Abstract
Chronic watery diarrhea poses a diagnostic and therapeutic challenge and is often a disabling condition for patients. Although acute diarrhea is likely to be caused by infection, the causes of chronic diarrhea (more than 4 weeks in duration) are more elusive. We review on the pathophysiology, diagnosis, and treatment of chronic diarrhea. Drawing on recent insights into the molecular mechanisms of intestinal epithelial transport and barrier function, we discuss how diarrhea can result from a decrease in luminal solute absorption, an increase in secretion, or both, as well as derangements in barrier properties. We also describe the various extra-epithelial factors that activate diarrheal mechanisms. Finally, clinical evaluation and tests used in assessment of patients presenting with chronic diarrhea are reviewed, and an algorithm guiding therapeutic decisions and pharmacotherapy is presented.
Diarrhea poses a diagnostic and therapeutic challenge to clinicians, in part, because it has diverse etiologies. Diagnostic tests may be difficult or not readily available, a specific diagnosis may be elusive, and targeted treatment may be unavailable, leading to the need for trials of empiric therapy.
Although diarrhea may be obvious to the patient, it is important to define the basic characteristics of the diarrhea: frequency and consistency. A more quantitative approach is to determine stool weight/24 hrs in a timed collection. A rational classification for evaluation of diarrhea considers acute and chronic (more than 4 weeks) forms. This approach emphasizes the likelihood of an infectious etiology for acute conditions, whereas chronic diarrhea is much less likely to be infectious, and other causes should be considered. An alternative classification for diarrhea is based on the appearance of the stool: fatty, inflammatory (associated with blood in the stool) or watery, as detailed elsewhere.1
In this article, we focus exclusively on chronic watery diarrhea, reviewing the basic pathophysiology and recent advances in our understanding of intestinal mechanisms that control fluid and electrolyte transport, as well as providing a rational and parsimonious approach to the clinical evaluation of watery diarrhea and a discussion of therapeutic options.
Intestinal Cellular Mechanisms of Fluid and Ion Transport
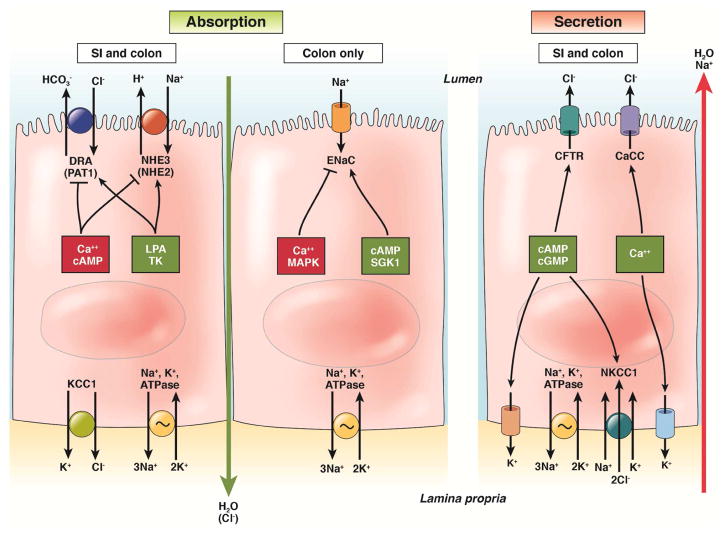
The amount of fluid in the stool is determined by its content of solutes. In patients with watery diarrhea, solutes are not being sufficiently absorbed, are being actively secreted into the lumen, or both. The ability, normally, to dehydrate the stool also depends on epithelial barrier function, to prevent the back diffusion of electrolytes and other solutes once they have been absorbed across the epithelium. To provide a foundation for understanding the pathophysiology of chronic diarrhea, we review our understanding of epithelial transport and barrier functions in the small intestine and colon. We confine the discussion to a consideration of the mechanisms that, when abnormal, have to contribute to diarrheal symptoms (Figure 1).
Figure 1. Cellular mechanisms accounting for intestinal absorption and secretion.
Factors that reduce the amount or function of a specific transporter are shown in red boxes; those that increase levels of activity are shown in green boxes. Long vertical arrows indicate the paracellular absorption or secretion of water, with or without an appropriate counterion.
NaCl absorption
The coupled absorption of sodium and chloride ions is a prominent mechanism for the reclamation of fluid and electrolytes throughout the small and large intestines, particularly (for the former) in the period between meals.2 The transport mechanism depends on paired transporters expressed on the apical membrane of villous (or surface) epithelial cells—a member of the solute carrier 9 (SLC9) family of sodium–hydrogen exchangers (NHE) and a member of the SLC26 family of anion exchangers. Depending on the precise gut segment, either SLC9A2 (also called NHE2) or SLC9A3 (also known called NHE3) mediate sodium–hydrogen exchange, whereas SLC26A3 (also called DRA, for down regulated in adenoma) or SLC26A6 (also called PAT1, for putative anion transporter 1) mediate chloride–bicarbonate exchange. Through these transporters, sodium and chloride ions enter the cell cytosol and can then be exported across the basolateral membrane via the Na+/K+ ATPase and a potassium chloride co-transporter, respectively.2
The activity of the apical SLC9 and SLC26 transporters is coordinately regulated, such that NHE inhibitors reduce chloride–bicarbonate exchange, and vice versa.3 Similarly, neurohumoral signals either upregulate or downregulate both transporters in parallel. This functional coupling results, in part, from localization of the transporters to the apical membrane in the form of macromolecular complexes that are linked (via PDZ-domain binding) to cytoplasmic regulatory proteins known as NHE regulatory factors.3 NHE regulatory factors also contain sites for phosphorylation by intracellular kinases, such as protein kinase A, which control membrane abundance of NHEs via their regulated trafficking into and out of the apical membrane.4 In general, SLC9 and SLC26 transporter activity is reduced by hormones that increase intracellular levels of cAMP, cGMP, or calcium, whereas it is increased by agents such as epidermal growth factor, which induce tyrosine kinase-dependent signaling.5 Lysophosphatidic acid also increases SLC26A3 expression and trafficking of this transporter as well as SLC9A3 to the apical membrane.5,6
Mice lacking SLC9A3 develop the equivalent of chronic diarrhea.7 The function and/or expression of intestinal SLC9 and SLC26 transporters are also downregulated by a variety of inflammatory cytokines, and this may contribute to diarrhea in patients with inflammatory bowel diseases (IBD).8 Similarly, single nucleotide polymorphisms in SLC9A3 that reduce activity of the transporter could increase susceptibility to chronic diarrhea,9 though this hypothesis should be tested in patients.
Electrogenic Na+ absorption
In the distal colon, sodium ions are additionally absorbed without concomitant uptake of chloride. Rather, they enter the apical membranes of surface colonocytes via the heterotrimeric epithelial sodium channel (ENaC), and then they exit the colonocytes at the basolateral membrane via Na+/K+ ATPase. ENaC channel opening and/or membrane abundance are stimulated by neurohumoral agents that elevate cAMP, but the channel is inhibited by those that increase levels of cytoplasmic calcium and/or activate mitogen-activated protein kinases.10,11 Acute regulation of the ENaC is mediated in large part by NEDD4-2, a ubiquitin ligase whose activity results in internalization of the ENaC and its degradation by the proteasome.11 Phosphorylation of NEDD4-2, either by protein kinase A or by the serum and glucocorticoid-inducible kinase, which is activated by aldosterone, reduces its binding to the ENaC and thereby increases the residence time of the channel in the apical membrane.12 The channel’s activity is also positively influenced by channel-activating protease 1, a membrane-bound protease that acts on the extracellular domains of ENaC subunits to increase the probability that the channel will be open.13 ENaC activity can also be chronically upregulated when its expression is increased by aldosterone (in response to a low-salt diet, for example) or glucocorticoids.14,15 There is also evidence for sodium channels other than the ENaC that contribute to absorption of the cation in the human colon.16
Mice that lack ENaCs specifically in the distal colon lose significantly greater amounts of sodium in their feces than control animals, but do not have diarrhea.14 So, the ENaC-dependent mechanism for sodium absorption in the colon appears to be a salvage pathway that does not contribute significantly to water balance in health, presumably because NaCl absorption and sodium-coupled nutrient uptake are sufficient for fluid reabsorption. However, if fluid is not adequately reclaimed upstream, ENaC-dependent sodium absorption may become functionally relevant. Furthermore, expression and function of ENaC are downregulated in mice with colitis, (serving as a model of human IBD) as well as in patients with IBD (even in macroscopically normal regions); ENaC is also downregulated in microscopic colitis, which is increasingly appreciated as a cause of chronic watery diarrhea.17–21
Chloride secretion
Although the net vector for electrolyte transport in the healthy gut is absorptive, there is ongoing secretion to provide appropriate fluidity of luminal content to support digestion, absorption, and movement of intestinal contents along the digestive tract. Indeed, it has been estimated that the intestine itself provides approximately 1L/day of the 8–9 L of fluid that typically traverse the gastrointestinal system. If a meal is hypertonic, it will initially draw fluid into the intestinal lumen by osmosis, as the stomach does not sufficiently control the emptying of the osmotically-active meal, despite the increase in pyloric resistance.22 However, secretory fluxes are driven by the active secretion of chloride ions, which occurs predominantly across crypt epithelial cells and is regulated by specific neurohormonal triggers.23
The chloride secretory mechanism23 involves uptake of chloride across the basolateral membrane via a sodium-potassium-2 chloride co-transporter, NKCC1. The activity of NKCC1 is driven by the low intracellular sodium concentration established by extrusion of sodium ions by the basolateral Na+, K+ ATPase. Potassium ions are also recycled across the basolateral membrane by cAMP- or calcium-activated channels; this serves to maintain the favorable electrical gradient that drives chloride exit across the apical membrane. Chloride ions that accumulate in the cytosol exit apically via regulated chloride conductances. The major pathway is via cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels, but there can also be a contribution from calcium-activated chloride channels, although the molecular identity of such channels remains controversial.24 No matter the precise exit pathway, the net effect is to transfer chloride from the bloodstream to the intestinal lumen, with water (and sodium ions) following through the paracellular route.
Chloride secretion is stimulated by neurohumoral agents that increase levels of cAMP, cGMP, or calcium, although the characteristics of the responses differ.23 Cyclic nucleotide-stimulated secretion is large and sustained while the stimulus persists. Calcium-dependent secretion, on the other hand, is smaller and transient, apparently due to a variety of inhibitory pathways intrinsic and extrinsic to the epithelium. This may allow for lubrication of the epithelium, without risking dehydration. On the other hand, when the epithelium is exposed to agonists acting through both cAMP and calcium-dependent pathways, a synergistic enhancement of secretion results. Many agents that stimulate chloride secretion simultaneously inhibit NaCl absorption, which may be relevant for the pathogenesis of secretory diarrhea.
