Abstract
Subsequent neoplasms (SN) following hematopoietic cell transplantation (HCT) cause significant patient morbidity and mortality. Risks for specific SN types vary substantially, with particularly elevated risks for post-transplant lymphoproliferative disorders, myelodysplastic syndrome/acute myeloid leukemia, and squamous cell malignancies. This consensus document provides an overview of the current state of knowledge regarding SN after HCT and recommends priorities and approaches to overcome challenges and gaps in understanding. Numerous factors have been suggested to impact risk, including patient-related (e.g., age), primary disease-related (e.g., disease type, pre-HCT therapies), and HCT-related characteristics (e.g., type and intensity of conditioning regimen, stem cell source, development of graft-versus-host disease). However, gaps in understanding remain for each of these risk factors, particularly for patients receiving HCT in the current era due to substantial advances in clinical transplantation practices. Additionally, the influence of non-transplant-related risk factors (e.g., germline genetic susceptibility, oncogenic viruses, lifestyle factors) is poorly understood. Clarification of the magnitude of SN risks and identification of etiologic factors will require large-scale, long-term, systematic follow-up of HCT survivors with detailed clinical data. Most investigations of the mechanisms of SN pathogenesis after HCT have focused on immune drivers. Expansion of our understanding in this area will require interdisciplinary laboratory collaborations utilizing measures of immune function and availability of archival tissue from SN diagnoses. Consensus-based recommendations for optimal preventative, screening, and therapeutic approaches have been developed for certain SN after HCT, whereas for other SN, general population guidelines are recommended. Further evidence is needed to specifically tailor preventative, screening, and therapeutic guidelines for SN after HCT, particularly for unique patient populations. Accomplishment of this broad research agenda will require increased investment in systematic data collection with engagement from patients, clinicians, and interdisciplinary scientists in order to reduce the burden of SN in the rapidly growing population of HCT survivors.
Keywords: subsequent neoplasms, second malignancies, hematopoietic cell transplantation, long-term follow-up, NIH consensus, late effects
BACKGROUND AND PURPOSE
Hematopoietic cell transplantation (HCT) is an important component of treatment for many malignant and nonmalignant diseases. Over the last several decades, the number of HCTs performed annually has increased, surpassing 18,000 in the United States in 2014.1 With improvements in clinical approaches to HCT and supportive care, survival following HCT has improved dramatically.2–4 The current population of >100,000 survivors in the United States is expected to increase five-fold by 2030, with 14% of the population aged <18 years and 25% aged ≥60 years at transplant.5
Subsequent neoplasms (SN) are a leading cause of non-relapse mortality among HCT survivors.3,6–8 SN are typically grouped into three categories: B- and T-cell malignancies (including post-transplant lymphoproliferative disorder [PTLD]), myelodysplastic syndrome/acute myeloid leukemia (MDS/AML), and solid tumors. The time course for development of SN after HCT is variable, with lymphomas and leukemias developing relatively early, whereas solid tumors tend to have a longer latency.6,9–14
Here we summarize what is currently known about the magnitude of risk for SN and their pathogenesis, transplant- and non-transplant-related risk factors, and outcomes, as well as current guidelines for prevention and screening. When appropriate, autologous and allogeneic HCT are considered separately. To summarize existing knowledge, we searched the databases of PubMed and Embase spanning articles published during 1995–2015 using medical subject headings including “Neoplasms, Second Primary,” “Bone Marrow Transplantation,” and “Hematopoietic Stem Cell Transplantation,” as well as additional headings relevant for specific topics (e.g., “Transplantation Conditioning,” “Graft-versus-Host Disease [GVHD]”). A working group consensus over a 12-month period then defined significant gaps in understanding and developed recommendations for future research regarding SN after HCT. The highest priority recommendations are summarized in the text box. Additional information on research gaps and recommendations are found within each section below.
MAGNITUDE OF RISK
We identified 62 publications that quantified SN risk (Supplementary Table 1). About half (N=34) of the studies included patients undergoing HCT for different indications,15–48 whereas the remainder focused on specific diseases, including myeloid malignancies (N=1),11 lymphoid malignancies (N=15),49–63 plasma cell disorders (N=3),64–66 and benign hematologic disorders such as aplastic anemia and Fanconi anemia (FA) (N=9).67–76 Only three were prospective.34,36,51
Few studies included a comparison of SN occurrence to that expected in the general population. The largest study quantified risks of solid tumors by anatomic site in an international collaborative effort including 28,874 patients who received allogeneic HCT during 1964–1994.9 Overall, SN occurred more than twice as often as expected in the general population. Those SN with the greatest excess risks included lip (standardized incidence ratio [SIR]=26.8), salivary gland (SIR=14.2), tongue (SIR=13.3), bone (SIR=8.5), soft tissue (SIR=6.5), and liver (SIR=6.3). The SIRs for most solid tumors increased with increasing time since transplantation, with the highest SIRs observed for tumors occurring ≥10 years after transplantation. The most notable exception to this pattern was for melanomas and thyroid cancers, where risks were persistently elevated from the first year through ≥10 years after transplantation. Similar findings were observed in an Australian cohort of 3273 adults who received allogeneic HCT during 1992–2007.77 Risks for several types of skin cancer are also strikingly elevated after HCT,78,79 as reported in the Danish study of 3302 HCT recipients during 1999–2014 that leveraged the comprehensive reporting of all cutaneous malignancies [including basal and squamous cell carcinomas (SCC)] to general population cancer registries.77
Quantifying hematologic SN risks after HCT is more complicated than for solid tumors for two reasons. First, hematologic malignancies are the most common indication for HCT, thus relapsed disease must be distinguished from SN of donor origin. Distinguishing a donor-derived SN ideally utilizes sensitive molecular genetic analysis,37 though in the case of an opposite sex donor, cytogenetic analysis has been used. Second, certain hematologic SN such as PTLD are not directly comparable to lymphoid malignancies that occur in the general population. Nevertheless, several large-scale studies have quantified risk of hematologic SN after HCT. In an international study of 26,901 allogeneic HCT recipients during 1964–1994, the observed to expected ratio of PTLD was 29.7.13,80 MDS/AML risk after allogeneic HCT is thought to be comparable to that of other cancer survivors who have received particular cytotoxic chemotherapeutic agents and radiotherapy, with such risks often exceeding 10-fold.81 There is also some evidence that MDS/AML risks may be even higher after autologous HCT because of the substantial cumulative doses received during pre-transplant chemotherapy and radiotherapy, chemotherapy-based stem cell mobilization and HCT conditioning.62,82,83
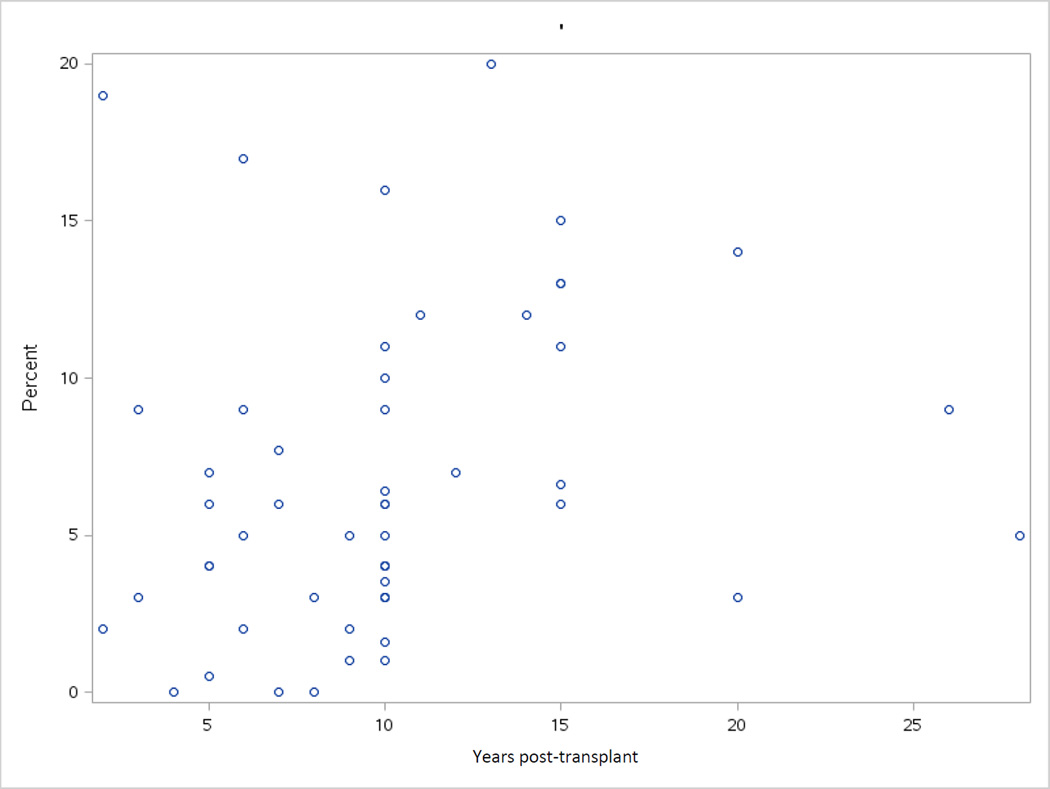
Cumulative incidence is particularly useful for quantifying the clinical burden of SNs. Although most studies reported the cumulative incidence of SN, substantial differences in the populations studied in terms of primary disease, age, and HCT approach, as well as the wide range in sample size and duration of follow-up, result in significant heterogeneity in the reported incidence, hampering comparison of studies and interpretation of results (Figure 1, Supplementary Table 1).
Figure 1.
Cumulative incidence of SN (percent) after HCT from reported studies demonstrates wide variability.
Research Gaps and Recommendations
-
Large-scale, long-term (>10 years), systematic follow-up of HCT recipients for SN occurrence is essential to better define the magnitude of risks for specific SN types. The full spectrum of SN should be considered.
Substantial efforts will be required to minimize loss-to-follow-up and ensure comprehensive ascertainment of SN diagnoses.
Detailed patient and clinical data should be collected to investigate modifiers of SN risks, including information on patient characteristics, primary disease, pre-transplant treatment exposures, conditioning regimen, transplant-related factors, and other clinical factors. Standardized reporting of data elements would facilitate future research, as multi-institution collaboration will be essential to achieve statistical power for certain analyses. For certain data elements, longitudinal data collection is critical (e.g., clinical factors such as GVHD).
When possible, studies should consider SN risks relative to the general population and absolute risks to quantify the clinical burden of SNs.
Interdisciplinary laboratory studies are needed to better characterize donor-derived SN.
