1007/s10803-012-1540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, Croen LA. Maternal Infection During Pregnancy and Autism Spectrum Disorders. Journal of autism and developmental disorders. 2015;45:4015–4025. doi: 10.1007/s10803-013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mandal M, Donnelly R, Elkabes S, Zhang P, Davini D, David BT, et al. Maternal immune stimulation during pregnancy shapes the immunological phenotype of offspring. Brain Behav Immun. 2013;33:33–45. doi: 10.1016/j.bbi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 100.Weir RK, Forghany R, Smith SE, Patterson PH, McAllister AK, Schumann CM, et al. Preliminary evidence of neuropathology in nonhuman primates prenatally exposed to maternal immune activation. Brain Behav Immun. 2015;48:139–146. doi: 10.1016/j.bbi.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Theije CG, Koelink PJ, Korte-Bouws GA, Lopes da Silva S, Korte SM, Olivier B, et al. Intestinal inflammation in a murine model of autism spectrum disorders. Brain Behav Immun. 2014;37:240–247. doi: 10.1016/j.bbi.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 102.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G. Cesarean delivery may affect the early biodiversity of intestinal bacteria. The Journal of nutrition. 2008;138:1796S–1800S. doi: 10.1093/jn/138.9.1796S. [DOI] [PubMed] [Google Scholar]
- 104.Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early human development. 2010;86(Suppl 1):13–15. doi: 10.1016/j.earlhumdev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 105.Golubeva AV, Crampton S, Desbonnet L, Edge D, O’Sullivan O, Lomasney KW, et al. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology. 2015;60:58–74. doi: 10.1016/j.psyneuen.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 106.Jasarevic E, Howerton CL, Howard CD, Bale TL. Alterations in the Vaginal Microbiome by Maternal Stress Are Associated With Metabolic Reprogramming of the Offspring Gut and Brain. Endocrinology. 2015;156:3265–3276. doi: 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.De Palma G, Blennerhassett P, Lu J, Deng Y, Park AJ, Green W, et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nature communications. 2015;6:7735. doi: 10.1038/ncomms8735. [DOI] [PubMed] [Google Scholar]
- 108.Curran EA, Dalman C, Kearney PM, Kenny LC, Cryan JF, Dinan TG, et al. Association Between Obstetric Mode of Delivery and Autism Spectrum Disorder: A Population-Based Sibling Design Study. JAMA psychiatry. 2015;72:935–942. doi: 10.1001/jamapsychiatry.2015.0846. [DOI] [PubMed] [Google Scholar]
- 109.Curran EA, O’Neill SM, Cryan JF, Kenny LC, Dinan TG, Khashan AS, et al. Research review: Birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Journal of child psychology and psychiatry, and allied disciplines. 2015;56:500–508. doi: 10.1111/jcpp.12351. [DOI] [PubMed] [Google Scholar]
- 110.Curran EA, Cryan JF, Kenny LC, Dinan TG, Kearney PM, Khashan AS. Obstetrical Mode of Delivery and Childhood Behavior and Psychological Development in a British Cohort. Journal of autism and developmental disorders. 2016;46:603–614. doi: 10.1007/s10803-015-2616-1. [DOI] [PubMed] [Google Scholar]
- 111.Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nature medicine. 2016;22:250–253. doi: 10.1038/nm.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8:343ra382. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yassour M, Vatanen T, Siljander H, Hamalainen AM, Harkonen T, Ryhanen SJ, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8:343ra381. doi: 10.1126/scitranslmed.aad0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Niehus R, Lord C. Early medical history of children with autism spectrum disorders. Journal of developmental and behavioral pediatrics : JDBP. 2006;27:S120–S127. doi: 10.1097/00004703-200604002-00010. [DOI] [PubMed] [Google Scholar]
- 115.Verdu EF, Bercik P, Verma-Gandhu M, Huang XX, Blennerhassett P, Jackson W, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55:182–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Adams JB, Romdalvik J, Ramanujam VM, Legator MS. Mercury, lead, and zinc in baby teeth of children with autism versus controls. Journal of toxicology and environmental health Part A. 2007;70:1046–1051. doi: 10.1080/15287390601172080. [DOI] [PubMed] [Google Scholar]
- 117.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. Journal of psychiatric research. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 118.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. e2101. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 119.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 120.McKernan DP, Fitzgerald P, Dinan TG, Cryan JF. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2010;22:1029–1035. e1268. doi: 10.1111/j.1365-2982.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- 121.Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 122.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. The British journal of nutrition. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 125.Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut microbes. 2011;2:256–261. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- 126.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Digestive diseases and sciences. 2012;57:2096–2102. doi: 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- 127.D’Eufemia P, Celli M, Finocchiaro R, Pacifico L, Viozzi L, Zaccagnini M, et al. Abnormal intestinal permeability in children with autism. Acta paediatrica. 1996;85:1076–1079. doi: 10.1111/j.1651-2227.1996.tb14220.x. [DOI] [PubMed] [Google Scholar]