Stimulation of active chloride secretion is an underlying pathophysiological mechanism in some acute, infectious diarrheas, such as cholera or rotavirus infection (predominantly related to excessive activation of CFTR and calcium-activated chloride channels, respectively).25,26 However, there is evidence that certain forms of chronic watery diarrhea share a secretory component, as illustrated by the effectiveness of a chloride channel-directed therapeutic, crofelemer, in patients with HIV receiving retroviral therapy.27 Chloride secretion is also implicated in diarrhea associated with some neuroendocrine tumors, such as those secreting vasoactive intestinal polypeptide or serotonin (5-hydroxytryptamine, 5-HT). Chronic diarrhea in a Norwegian cohort was attributed to a gain-of-function mutation in the guanylate cyclase 2C gene, which encodes the receptor for endogenous chloride secretagogues, such as guanylin and uroguanylin. Gain of function mutations in this protein would be expected to cause continual increases in cGMP.28
Excess bile acids can also activate chloride secretion.29 Bile acid malabsorption may account for a significant proportion of patients diagnosed with diarrhea-predominant IBS (IBS-D).30,31
Intestinal Barrier Function
The physiologic function of the gut as a portal for the uptake of beneficial substances, while excluding pathogens and toxins, requires that paracellular transport of solutes be carefully controlled. Barrier function is predominantly provided by the tight junctions that link adjacent epithelial cells, although other intercellular junctions also contribute. The barrier is not only dynamic, but may also allow for the selective permeation of solutes through tight-junction pores.
It is beyond the scope of this article to discuss all aspects of tight junction biology, and the reader is referred to recent reviews on this topic.32,33 Nevertheless, some general observations can be made. A family of claudin molecules is critical for establishing the actual permeability properties of the tight junctions, forming homotypic and heterotypic bonds with partner claudins on adjacent cells. Depending on the range of claudins expressed and their levels of expression, the junctions will be more or less leaky, or may contain charge-selective pores.34 Other membrane-bound junctional components, such as occludin and tricellulin, are important in limiting macromolecular permeability across the epithelium.32 The membrane-bound components of the tight junctions interact, via their cytoplasmic domains, with a number of adapter and regulatory proteins that influence junctional permeability.35 For example, contraction of the actomyosin ring that encircles epithelial cells just below their apical poles can exert strain on the junctions that increase their permeability. Inappropriate activation of myosin light chain kinase, which increases such contraction, may contribute to epithelial leakiness in the setting of inflammation.35
Reduced effectiveness of the epithelial barrier is not sufficient to cause significant diarrhea. However, if it is coupled with defective ion transport and fluid accumulation in the lumen, the efficiency of absorptive transport may be compromised by a paracellular leak of absorbed solutes back into the luminal compartment. The resulting leak-flux diarrhea has been proposed as mechanism of pathogenesis of IBD, but presumably could contribute to chronic watery diarrhea as well.32,36 Reduced tight junction expression of claudin-1 (called the sealing claudin) in mucosa from human ileum and ascending colon have been reported in patients with IBS-D (and conversely, levels of claudin-1 are increased in patients with constipation).37 Tight junction dysfunction also appears to contribute to development of diarrhea following infection with Giardia lamblia, as well as post-infectious sequelae, at least in an animal model.38 However, measurement of intestinal permeability39,40 in patients with chronic diarrhea should be considered as only a research tool, rather than for use in the clinic.
Secretory or Osmotic Diarrhea
Watery diarrheas are categorized as either osmotic or secretory. In patients with secretory diarrhea, stool osmolality is almost entirely accounted for by electrolytes (Na+, K+, and accompanying anions). In osmotic diarrhea, there is an unaccounted gap between the stool water electrolytes and the measured osmolality. This gap is due to poorly absorbed molecules (e.g. lactose in lactase deficiency) that draw fluid into the lumen.41 This classification has never been validated in a clinical study. A retrospective review of patients at a tertiary referral center recently demonstrated the utility of measuring stool electrolytes and osmolality.42
Watery diarrhea involving motility, inflammatory, and combinations of mechanisms
The importance of motility in the pathogenesis of diarrhea has been difficult to fully evaluate. There are elegant basic science techniques to elucidate the molecular mechanisms of ion transport, but only recent studies provide insight into potential motor mechanisms involved in the development of diarrhea.43 In most discussions about the basic mechanisms of diarrhea, motility is generally mentioned only briefly. Clinical studies have indicated that chronic watery diarrhea frequently has a major motility component, typically rapid transit through the gut, limiting the contact time between theoretically absorbable solutes and a normal epithelium.
There are other examples where multiple mechanisms contribute to the development of watery diarrhea. For example, 5-hydroxytryptamine (5-HT), which is primarily produced in gut, mediates intrinsic reflexes (e.g. stimulates motility, secretion, and vasodilation) and may promote inflammation that contributes to the development of diarrhea.44
Mechanisms of pathogenesis
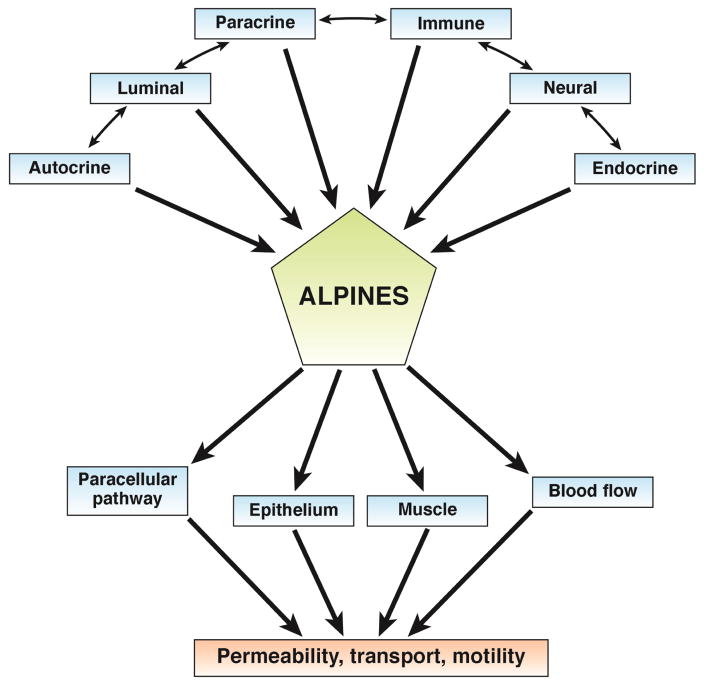
A model of chronic diarrhea (see Figure 2) includes effects of autocrine, luminal, paracrine, immune, neural, and endocrine factors on the paracellular pathway, epithelium, muscle, and vasculature; these can alter intestinal permeability, ion transport, and motility. Studies are underway to evaluate the effects of the microbiota on electrolyte transport and motility.
Figure 2. Interaction of mechanisms in chronic watery diarrhea.
Although most reductionist research models focus on a single parameter of intestinal function, in fact there is an intricate network of agonists and effectors with a promiscuous interaction among autocrine, luminal, paracrine, immune, neural and endocrine (ALPINES) inputs that may alter the paracellular pathway, epithelial cell function, intestinal smooth muscle and blood flow. Diarrhea can result from a change in one or many of these pathways and alterations in permeability, transport, and motility. Figure adapted from ref. 187, Schiller LR, Sellin JH. Diarrhea. In: Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, 10th ed. Feldman M, Friedman LS, Brandt LJ, Eds. Elsevier:New York, 2015, pp. 221–241 (permission not required by Elsevier, as Dr. Sellin is author of original work and of this work).
Although the archetype of chronic watery diarrhea is that produced by neuroendocrine tumors, these are exceedingly rare. More subtle abnormalities may contribute to functional diarrhea and IBS-D. IBS-D is a functional bowel disorder in which recurrent abdominal pain is associated with defecation or a change in bowel habits. Functional diarrhea is characterized by recurrent passage of loose or watery stools; patients with functional diarrhea should not meet criteria for IBS. Although abdominal pain and/or bloating may be present, they are not predominant symptoms.45 Chronic pancreatitis is usually associated with a history of heavy intake of alcohol leading to significant steatorrhea; we do not discuss this process in our review of chronic watery diarrhea. If the initial screening tests provide evidence for steatorrhea, it should be included in the differential diagnosis of chronic diarrhea.
Peptides and amines produced by enteroendocrine cells, mast cells, or submucosal neurons
Several peptides and amines, such as serotonin and granins, are released from enteroendocrine cells by dietary components, bacterial metabolites of nutrients such as short chain fatty acids (SCFAs), and endogenous chemicals such as bile acids.46,47 Table 1 summarizes enteroendocrine mediators that cause net fluid flux towards the lumen, either by inducing intestinal secretion or by inhibiting absorption, or both (reviewed in detail in ref. 48). In addition, Table 1 summarizes information that supports the potential role of intestinal secretion in development of IBS and the neuroendocrine tumors that result in diarrhea.
Table 1.