PATHOGENESIS
Substantial advances in technologies related to genomics and assessment of immune function have provided insight into tumor pathogenesis in the general population. In particular, comprehensive molecular profiling of tumors has identified new disease subtypes and signatures reflective of particular exposures.84 However, much less is known about pathogenesis of SN. Most previous research has focused on immune drivers because the post-HCT milieu is associated with unique alterations that may promote the development of SN. After both autologous and allogeneic HCT, but especially after allogeneic HCT, a period of lymphopenia and cell-mediated immune deficiency affecting T- and NK-cells and antibody production occurs. This period can persist for months and is exacerbated by well-defined factors, including acute and chronic GVHD (aGVHD, cGVHD) as well as immunosuppressive therapy. The surge of interleukin (IL)-15 after HCT promotes rapid T-cell clonal expansion leading to a restricted T-cell repertoire.85,86 Expanding donor T-cells often have the phenotype of large granular lymphocytes, but occasionally a true clonal expansion of large granular lymphocyte disease can develop.87–92 Defective T-cell immunity permits reactivation of Epstein-Barr virus (EBV) and other oncogenic viruses, but post-HCT immune deficiency may increase the risk of other SN, as demonstrated in solid organ transplant recipients.93,94 Solid tumors also may arise due to the DNA damaging effects of cytotoxic chemotherapy and radiotherapy used as pre-transplant therapies or in conditioning regimens, although the exact carcinogenic mechanisms are less well understood. Additionally, post-allogeneic HCT treatment with voriconazole to prevent infectious complications has been associated with increased risk of cutaneous SCC, paralleling observations among solid organ transplant recipients.95
Hematologic malignancies may arise through accidental transplantation from an affected donor or develop in donated cells after transplantation. Donor-derived hematologic malignancies have particularly been reported following umbilical cord blood (UCB) transplants,37,96–101 with support for the likely importance of the post-HCT milieu in promoting neoplasm development. Despite a rate of up to 6% donor-derived MDS/AML in the recipient, there have been no instances of leukemia in the UCB donor.102 Similarly, although the occurrence of multiple myeloma after allogeneic HCT may be due to transfer of malignant cells with the transplant,103–106 the absence of reports of disease in the donor suggests that the recipient milieu is especially favorable to multiple myeloma. There are several reports from adult and UCB transplants that donor-derived leukemias do not always develop even with the transfer of hematopoietic progenitors bearing gene mutations.107–111 Finally, research into the allogeneic graft-versus-leukemia effect reveals donor T-cell responses to tumor antigens and minor antigens, and that disease relapse can be provoked by immunosuppressive therapy.112
Donor-derived leukemia may be driven by telomere shortening consequent upon the rapid and intense expansion of the graft.108 Following allogeneic HCT, the reduction in telomere length of donor hematopoietic cells is equivalent to approximately 15 years, and in some instances 40–60 years, of aging.113 In addition, telomere erosion is well recognized to cause significant genomic instability, leading to malignant transformation.114
The reports of solid tumors containing donor cells indicate that donor cells may either influence malignant change or more inexplicably transform into cancer cells.115–118 In favor of the transfer of non-hematopoietic cells in the graft are several publications describing donor cells participating in non-hematopoietic tissues.119,120 Techniques for identifying donor derived cancer cells relying on in situ hybridization have led to both positive and negative findings in animals121 and in humans.122
Research Gaps and Recommendations
Investigations of the pathogenesis of SN will require interdisciplinary laboratory collaboration, utilizing banked cryopreserved donor and recipient blood and marrow cells and SN tissues. Samples should be annotated with detailed patient and clinical data (e.g., stem cell source to investigate why UCB transplants have such a high propensity to develop donor malignancy).
Longitudinal biomarker studies are needed to investigate the interaction of the donor’s immune system with donor hematopoiesis or against recipient malignancies arising in non-hematopoietic tissue. Unanswered questions include mechanisms underlying viral induced SN, factors that control the development of T-large granular lymphocytes, which changes in the recipient milieu and tissue niches promote SN and how, and whether donor cells found in non-hematologic malignancies cause the malignancy or in some way promote its development.
DISEASE- AND TRANSPLANT-RELATED RISK FACTORS
Primary disease and disease status
Several reports in both autologous and allogeneic HCT have suggested that SN risk may vary by primary disease type or status. Among 674 pediatric patients undergoing allogeneic HCT, a primary diagnosis of non-Hodgkin lymphoma (NHL) was associated with a four-fold increase in the risk of solid cancers.17 Among 28,874 patients undergoing allogeneic HCT, those transplanted for chronic myelogenous leukemia (CML) had a lower risk of solid cancers than patients with acute leukemia,39 but this has not been confirmed in other studies.37,38 Two studies reported increased SN risks with more advanced disease, including adults with lymphoid malignances undergoing autologous HCT (N=300)59 and patients with FA undergoing allogeneic HCT (N=795).69 However, disease status was not associated with SN risk in 1487 pediatric autologous HCT recipients with a variety of primary diagnoses.24
Pre-HCT therapy and conditioning regimens
Among cancer survivors who have not received HCT, radiotherapy and certain systemic therapies have been associated with SN risks.123 Similar studies after HCT are complicated by the complexity of treatment exposures patients receive as part of pre-HCT therapies and conditioning regimens.
In 268 adult patients with lymphoid cancers undergoing autologous HCT, pre-HCT nitrogen mustard, oncovin (vincristine), procarbazine and prednisone (MOPP) chemotherapy was associated with a six-fold higher rate of developing any SN compared to other pre-HCT regimens.54 An almost eight-fold increase in risk of SN was reported after etoposide stem cell priming in one study.62 In contrast, two other studies found no association between pre-HCT chemotherapy and risk of SN.41,62 Pre-HCT radiotherapy has been shown to be an adverse risk factor for SN.59,60,62 Among 133 patients with aplastic anemia undergoing allogeneic HCT, pre-HCT immunosuppressive therapy with cyclosporine (a group 1 carcinogen according to the International Agency for Research on Cancer)124 was associated with five-fold higher rates of SN.75
Investigations of the role of conditioning regimens have largely focused on exposure to total body irradiation (TBI). The data suggest an interaction with age, with higher risk of SN in younger recipients exposed to TBI compared with older patients.39,125 However, the role of TBI in SN risk remains unresolved, with six publications providing strong evidence of increased SN risk from TBI exposure,16,17,32,46,49,60 and eight studies failing to identify an association between TBI and SN.22,24,33,37,38,41,59,69
In the non-TBI setting, patients undergoing allogeneic HCT with busulfan/cyclophosphamide (Bu/Cy) conditioning demonstrated 1.4-fold increased SN risk compared with the general population.11 In the autologous setting, etoposide as part of the conditioning regimen was not associated with SN among pediatric patients.24
Allogeneic donor source
In recent years, allogeneic transplantation practices have expanded to include unrelated donors (URD), haplo-identical related donors and stem cell sources other than bone marrow (e.g., peripheral blood, UCB). Because these practices are relatively new, their impact on risk for SN is poorly understood. Most studies have shown no association,22,36,38,41 but they are generally small with limited follow-up.
A comparison of 355 human leukocyte antigen (HLA)-matched sibling donor (MSD) and 108 URD HCT recipients surviving >2 years (median follow-up=10 years) did not show an effect of donor source on SN risk.126 Pre-HCT manipulation of donor stem cells (e.g., ex-vivo T-cell depletion) has been associated with increased risk of PTLD, with particularly elevated risks when using anti-thymocyte globulin or alemtuzumab in the preparative regimen, but no difference when using MSD or URD products.127,128 A European Society for Blood and Marrow Transplantation (EBMT) study of donor cell leukemia after 10,489 allogeneic HCTs performed during 1975–1998 found 14 cases, of which 12 were from matched related donors and two from URD.48 However, the small sample size and the paucity of URD in the study period limit any conclusions relating donor type to SN risk, and the only other data derive from case reports. A study from EBMT of 1036 recipients surviving >5 years (median follow-up=10.7 years, range 5–22) did not find donor-recipient histocompatibility to be a significant risk factor for development of new malignancies.129 Unpublished data from the Fred Hutchinson Cancer Research Center (FHCRC) found that the relative risk of SN in allogeneic HCT recipients, after adjustment for both aGVHD and cGVHD, was higher in those recipients who received peripheral blood stem cell (PBSC) grafts compared to bone marrow (RR=1.6, 95%CI=1.2–2.10, p=0.001). The fewer number of UCB transplants and their shorter follow-up precluded an accurate assessment of risk in UCB recipients. In a cohort of 98 adult patients who received UCB grafts, the cumulative incidence of 19% SN at two years appeared high, with PTLD being the most common.37 In autologous HCT recipients, risk for MDS/AML has been reported to be three-fold higher with PBSC grafts.128
Post-HCT maintenance
Post-autologous HCT maintenance therapy with lenalidomide for multiple myeloma has been associated in some studies with an increased risk for SN, but the magnitude of risk varied and detailed analysis of other potential risk factors was lacking.130–133 A meta-analysis of 3254 newly diagnosed patients treated on randomized controlled trials suggested that exposure to lenalidomide plus oral melphalan significantly increased the risk for hematologic malignancies but not solid tumors.134 Retrospective reports from single institutions are difficult to interpret because of heterogeneity in patient populations.135,136 A Center for International Blood and Marrow Transplant Research (CIBMTR) registry analysis did not demonstrate an increased risk in new malignancies with lenalidomide.64 There are no data for more recently utilized maintenance therapies (e.g., Flt3 inhibitors).
GVHD
Patients typically receive some type of prophylactic systemic immunosuppressive therapy or manipulated graft (e.g., CD34 selection, T-cell depletion) to prevent GVHD. Those diagnosed with GVHD are primarily treated with immunosuppressive therapy and may continue therapy for an extended time. Combinations of graft manipulation, immunosuppressive therapy, and GVHD-related immune dysregulation result in long-term immune impairment and an environment that may promote SN development.
Chronic GVHD and SN risk
A number of studies found that patients with cGVHD developed SN overall at 2–3-fold higher rates than the general population (Supplementary Table 2).11,19,33,46,69,137–142 Risks associated with cGVHD vary by malignancy type (Supplementary Table 3). SCC is the most commonly reported SN. One of the largest studies of SN found a nearly three-fold increased risk for SCC associated with cGVHD.10 Risks increased slightly when cGVHD was preceded by aGVHD but more strikingly – to nearly 10-fold – for severe cGVHD. Although some studies found a similar overall risk estimate for SCC after cGVHD;79 slightly higher risk estimates of approximately five-fold also have been reported for any SCC, regardless of time after transplant.9 Several studies have established that cGVHD increases risk in specific organs. The most frequently reported organs include skin, buccal cavity, and esophagus.5,10,139,140 Less is known about risks for other malignancies, though elevated risks for thyroid cancer (RR=2.94)46 and basal cell carcinoma (RR=1.6)79 were reported after cGVHD in allogeneic HCT recipients.