- 128.Pavone L, Fiumara A, Bottaro G, Mazzone D, Coleman M. Autism and celiac disease: failure to validate the hypothesis that a link might exist. Biol Psychiatry. 1997;42:72–75. doi: 10.1016/S0006-3223(97)00267-9. [DOI] [PubMed] [Google Scholar]
- 129.Furlano RI, Anthony A, Day R, Brown A, McGarvey L, Thomson MA, et al. Colonic CD8 and gamma delta T-cell infiltration with epithelial damage in children with autism. The Journal of pediatrics. 2001;138:366–372. doi: 10.1067/mpd.2001.111323. [DOI] [PubMed] [Google Scholar]
- 130.Torrente F, Anthony A, Heuschkel RB, Thomson MA, Ashwood P, Murch SH. Focal-enhanced gastritis in regressive autism with features distinct from Crohn’s and Helicobacter pylori gastritis. The American journal of gastroenterology. 2004;99:598–605. doi: 10.1111/j.1572-0241.2004.04142.x. [DOI] [PubMed] [Google Scholar]
- 131.Atladottir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124:687–694. doi: 10.1542/peds.2008-2445. [DOI] [PubMed] [Google Scholar]
- 132.de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. Journal of pediatric gastroenterology and nutrition. 2010;51:418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- 133.Coury DL, Ashwood P, Fasano A, Fuchs G, Geraghty M, Kaul A, et al. Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics. 2012;130(Suppl 2):S160–S168. doi: 10.1542/peds.2012-0900N. [DOI] [PubMed] [Google Scholar]
- 134.Ludvigsson JF, Reichenberg A, Hultman CM, Murray JA. A nationwide study of the association between celiac disease and the risk of autistic spectrum disorders. JAMA psychiatry. 2013;70:1224–1230. doi: 10.1001/jamapsychiatry.2013.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lau NM, Green PH, Taylor AK, Hellberg D, Ajamian M, Tan CZ, et al. Markers of Celiac Disease and Gluten Sensitivity in Children with Autism. PloS one. 2013;8:e66155. doi: 10.1371/journal.pone.0066155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dalton N, Chandler S, Turner C, Charman T, Pickles A, Loucas T, et al. Gut permeability in autism spectrum disorders. Autism research : official journal of the International Society for Autism Research. 2014;7:305–313. doi: 10.1002/aur.1350. [DOI] [PubMed] [Google Scholar]
- 137.Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. Journal of child neurology. 1999;14:388–394. doi: 10.1177/088307389901400608. [DOI] [PubMed] [Google Scholar]
- 138.Jyonouchi H, Sun S, Itokazu N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology. 2002;46:76–84. doi: 10.1159/000065416. [DOI] [PubMed] [Google Scholar]
- 139.Connolly AM, Chez M, Streif EM, Keeling RM, Golumbek PT, Kwon JM, et al. Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol Psychiatry. 2006;59:354–363. doi: 10.1016/j.biopsych.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 140.Ashwood P, Wakefield AJ. Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. Journal of neuroimmunology. 2006;173:126–134. doi: 10.1016/j.jneuroim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 141.Heuer L, Ashwood P, Schauer J, Goines P, Krakowiak P, Hertz-Picciotto I, et al. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism research : official journal of the International Society for Autism Research. 2008;1:275–283. doi: 10.1002/aur.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, et al. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Enstrom A, Krakowiak P, Onore C, Pessah IN, Hertz-Picciotto I, Hansen RL, et al. Increased IgG4 levels in children with autism disorder. Brain Behav Immun. 2009;23:389–395. doi: 10.1016/j.bbi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2009;23:64–74. doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rossi CC, Van de Water J, Rogers SJ, Amaral DG. Detection of plasma autoantibodies to brain tissue in young children with and without autism spectrum disorders. Brain Behav Immun. 2011;25:1123–1135. doi: 10.1016/j.bbi.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. Journal of neuroimmunology. 2011;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Onore C, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen R, Van de Water J, et al. Decreased cellular IL-23 but not IL-17 production in children with autism spectrum disorders. Journal of neuroimmunology. 2009;216:126–129. doi: 10.1016/j.jneuroim.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jyonouchi H, Geng L, Streck DL, Toruner GA. Immunological characterization and transcription profiling of peripheral blood (PB) monocytes in children with autism spectrum disorders (ASD) and specific polysaccharide antibody deficiency (SPAD): case study. Journal of neuroinflammation. 2012;9:4. doi: 10.1186/1742-2094-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Akintunde ME, Rose M, Krakowiak P, Heuer L, Ashwood P, Hansen R, et al. Increased production of IL-17 in children with autism spectrum disorders and co-morbid asthma. Journal of neuroimmunology. 2015;286:33–41. doi: 10.1016/j.jneuroim.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]