Altered Functions of Peripheral Hormones, Amines, and Peptides in Patients with Chronic Diarrhea
| Mechanism | Pathophysiology | Release, distribution, action | Biological and clinical correlates in IBS | Tumors causing diarrhea | References |
|---|---|---|---|---|---|
| Granins | Cgs and Sg in secretory granules mobilize release of peptide hormones from enteroendocrine cells | Release of, for example, 5-HT, PYY, somatostatin (SS) from secretory granules | IBS-D or IBS-alternating: higher fecal CgA, SGII and III and duodenal CgA cell density; changes not specific for IBS; higher CgA and SG associated with faster colonic transit and weakly with symptoms | 49 Montero-Hadjadje 2009; 50 Ohman 2012; 51 El-Salhy 2012 | |
| Serotonin | Derived primarily from EC and neurons: mediates intrinsic reflexes that stimulate motility, secretion and vasodilation; activates extrinsic afferents that mediate extrinsic reflexes and sensation | Circulating 5-HT represents 5-HT that does not undergo re- uptake by the serotonin transporter (SERT) in epithelial cells or platelets | Plasma postprandial 5-HT elevated in IBS-D and IBS- PI; reduced in IBS-C; Platelet SERT uptake disrupted in IBS-D; Mucosal 5-HT elevated in IBS-C and IBS-PI; Mucosal SERT mRNA expression and immune- reactivity varies between studies | Carcinoid diarrhea arises when tumor mass produces sufficient 5-HT or other peptides to induce secretion, accelerated transit and colonic hypermotility | 60 Donowitz and Binder 1975; 65 Spiller 2000; 59 Mawe, Hofmann 2013; 63 Houghton 2003; 62 Atkinson 2006; 176 Bellini 2003; 177 Franke 2010; 178 Foley 2011; 52 El-Salhy 2013; 61 von der Ohe 1993 |
| Substance P | Derived primarily from EC and neurons | Excitatory neurotransmitter stimulating motility | Co-secreted with 5-HT from carcinoid tumors | 73 Buhner, Schemann 2009 | |
| Prostaglandins | Derived primarily from immune cells and subepithelial myofibroblasts | Stimulate fluid secretion and motility | Co-secreted with 5-HT from carcinoid tumors | ||
| Peptide YY (PYY) | Derived primarily from EC | Intraluminal PYY induces small bowel and colon fluid/electrolyte absorption | Rectal biopsy PYY elevated during acute Campylobacter enteritis; normal IBS-PI by 12 weeks; lower PYY in colonic mucosa in IBS | 65 Spiller 2000; 179 Playford 1990; 180 Bilchik 1993; 181 Bilchik 1994; 182 Liu 1997 | |
| Neuropeptide Y (NPY) | Derived from enteric neurons | NPY Y2 receptor agonists reduce intestinal fluid secretion (mice) | NPY levels in both plasma and the sigmoid slower in IBS patients than controls | 183 Zhang 2008; 184 Moriya 2010 | |
| Somatostatin (SS) | Derived primarily from EC and neurons | SS inhibits NHE1 (basolateral in enterocytes), involved in secretion of HCO3− | Expression of SS in serum and colonic or rectal mucosa of IBS higher compared with controls; SS in mucosa in IBS-C greater than in IBS-D | 185 Han 2013; 5 Zachos 2005 | |
| Vasoactive intestinal peptide | Derived mainly from gut secretomotor neurons | Increases secretion and vasodilatation | Sigmoid mucosa and plasma levels of vasoactive intestinal peptide higher in patients with IBS than controls; rectosigmoid mucosal expression of vasoactive intestinal peptide increased, based on mRNA analysis | Vasoactive intestinal peptide-associated tumors (usually pancreatic) cause watery diarrhea, hypokalemia, achlorhydria | 183 Zhang 2005; 185 Han 2013; 98 Camilleri 2014; 77 Bloom 1988 |
| Gastrin | Derived from parietal cells and pancreatic tumors | Intestinal secretion, some malabsorption caused by altered duodenal pH | Gastrinoma diarrhea | 76 Barbezat and Grossman 1971 | |
| Calcitonin | Derived from thyroid parafollicular C cells | Intestinal secretion | Medullary cancer diarrhea | 78 Cox 1979 | |
| Serine proteases | Derived from mast cells and other immune cells | Visceral hypersensitivity | Increased mast cell numbers and greater visceral afferent sensitivity to proteases from mucosa of IBS patients | Systemic mastocytosis | 73 Buhner 2009; 186 Barbara 2007 |
| Purines | P1 and P2 receptors activated by adenosine and extra- cellular nucleotides e.g., ATP | P1A2B receptor regulates colonic Cl− and water secretion; P2Y activates K+, Cl−, HCO3− secretion; inhibits Na+ absorption | Rectosigmoid mucosal expression of P2RY4 mRNA | 98 Camilleri 2014 |
Note: (adapted from reference 44, Camilleri M. J Physiol 2014;592:2967-2980)
Enteroendocrine cells (EC), serotonin (5-HT), peptide YY (PYY), somatostatin (SS), serotonin transporter (SERT), post-infectious IBS (IBS-PI), neuropeptide Y (NPY), sodium-hydrogen antiporter 1 (NHE1), bicarbonate (HCO3), constipation-predominant irritable bowel syndrome (IBS-C), vasoactive intestinal peptide (VIP)
Roles of hormones and transmitters in functional diarrhea and IBS-D
Chromogranins (Cg) and secretogranins (Sg) are present in secretory vesicles of nervous, endocrine, and immune cells. CgA supports the formation of secretory granules and the sorting of amine or peptide hormones to these in enteroendocrine cells, but it also exerts independent biological actions.49 Granin release is stimulated via nicotinic cholinergic receptors. Patients with IBS with faster colonic transit have higher levels of fecal CgA, SgII, and SgIII, but lower levels of CgB compared with healthy controls;50 patients with IBS have increased duodenal CgA cell density.51,52 CgA-positive cells also express free fatty-acid receptors that respond to SCFAs.53 The role for granins in sorting and packaging neuropeptides indicates they can also affect colonic secretion and motility indirectly.54,55
To provide an example of the cell products mediating intestinal secretion, we chose the amine, 5-HT, which is synthesized primarily (95%) in the gastrointestinal tract, stored in mucosal enterochromaffin cells,56 and released in response to mechanical and chemical stimulation. 5-HT mediates intrinsic reflexes (e.g., stimulation of propulsive and segmentation motility, epithelial secretion and vasodilation) and activates extrinsic vagal and spinal afferents.57–59 Effects of 5-HT on intestinal secretion and colonic transit have been well documented in the carcinoid syndrome.60,61 Postprandial plasma 5-HT levels are increased in patients with IBS-D62,63 or post-infectious IBS (IBS-PI),64 and are reduced64 or unchanged in patients with constipation-predominant IBS .62 Rectal or colonic mucosal levels of 5-HT are increased in patients with IBS-PI.65
Other biogenic peptides that may influence intestinal secretion or absorption (detailed in Table 1) include somatostatin, peptide YY and neuropeptide Y, all of which increase fluid absorption and activate the ileal brake, retarding small intestinal transport and allowing greater absorption of solutes, fluids, and electrolytes.66,67 Their expression is generally reduced in patients with IBS-D. Conversely, IBS-D is associated with increased mucosal expression of vasoactive intestinal polypeptide and purinergic receptors, which are associated with intestinal secretion.
Serine proteases in IBS-D
IBS is associated with increased numbers of mast cells and increased effects on afferent nerves of extracts, including proteases, from mucosal biopsies of patients with IBS. Increased infiltration of mast cells in the gut mucosa of IBS patients has been reported in many, but not all, studies, as summarized elsewhere.40 Higher levels of mast cell mediators, such as histamine or tryptase, have been observed in supernatants from colonic and jejunal biopsies of patients with IBS-D, irrespective of mast cell numbers.40 The proteases may increase intestinal permeability68 and thereby contribute to diarrhea.69,70 Serine protease activity in fecal71 and colonic mucosal72 supernatants from biopsies of patients with IBS-D can activate visceral afferents via proteinase-activated receptor 2,73 as well as tachykininergic mechanisms74. This process could activate secretory or motor mechanisms that lead to diarrhea, although they have not been extensively studied.
Hormone-related diarrhea in patients with neuroendocrine tumor syndromes
Patients with neuroendocrine tumors may present with chronic diarrhea that results from an increase in intestinal secretion.75 These patients have carcinoid syndrome (predominantly through effects of 5-HT and possibly substance P on intestinal secretion60 and motility61), Zollinger-Ellison syndrome (gastrin),76 Verner-Morrison syndrome,77 medullary carcinoma of the thyroid (calcitonin),78 or systemic mastocytosis. Systemic mastocytosis is a rare myeloproliferative disorder characterized by excessive numbers of mast cells with release of serine proteases (e.g., tryptase) and histamine, resulting in mildly impaired intestinal absorption.79 Typically, diarrhea occurs when there is a sufficient mass of metastatic tumor that produces the relevant secretagogue.
Intraluminal factors: bile acids and SCFAs
Enterohepatic circulation of bile acids is commonly observed in patients with ileal disease (such as Crohn’s disease and radiation enteritis), patients who have undergone ileal resection, or in patients with deficiencies in production of fibroblast growth factor-19 by ileal enterocytes. Malabsorption of bile acids increases the bile acid concentration in the colon and leads to a series of effects that could result in watery diarrhea. Bile acids stimulate colonic motility80 and transit,81 and increase colonic mucosal permeability,82,83 colonocyte chloride secretion, and apical Cl−/OH− exchange.84 Many of the effects of bile are mediated by the receptor GPBAR1 (also known as TGR5),85 which is expressed in enteric neurons, enteroendocrine cells,86 and primary spinal afferent and spinal neurons involved in sensory transduction.87 GPBAR1 mediates the prokinetic actions of intestinal bile acids, is required for normal defecation in mice, and mediates colonic fluid secretion.88 Variations in GPBAR1 genotype were significantly associated with accelerated colonic transit at 48 hours.89
Even in healthy adults, up to 20% of dietary starch escapes absorption in the small bowel,90 resulting in generation of SCFAs (less than 6 carbon chain length) by colonic bacteria and increased delivery of water to the colon.91 SCFAs stimulate colonic release of 5-HT92 from enteroendocrine cells in rats;93 propionate induced chloride secretion across guinea pig distal colonic mucosa in vitro.53 However, overall, SCFAs are rapidly absorbed in the colon and mostly stimulate absorption rather than secretion. SCFA profiles in fecal samples from patients with IBS-D are characterized by lower total SCFAs, acetate, and propionate and higher n-butyrate.94
Abnormalities in processes that regulate secretion in the colon
Increased expression of ion secretory mechanisms has been detected in the colorectal mucosa of patients with IBS-D95. Guanylate cyclase 2B (GUC2AB) mediates chloride secretion in response to uroguanylin. The protein PDZ domain containing 3 (PDZD3) associates with guanylate cyclase C and regulates cGMP production following receptor stimulation.96 Levels of GUC2AB mRNA and PDZD3 protein are increased in mucosal biopsies from patients with IBS-D.97
Role of the microbiome in chronic diarrhea
Much of the research on the microbiome has established associations, and clinical relevance of the microbiome in health and disease requires further study of the complex interactions among the microbiota, intestinal mucosa, diet, and bacterial metabolites such as butyrate and secondary bile acids.
Recent studies in animal models have provided evidence that commensal microbe byproducts can modulate the immune system, regulating intestinal inflammation. Specifically, butyrate increased generation of anti-inflammatory regulatory T cells and decreased colonic inflammation.98 Devkota et al demonstrated that a diet high in saturated milk fat promotes taurine conjugation of bile acids, increasing the luminal availability of organic sulfur and allowing expansion of a specific sulfite-reducing bacteria (Bilophila wadsworthia). These bacteria were associated with an inflammatory response, mediated by cytokines from T-helper type 1 lymphocytes that increased the incidence of colitis in genetically susceptible mice.99
Chronic diarrhea in cats, dogs, and humans has been associated with an overall shift in the composition of the microbiota and its metabolic capacity.100–102 This so-called dysbiosis has been observed in a number of other gastrointestinal as well as systemic diseases, although most studies to date have shown correlations, rather than causality. Nevertheless, the ability of specific commensals and probiotics to beneficially influence epithelial transport and barrier properties indicates that reversing dysbiosis may be of value in chronic diarrhea.103,104 Dynamic changes in the diet, microbiome, bacterial metabolism, intestinal mucosa, and the immune system could all have protean effects on the gastrointestinal tract.105
Motility-related diarrhea
Motility disorders cause diarrhea by either accelerating gastrointestinal transit (e.g. post-vagotomy diarrhea) or by slowing transit, thereby predisposing to small intestinal bacterial overgrowth (SIBO; e.g. scleroderma). Motility-related diarrhea can be either secretory or osmotic. The most prevalent forms of motility-related diarrheas are autonomic neuropathy106 or IBS-D, associated with either accelerated colonic transit107 or increased numbers of high-amplitude propagated contractions.108
Rapid small bowel transit is a relatively common finding in patients with diabetes or autonomic neuropathies, or patients who have undergone upper gastrointestinal tract surgery. A diagnosis can be made based on combined results from a hydrogen breath test and nuclear scintigraphy small bowel transit test.109 There is controversy over whether SIBO can cause chronic diarrhea. Studies have questioned a diagnosis of SIBO based on breath hydrogen or methane measurements after glucose or lactulose oral load,110 or small intestinal total bacterial counts of not more than 105cfu/mL, or anerobic counts not above 104cfu/mL.111
Management of Patients with Chronic Watery Diarrhea
Although an exact etiology can usually be determined for steatorrhea and inflammation- associated diarrhea, the causes of watery diarrhea are not always clear. Watery diarrhea can therefore present ongoing challenges for management. Patient history should adequately characterize chronic watery diarrhea. If there are any uncertainties, fecal fat should be quantified; the Sudan stain approach to quantification identifies patients with fatty diarrhea with 76% sensitivity and 99% specificity. The sensitivity of detection can be increased to 94% and the specificity to 95% by counting and size measurement of fat globules.112
Patients should also undergo testing for inflammation-associated diarrhea, which can be detected based on serum level of c-reactive protein (CRP) and fecal levels of calprotectin and lactoferrin. A systematic review reported that CRP detected inflammation-associated diarrhea with a pooled sensitivity value of 49% and specificity of 73%, whereas fecal calprotectin identified this disorder with a pooled sensitivity value of 92% and specificity of 82%. Tests for stool lactoferrin identified inflammation-associated diarrhea with a pooled sensitivity value of 88% and 79% specificity.113
If the results from these screening tests are negative, the patient is likely to have chronic watery diarrhea. The clinician can then focus on the most likely or common causes, such as microscopic colitis, celiac disease, IBS-D, functional diarrhea, bile acid malabsorption, diet, motility disorders, and perhaps SIBO. Rarer conditions, such as autoimmune enteritis or Addison’s disease presenting with chronic diarrhea, are beyond the scope of this discussion.