GVHD treatment and SN risk
Studies of the influence of immunosuppressive therapy for GVHD on the risk of SN after allogeneic HCT have been infrequent, largely due to their inherent need for well-annotated cohorts. Nevertheless, there are reports in which immunosuppressive therapy was associated with increased risk of SN.10,19 Chien et al. reported a 2.5-fold increased SN risk with use of azathioprine, at one point a relatively common drug used to treat cGVHD, and 5.5-fold non-significantly increased risk with sirolimus. (Supplementary Table 4).19
Curtis et al. reported several associations of immunosuppressive therapy with an increased risk of SCC for specific SN (Supplementary Table 5).10 When all systemic immunosuppressive therapy, both for GVHD prophylaxis and treatment, was considered, longer duration of immunosuppressive therapy increasingly raised the risk of SCC (2–4 years: RR=4.75; ≥4 years: RR=6.2), with particularly elevated risks for SCC in the oral cavity and skin. These associations generally persisted when only immunosuppressive therapy for treatment of cGVHD was considered. SCC risks also were increased five-fold for recipients of azathioprine given with steroids, and the addition of cyclosporine further increased risk, especially after one year (any duration: RR=18.61; ≥12 months: RR=38.71).
Research Gaps and Recommendations
Understanding disease- and transplant-related risk factors for SN is critical for future efforts to reduce risks as well as identify patients at highest risk who would most benefit from prevention and surveillance efforts.
-
Detailed data collected at consistent time points should include pre-transplant exposures, transplant-related factors, and clinical data during long-term follow-up. Specifically:
Studies that investigate the role of primary disease in SN risk should consider inherent biologic susceptibility related to the natural history of the primary disease, underlying genetic susceptibility to cancer, patient-related correlates (e.g., age), and cumulative carcinogenic effect of pre-transplant therapies.
Transplant-related variables should include degree of HLA matching, donor and graft source, graft manipulation, and conditioning regimens.
Post-HCT clinical variables should be collected longitudinally, including: post-HCT maintenance; GVHD prophylaxis, therapy, type, specific organ involvement and severity; and therapy for malignancy relapse.
Future studies that include patients who received transplants more recently are critical for defining whether the burden of SN has been altered with newer HCT and GVHD therapy approaches, even as follow-up is extended for patients transplanted in the past to identify long-term risks.
NON-TRANSPLANT-RELATED RISK FACTORS
The role of non-transplant-related risk factors in the development of SN is relatively unexplored. Below, we consider a wide range of potential risk factors, from genetic susceptibility to viruses and other medical history factors as well as lifestyle factors.
Advances in microarray and sequencing technologies have rapidly advanced our understanding of genetic susceptibility to cancer in the non-HCT setting. Allogeneic HCT is the treatment of choice for severe aplastic anemia or hematological malignancies arising in patients with variety of inherited cancer susceptibility syndromes. However, risk of SN after allogeneic HCT in individuals with rare inherited cancer predisposition syndromes is not well studied. In 157 patients with FA who survived ≥2 years after HCT, the incidence of oral cavity cancers, mainly SCC, increased with time (8% and 14% at 10 and 15 years post-HCT, respectively), and SCC accounted for 40% of deaths.76 In another study of 262 patients with FA, the 117 HCT recipients had a four-fold increased risk of developing SCC.143
The importance of studying genetic susceptibility outside the context of rare inherited cancer predisposition syndromes is highlighted by literature on risk for therapy-related MDS/AML (tMDS/AML) after autologous HCT or following conventional chemotherapy. Initial studies on inter-individual variability in risk of tMDS/AML using a candidate gene approach have identified common polymorphisms in low penetrance genes that regulate the availability of active drug metabolite or those responsible for DNA repair. Associations have been observed for glutathione S-transferase genes (GSTM1, GSTT1 and GSTP1), particularly among those with prior exposure to chemotherapeutic agents that are known GSTP1 substrates,144 and for P-glycoprotein (encoded by MDR1), a cell membrane protein that impacts response to numerous chemotherapeutic agents.145 A number of studies also have evaluated key DNA repair genes, including mismatch repair family members MLH1146,147 and MSH2,148,149 RAD51,150 and XRCC3.151–153 Other polymorphisms in candidate genes from DNA repair pathways include ERCC2 of the nucleotide excision repair pathway154 and two common functional p53-pathway variants.155
As technology for larger-scale genomic studies has advanced, Knight et al. conducted a case-control genome-wide association study, comparing patients who had developed tAML and healthy controls.156 The investigators identified three polymorphisms (rs1394384, intronic to ACCN1; rs1381392, intergenic; and rs1199098, in linkage disequilibrium with IPMK) to be associated with tMDS/AML with chromosome 5 and/or 7 abnormalities. Although the investigators were able to confirm findings in an independent replication cohort, the utilization of a non-cancer healthy control group raises concerns about the case-control differences being generated by the genetics of the primary cancer versus tMDS/AML.
Telomeres, the long TTAGGG nucleotide repeats and protein complexes at the end of chromosomes, are markers of cellular replicative capacity and aging.157 Mutations in telomere genes cause dyskeratosis congenita, a rare inherited marrow failure and cancer susceptibility syndrome.158 In the general population, genetic variations in telomere-biology genes, namely TERT159 and RTEL1,160 have been associated with the development of several cancers, and short telomere length with cancer risk and mortality in large epidemiological studies.161,162 Although no studies have investigated telomere length and cancer risk after allogeneic HCT, several studies suggest that it may be important. Telomere shortening in the transplanted hematopoietic cells appeared to accelerate post-transplant when compared with age-matched controls or matched donors (reviewed in163). Short telomeres after HCT were more common in females and those with cGVHD,164 and pre-HCT short telomere length in recipients has been associated with transplant-related mortality.165,166 Finally, in a longitudinal study including 287 lymphoma patients, accelerated telomere shortening was associated with MDS/AML risk after autologous HCT.167
For a number of specific SN that occur in excess in allogeneic HCT recipients, there is substantial understanding of the key lifestyle/medical history etiologic factors in the general population. Examples include the role of tobacco, alcohol, and human papilloma virus (HPV) infections in oral cavity cancers;168,169 sun exposure in melanoma;170 and hepatitis C virus (HCV) infections, autoimmune diseases, and sun exposure in certain NHLs.171 However, the role of these risk factors in SN after allogeneic HCT is largely unknown. In a large analysis of outcomes of 4161 patients with plasma cell disorders undergoing autologous transplantation, obesity was associated with a nearly two-fold increase in SN risk.64
Investigations should also consider whether other cancer risk factors have an additive or multiplicative effect with transplant-related risk factors. For example, immune activation or chronic immune damage of tissues from GVHD could act synergistically with viruses to impact risk of cancers such as oral cavity SCC.80,172–175 Similarly, immunosuppression could lead to the reappearance of latent HPV infection, affecting risk of cutaneous SCC or cervical cancer.176,177
Additional studies are needed to confirm the role of oncogenic viruses in cancer risk after allogeneic HCT. Studies of cancer occurrence and cancer risk factors in other immunosuppressed populations, particularly solid organ transplant recipients and individuals with human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS), support the idea that immunosuppression contributes to cancer risk in part through loss of immune control of oncogenic viruses.93,178 PTLD is the most common virus-related cancer in allogeneic HCT recipients, and is caused by EBV.179 EBV also has been associated with gastric adenocarcinoma after allogeneic HCT.180 Other virus-related cancers are rare, but observed elevations in risk for cervical cancer (HPV) and liver cancer (HCV, hepatitis B virus [HBV]) support the likely importance of oncogenic viruses in cancer risk after allogeneic HCT.24,39,43,181 A single case reports suggests the possibility of donor T-cells acquiring human T-lymphotropic virus (HTLV1).182 Polyomaviruses also represent an emerging group of viruses requiring study because of their potential to cause epithelial malignancy.183 The recently described Merkel cell skin cancer occurring in immune deficient states is driven by the Merkel cell polyomavirus, which is closely related to BK and JC viruses, both of which reactivate after HCT.184
Research Gaps and Recommendations
Research is needed to investigate whether established cancer risk factors in de novo malignancies exhibit similar associations for post-HCT SN, and whether established cancer risk factors interact with transplant-related risk factors.
With advances in technology for evaluating inherited genetic variation, there is great opportunity to identify genetic risk factors for SN after allogeneic HCT. Studies should consider a range of genetic variation, from rare inherited cancer predisposition syndromes or other rare variants to common genetic variants.
More work is also needed to understand the role telomeres play in post-HCT cancer development and the nature of donor-recipient interactions.
OUTCOMES
Although data from other studies of SN have suggested that survival may be poorer,185–187 relatively little is known about the outcomes of SN occurring in HCT recipients. Post-HCT PTLD has been reported to have an aggressive, immunoblastic phenotype,188 and can be a major contributor to death.13 Therapy-related MDS/AML is more likely to have adverse cytogenetic characteristics than sporadic MDS/AML,82,189 and prognosis has been shown to be poorer even after controlling for cytogenetic abnormalities.190 For solid tumors, outcomes after allogeneic HCT appear to vary by tumor type, with poorer prognosis compared with the general population for female reproductive, bone and joint, lower gastrointestinal tract, and central nervous system (CNS) tumors, but comparable outcomes for thyroid, testis, breast, oral cavity, and soft tissue tumors, as well as melanoma.191
Research Gaps and Recommendations
Further research is needed to better understand the prognosis following SN in the HCT setting. Studies with systematic long-term follow-up should include comprehensive information on prior treatments, tumor subtype, stage and grade, tumor molecular characteristics, and the therapeutic approach selected for the transplant recipient. Comparisons with comparable survivors in the general population are essential.
Studies are needed to identify optimal treatment approaches for SN after HCT. Treatment options may be limited by prior therapeutic exposures, including both pre-HCT treatments and conditioning regimens. Examples of these concerns include patients who have already received cumulative maximum anthracycline doses or radiotherapy to a particular region of the body. Optimal treatment approaches should explore most recent advances including pharmacological and cellular immunotherapy approaches (e.g., donor-derived EBV-specific lymphocytes to manage PTLD192,193).
OPTIMAL SCREENING AND PREVENTATIVE PRACTICES
Screening
The development of screening guidelines has been the focus of several efforts by professional organizations.194–196 The Children’s Oncology Group (COG) created long-term follow-up guidelines (www.survivorshipguidelines.org) for children, adolescent, and young adults who underwent HCT. 197 Consensus-based recommendations for screening and prevention of solid cancers were adapted from those for the general population through collaboration of the CIBMTR Late Effects and Quality of Life Working Committee and the EBMT Complications and Quality of Life Working Party for children and adults.198
Given the heterogeneity in the types of SN developing after HCT, cancer screening has never been adequately studied in this population to determine if it provides any benefit in terms of survival or cost effectiveness. The issue of when to initiate screening is also unclear, particularly for patients transplanted as children (the risk of SN is higher at younger ages compared to a relatively low risk in the general population), and older adults undergoing HCT (as the risk of malignancies increases with age in the general population).