Patient history
It is important to collect a detailed history of the characteristics of a patient’s diarrhea, to select the best course of treatment. Although abdominal pain and diarrhea may be a common capsule summary of a case, there are substantial differences among patients with pain relieved by bowel movements (suggesting IBS-D) vs those with bloating, gas, and discomfort after meals (suggesting maldigestion and/or SIBO) or post-prandial urgency and discomfort (suggesting rapid transit) (see Table 2). Patients often focus on dietary factors that induce symptoms.
Table 2.
Clinical Features in Chronic Watery Diarrhea
| Factor | Potential Implications |
|---|---|
| HISTORY | |
| Family history of celiac disease, IBD, or MEN2B | Celiac disease, IBD, Hormone-induced diarrhea |
| Drugs (including olmesartan) | Celiac-like disease |
| Surgery/Radiation | |
| cholecystectomy | Bile acid diarrhea (BAD) |
| intestinal resection | Short bowel syndrome, BAD |
| abdominal radiation | Radiation enteritis (BAD) |
| vagotomy, bariatric surgery | Rapid transit, motility disorder |
| Travel | Infections e.g. parasitic |
| Immune status | Opportunistic/uncommon infections |
| Common variable immunodeficiency | Chronic giardiasis/Norwalk virus infection |
| Diabetes | Rapid transit, SIBO, Celiac disease, pancreatic insufficiency |
| Associated with diabetes medications and alternative sweeteners | Metformin, acarbose, sorbitol, sugar alcohols |
| PHYSICAL EXAMINATION | |
| Orthostasis, hypotension | Autonomic neuropathy (diabetes/amyloid) |
| Urticaria pigmentosa, dermatographism | Mast cell disease (mastocytosis) |
| Pinch purpura, macroglossia | Amyloidosis |
| Migratory necrotizing erythema | Glucagonoma |
| Leonine Facies, flushing, heart murmur, wheezing | Carcinoid syndrome |
| Dermatitis herpetiformis | Celiac disease |
| Thyroid nodule, lymphadenopathy | Medullary carcinoma of the thyroid |
| Tremor, lid lag | Hyperthyroidism |
| Lymphadenopathy | HIV, lymphoma, cancer |
| Abdominal bruit | Chronic mesenteric ischemia |
| Hepatomegaly | Neuroendocrine tumor, amyloidosis |
| Anal sphincter weakness | Fecal incontinence |
Note: Adapted from reference 1, Schiller LR, et al. J Gastroenterol Hepatol 2014;29:6-25
Specific foods are often incriminated as causes of diarrhea, some with good evidence and others less so.114 Patients are frequently concerned about how diet may precipitate symptoms.115 In assessing associations with foods, it is important to consider substances that, in sufficient quantities, cause diarrhea in a normal gut (e.g., fructose), foods that cause diarrhea because of an underlying condition (e.g., dairy products in lactase deficiency), gastrointestinal diseases that limit digestion or absorption (e.g. short bowel), idiosyncratic food intolerances, and true food allergies (uncommon in adults). A food diary may aid in the identification of a dietary cause of diarrhea.
Poorly-absorbed carbohydrates are commonly linked to diarrhea.114 Some monosaccharides are absorbed by facilitated diffusion with limited capacity; when the amount ingested exceeds capacity, malabsorption and diarrhea occur. Disaccharides must be split by disaccharidases, such as sucrase or lactase, which may be insufficient due to mucosal disease or genetic down-regulation. Unabsorbed carbohydrates lead to osmotic retention of fluid in the intestine and bacterial fermentation to gases.116 Therefore, flatus or bloating are important clues that indicate carbohydrate malabsorption. Lactose is a common cause of diet-induced diarrhea.117,118 Fructose is absorbed by limited capacity facilitated diffusion.119 Although it may be difficult to exceed absorptive capacity with natural foods, consumption of high fructose corn syrup may exceed the absorptive capacity of the gut.120,121
Sugar alcohol malabsorption is an increasingly recognized cause of diarrhea. Sorbitol, mannitol, and xylitol are poorly absorbed nonnutritive sweeteners in items such as sugar-free chewing gum and candy; excessive intake may cause osmotic diarrhea.122–124 It is also important to carefully quantify the amount of caffeine consumed in coffee and energy drinks,125 since caffeine may induce diarrhea via effects on motility and cAMP-induced secretion.
The recognition that carbohydrates can cause diarrhea and other symptoms led to development of the diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs), which can reduce ingestion.126 In a randomized trial, a diet low in FODMAP alleviated intestinal symptoms in 75% of patients with IBS.127 However, a more recent randomized, controlled trial concluded that a sensible diet was as efficacious as a low-FODMAP diet in reducing symptoms of IBS.128 A systematic review showed that a diet low in FODMAPs reduced IBS symptom severity, pain, bloating and overall symptoms, but had no definitive benefit on diarrhea.129
All patients with chronic diarrhea should be screened for celiac disease. Celiac disease is diagnosed based on symptoms, serology, and intestinal histology, preferably before the start of a gluten-free diet.130 Patients can have gluten-responsive symptoms without positive results from serologic tests for celiac disease, or findings of low disease severity from pathology analysis (Marsh scores of 1 or 2).131–133 It is not clear when a gluten-free diet should be considered for patients with diarrhea who do not have celiac disease.134
Fatty and fried foods are frequently implicated in the pathogenesis of watery diarrhea.135 It is paradoxical that some foods with the highest fat content, such as ice cream, are rarely implicated in fatty food intolerance. Although fat malabsorption stimulates colonic secretion to cause diarrhea,136 it seems that fat may also precipitate symptoms without demonstrable steatorrhea.
Food allergies (an immune response to specific foods) can cause diarrhea and other symptoms. Food intolerances are not immune-based and are more common.137 Epidemiology studies reported that 1%–2% of adults have a bona fide food allergy; children have a higher incidence.138–141 Certain foods more frequently cause allergic reactions. Recent studies have linked banana, avocado, walnut, and kiwi to a latex-food allergy syndrome.142,143 Although true food allergy is uncommon in adults, it should be considered when other allergic features are present, such as hives. Some patients who are allergic to food have increased fecal levels of tryptase and eosinophilic cationic protein, without increased levels of fecal calprotectin.144,145
Physical examination
Results from physical examination of patients with watery diarrhea are usually unremarkable, but there may be occasional findings that can lead to a specific diagnosis (see Table 2). In the absence of alarm signs such as weight loss, clinicians face the dilemma of how far to pursue a potential diagnosis through testing before making a diagnosis of IBS-D and/or functional diarrhea based solely on symptom criteria. Whether (and when or how many) tests are worthwhile depends on progression of the disorder. The literature is replete with arguments for and against testing for thyroid status, lactose intolerance, and celiac disease,146,147 whereas consensus favors testing for celiac disease in all patients with chronic diarrhea. One challenge in evaluating chronic watery diarrhea is that, beyond some basic tests, many published testing strategies are not readily available. We focus on the most clinically relevant tests.
Colonoscopy and endoscopy
The diagnostic yield of colonoscopy for chronic watery diarrhea has been reported to range from 2% to 15%.148–150 The most common diagnosis is microscopic colitis, with IBD generally a distant second. Although a recent meta-analysis suggested that colonoscopy may have limited benefit in detecting microscopic colitis in patients with IBS,151 scoring systems have been developed that can guide the clinician to supect the diagnosis.152,153 These include factors such as patient age, sex, and use of high risk medications such as proton-pump inhibitors, non-steroidal anti-inflammatory drugs, and serotonin reuptake inhibitors. Although there is debate about whether isolated right-sided microscopic colitis is a real or common entity, it is reasonable to perform a complete colonoscopy and to take random right- and left-sided biopsies even with normal-appearing colonic mucosa. This practice may need to be revisited after the clinical introduction of tests for bile acid-associated diarrhea, since microscopic colitis (and to a lesser extent, collagenous colitis) are associated with bile acid malabsorption,154 and such patients appear to respond well to bile acid sequestration.155
Patients are rarely examined by upper gastrointestinal endoscopy for chronic watery diarrhea. In a pathology review of 28,000 duodenal biopsies, Carmack and Genta found only celiac disease and mild lymphoctic duodenitis—none of the rare causes of chronic diarrhea that may be diagnosed based only on small bowel biopsy, such as Whipple’s disease.156 Therefore, if results are negative from serologic tests for tissue transglutaminase-IgA (assuming the patient is not IgA-deficient), upper gastrointestinal endoscopy with biopsy analysis would provide only limited benefit. Patients with villous atrophy limited to the duodenal bulb are significantly less likely to present with diarrhea than traditional celiac disease.157
Timed stool collection
Neither patients nor laboratory technicians relish in the timed stool test (48–72 hrs of stool collection; normal stool weight is 200g/24 hrs with less than 7g fat), yet this is the standard for assessing steatorrhea. A fresh stool sample is necessary to differentiate secretory from osmotic diarrhea. Stool weight greater than 1000g/24 hrs leads to a different diagnostic approach (a search for a possible neuroendocrine cause) than a value of 300g/24 hrs. Approximately 25% of patients referred specifically for a diarrhea evaluation actually have normal stool weight (Sellin J, unpublished observations). The 48 hr stool collection test can also be used to measure fecal bile acids.158
Blood tests for neuroendocrine tumors
Hormone-secreting tumors are rare causes of secretory diarrhea, typically detected by measuring serum levels of chromogranin, gastrin, vasoactive intestinal polypeptide, or calcitonin, as well as urine level of 5-hydroxyindoleacetic acid. However, due to the rarity of these tumors and low pretest probability, many positive results from these tests (especially borderline results) turn out to be false positives.159 These tests should therefore almost never be considered early in the course of an evaluation.