Preventative practices
Several studies have described adherence to preventative health behaviors and recommended screening guidelines for SN in HCT survivors.199–201 In a study of 1549 adult survivors of autologous HCT, those concerned about medical costs, non-white race, male gender, lower physical functioning, no chronic GHVD, longer time since HCT and lack of knowledge about recommended tests were factors associated with lower adherence to recommended preventive care. A cross-sectional study (N=662) reported that autologous HCT survivors were more likely than allogeneic to undergo screening for breast and cervical cancer but less likely to have undergone a skin examination in the previous year.200 The Bone Marrow Transplant Survivor Study examined health behaviors and cancer screening practices in HCT recipients who underwent HCT during 1976–1998.201 Multivariate regression analysis revealed younger age (<35 years) at study participation and lower education (<college) to be significantly associated with high-risk health behaviors for SN. Female survivors were more likely to have had a screening mammogram when compared to gender-matched sibling controls.
Interventions
It is imperative that survivors and clinicians be educated on recommended preventative practices and screening guidelines for SN. Studies suggest that as few as 20% of providers talk to cancer survivors about health behaviors,202 and only 10% of survivors report their physicians talking to them about smoking, exercise, and diet.203 Similar data are lacking for HCT survivors. Care of HCT survivors is complex, requires a multi-disciplinary approach, and may be expensive. The optimal health care delivery model for cost-effective education on preventative practices and screening for SN deserves greater study, with a particular focus on education and care delivery to socioeconomically-challenged patient populations.
Research Gaps and Recommendations
Although the current consensus screening recommendations represent an important step forward, stronger evidence is needed to support their validity and cost-effectiveness in the HCT population. Additionally, the magnitude of risk reduction, optimal screening techniques and timing of initiation, and effect on outcomes are unknown. Future recommendations should account for host factors, underlying disease, therapeutic exposures, and donor source, as appropriate.
As cancer prevention interventions are implemented in the general population, such as vaccination against HPV,173,177 their efficacy in HCT survivors should be evaluated.
SUMMARY
To reduce the burden of SN and improve the long-term health of HCT survivors, it is critical to identify the magnitude of risk, risk factors and mechanisms of pathogenesis for SN and to determine optimal preventative, screening, and therapeutic approaches. Research in these areas is challenging because of the complex clinical history of many transplant survivors, the need for long-term follow-up, and diversity of timing, behavior, and origin of SN. With substantial changes in clinical transplantation practices, risks for patients receiving HCT in the current era are poorly understood. Hence, the need for establishing a robust and collaborative, multidisciplinary infrastructure for SN research has never been greater. Clinical data must be consistently and uniformly collected on patient, disease, and pre-, transplant, and post-HCT variables within the context of large-scale, long-term (>10 years) studies of HCT recipients undergoing systematic follow-up in conjunction with recommended screening practices. Resources, particularly financial and other funding mechanisms, designed to support banking of cryopreserved donor and recipient blood and marrow cells and SN tissues with detailed patient and clinical data, as well as resources that provide support for longitudinal biomarker studies will be critical to advancing our understanding of the pathogenesis of SN and identify effective approaches for prevention and management.
Supplementary Material
HIGH PRIORITY RECOMMENDATIONS.
Conduct large-scale, long-term (>10 years), systematic follow-up of HCT recipients to better define the magnitude of risks for specific SN types, including both common and rarer tumors. The full spectrum of SN should be considered, including certain benign tumors (e.g., meningioma) as well as all types of cutaneous malignancies.
Collect detailed patient and clinical data (e.g., primary disease, pre- and post-transplant therapies, donor source, GVHD type/severity/therapy, adherence to screening recommendations, exposure to key cancer risk factors such as tobacco) in a standardized format to investigate determinants of SN risks.
Bank cryopreserved donor and recipient blood and marrow cells and SN tissues to enable interdisciplinary clinical and laboratory investigations of SN susceptibility and pathogenesis.
Acknowledgments
We thank the members of the NIH Late Effects Initiative Steering Committee (Minoo Battiwalla, Shahrukh Hashmi, Navneet Majhail, Steven Pavletic, Bipin Savani, and Nonniekaye Shelburne) for their leadership of this initiative and critical review of the manuscript. We thank Mary Flowers and Gérard Socié for their critical review of the manuscript. We thank Barbara Brandys, National Institutes of Health (NIH) Library, for conducting the literature database searches, and we thank Filip Pirsl for his assistance reviewing literature and preparing supplementary tables regarding GVHD. This initiative was sponsored jointly by the National Heart, Lung and Blood Institute (NHLBI) and the National Cancer Institute (NCI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure statement: The authors have no disclosures.
DISCLAIMER
The opinions expressed here are those of the authors and do not represent the official position of the NIH or the United States Government.
REFERENCES
- 1.Pasquini MC, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2015;20162015 [Google Scholar]
- 2.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29:2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105:4215–4222. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majhail NS, Tao L, Bredeson C, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. 2013;19:1498–1501. doi: 10.1016/j.bbmt.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Socie G, Baker KS, Bhatia S. Subsequent malignant neoplasms after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:S139–S150. doi: 10.1016/j.bbmt.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin PJ, Counts GW, Jr, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28:1011–1016. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis RE, Metayer C, Rizzo JD, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105:3802–3811. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majhail NS, Brazauskas R, Rizzo JD, et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117:316–322. doi: 10.1182/blood-2010-07-294629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21:1352–1358. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 13.Curtis RE, Travis LB, Rowlings PA, et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood. 1999;94:2208–2216. [PubMed] [Google Scholar]
- 14.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 15.Boztug H, Sykora KW, Slatter M, et al. European Society for Blood and Marrow Transplantation Analysis of Treosulfan Conditioning Before Hematopoietic Stem Cell Transplantation in Children and Adolescents With Hematological Malignancies. Pediatr Blood Cancer. 2016;63:139–148. doi: 10.1002/pbc.25764. [DOI] [PubMed] [Google Scholar]
- 16.Wilhelmsson M, Vatanen A, Borgstrom B, et al. Adverse health events and late mortality after pediatric allogeneic hematopoietic SCT-two decades of longitudinal follow-up. Bone Marrow Transplant. 2015;50:850–857. doi: 10.1038/bmt.2015.43. [DOI] [PubMed] [Google Scholar]
- 17.Nelson AS, Ashton LJ, Vajdic CM, et al. Second cancers and late mortality in Australian children treated by allogeneic HSCT for haematological malignancy. Leukemia. 2015;29:441–447. doi: 10.1038/leu.2014.203. [DOI] [PubMed] [Google Scholar]
- 18.Gifford G, Gilroy N, Dyer G, et al. The experience of survival following allogeneic haematopoietic stem cell transplantation in New South Wales, Australia. Bone Marrow Transplant. 2016 doi: 10.1038/bmt.2016.135. [DOI] [PubMed] [Google Scholar]
- 19.Chien SH, Liu CJ, Hong YC, et al. Use of azathioprine for graft-vs-host disease is the major risk for development of secondary malignancies after haematopoietic stem cell transplantation: a nationwide population-based study. Br J Cancer. 2015;112:177–184. doi: 10.1038/bjc.2014.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamora-Ortiz G, Velazquez-Sanchez-de-Cima S, Ponce-de-Leon S, et al. Secondary malignancies after allogeneic hematopoietic stem cell transplantation using reduced-intensity conditioning and outpatient conduction. Hematology. 2014;19:435–440. doi: 10.1179/1607845414Y.0000000154. [DOI] [PubMed] [Google Scholar]
- 21.Ringden O, Brazauskas R, Wang Z, et al. Second solid cancers after allogeneic hematopoietic cell transplantation using reduced-intensity conditioning. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20:1777–1784. doi: 10.1016/j.bbmt.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michelis FV, Kotchetkov R, Grunwald RM, et al. Incidence of secondary malignancies following allogeneic hematopoietic cell transplantation: A single center experience. Blood. 2014:124. doi: 10.1016/j.bbmt.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Gifford G, Sim J, Horne A, Ma D. Health status, late effects and long-term survivorship of allogeneic bone marrow transplantation: A retrospective study. Internal Medicine Journal. 2014;44:139–147. doi: 10.1111/imj.12336. [DOI] [PubMed] [Google Scholar]
- 24.Danner-Koptik KE, Majhail NS, Brazauskas R, et al. Second malignancies after autologous hematopoietic cell transplantation in children. Bone Marrow Transplant. 2013;48:363–368. doi: 10.1038/bmt.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omori M, Yamashita H, Shinohara A, et al. Eleven secondary cancers after hematopoietic stem cell transplantation using a total body irradiation-based regimen in 370 consecutive pediatric and adult patients. SpringerPlus. 2013;2:424. doi: 10.1186/2193-1801-2-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun C-L, Kersey JH, Francisco L, et al. Burden of Morbidity in 10+ Year Survivors of Hematopoietic Cell Transplantation: Report from the Bone Marrow Transplantation Survivor Study. Biology of Blood and Marrow Transplant. 2013;19:1073–1080. doi: 10.1016/j.bbmt.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang JC, Boulad F, Sklar CA, Friedman DN, Wolden SL. Total body irradiation in very young children. International Journal of Radiation Oncology Biology Physics. 2012;84:S161. [Google Scholar]
- 28.Muftuoglu M, Gurses N, Sargin F, Kalayoglu-Besisik S. Secondary cancers following stem cell transplantation: A single center experience. Haematologica. 2012;97:721. [Google Scholar]
- 29.Kauppila M, Remes K, Putkonen M, Lähteenmäki P, Salmenniemi U, Itälä-Remes M. Malignancies after allogeneic haematopoietic stem cell transplantation-a single-centre analysis of 370 patients. Bone Marrow Transplantation. 2012;47:S351. [Google Scholar]
- 30.Sorror ML, Storb R, Sandmaier BM, et al. Impact of comorbidities on early and late mortalities after allogeneic Hematopoietic Cell Transplantation (HCT) Blood. 2011:118. [Google Scholar]
- 31.Clavert A, Zina P, Cahu X, et al. Long-term complications (LTC) and quality of life (QOL) after reduced-intensity conditioning (RIC) allogeneic stem cell transplantation (allo-SCT) Blood. 2011:118. [Google Scholar]
- 32.Bieri S, Roosnek E, Ozsahin H, et al. Outcome and risk factors for late-onset complications 24 months beyond allogeneic hematopoietic stem cell transplantation. Eur J Haematol. 2011;87:138–147. doi: 10.1111/j.1600-0609.2011.01638.x. [DOI] [PubMed] [Google Scholar]
- 33.Atsuta Y, Suzuki R, Yamashita T, et al. Risk and risk factors of secondary solid cancers after allogeneic hematopoietic stem cell transplantation in adult recipients. Biology of Blood and Marrow Transplantation. 2011;17:S174. [Google Scholar]
- 34.Michallet M, Sobh M, Morisset S, et al. Phase II prospective multicenter study of treosulfan based reduced intensity conditioning in allogeneic hematopoietic stem cell transplantation for hematological malignancies from 10/10 hla identical unrelated donor. Blood. 2010:116. [Google Scholar]
- 35.Ghavamzadeh A, Alimoghaddam K, Jahani M, et al. The incidence of secondary malignancy post stem cell transplantation: Importance of conditioning regimens without total body irradiation. Bone Marrow Transplantation. 2010;45:S96–S97. [Google Scholar]
- 36.Friedrichs B, Tichelli A, Bacigalupo A, et al. Long-term outcome and late effects in patients transplanted with mobilised blood or bone marrow: A randomised trial. The Lancet Oncology. 2010;11:331–338. doi: 10.1016/S1470-2045(09)70352-3. [DOI] [PubMed] [Google Scholar]
- 37.Ballen KK, Cutler C, Yeap BY, et al. Donor-derived second hematologic malignancies after cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:1025–1031. doi: 10.1016/j.bbmt.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abou-Mourad YR, Lau BC, Barnett MJ, et al. Long-term outcome after allo-SCT: close follow-up on a large cohort treated with myeloablative regimens. Bone Marrow Transplant. 2010;45:295–302. doi: 10.1038/bmt.2009.128. [DOI] [PubMed] [Google Scholar]
- 39.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricardi U, Filippi AR, Biasin E, et al. Late toxicity in children undergoing hematopoietic stem cell transplantation with TBI-containing conditioning regimens for hematological malignancies. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft … [et al] 2009;185(Suppl 2):17–20. doi: 10.1007/s00066-009-1008-x. [DOI] [PubMed] [Google Scholar]
- 41.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 42.Kulkarni S, Powles R, Treleaven J, et al. Melphalan/TBI is not more carcinogeneic than cyclophosphamide/TBI for transplant conditioning: follow-up of 725 patients from a single centre over a period of 26 years. Bone Marrow Transplant. 2000;25:365–370. doi: 10.1038/sj.bmt.1702148. [DOI] [PubMed] [Google Scholar]
- 43.Kolb HJ, Socie G, Duell T, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med. 1999;131:738–744. doi: 10.7326/0003-4819-131-10-199911160-00004. [DOI] [PubMed] [Google Scholar]
- 44.Lowsky R, Lipton J, Fyles G, et al. Secondary malignancies after bone marrow transplantation in adults. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1994;12:2187–2192. doi: 10.1200/JCO.1994.12.10.2187. [DOI] [PubMed] [Google Scholar]
- 45.Hasegawa W, Pond GR, Rifkind JT, et al. Long-term follow-up of secondary malignancies in adults after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2005;35:51–55. doi: 10.1038/sj.bmt.1704706. [DOI] [PubMed] [Google Scholar]
- 46.Cohen A, Rovelli A, Merlo DF, et al. Risk for secondary thyroid carcinoma after hematopoietic stem-cell transplantation: an EBMT Late Effects Working Party Study. J Clin Oncol. 2007;25:2449–2454. doi: 10.1200/JCO.2006.08.9276. [DOI] [PubMed] [Google Scholar]
- 47.Au WY, Chan EC, Pang A, et al. Nonhematologic malignancies after allogeneic hematopoietic stem cell transplantation: incidence and molecular monitoring. Bone Marrow Transplant. 2004;34:981–985. doi: 10.1038/sj.bmt.1704674. [DOI] [PubMed] [Google Scholar]
- 48.Hertenstein B, Hambach L, Bacigalupo A, et al. Development of leukemia in donor cells after allogeneic stem cell transplantation--a survey of the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2005;90:969–975. [PubMed] [Google Scholar]
- 49.Sumaili H, Al-Kofide A, Al-Seraihi A, et al. Outcome of pediatric patients with lymphoma following stem cell transplant: A single institution report. Leukemia and Lymphoma. 2015;56:1327–1334. doi: 10.3109/10428194.2014.951846. [DOI] [PubMed] [Google Scholar]
- 50.Kato M, Hasegawa D, Koh K, et al. Allogeneic haematopoietic stem cell transplantation for infant acute lymphoblastic leukaemia with KMT2A (MLL) rearrangements: a retrospective study from the paediatric acute lymphoblastic leukaemia working group of the Japan Society for Haematopoietic Cell Transplantation. Br J Haematol. 2015;168:564–570. doi: 10.1111/bjh.13174. [DOI] [PubMed] [Google Scholar]
- 51.Shah NA, Rauenzahn S, Wen S, et al. Long term outcomes of autologous hematopoietic cell transplant (AHCT) following thiotepa-based high-dose therapy (HDT) in patients with non-hodgkin lymphoma (NHL) Biology of Blood and Marrow Transplantation. 2014;20:S117. doi: 10.1038/bmt.2016.275. [DOI] [PubMed] [Google Scholar]
- 52.Sureda A, Boumendil A, Sieniawski M, et al. Secondary malignancies after autologous stem cell transplantation in patients with relapsed/refractory hodgkin’s lymphoma. A retrospective analysis on behalf of the lymphoma working party of the European society for blood and marrow transplantation (EBMT) Blood. 2013:122. [Google Scholar]
- 53.Nieto Y, Popat U, Anderlini P, et al. Autologous stem cell transplantation for refractory or poor-risk relapsed Hodgkin’s lymphoma: effect of the specific high-dose chemotherapy regimen on outcome. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19:410–417. doi: 10.1016/j.bbmt.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Czyz A, Łojko-Dankowska A, Matuszak M, et al. Second malignancies after autologous hematopoietic stem cell transplantation following a uniform conditioning regimen in patients with lymphoma-characteristics and risk factors analysis. Acta Haematologica Polonica. 2013;44:63–64. doi: 10.5114/wo.2013.34626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim G, Lee JH, Jung MJ, et al. Hematopoietic stem cell transplantation with total body irradiation conditioning in childhood acute Lymphoblastic Leukemia patients with relapsed or high risk group. European Journal of Cancer. 2011;47:S289. [Google Scholar]
- 56.Viviani S, Di Nicola M, Bonfante V, et al. Long-term results of high-dose chemotherapy with autologous bone marrow or peripheral stem cell transplant as first salvage treatment for relapsed or refractory Hodgkin lymphoma: A single institution experience. Leukemia and Lymphoma. 2010;51:1251–1259. doi: 10.3109/10428194.2010.486090. [DOI] [PubMed] [Google Scholar]
- 57.Rubio MT, Boumendil A, Luan JJ, et al. Is there still a place for total body irradiation (TBI) in the conditioning regimen of autologous stem cell transplantation in mantle cell lymphoma ?: A retrospective study from the lymphoma working party of the EBMT. Blood. 2010:116. [Google Scholar]
- 58.Czyz A, Lojko A, Gil L, et al. Autologous hematopoietic stem cell transplantation following a uniform modified BEAM conditioning regimen for Hodgkin’s lymphoma - Prognostic factors and long-term outcomes. Blood. 2009:114. doi: 10.1007/s12032-013-0611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wheeler C, Khurshid A, Ibrahim J, et al. Incidence of post transplant myelodysplasia/acute leukemia in non-Hodgkin’s lymphoma patients compared with Hodgkin’s disease patients undergoing autologous transplantation following cyclophosphamide, carmustine, and etoposide (CBV) Leukemia & lymphoma. 2001;40:499–509. doi: 10.3109/10428190109097649. [DOI] [PubMed] [Google Scholar]
- 60.Sureda A, Arranz R, Iriondo A, et al. Autologous stem-cell transplantation for Hodgkin’s disease: results and prognostic factors in 494 patients from the Grupo Espanol de Linfomas/Transplante Autologo de Medula Osea Spanish Cooperative Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:1395–1404. doi: 10.1200/JCO.2001.19.5.1395. [DOI] [PubMed] [Google Scholar]
- 61.Park S, Brice P, Noguerra ME, et al. Myelodysplasias and leukemias after autologous stem cell transplantation for lymphoid malignancies. Bone Marrow Transplant. 2000;26:321–326. doi: 10.1038/sj.bmt.1702510. [DOI] [PubMed] [Google Scholar]
- 62.Krishnan A, Bhatia S, Slovak ML, et al. Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma: an assessment of risk factors. Blood. 2000;95:1588–1593. [PubMed] [Google Scholar]
- 63.Traweek ST, Slovak ML, Nademanee AP, Brynes RK, Niland JC, Forman SJ. Clonal karyotypic hematopoietic cell abnormalities occurring after autologous bone marrow transplantation for Hodgkin’s disease and non-Hodgkin’s lymphoma. Blood. 1994;84:957–963. [PubMed] [Google Scholar]
- 64.Mahindra A, Raval G, Mehta P, et al. New cancers after autotransplantations for multiple myeloma. Biol Blood Marrow Transplant. 2015;21:738–745. doi: 10.1016/j.bbmt.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vadikoliou C, Malouri D, Kaliou M, et al. Secondary malignancies in multiple myeloma. Clinical Lymphoma, Myeloma and Leukemia. 2015;15:e200. [Google Scholar]
- 66.Adekola KUA, Bashir Q, Shah N, et al. Characteristics of multiple myeloma patients with 6-year or longer progression-free survival after a single autologous transplant. Blood. 2013:122. [Google Scholar]
- 67.Koc¸ak Ü, Küpesiz A, Aksoylar S, et al. Outcome of allogeneic hematopoietic stem cell transplantation in pediatric patients with acquired severe aplastic anemia: A Turkish multicenter study. Bone Marrow Transplantation. 2015;50:S274–S275. [Google Scholar]