Tests for bile acid diarrhea
A systematic review of 36 studies (5028 patients) found that 22.5% of patients with chronic functional diarrhea or IBS-D have bile acid malabsorption. Bile acid malabsorption is identified based on the results of administration of a bile acid sequestrant, or when possible, measuring serum levels of 7α-hydroxy-4-cholesten-3-one or fibroblast growth factor 19, fecal level of 48 hr bile acid (available through reference laboratories), or 7-day retention of 75Se-labeled 23-seleno-25-homotaurocholic acid (available in some countries).160 Patients with bile acid malabsorption have increased small bowel permeability, borderline faster colonic transit, a higher proportion of chenodeoxycholic acid in stool bile acids, and higher fecal levels of fats compared to patients with IBS-D without increased bile acid excretion.161 The prevalence of bile-acid diarrhea is estimated to be similar to that of celiac disease, so it is logical to screen for this condition in patients with chronic watery diarrhea. Unfortunately, most of the tests for bile acid malabsorption are not available outside research settings.
Evaluation for SIBO
SIBO is generally caused by anatomic or functional abnormalities of the intestine, such as strictures, achlorhydria, motility disorders, or scleroderma. Diarrhea, bloating, and weight loss are classic symptoms related to SIBO. The diagnostic standard, quantitative culture of intestinal aspirates,162 is uncommonly performed in practice. Instead, patients are usually given hydrogen breath tests using glucose or lactulose as substrates. However, these tests detect SIBO with varying levels of sensitivity and specificity,162–166 so they are not reliable and can produce conflicting positive or negative results in patients with IBS-D.115,167–171 These tests do not have sufficient diagnostic accuracy for clinical decision making.
The sensitivity and specificity of tests for IBS-D detection can increased by simultaneous measurement of intestinal transit by scintigraphy, to determine whether the hydrogen signal arises from the small bowel or colon.111,114 However, such simultaneous testing is seldom performed.
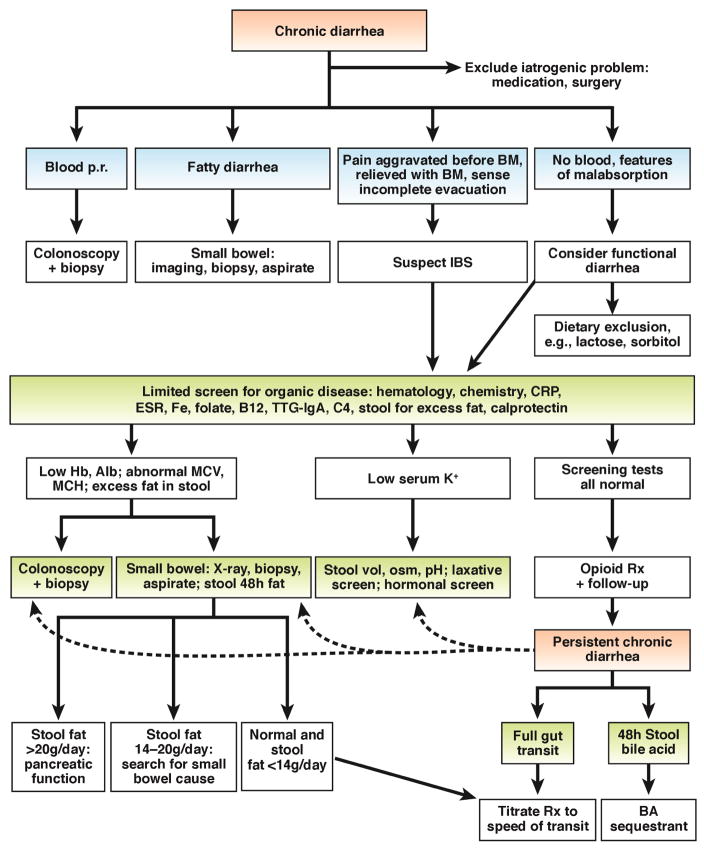
Algorithm for evaluation of chronic diarrhea
Figure 3 shows a proposed algorithm for diagnosis of chronic diarrhea. It is important to determine patients’ histories of rectal bleeding, features of malabsorption, or symptoms of IBS. If there are no blood or features of malabsorption, a limited screen for organic disease may include hematology analyses, chemical analyses, and tests to measure c-reactive protein, erythrocyte sedimentation rate, serum iron, folate, vitamin B12 (typically if there are abnormal red blood cell indices on hematology group), tissue transglutaminase-IgA (to detect celiac disease), serum level of 7α-hydroxy-4-cholesten-3-one or fibroblast growth factor 19 (if available, to detect bile acid diarrhea), and excess fecal fat and calprotectin. Colonoscopy and biopsy are usually performed according to recommendations for colorectal cancer screening. Intractable watery diarrhea may require colonic biopsy analysis, to exclude microscopic colitis. American Gastroenterological Association guidelines specify the importance of excluding celiac disease, hyperthyroidism, IBS, and medication use (e.g. non-steroidal anti-inflammatory drugs, aspirin, proton-pump inhibitors, clozapine, and acarbose) when considering the possibility of microscopic colitis.172
Figure 3. Algorithm for management of chronic diarrhea.
Patients undergo an initial evaluation based on different symptom presentations, leading to selection of patients for imaging, biopsy analysis, and limited screens for organic diseases.
The next steps in the management algorithm are guided by results of the initial screen for organic disease and include further specific tests when features indicate IBD or malabsorption. When results from all tests are normal and suggest chronic watery diarrhea, opioid therapy should be tested (e.g. loperamide, 2– 4 mg, as many as 4 times/day), with pre-prandial dosing for patients with prominent postprandial diarrhea. If the diarrhea persists, patients should be tested for bile acid malabsorption; measurement of colonic transit will make it easier to select subsequent therapies. Tests for SIBO should be considered when there is evidence of malabsorption from tests for organic disease.
Management based on pathogenesis of chronic diarrhea
The principles of management are accurate diagnosis and treatment of the specific factor that causes the chronic diarrhea. Dehydration and severe electrolyte abnormalities are uncommon in patients with chronic watery diarrhea, but, when they occur, should be addressed with oral rehydration therapy.
Treating the factors that cause the disorder is more specific, such as with budesonide for microscopic colitis or a bile acid sequestrant for patients with diarrhea, and is certainly more intellectually satisfying. However, when that is not possible, it is important to reduce symptoms with non-specific therapies that address the secretory and motor components of chronic diarrhea. Opioids are the mainstay of treatment and, when given in a scheduled regimen, are generally safe. However, a recent report found that high doses of loperamide can induce toxic cardiac arrhythmias and death.173
Deodorized tincture of opium and morphine are significantly more potent, but are necessarily prescribed with stringent precautions. Although these medications are generic, recent increases in price have limited their use. Clonidine has been used to relieve the autonomic neuropathy associated with diabetic diarrhea, but could provide only limited benefit, because of associated orthostatic hypotension. Chronic intermittent antibiotics are the mainstay of treatment for well-proven SIBO. Several antibiotics have been shown to be equally effective.111 Although rifaximin is frequently prescribed, its use is limited by its high cost and regulatory approval for 3 courses each of 2 weeks duration. Less-expensive alternatives, such as metronidazole or ciprofloxacin, should therefore be considered. Agents that act intraluminally (fiber, pectin, and calcium) may be particularly helpful in patients with small-volume diarrheas. In some cases, a cocktail of agents with different mechanisms is required. These and second-line approaches to use when first-line treatments fail are presented in Table 3.
Table 3.
Summary of Drugs used in Treatment of Chronic Watery Diarrhea
| Drug class | Agent | Dose |
|---|---|---|
| Opiates (μ-opiate receptor selective) | ||
| Diphenoxylate | 2.5–5 mg, 4 times/day | |
| Loperamide | 2–4 mg, 4 times/day | |
| Codeine | 15–60 mg, 4 times/day | |
| Opium tincture | 2–20 drops, 4 times/day | |
| Morphine | 2–20 mg, 4 times/day | |
| Eluxadoline | 100 mg twice daily (μ-opioid agonist and δ-opioid antagonist) for IBS-D | |
| Adrenergic α2 receptor agonist | ||
| Clonidine | 0.1–0.3 mg 3 times/day; Weekly patch | |
| Somatostatin analogue | ||
| Octreotide | 50–250 μg 3 times/day (subcutaneously) | |
| Bile acid-binding resin | ||
| Cholestyramine | 4 g daily or up to 4 times/day | |
| Colestipol | 4 g daily or up to 4 times/day | |
| Colesevelam | 1875 mg up to twice daily | |
| Fiber supplements | ||
| Calcium polycarbophil | 5–10 g daily | |
| Psyllium | 10–20 g daily | |
| Soluble fiber | Pectin | 2 capsules before meals |
| Calcium | 1000 mg twice or 3 times daily | |
| Serotonin 5-HT3 receptor antagonists | ||
| Alosetron | 0.5–1.0 mg twice daily | |
| Ondansetron | 2–8 mg twice daily | |
Future Directions
Despite diagnostic and therapeutic challenges, it is possible to manage most patients with chronic watery diarrhea. It is important to increase our understanding of mechanisms of pathogenesis, for patients and for the field of research. Many investigators have focused on what proportions of patients have specific factors, such as bile acid malabsorption, but the relative importance of individual etiologies of watery diarrheas cannot be resolved until a comprehensive study simultaneously assesses multiple factors. Recent research has demonstrated the complex interactions among these factors. For example, rapid colonic transit is associated with altered microbiota in rats174 and with dysbiosis175 and fecal bile acids161,175 in humans; further integrated research is required. Advances in our understanding of epithelial biology, as well as interactions of the microbiota with host physiology (including neurohormonal factors and organic anions), should yield targeted therapies for chronic diarrhea.
Acknowledgments
Funding: Work from the authors’ laboratories have been supported by grants from the National Institutes of Health (M. Camilleri: R01-DK92179; K. Barrett: AI077661). Dr. Barrett has received an unrestricted grant from the Estratest/Shape Up settlement fund.
Footnotes
Authors’ contributions: M.Camilleri: co-authorship and coordination of manuscript; primary author on pathophysiology and secondary author on clinical section; K.E. Barrett: co-authorship of manuscript, and primary author on basic and cellular mechanisms; J.H. Sellin: co-authorship of manuscript; primary author on clinical section; secondary author of pathophysiology.