- 68.Dufour C, Pillon M, Passweg J, et al. Outcome of aplastic anemia in adolescence: a survey of the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2014;99:1574–1581. doi: 10.3324/haematol.2014.106096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peffault de Latour R, Porcher R, Dalle JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122:4279–4286. doi: 10.1182/blood-2013-01-479733. [DOI] [PubMed] [Google Scholar]
- 70.Konopacki J, Porcher R, Robin M, et al. Long-term follow up after allogeneic stem cell transplantation in patients with severe aplastic anemia after cyclophosphamide plus antithymocyte globulin conditioning. Haematologica. 2012;97:710–716. doi: 10.3324/haematol.2011.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buchbinder D, Nugent DJ, Brazauskas R, et al. Late effects in hematopoietic cell transplant recipients with acquired severe aplastic anemia: a report from the late effects working committee of the center for international blood and marrow transplant research. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:1776–1784. doi: 10.1016/j.bbmt.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inamoto Y, Suzuki R, Kuwatsuka Y, et al. Long-term outcome after bone marrow transplantation for aplastic anemia using cyclophosphamide and total lymphoid irradiation as conditioning regimen. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14:43–49. doi: 10.1016/j.bbmt.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 73.Deeg HJ, Socie G, Schoch G, et al. Malignancies after marrow transplantation for aplastic anemia and fanconi anemia: a joint Seattle and Paris analysis of results in 700 patients. Blood. 1996;87:386–392. [PubMed] [Google Scholar]
- 74.Socie G, Henry-Amar M, Bacigalupo A, et al. Malignant tumors occurring after treatment of aplastic anemia. European Bone Marrow Transplantation-Severe Aplastic Anaemia Working Party. The New England journal of medicine. 1993;329:1152–1157. doi: 10.1056/NEJM199310143291603. [DOI] [PubMed] [Google Scholar]
- 75.Ades L, Mary JY, Robin M, et al. Long-term outcome after bone marrow transplantation for severe aplastic anemia. Blood. 2004;103:2490–2497. doi: 10.1182/blood-2003-07-2546. [DOI] [PubMed] [Google Scholar]
- 76.Bonfim C, Ribeiro L, Nichele S, et al. Long-term Survival, Organ Function, and Malignancy after Hematopoietic Stem Cell Transplantation for Fanconi Anemia. Biol Blood Marrow Transplant. 2016 doi: 10.1016/j.bbmt.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 77.Vajdic CM, Mayson E, Dodds AJ, et al. Second Cancer Risk and Late Mortality in Adult Australians Receiving Allogeneic Hematopoietic Stem Cell Transplantation: A Population-Based Cohort Study. Biol Blood Marrow Transplant. 2016;22:949–956. doi: 10.1016/j.bbmt.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 78.Omland SH, Gniadecki R, Haedersdal M, Helweg-Larsen J, Omland LH. Skin Cancer Risk in Hematopoietic Stem-Cell Transplant Recipients Compared With Background Population and Renal Transplant Recipients: A Population-Based Cohort Study. JAMA Dermatol. 2016;152:177–183. doi: 10.1001/jamadermatol.2015.3902. [DOI] [PubMed] [Google Scholar]
- 79.Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24:1119–1126. doi: 10.1200/JCO.2005.02.7052. [DOI] [PubMed] [Google Scholar]
- 80.Landgren O, Gilbert ES, Rizzo JD, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113:4992–5001. doi: 10.1182/blood-2008-09-178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leone G, Pagano L, Ben-Yehuda D, Voso MT. Therapy-related leukemia and myelodysplasia: susceptibility and incidence. Haematologica. 2007;92:1389–1398. doi: 10.3324/haematol.11034. [DOI] [PubMed] [Google Scholar]
- 82.Metayer C, Curtis RE, Vose J, et al. Myelodysplastic syndrome and acute myeloid leukemia after autotransplantation for lymphoma: a multicenter case-control study. Blood. 2003;101:2015–2023. doi: 10.1182/blood-2002-04-1261. [DOI] [PubMed] [Google Scholar]
- 83.Milligan DW, Ruiz De Elvira MC, Kolb HJ, et al. Secondary leukaemia and myelodysplasia after autografting for lymphoma: results from the EBMT. EBMT Lymphoma, Late Effects Working Parties European Group for Blood and Marrow Transplantation. Br J Haematol. 1999;106:1020–1026. doi: 10.1046/j.1365-2141.1999.01627.x. [DOI] [PubMed] [Google Scholar]
- 84.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Melenhorst JJ, Tian X, Xu D, et al. Cytopenia and leukocyte recovery shape cytokine fluctuations after myeloablative allogeneic hematopoietic stem cell transplantation. Haematologica. 2012;97:867–873. doi: 10.3324/haematol.2011.053363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thiant S, Moutuou MM, Leboeuf D, Guimond M. Homeostatic cytokines in immune reconstitution and graft-versus-host disease. Cytokine. 2016 doi: 10.1016/j.cyto.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 87.Rivet J, Moreau D, Daneshpouy M, et al. T-cell lymphoma with eosinophilia of donor origin occurring 12 years after allogeneic bone marrow transplantation for myeloma. Transplantation. 2001;72:965. doi: 10.1097/00007890-200109150-00040. [DOI] [PubMed] [Google Scholar]
- 88.Au WY, Lam CC, Lie AK, Pang A, Kwong YL. T-cell large granular lymphocyte leukemia of donor origin after allogeneic bone marrow transplantation. Am J Clin Pathol. 2003;120:626–630. doi: 10.1309/VA75-5A03-PVRV-9XDT. [DOI] [PubMed] [Google Scholar]
- 89.Chang H, Kamel-Reid S, Hussain N, Lipton J, Messner HA. T-cell large granular lymphocytic leukemia of donor origin occurring after allogeneic bone marrow transplantation for B-cell lymphoproliferative disorders. Am J Clin Pathol. 2005;123:196–199. [PubMed] [Google Scholar]
- 90.Fahy CM, Fortune A, Quinn F, et al. Development of mycosis fungoides after bone marrow transplantation for chronic myeloid leukaemia: transmission from an allogeneic donor. Br J Dermatol. 2014;170:462–467. doi: 10.1111/bjd.12647. [DOI] [PubMed] [Google Scholar]
- 91.Camp BJ, Busam KJ, Brownell I, Koehne G, Hedvat C, Pulitzer MP. Donor-derived lymphomatoid papulosis in a stem-cell transplantation recipient. J Clin Oncol. 2011;29:e855–e858. doi: 10.1200/JCO.2011.37.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Santos-Briz A, Romo A, Antunez P, et al. Primary cutaneous T-cell lymphoproliferative disorder of donor origin after allogeneic haematopoietic stem-cell transplantation. Clin Exp Dermatol. 2009;34:e778–e781. doi: 10.1111/j.1365-2230.2009.03509.x. [DOI] [PubMed] [Google Scholar]
- 93.Engels EA, Pfeiffer RM, Fraumeni JF, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morton LM, Gibson TM, Clarke CA, et al. Risk of myeloid neoplasms after solid organ transplantation. Leukemia. 2014 doi: 10.1038/leu.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wojenski DJ, Bartoo GT, Merten JA, et al. Voriconazole exposure and the risk of cutaneous squamous cell carcinoma in allogeneic hematopoietic stem cell transplant patients. Transpl Infect Dis. 2015;17:250–258. doi: 10.1111/tid.12367. [DOI] [PubMed] [Google Scholar]
- 96.Kusumoto S, Mori S, Nosaka K, et al. T-cell large granular lymphocyte leukemia of donor origin after cord blood transplantation. Clin Lymphoma Myeloma. 2007;7:475–479. doi: 10.3816/clm.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 97.Hayes C, Petersen B, Malone A. Donor Cell Myeloid Sarcoma in an Umbilical Cord Transplant Patient: A Case Report and a Review of the Literature. Case Rep Hematol. 2015;2015:186869. doi: 10.1155/2015/186869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matsunaga T, Murase K, Yoshida M, et al. Donor cell derived acute myeloid leukemia after allogeneic cord blood transplantation in a patient with adult T-cell lymphoma. Am J Hematol. 2005;79:294–298. doi: 10.1002/ajh.20349. [DOI] [PubMed] [Google Scholar]
- 99.Gustafsson B, Moell J, Leblanc K, Barbany G, Soderhall S, Winiarski J. Donor cell-derived acute myeloid leukemia after second allogenic cord blood transplantation in a patient with Fanconi anemia. Pediatr Transplant. 2012;16:E241–E245. doi: 10.1111/j.1399-3046.2011.01584.x. [DOI] [PubMed] [Google Scholar]
- 100.Fraser CJ, Hirsch BA, Dayton V, et al. First report of donor cell-derived acute leukemia as a complication of umbilical cord blood transplantation. Blood. 2005;106:4377–4380. doi: 10.1182/blood-2005-06-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shiozaki H, Yoshinaga K, Kondo T, et al. Donor cell-derived leukemia after cord blood transplantation and a review of the literature: differences between cord blood and BM as the transplant source. Bone Marrow Transplant. 2014;49:102–109. doi: 10.1038/bmt.2013.127. [DOI] [PubMed] [Google Scholar]
- 102.Nagamura-Inoue T, Kodo H, Takahashi TA, Mugishima H, Tojo A, Asano S. Four cases of donor cell-derived AML following unrelated cord blood transplantation for adult patients: experiences of the Tokyo Cord Blood Bank. Cytotherapy. 2007;9:727–728. doi: 10.1080/14653240701466339. [DOI] [PubMed] [Google Scholar]
- 103.Maestas E, Jain S, Stiff P. A 54-Year-Old Woman with Donor Cell Origin of Multiple Myeloma after Allogeneic Hematopoietic Stem Cell Transplantation for the Treatment of CML. Case Rep Hematol. 2016;2016:6751914. doi: 10.1155/2016/6751914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamazaki R, Nakasone H, Wada H, et al. Recurrence of monoclonal gammopathy associated with donor-derived myelodysplastic syndrome after cord blood stem cell transplantation. Exp Hematol. 2011;39:1119–1123. doi: 10.1016/j.exphem.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 105.Kim YI, Kim HR, Shin MG, et al. Donor cell origin of multiple myeloma occurring after allogeneic haematopoietic stem cell transplantation in a patient with refractory anaemia with ring sideroblast. J Clin Pathol. 2011;64:265–268. doi: 10.1136/jcp.2010.084731. [DOI] [PubMed] [Google Scholar]
- 106.Grey M, Townsend N, Lappin D, et al. IgA myeloma of donor origin arising 7 years after allogeneic renal transplant. Br J Haematol. 2000;108:592–594. doi: 10.1046/j.1365-2141.2000.01913.x. [DOI] [PubMed] [Google Scholar]
- 107.Aikawa V, Porter D, Luskin MR, Bagg A, Morrissette JJ. Transmission of an expanding donor-derived del(20q) clone through allogeneic hematopoietic stem cell transplantation without the development of a hematologic neoplasm. Cancer Genet. 2015;208:625–629. doi: 10.1016/j.cancergen.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 108.Dickson MA, Papadopoulos EB, Hedvat CV, Jhanwar SC, Brentjens RJ. Acute myeloid leukemia arising from a donor derived premalignant hematopoietic clone: A possible mechanism for the origin of leukemia in donor cells. Leuk Res Rep. 2014;3:38–41. doi: 10.1016/j.lrr.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chonabayashi K, Kondo T, Yamamoto K, et al. Successful use of second cord blood transplantation to achieve long-term remission in cord blood donor cell-derived AML harboring a FLT3-ITD and an NPM1 mutation. Bone Marrow Transplant. 2012;47:1252–1253. doi: 10.1038/bmt.2011.256. [DOI] [PubMed] [Google Scholar]
- 110.Rodriguez-Macias G, Martinez-Laperche C, Gayoso J, et al. Mutation of the NPM1 gene contributes to the development of donor cell-derived acute myeloid leukemia after unrelated cord blood transplantation for acute lymphoblastic leukemia. Hum Pathol. 2013;44:1696–1699. doi: 10.1016/j.humpath.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 111.Yasuda T, Ueno T, Fukumura K, et al. Leukemic evolution of donor-derived cells harboring IDH2 and DNMT3A mutations after allogeneic stem cell transplantation. Leukemia. 2014;28:426–428. doi: 10.1038/leu.2013.278. [DOI] [PubMed] [Google Scholar]
- 112.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 113.Lee J, Kook H, Chung I, et al. Telomere length changes in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;24:411–415. doi: 10.1038/sj.bmt.1701923. [DOI] [PubMed] [Google Scholar]
- 114.Notaro R, Cimmino A, Tabarini D, Rotoli B, Luzzatto L. In vivo telomere dynamics of human hematopoietic stem cells. Proc Natl Acad Sci U S A. 1997;94:13782–13785. doi: 10.1073/pnas.94.25.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Avital I, Moreira AL, Klimstra DS, et al. Donor-derived human bone marrow cells contribute to solid organ cancers developing after bone marrow transplantation. Stem Cells. 2007;25:2903–2909. doi: 10.1634/stemcells.2007-0409. [DOI] [PubMed] [Google Scholar]
- 116.Munakata W, Nomoto J, Takahashi N, et al. Carcinoma of donor origin after allogeneic peripheral blood stem cell transplantation. Am J Surg Pathol. 2012;36:1376–1384. doi: 10.1097/PAS.0b013e318261089c. [DOI] [PubMed] [Google Scholar]
- 117.Janin A, Murata H, Leboeuf C, et al. Donor-derived oral squamous cell carcinoma after allogeneic bone marrow transplantation. Blood. 2009;113:1834–1840. doi: 10.1182/blood-2008-07-171702. [DOI] [PubMed] [Google Scholar]
- 118.Arai Y, Arai H, Aoyagi A, et al. A solid tumor of donor cell-origin after allogeneic peripheral blood stem cell transplantation. Am J Transplant. 2006;6:3042–3043. doi: 10.1111/j.1600-6143.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- 119.Shirai K, Sera Y, Bulkeley W, et al. Hematopoietic stem cell origin of human fibroblasts: cell culture studies of female recipients of gender-mismatched stem cell transplantation and patients with chronic myelogenous leukemia. Exp Hematol. 2009;37:1464–1471. doi: 10.1016/j.exphem.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nemeth K, Key S, Bottlik G, Masszi T, Mezey E, Karpati S. Analyses of donor-derived keratinocytes in hairy and nonhairy skin biopsies of female patients following allogeneic male bone marrow transplantation. Stem Cells Dev. 2012;21:152–157. doi: 10.1089/scd.2010.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang C, Gu L, Deng D. Bone marrow-derived cells may not be the original cells for carcinogen-induced mouse gastrointestinal carcinomas. PLoS One. 2013;8:e79615. doi: 10.1371/journal.pone.0079615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smith MJ, van Cleef PH, Schattenberg AV, van Krieken JH. The origin of epithelial neoplasms after allogeneic stem cell transplantation. Haematologica. 2006;91:283–284. [PubMed] [Google Scholar]
- 123.Morton LM, Swerdlow AJ, Schaapveld M, et al. Current knowledge and future research directions in treatment-related second primary malignancies. EJC Suppl. 2014;12:5–17. doi: 10.1016/j.ejcsup.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grosse Y, Baan R, Straif K, et al. A review of human carcinogens-Part A: pharmaceuticals. Lancet Oncol. 2009;10:13–14. doi: 10.1016/s1470-2045(08)70286-9. [DOI] [PubMed] [Google Scholar]
- 125.Schwartz JL, Kopecky KJ, Mathes RW, Leisenring WM, Friedman DL, Deeg HJ. Basal cell skin cancer after total-body irradiation and hematopoietic cell transplantation. Radiation research. 2009;171:155–163. doi: 10.1667/RR1469.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hows JM, Passweg JR, Tichelli A, et al. Comparison of long-term outcomes after allogeneic hematopoietic stem cell transplantation from matched sibling and unrelated donors. Bone Marrow Transplant. 2006;38:799–805. doi: 10.1038/sj.bmt.1705531. [DOI] [PubMed] [Google Scholar]
- 127.Ortega JJ, Olivé T, de Heredia CD, Llort A. Secondary malignancies and quality of life after stem cell transplantation. Bone Marrow Transplantation. 2005;35:S83–S87. doi: 10.1038/sj.bmt.1704854. [DOI] [PubMed] [Google Scholar]
- 128.Baker K, DeFor T, Burns L, Ramsay N, Neglia J, Robison L. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21:1352–1358. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 129.Kolb HJ, Socié G, Duell T, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Annals of Internal medicine. 1999;131:738–744. doi: 10.7326/0003-4819-131-10-199911160-00004. [DOI] [PubMed] [Google Scholar]
- 130.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 132.Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 133.Gay F, Oliva S, Petrucci MT, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1617–1629. doi: 10.1016/S1470-2045(15)00389-7. [DOI] [PubMed] [Google Scholar]
- 134.Palumbo A, Bringhen S, Kumar SK, et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol. 2014;15:333–342. doi: 10.1016/S1470-2045(13)70609-0. [DOI] [PubMed] [Google Scholar]
- 135.Usmani SZ, Sexton R, Hoering A, et al. Second malignancies in total therapy 2 and 3 for newly diagnosed multiple myeloma: influence of thalidomide and lenalidomide during maintenance. Blood. 2012;120:1597–1600. doi: 10.1182/blood-2012-04-421883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Krishnan AY, Mei M, Sun CL, et al. Second primary malignancies after autologous hematopoietic cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2013;19:260–265. doi: 10.1016/j.bbmt.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee HJ, Kim HK, Cho B, Chung SM. Second solid cancers after total body irradiation conditioning in children undergoing hematopoietic stem cell transplant. European Journal of Cancer. 2015;51:S653. [Google Scholar]
- 138.Kim J, Chung MJ, Lee JH, Choi BO, Chung JS. Second cancers after hematopoietic stem cell transplantation with total body irradiation conditioning regimen in hematologic disorders. Journal of Clinical Oncology. 2011:29. [Google Scholar]
- 139.Yokota A, Ozawa S, Masanori T, et al. Secondary solid tumors after allogeneic hematopoietic SCT in Japan. Bone Marrow Transplant. 2012;47:95–100. doi: 10.1038/bmt.2011.23. [DOI] [PubMed] [Google Scholar]
- 140.Atsuta Y, Suzuki R, Yamashita T, et al. Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann Oncol. 2014;25:435–441. doi: 10.1093/annonc/mdt558. [DOI] [PubMed] [Google Scholar]
- 141.Shimada K, Yokozawa T, Atsuta Y, et al. Solid tumors after hematopoietic stem cell transplantation in Japan: incidence, risk factors and prognosis. Bone Marrow Transplant. 2005;36:115–121. doi: 10.1038/sj.bmt.1705020. [DOI] [PubMed] [Google Scholar]
- 142.Shimoni A, Shem-Tov N, Chetrit A, et al. Secondary malignancies after allogeneic stem-cell transplantation in the era of reduced-intensity conditioning; the incidence is not reduced. Leukemia. 2013;27:829–835. doi: 10.1038/leu.2012.299. [DOI] [PubMed] [Google Scholar]
- 143.Rosenberg PS, Socie G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105:67–73. doi: 10.1182/blood-2004-04-1652. [DOI] [PubMed] [Google Scholar]
- 144.Allan JM, Wild CP, Rollinson S, et al. Polymorphism in glutathione S-transferase P1 is associated with susceptibility to chemotherapy-induced leukemia. Proc Natl Acad Sci U S A. 2001;98:11592–11597. doi: 10.1073/pnas.191211198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rund D, Krichevsky S, Bar-Cohen S, et al. Therapy-related leukemia: clinical characteristics and analysis of new molecular risk factors in 96 adult patients. Leukemia. 2005;19:1919–1928. doi: 10.1038/sj.leu.2403947. [DOI] [PubMed] [Google Scholar]
- 146.Casorelli I, Offman J, Mele L, et al. Drug treatment in the development of mismatch repair defective acute leukemia and myelodysplastic syndrome. DNA Repair (Amst) 2003;2:547–559. doi: 10.1016/s1568-7864(03)00020-x. [DOI] [PubMed] [Google Scholar]
- 147.Seedhouse CH, Das-Gupta EP, Russell NH. Methylation of the hMLH1 promoter and its association with microsatellite instability in acute myeloid leukemia. Leukemia. 2003;17:83–88. doi: 10.1038/sj.leu.2402747. [DOI] [PubMed] [Google Scholar]
- 148.Zhu YM, Das-Gupta EP, Russell NH. Microsatellite instability and p53 mutations are associated with abnormal expression of the MSH2 gene in adult acute leukemia. Blood. 1999;94:733–740. [PubMed] [Google Scholar]
- 149.Horiike S, Misawa S, Kaneko H, et al. Distinct genetic involvement of the TP53 gene in therapy-related leukemia and myelodysplasia with chromosomal losses of Nos 5 and/or 7 and its possible relationship to replication error phenotype. Leukemia. 1999;13:1235–1242. doi: 10.1038/sj.leu.2401466. [DOI] [PubMed] [Google Scholar]
- 150.Seedhouse C, Faulkner R, Ashraf N, Das-Gupta E, Russell N. Polymorphisms in genes involved in homologous recombination repair interact to increase the risk of developing acute myeloid leukemia. Clin Cancer Res. 2004;10:2675–2680. doi: 10.1158/1078-0432.ccr-03-0372. [DOI] [PubMed] [Google Scholar]
- 151.Shen MR, Jones IM, Mohrenweiser H. Nonconservative amino acid substitution variants exist at polymorphic frequency in DNA repair genes in healthy humans. Cancer Res. 1998;58:604–608. [PubMed] [Google Scholar]
- 152.Matullo G, Palli D, Peluso M, et al. XRCC1, XRCC3, XPD gene polymorphisms, smoking and (32)P-DNA adducts in a sample of healthy subjects. Carcinogenesis. 2001;22:1437–1445. doi: 10.1093/carcin/22.9.1437. [DOI] [PubMed] [Google Scholar]
- 153.Au WW, Salama SA, Sierra-Torres CH. Functional characterization of polymorphisms in DNA repair genes using cytogenetic challenge assays. Environ Health Perspect. 2003;111:1843–1850. doi: 10.1289/ehp.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Allan JM, Smith AG, Wheatley K, et al. Genetic variation in XPD predicts treatment outcome and risk of acute myeloid leukemia following chemotherapy. Blood. 2004;104:3872–3877. doi: 10.1182/blood-2004-06-2161. [DOI] [PubMed] [Google Scholar]