Disclosures: Dr. Camilleri has received support for single-center research studies from Salix, Novartis, and NGM Biopharmaceuticals for studies in diarrheal diseases. Dr. Barrett has nothing to disclose. Dr. Sellin discloses participation in clinical trials related to inflammatory bowel diseases.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schiller LR, Pardi DS, Spiller R, Spiller R, Semrad CE, Surawicz CM, Giannella RA, Krejs GJ, Farthing MJ, Sellin JH. Gastro 2013 APDW/WCOG Shanghai working party report: chronic diarrhea: definition, classification, diagnosis. J Gastroenterol Hepatol. 2014;29:6–25. doi: 10.1111/jgh.12392. [DOI] [PubMed] [Google Scholar]
- 2.Barrett KE, Keely SJ. Integrative physiology and pathophysiology of intestinal electrolyte transport. In: Johnson LR, Barrett KE, Gishan FK, Merchant JL, Said HM, Wood JD, editors. Physiology of the Gastrointestinal Tract. 4. Elsevier; Philadelphia: 2006. pp. 1931–1951. [Google Scholar]
- 3.Kato A, Romero MF. Regulation of electroneutral NaCl absorption by the small intestine. Annu Rev Physiol. 2011;73:261–281. doi: 10.1146/annurev-physiol-012110-142244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donowitz M, Cha B, Zachos NC, Brett CL, Sharma A, Tse CM, Li X. NHERF family and NHE3 regulation. J Physiol. 2005;567:3–11. doi: 10.1113/jphysiol.2005.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol. 2005;67:411–443. doi: 10.1146/annurev.physiol.67.031103.153004. [DOI] [PubMed] [Google Scholar]
- 6.Singla A, Dwivedi A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of lysophosphatidic acid (LPA) mediated stimulation of intestinal apical Cl-/OH- exchange. Am J Physiol Gastrointest Liver Physiol. 2010;298:G182–G189. doi: 10.1152/ajpgi.00345.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 8.Priyamvada S, Gomes R, Gill RK, Saksena S, Alrefai WA, Dudeja PK. Mechanisms underlying dysregulation of electrolyte absorption in inflammatory bowel disease-associated diarrhea. Inflamm Bowel Dis. 2015;21:2926–2935. doi: 10.1097/MIB.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu XC, Sarker R, Horton JR, Chakraborty M, Chen TE, Tse CM, Cha B, Donowitz M. Nonsynonymous single nucleotide polymorphisms of NHE3 differentially decrease NHE3 transporter activity. Am J Physiol Cell Physiol. 2015;308:C758–C766. doi: 10.1152/ajpcell.00421.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alli AA, Bao HF, Liu BC, Yu L, Aldrugh S, Montgomery DS, Ma HP, Eaton DC. Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am J Physiol Renal Physiol. 2015;309:F456–F463. doi: 10.1152/ajprenal.00631.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashlan OB, Kleyman TR. Epithelial Na(+) channel regulation by cytoplasmic and extracellular factors. Exp Cell Res. 2012;318:1011–1019. doi: 10.1016/j.yexcr.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na(+) channel through convergent phosphorylation of Nedd4-2. J Biol Chem. 2004;279:45753–45758. doi: 10.1074/jbc.M407858200. [DOI] [PubMed] [Google Scholar]
- 13.Planes C, Caughey GH. Regulation of the epithelial Na+ channel by peptidases. Curr Top Dev Biol. 2007;78:23–46. doi: 10.1016/S0070-2153(06)78002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malsure S, Wang Q, Charles RP, Sergi C, Perrier R, Christensen BM, Maillard M, Rossier BC, Hummler E. Colon-specific deletion of epithelial sodium channel causes sodium loss and aldosterone resistance. J Am Soc Nephrol. 2014;25:1453–1464. doi: 10.1681/ASN.2013090936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergann T, Zeissig S, Fromm A, Richter JF, Fromm M, Schulzke JD. Glucocorticoids and tumor necrosis factor-alpha synergize to induce absorption by the epithelial sodium channel in the colon. Gastroenterology. 2009;136:933–942. doi: 10.1053/j.gastro.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Sellin JH, De Soignie R. Ion transport in human colon in vitro. Gastroenterology. 1987;93:441–448. doi: 10.1016/0016-5085(87)90904-8. [DOI] [PubMed] [Google Scholar]
- 17.Barmeyer C, Erko I, Fromm A, Bojarski C, Loddenkemper C, Dames P, Kerick M, Siegmund B, Fromm M, Schweiger MR, Schulzke JD. ENaC dysregulation through activation of MEK1/2 contributes to impaired Na+ absorption in lymphocytic colitis. Inflamm Bowel Dis. 2016;22:539–547. doi: 10.1097/MIB.0000000000000646. [DOI] [PubMed] [Google Scholar]
- 18.McCole DF, Rogler G, Varki N, Barrett KE. Epidermal growth factor partially restores colonic ion transport responses in mouse models of chronic colitis. Gastroenterology. 2005;129:591–608. doi: 10.1016/j.gastro.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Greig E, Sandle GI. Diarrhea in ulcerative colitis. The role of altered colonic sodium transport. Ann N Y Acad Sci. 2000;915:327–332. doi: 10.1111/j.1749-6632.2000.tb05260.x. [DOI] [PubMed] [Google Scholar]
- 20.Greig ER, Boot-Handford RP, Mani V, Sandle GI. Decreased expression of apical Na+ channels and basolateral Na+, K+-ATPase in ulcerative colitis. J Pathol. 2004;204:84–92. doi: 10.1002/path.1613. [DOI] [PubMed] [Google Scholar]
- 21.Zeissig S, Bergann T, Fromm A, Bojarski C, Heller F, Guenther U, Zeitz M, Fromm M, Schulzke JD. Altered ENaC expression leads to impaired sodium absorption in the noninflamed intestine in Crohn's disease. Gastroenterology. 2008;134:1436–1447. doi: 10.1053/j.gastro.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 22.Paraskevopoulos JA1, Houghton LA, Eyre-Brooke I, Johnson AG, Read NW. Effect of composition of gastric contents on resistance to emptying of liquids from stomach in humans. Dig Dis Sci. 1988;33:914–918. doi: 10.1007/BF01535984. [DOI] [PubMed] [Google Scholar]
- 23.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- 24.Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2011;286:2365–2374. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiagarajah JR, Verkman AS. Chloride channel-targeted therapy for secretory diarrheas. Curr Opin Pharmacol. 2013;13:888–894. doi: 10.1016/j.coph.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thiagarajah JR, Ko EA, Tradtrantip L, Donowitz M, Verkman AS. Discovery and development of antisecretory drugs for treating diarrheal diseases. Clin Gastroenterol Hepatol. 2014;12:204–209. doi: 10.1016/j.cgh.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel TS, Crutchley RD, Tucker AM, Cottreau J, Garey KW. Crofelemer for the treatment of chronic diarrhea in patients living with HIV/AIDS. HIV AIDS (Auckl) 2013;5:153–162. doi: 10.2147/HIV.S30948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiskerstrand T, Arshad N, Haukanes BI, Tronstad RR, Pham KD, Johansson S, Håvik B, Tønder SL, Levy SE, Brackman D, Boman H, Biswas KH, Apold J, Hovdenak N, Visweswariah SS, Knappskog PM. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med. 2012;366:1586–1595. doi: 10.1056/NEJMoa1110132. [DOI] [PubMed] [Google Scholar]
- 29.Keating N, Keely SJ. Bile acids in regulation of intestinal physiology. Curr Gastroenterol Rep. 2009;11:375–382. doi: 10.1007/s11894-009-0057-8. [DOI] [PubMed] [Google Scholar]
- 30.Camilleri M. Bile Acid diarrhea: prevalence, pathogenesis, and therapy. Gut Liver. 2015;9:332–339. doi: 10.5009/gnl14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. 2015;42:3–11. doi: 10.1111/apt.13227. [DOI] [PubMed] [Google Scholar]
- 32.Krug SM, Schulzke JD, Fromm M. Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol. 2014;36:166–176. doi: 10.1016/j.semcdb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Z, Ding L, Lu Q, Chen YH. Claudins in intestines: Distribution and functional significance in health and diseases. Tissue Barriers. 2013;1:e24978. doi: 10.4161/tisb.24978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner JR, Buschmann MM, Romero-Calvo I, Sailer A, Shen L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol. 2014;36:204–212. doi: 10.1016/j.semcdb.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 37.Cheng P, Yao J, Wang C, Zhang L, Kong W. Molecular and cellular mechanisms of tight junction dysfunction in the irritable bowel syndrome. Mol Med Rep. 2015;12:3257–3264. doi: 10.3892/mmr.2015.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halliez MC, Motta JP, Feener TD, Guérin G, LeGoff L, François A, Colasse E, Favennec L, Gargala G, Lapointe TK, Altier C, Buret AG. Giardia duodenalis induces paracellular bacterial translocation and causes postinfectious visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2016;310:G574–G585. doi: 10.1152/ajpgi.00144.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teshima CW, Meddings JB. The measurement and clinical significance of intestinal permeability. Curr Gastroenterol Rep. 2008;10:443–449. doi: 10.1007/s11894-008-0083-y. [DOI] [PubMed] [Google Scholar]
- 40.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775–G785. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 41.Eherer AJ, Fordtran JS. Fecal osmotic gap and pH in experimental diarrhea of various causes. Gastroenterology. 1992;103:545–551. doi: 10.1016/0016-5085(92)90845-p. [DOI] [PubMed] [Google Scholar]
- 42.Steffer KJ, Santa Ana CA, Cole JA, Fordtran JS. The practical value of comprehensive stool analysis in detecting the cause of idiopathic chronic diarrhea. Gastroenterol Clin North Am. 2012;41:539–560. doi: 10.1016/j.gtc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Camilleri M, Linden DR. Measurement of gastrointestinal and colonic motor functions in humans and animals. Cell Molec Gastroenterol Hepatol. 2016 doi: 10.1016/j.jcmgh.2016.04.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camilleri M. Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol. 2014;592:2967–2980. doi: 10.1113/jphysiol.2014.270892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016 Feb 18; doi: 10.1053/j.gastro.2016.02.031. pii: S0016–5085(16)00222-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol. 2008;295:G260–G272. doi: 10.1152/ajpgi.00056.2008. [DOI] [PubMed] [Google Scholar]
- 47.Peregrin AT, Ahlman H, Jodal M, Lundgren O. Involvement of serotonin and calcium channels in the intestinal fluid secretion evoked by bile salt and cholera toxin. Br J Pharmacol. 1999;127:887–894. doi: 10.1038/sj.bjp.0702615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Camilleri M. Intestinal secretory mechanisms in irritable bowel syndrome-diarrhea. Clin Gastroenterol Hepatol. 2015;13:1051–1057. doi: 10.1016/j.cgh.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montero-Hadjadje M, Elias S, Chevalier L, Benard M, Tanguy Y, Turquier V, Galas L, Yon L, Malagon MM, Driouich A, Gasman S, Anouar Y. Chromogranin A promotes peptide hormone sorting to mobile granules in constitutively and regulated secreting cells: role of conserved N- and C-terminal peptides. J Biol Chem. 2009;284:12420–12431. doi: 10.1074/jbc.M805607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Öhman L, Stridsberg M, Isaksson S, Jerlstad P, Simren M. Altered levels of fecal chromogranins and secretogranins in IBS: relevance for pathophysiology and symptoms? Am J Gastroenterol. 2012;107:440–447. doi: 10.1038/ajg.2011.458. [DOI] [PubMed] [Google Scholar]
- 51.El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18:5151–5163. doi: 10.3748/wjg.v18.i37.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Salhy M, Wendelbo I, Gundersen D. Serotonin and serotonin transporter in the rectum of patients with irritable bowel disease. Mol Med Rep. 2013;8:451–455. doi: 10.3892/mmr.2013.1525. [DOI] [PubMed] [Google Scholar]