- 155.Ellis NA, Huo D, Yildiz O, et al. MDM2 SNP309 and TP53 Arg72Pro interact to alter therapy-related acute myeloid leukemia susceptibility. Blood. 2008;112:741–749. doi: 10.1182/blood-2007-11-126508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Knight JA, Skol AD, Shinde A, et al. Genome-wide association study to identify novel loci associated with therapy-related myeloid leukemia susceptibility. Blood. 2009;113:5575–5582. doi: 10.1182/blood-2008-10-183244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 158.Ballew BJ, Savage SA. Updates on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert.Rev.Hematol. 2013;6:327–337. doi: 10.1586/ehm.13.23. [DOI] [PubMed] [Google Scholar]
- 159.Baird DM. Variation at the TERT locus and predisposition for cancer. Expert Rev Mol Med. 2010;12:e16. doi: 10.1017/S146239941000147X. [DOI] [PubMed] [Google Scholar]
- 160.Rajaraman P, Melin BS, Wang Z, et al. Genome-wide association study of glioma and meta-analysis. Hum Genet. 2012;131:1877–1888. doi: 10.1007/s00439-012-1212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The Association of Telomere Length and Cancer: a Meta-analysis. Cancer Epidemiol.Biomarkers Prev. 2011 doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Willeit P, Willeit J, Mayr A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304:69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 163.Gadalla SM, Savage SA. Telomere biology in hematopoiesis and stem cell transplantation. Blood reviews. 2011;25:261–269. doi: 10.1016/j.blre.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baerlocher GM, Rovo A, Muller A, et al. Cellular senescence of white blood cells in very long-term survivors after allogeneic hematopoietic stem cell transplantation: the role of chronic graft-versus-host disease and female donor sex. Blood. 2009;114:219–222. doi: 10.1182/blood-2009-03-209833. [DOI] [PubMed] [Google Scholar]
- 165.Peffault de Latour R, Calado RT, Busson M, et al. Age-adjusted recipient pretransplantation telomere length and treatment-related mortality after hematopoietic stem cell transplantation. Blood. 2012;120:3353–3359. doi: 10.1182/blood-2012-01-403337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gadalla SM, Wang T, Haagenson M, et al. Association between donor leukocyte telomere length and survival after unrelated allogeneic hematopoietic cell transplantation for severe aplastic anemia. JAMA. 2015;313:594–602. doi: 10.1001/jama.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chakraborty S, Sun CL, Francisco L, et al. Accelerated telomere shortening precedes development of therapy-related myelodysplasia or acute myelogenous leukemia after autologous transplantation for lymphoma. J Clin Oncol. 2009;27:791–798. doi: 10.1200/JCO.2008.17.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Secretan B, Straif K, Baan R, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 169.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 170.El Ghissassi F, Baan R, Straif K, et al. A review of human carcinogens--part D: radiation. Lancet Oncol. 2009;10:751–752. doi: 10.1016/s1470-2045(09)70213-x. [DOI] [PubMed] [Google Scholar]
- 171.Morton LM, Slager SL, Cerhan JR, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr. 2014;2014:130–144. doi: 10.1093/jncimonographs/lgu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sri T, Merideth MA, Pulanic TK, Childs R, Stratton P. Human papillomavirus reactivation following treatment of genital graft-versus-host disease. Transplant infectious disease : an official journal of the Transplantation Society. 2013;15:E148–E151. doi: 10.1111/tid.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Battiwalla MSD, Anandi P, Grant C, Bachi A, Vyas N, Pophali P, Koklanaris E, Ito S, Savani B, Barrett J, Stratton P. Genital human papillomavirus (HPV) reactivation patterns in female allotransplant survivors support HPV vaccination. European Society for Blood and Marrow Transplantation Annual Meeting; Valencia, Spain. 2016. [Google Scholar]
- 174.Zhang L, Epstein JB, Poh CF, et al. Comparison of HPV infection, p53 mutation and allelic losses in post-transplant and non-posttransplant oral squamous cell carcinomas. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2002;31:134–141. doi: 10.1034/j.1600-0714.2002.310302.x. [DOI] [PubMed] [Google Scholar]
- 175.Demarosi F, Lodi G, Carrassi A, Soligo D, Sardella A. Oral malignancies following HSCT: graft versus host disease and other risk factors. Oral Oncology. 2005;41:865–877. doi: 10.1016/j.oraloncology.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 176.Aldabagh B, Angeles JG, Cardones AR, Arron ST. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.] 2013;39:1–23. doi: 10.1111/j.1524-4725.2012.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Savani BN, Goodman S, Barrett AJ. Can routine posttransplant HPV vaccination prevent commonly occurring epithelial cancers after allogeneic stem cell transplantation? Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:2219–2221. doi: 10.1158/1078-0432.CCR-08-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 179.Rasche L, Kapp M, Einsele H, Mielke S. EBV-induced post transplant lymphoproliferative disorders: a persisting challenge in allogeneic hematopoetic SCT. Bone Marrow Transplant. 2014;49:163–167. doi: 10.1038/bmt.2013.96. [DOI] [PubMed] [Google Scholar]
- 180.Au WY, Pang A, Chan EC, Chu KM, Shek TW, Kwong YL. Epstein-barr virus-related gastric adenocarcinoma: an early secondary cancer post hemopoietic stem cell transplantation. Gastroenterology. 2005;129:2058–2063. doi: 10.1053/j.gastro.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 181.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 182.Taguchi M, Imaizumi Y, Taguchi J, et al. Transient proliferation of donor-derived ATL cell-like lymphocytes early after allogeneic stem cell transplantation in an adult T-cell leukemia/lymphoma patient. Blood. 2013;121:4428–4430. doi: 10.1182/blood-2013-02-487652. [DOI] [PubMed] [Google Scholar]
- 183.Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509–515. doi: 10.1016/j.chom.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tothill R, Estall V, Rischin D. Merkel cell carcinoma: emerging biology, current approaches, and future directions. American Society of Clinical Oncology educational book / ASCO. American Society of Clinical Oncology. Meeting. 2015:e519–e526. doi: 10.14694/EdBook_AM.2015.35.e519. [DOI] [PubMed] [Google Scholar]
- 185.Ashton LJ, Le Marsney RE, Dodds AJ, et al. A population-based cohort study of late mortality in adult autologous hematopoietic stem cell transplant recipients in Australia. Biol Blood Marrow Transplant. 2014;20:937–945. doi: 10.1016/j.bbmt.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 186.Socie G, Henry-Amar M, Devergie A, et al. Poor clinical outcome of patients developing malignant solid tumors after bone marrow transplantation for severe aplastic anemia. Leuk Lymphoma. 1992;7:419–423. doi: 10.3109/10428199209049797. [DOI] [PubMed] [Google Scholar]
- 187.Friedberg JW, Neuberg D, Stone RM, et al. Outcome in patients with myelodysplastic syndrome after autologous bone marrow transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 1999;17:3128–3135. doi: 10.1200/JCO.1999.17.10.3128. [DOI] [PubMed] [Google Scholar]
- 188.Orazi A, Hromas RA, Neiman RS, et al. Posttransplantation lymphoproliferative disorders in bone marrow transplant recipients are aggressive diseases with a high incidence of adverse histologic and immunobiologic features. Am J Clin Pathol. 1997;107:419–429. doi: 10.1093/ajcp/107.4.419. [DOI] [PubMed] [Google Scholar]
- 189.Howe R, Micallef IN, Inwards DJ, et al. Secondary myelodysplastic syndrome and acute myelogenous leukemia are significant complications following autologous stem cell transplantation for lymphoma. Bone Marrow Transplant. 2003;32:317–324. doi: 10.1038/sj.bmt.1704124. [DOI] [PubMed] [Google Scholar]
- 190.Boehm A, Sperr WR, Leitner G, et al. Comorbidity predicts survival in myelodysplastic syndromes or secondary acute myeloid leukaemia after allogeneic stem cell transplantation. Eur J Clin Invest. 2008;38:945–952. doi: 10.1111/j.1365-2362.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- 191.Ehrhardt MJ, Brazauskas R, He W, Rizzo JD, Shaw BE. Survival of patients who develop solid tumors following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51:83–88. doi: 10.1038/bmt.2015.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bollard CM, Rooney CM, Heslop HE. T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nature reviews. Clinical Oncology. 2012;9:510–519. doi: 10.1038/nrclinonc.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ricciardelli I, Brewin J, Lugthart G, Albon SJ, Pule M, Amrolia PJ. Rapid generation of EBV-specific cytotoxic T lymphocytes resistant to calcineurin inhibitors for adoptive immunotherapy. Am J Transplant. 2013;13:3244–3252. doi: 10.1111/ajt.12475. [DOI] [PubMed] [Google Scholar]
- 194.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47:337–341. doi: 10.1038/bmt.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pulsipher MA, Skinner R, McDonald GB, et al. National Cancer Institute, National Heart, Lung and Blood Institute/Pediatric Blood and Marrow Transplantation Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: the need for pediatric-specific long-term follow-up guidelines. Biol Blood Marrow Transplant. 2012;18:334–347. doi: 10.1016/j.bbmt.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12:138–151. doi: 10.1016/j.bbmt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 197.Chow EJ, Anderson L, Baker KS, et al. Late Effects Surveillance Recommendations among Survivors of Childhood Hematopoietic Cell Transplantation: A Children’s Oncology Group Report. Biol Blood Marrow Transplant. 2016;22:782–795. doi: 10.1016/j.bbmt.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Inamoto Y, Shah NN, Savani BN, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50:1013–1023. doi: 10.1038/bmt.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Khera N, Chow EJ, Leisenring WM, et al. Factors associated with adherence to preventive care practices among hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant. 2011;17:995–1003. doi: 10.1016/j.bbmt.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16:207–214. doi: 10.1016/j.bbmt.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Armenian SH, Sun CL, Francisco L, et al. Health behaviors and cancer screening practices in long-term survivors of hematopoietic cell transplantation (HCT): a report from the BMT Survivor Study. Bone Marrow Transplant. 2012;47:283–290. doi: 10.1038/bmt.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sabatino SA, Coates RJ, Uhler RJ, Pollack LA, Alley LG, Zauderer LJ. Provider counseling about health behaviors among cancer survivors in the United States. J Clin Oncol. 2007;25:2100–2106. doi: 10.1200/JCO.2006.06.6340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.