- 53.Karaki S, Kuwahara A. Propionate-induced epithelial K(+) and Cl(−)/HCO3(−) secretion and free fatty acid receptor 2 (FFA2, GPR43) expression in the guinea pig distal colon. Pflugers Arch. 2011;461:141–152. doi: 10.1007/s00424-010-0889-y. [DOI] [PubMed] [Google Scholar]
- 54.Iacangelo AL, Eiden LE. Chromogranin A: current status as a precursor for bioactive peptides and a granulogenic/sorting factor in the regulated secretory pathway. Regul Pept. 1995;58:65–88. doi: 10.1016/0167-0115(95)00069-n. [DOI] [PubMed] [Google Scholar]
- 55.Camilleri M. Editorial: fecal granins in IBS: cause or indicator of intestinal or colonic irritation? Am J Gastroenterol. 2012;107:448–450. doi: 10.1038/ajg.2011.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spiller RC. Effects of serotonin on intestinal secretion and motility. Curr Opin Gastroenterol. 2001;17:99–103. doi: 10.1097/00001574-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 57.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Hoffman JM, Tyler K, Maceachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, Sharkey KA, Greenwood-Van Meerveld B, Mawe GM. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–854. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Donowitz M, Binder HJ. Jejunal fluid and electrolyte secretion in carcinoid syndrome. Am J Dig Dis. 1975;20:1115–1122. doi: 10.1007/BF01070754. [DOI] [PubMed] [Google Scholar]
- 61.von der Ohe M, Camilleri M, Kvols LK, Thomforde GM. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med. 1993;329:1073–1078. doi: 10.1056/NEJM199310073291503. [DOI] [PubMed] [Google Scholar]
- 62.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5 hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 63.Houghton LA, Atkinson W, Whitaker RP, Whorwell PJ, Rimmer MJ. Increased platelet depleted plasma 5 hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52:663–670. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 65.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ, Silk DB. The ileal brake--inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25:365–374. doi: 10.1136/gut.25.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spiller RC, Trotman IF, Adrian TE, Bloom SR, Misiewicz JJ, Silk DB. Further characterisation of the 'ileal brake' reflex in man--effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide YY. Gut. 1988;29:1042–1051. doi: 10.1136/gut.29.8.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JW, Park JH, Park DI, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. Subjects with diarrhea-predominant IBS have increased rectal permeability responsive to tryptase. Dig Dis Sci. 2010;55:2922–2928. doi: 10.1007/s10620-009-1094-8. [DOI] [PubMed] [Google Scholar]
- 69.Martinez C, Lobo B, Pigrau M, Ramos L, González-Castro AM, Alonso C, Guilarte M, Guilá M, de Torres I, Azpiroz F, Santos J, Vicario M. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160–1168. doi: 10.1136/gutjnl-2012-302093. [DOI] [PubMed] [Google Scholar]
- 70.Martinez C, Vicario M, Ramos L, Lobo B, Mosquera JL, Alonso C, Sánchez A, Guilarte M, Antolín M, de Torres I, González-Castro AM, Pigrau M, Saperas E, Azpiroz F, Santos J. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol. 2012;107:736–746. doi: 10.1038/ajg.2011.472. [DOI] [PubMed] [Google Scholar]
- 71.Gecse K, Roka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztóczy A, Izbéki F, Fioramonti J, Wittmann T, Bueno L. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591–599. doi: 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]
- 72.Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buhner S, Li Q, Vignali S, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H, Michel K, Schemann M. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425–1434. doi: 10.1053/j.gastro.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, Lee HL, Yoon BC, Choi HS, Sim J, Jang KS. Mast cell number, substance P and vasoactive intestinal peptide in irritable bowel syndrome with diarrhea. Scand J Gastroenterol. 2014;49:43–51. doi: 10.3109/00365521.2013.857712. [DOI] [PubMed] [Google Scholar]
- 75.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barbezat GO, Grossman MI. Intestinal secretion: stimulation by peptides. Science. 1971;174:422–424. doi: 10.1126/science.174.4007.422. [DOI] [PubMed] [Google Scholar]
- 77.Bloom SR, Yiangou Y, Polak JM. Vasoactive intestinal peptide secreting tumors. Pathophysiological and clinical correlations. Ann NY Acad Sci. 1988;527:518–527. doi: 10.1111/j.1749-6632.1988.tb27005.x. [DOI] [PubMed] [Google Scholar]
- 78.Cox TM, Fagan EA, Hillyard CJ, Allison DJ, Chadwick VS. Role of calcitonin in diarrhoea associated with medullary carcinoma of the thyroid. Gut. 1979;20:629–633. doi: 10.1136/gut.20.7.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cherner JA, Jensen RT, Dubois A, O'Dorisio TM, Gardner JD, Metcalfe DD. Gastrointestinal dysfunction in systemic mastocytosis. A prospective study. Gastroenterology. 1988;95:657–667. doi: 10.1016/s0016-5085(88)80012-x. [DOI] [PubMed] [Google Scholar]
- 80.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol. 2002;282:G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 81.Rao AS, Wong BS, Camilleri M, Odunsi-Shiyanbade ST, McKinzie S, Ryks M, Burton D, Carlson P, Lamsam J, Singh R, Zinsmeister AR. Chenodeoxycholate in females with irritable bowel syndrome-constipation: A pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549–1558. doi: 10.1053/j.gastro.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chadwick VS, Gaginella TS, Carlson GL, Debongnie JC, Phillips SF, Hofmann AF. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979;94:661–674. [PubMed] [Google Scholar]
- 83.Gullikson GW, Cline WS, Lorenzsonn V, Benz L, Olsen WA, Bass P. Effects of anionic surfactants on hamster small intestinal membrane structure and function: relationship to surface activity. Gastroenterology. 1977;73:501–511. [PubMed] [Google Scholar]
- 84.Alrefai WA, Saksena S, Tyagi S, Gill RK, Ramaswamy K, Dudeja PK. Taurodeoxycholate modulates apical Cl-/OH- exchange activity in Caco2 cells. Dig Dis Sci. 2007;52:1270–1278. doi: 10.1007/s10620-006-9090-8. [DOI] [PubMed] [Google Scholar]
- 85.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 86.Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22:814–825. e227–228. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lieu T, Jayaweera G, Bunnett NW. GPBA: A G protein-coupled receptor for bile acids and an emerging therapeutic target for disorders of digestion and sensation. Br J Pharmacol. 2014;171:1156–1166. doi: 10.1111/bph.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, Bunnett NW, Corvera CU. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Camilleri M, Shin A, Busciglio I, Carlson P, Acosta A, Bharucha AE, Burton D, Lamsam J, Lueke A, Donato LJ, Zinsmeister AR. Genetic variation in GPBAR1 predisposes to quantitative changes in colonic transit and bile acid excretion. Am J Physiol. 2014;307:G508–G516. doi: 10.1152/ajpgi.00178.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stephen AM, Haddad AC, Phillips SF. Passage of carbohydrate into the colon. Direct measurements in humans. Gastroenterology. 1983;85:589–595. [PubMed] [Google Scholar]
- 91.Barrett JS, Gearry RB, Muir JG, Irving PM, Rose R, Rosella O, Haines ML, Shepherd SJ, Gibson PR. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31:874–882. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 92.Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 93.Mitsui R, Ono S, Karaki S, Kuwahara A. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol Motil. 2005;17:585–594. doi: 10.1111/j.1365-2982.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 94.Treem WR, Ahsan N, Kastoff G, Hyams JS. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr. 1996;23:280–286. doi: 10.1097/00005176-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 95.Camilleri M, Carlson P, Acosta A, Busciglio I, Nair AA, Gibbons SJ, Farrugia G, Klee EW. RNA sequencing shows transcriptomic changes in rectosigmoid mucosa in patients with irritable bowel syndrome-diarrhea: a pilot case-control study. Am J Physiol. 2014;306:G1089–G1098. doi: 10.1152/ajpgi.00068.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lamprecht G, Seidler U. The emerging role of PDZ adapter proteins for regulation of intestinal ion transport. Am J Physiol Gastrointest Liver Physiol. 2006;291:G766–G777. doi: 10.1152/ajpgi.00135.2006. [DOI] [PubMed] [Google Scholar]
- 97.Camilleri M, Carlson P, Acosta A, Busciglio I. Colonic mucosal gene expression and genotype in irritable bowel syndrome patients with normal or elevated fecal bile acid excretion. Am J Physiol Gastrointest Liver Physiol. 2015;309:G10–G20. doi: 10.1152/ajpgi.00080.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary fat induced taurocholic acid promotes pathobiont expansion and colitis in il10 −/− mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jia J, Frantz N, Khoo C, Gibson GR, Rastall RA, McCartney AL. Investigation of the faecal microbiota associated with canine chronic diarrhoea. FEMS Microbiol Ecol. 2010;71:304–312. doi: 10.1111/j.1574-6941.2009.00812.x. [DOI] [PubMed] [Google Scholar]
- 101.Suchodolski JS, Foster ML, Sohail MU, Leutenegger C, Queen EV, Steiner JM, Marks SL. The fecal microbiome in cats with diarrhea. PLoS One. 2015;10:e0127378. doi: 10.1371/journal.pone.0127378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alrefai WA, Tyagi S, Nazir TM, Barakat J, Anwar SS, Hadjiagapiou C, Bavishi D, Sahi J, Malik P, Goldstein J, Layden TJ, Ramaswamy K, Dudeja PK. Human intestinal anion exchanger isoforms: expression, distribution, and membrane localization. Biochim Biophys Acta. 2001;1511:17–27. doi: 10.1016/s0005-2736(00)00366-7. [DOI] [PubMed] [Google Scholar]
- 103.Resta-Lenert SC, Barrett KE. Modulation of intestinal barrier properties by probiotics: role in reversing colitis. Ann N Y Acad Sci. 2009;1165:175–182. doi: 10.1111/j.1749-6632.2009.04042.x. [DOI] [PubMed] [Google Scholar]
- 104.Borody T, Fischer M, Mitchell S, Campbell J. Fecal microbiota transplantation in gastrointestinal disease: 2015 update and the road ahead. Expert Rev Gastroenterol Hepatol. 2015;9:1379–1391. doi: 10.1586/17474124.2015.1086267. [DOI] [PubMed] [Google Scholar]
- 105.Devkota S, Chang EB. Interactions between diet, bile acid metabolism, gut microbiota, and inflammatory bowel diseases. Dig Dis. 2015;33:351–356. doi: 10.1159/000371687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choi MG, Camilleri M, O'Brien MD, Kammer PP, Hanson RB. A pilot study of motility and tone of the left colon in patients with diarrhea due to functional disorders and dysautonomia. Am J Gastroenterol. 1997;92:297–302. [PubMed] [Google Scholar]
- 107.Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499–1506. doi: 10.1111/j.1572-0241.2001.03804.x. [DOI] [PubMed] [Google Scholar]
- 109.Sellin JH. A breath of fresh air. Clin Gastroenterol Hepatol. 2016;14:209–211. doi: 10.1016/j.cgh.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 110.Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011;60:334–340. doi: 10.1136/gut.2009.205476. [DOI] [PubMed] [Google Scholar]
- 111.Posserud I, Stotzer PO, Björnsson ES, Abrahamsson H, Simrén M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802–808. doi: 10.1136/gut.2006.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fine KD, Ogunji F. A new method of quantitative fecal fat microscopy and its correlation with chemically measured fecal fat output. Am J Clin Pathol. 2000;113:528–534. doi: 10.1309/0T2W-NN7F-7T8Q-5N8C. [DOI] [PubMed] [Google Scholar]
- 113.Mosli MH, Zou G, Garg SK, Feagan SG, MacDonald JK, Chande N, Sandborn WJ, Feagan BG. C-Reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:802–819. doi: 10.1038/ajg.2015.120. [DOI] [PubMed] [Google Scholar]
- 114.Schiller LR. Nutrition management of chronic diarrhea and malabsorption. Nutr Clin Pract. 2006;21:34–39. doi: 10.1177/011542650602100134. [DOI] [PubMed] [Google Scholar]
- 115.Sellin J. Dietary dilemmas, delusions, and decisions. Clin Gastroenterol Hepatol. 2014;12:1601–1604. doi: 10.1016/j.cgh.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 116.Hammer HF, Fine KD, Santa Ana CA, Porter JL, Schiller LR, Fordtran JS. Carbohydrate malabsorption. Its measurement and its contribution to diarrhea. J Clin Invest. 1990;86:1936–1944. doi: 10.1172/JCI114927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hammer HF, Hammer J. Diarrhea caused by carbohydrate malabsorption. Gastroenterol Clin North Am. 2012;41:611–627. doi: 10.1016/j.gtc.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 118.Mattar R, de Campos Mazo DF, Carrilho FJ. Lactose intolerance: diagnosis, genetic, and clinical factors. Clin Exp Gastroenterol. 2012;5:113–121. doi: 10.2147/CEG.S32368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jones HF, Butler RN, Brooks DA. Intestinal fructose transport and malabsorption in humans. Am J Physiol Gastrointest Liver Physiol. 2011;300:G202–G206. doi: 10.1152/ajpgi.00457.2010. [DOI] [PubMed] [Google Scholar]
- 120.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- 121.Park YK, Yetley EA. Intakes and food sources of fructose in the United States. Am J Clin Nutr. 1993;58(5 Suppl):737s–747s. doi: 10.1093/ajcn/58.5.737S. [DOI] [PubMed] [Google Scholar]
- 122.Bauditz J, Norman K, Biering H, Lochs H, Pirlich M. Severe weight loss caused by chewing gum. BMJ. 2008;336:96–97. doi: 10.1136/bmj.39280.657350.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Breitenbach RA. 'Halloween diarrhea'. An unexpected trick of sorbitol-containing candy. Postgrad Med. 1992;92:63–66. doi: 10.1080/00325481.1992.11701482. [DOI] [PubMed] [Google Scholar]
- 124.Fernandez-Banares F, Esteve M, Viver JM. Fructose-sorbitol malabsorption. Curr Gastroenterol Rep. 2009;11:368–374. doi: 10.1007/s11894-009-0056-9. [DOI] [PubMed] [Google Scholar]
- 125.Wagner SM, Mekhjian HS, Caldwell JH, Thomas FB. Effects of caffeine and coffee on fluid transport in the small intestine. Gastroenterology. 1978;75:379–381. [PubMed] [Google Scholar]
- 126.Shepherd SJ, Lomer MC, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:707–717. doi: 10.1038/ajg.2013.96. [DOI] [PubMed] [Google Scholar]
- 127.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765–771. doi: 10.1016/j.cgh.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 128.Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, Törnblom H, Simrén M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149:1399–1407. e2. doi: 10.1053/j.gastro.2015.07.054. [DOI] [PubMed] [Google Scholar]
- 129.Marsh A, Eslick EM, Eslick GD. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur J Nutr. 2016;55:897–906. doi: 10.1007/s00394-015-0922-1. [DOI] [PubMed] [Google Scholar]
- 130.Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA American College of Gastroenterology. ACG Clinical Guidelines: Diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656–676. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wahnschaffe U, Ullrich R, Riecken EO, Schulzke JD. Celiac disease-like abnormalities in a subgroup of patients with irritable bowel syndrome. Gastroenterology. 2001;121:1329–1338. doi: 10.1053/gast.2001.29572. [DOI] [PubMed] [Google Scholar]
- 132.Wahnschaffe U, Schulzke JD, Zeitz M, Ulrich R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:844–850. doi: 10.1016/j.cgh.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 133.Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, Shepherd SJ, Muir JG, Gibson PR. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106:508–514. doi: 10.1038/ajg.2010.487. [DOI] [PubMed] [Google Scholar]
- 134.Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the "no man's land" of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–1594. doi: 10.1038/ajg.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Böhn L, Störsrud S, Tornblom H, Bengtsson U, Simrén M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108:634–641. doi: 10.1038/ajg.2013.105. [DOI] [PubMed] [Google Scholar]
- 136.Ramakrishna BS, Mathan M, Mathan VI. Alteration of colonic absorption by long-chain unsaturated fatty acids. Influence of hydroxylation and degree of unsaturation. Scand J Gastroenterol. 1994;29:54–58. doi: 10.3109/00365529409090437. [DOI] [PubMed] [Google Scholar]
- 137.NIAID-Sponsored Expert Panel1. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA, Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Young E, Stoneham MD, Petruckevitch A, Barton J, Rona R. A population study of food intolerance. Lancet. 1994;343(8906):1127–1130. doi: 10.1016/s0140-6736(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 139.Schafer T, Bohler E, Ruhdorfer S, Weigl L, Wessner D, Heinrich J, Filipiak B, Wichmann HE, Ring J. Epidemiology of food allergy/food intolerance in adults: associations with other manifestations of atopy. Allergy. 2001;56:1172–1179. doi: 10.1034/j.1398-9995.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 140.Bischoff S, Crowe SE. Gastrointestinal food allergy: new insights into pathophysiology and clinical perspectives. Gastroenterology. 2005;128:1089–1113. doi: 10.1053/j.gastro.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 141.Nowak-Wegrzyn A, Conover-Walker MK, Wood RA. Food-allergic reactions in schools and preschools. Arch Pediatr Adolesc Med. 2001;155:790–795. doi: 10.1001/archpedi.155.7.790. [DOI] [PubMed] [Google Scholar]
- 142.Yagami T. Allergies to cross-reactive plant proteins. Latex-fruit syndrome is comparable with pollen-food allergy syndrome. Int Arch Allergy Immunol. 2002;128:271–279. doi: 10.1159/000063859. [DOI] [PubMed] [Google Scholar]
- 143.Gawchik SM. Latex allergy. The Mount Sinai J Med. 2011;78:759–772. doi: 10.1002/msj.20281. [DOI] [PubMed] [Google Scholar]
- 144.Carroccio A, Brusca I, Mansueto P, Soresi M, D'Alcamo A, Ambrosiano G, Pepe I, Iacono G, Lospalluti ML, La Chiusa SM, Di Fede G. Fecal assays detect hypersensitivity to cow's milk protein and gluten in adults with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2011;9:965–971. doi: 10.1016/j.cgh.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 145.Simren M, Mansson A, Langkilde AM, Svedlund J, Abrahamsson H, Bengtsson U, Björnsson ES. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–115. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 146.Ford AC, Chey WD, Talley NJ, Malhotra A, Spiegel BM, Moayyedi P. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med. 2009;169:651–658. doi: 10.1001/archinternmed.2009.22. [DOI] [PubMed] [Google Scholar]
- 147.Cash BD, Rubenstein JH, Young PE, Gentry A, Nojkov B, Lee D, Andrews AH, Dobhan R, Chey WD. The prevalence of celiac disease among patients with nonconstipated irritable bowel syndrome is similar to controls. Gastroenterology. 2011;141:1187–1193. doi: 10.1053/j.gastro.2011.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fine KD, Seidel RH, Do K. The prevalence, anatomic distribution, and diagnosis of colonic causes of chronic diarrhea. Gastrointest Endosc. 2000;51:318–326. doi: 10.1016/s0016-5107(00)70362-2. [DOI] [PubMed] [Google Scholar]
- 149.Shah RJ, Fenoglio-Preiser C, Bleau BL, Giannella RA. Usefulness of colonoscopy with biopsy in the evaluation of patients with chronic diarrhea. Am J Gastroenterol. 2001;96:1091–1095. doi: 10.1111/j.1572-0241.2001.03745.x. [DOI] [PubMed] [Google Scholar]
- 150.Chey WD, Nljkov B, Rubenstein JH, Dobhan RR, Greenson JK, Cash BD. The yield of colonoscopy in patients with non-constipated irritable bowel syndrome: results from a prospective controlled US trial. Am J Gastroenterol. 2010;105:859–865. doi: 10.1038/ajg.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kamp EJCA, Kane JS, Ford AC. Irritable bowel syndrome and microscopic colitis: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:659–668. doi: 10.1016/j.cgh.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 152.Kane JS, Rotimi O, Everett SM, Samji S, Michelotti F, Ford AC. Developm,ent and validation of a socring system to identify patients with microscopic colitis. Clin Gastroenterol Hepatol. 2015;13:1125–1131. doi: 10.1016/j.cgh.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 153.Macaigne G, Lahmek P, Locher C, Lesgourgues B, Costes L, Nicolas MP, Courillon-Mallet A, Ghilain JM, Bellaïche G, de Montigny-Lehnardt S, Barjonet G, Vitte RL, Faroux R, Lambare B, Fleury A, Pariente A, Nahon S. Microscpic colitis or functional bowel disease: A French prospective multicenter study. Am J Gastroenterol. 2014;109:1461–1470. doi: 10.1038/ajg.2014.182. [DOI] [PubMed] [Google Scholar]
- 154.Fernandez-Bañares F, Esteve M, Salas A, Forné TM, Espinos JC, Martín-Comin J, Viver JM. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci. 2001;46:2231–2238. doi: 10.1023/a:1011927302076. [DOI] [PubMed] [Google Scholar]
- 155.Fernández-Bañares F, Salas A, Esteve M, Espinós J, Forné M, Viver JM. Collagenous and lymphocytic colitis. evaluation of clinical and histological features, response to treatment, and long-term follow-up. Am J Gastroenterol. 2003;98:340–347. doi: 10.1111/j.1572-0241.2003.07225.x. [DOI] [PubMed] [Google Scholar]
- 156.Carmack SW, Genta RM. The diagnostic value of the duodenal biopsy: a clinico- pathologic analysis of 28,000 patients. Dig Liver Dis. 2010;42:485–489. doi: 10.1016/j.dld.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 157.Mooney PD, Kurien M, Evans KE, Rosario E3, Cross SS4, Vergani P5, Hadjivassiliou M6, Murray JA7, Sanders DS. Clinical and immunologic features of ultra-short celiac disease. Gastroenterology. 2016;150:1125–1134. doi: 10.1053/j.gastro.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 158.Wong BS, Camilleri M, Carlson P, McKinzie S, Busciglio I, Bondar O, Dyer RB, Lamsam J, Zinsmeister AR. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10:1009–1015. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schiller LR, Rivera LM, Santangelo WC, Little KH, Fordtran JS. Diagnostic value of fasting plasma peptide concentrations in patients with chronic diarrhea. Dig Dis Sci. 1994;39:2216–2222. doi: 10.1007/BF02090374. [DOI] [PubMed] [Google Scholar]
- 160.Valentin N, Camilleri M, Altayar O, Vijayvargiya P, Acosta A, Nelson AD, Murad MH. Biomarkers for bile acid diarrhea in functional bowel disorder with diarrhea: a systematic review and meta-analysis. Gut. 2015 Sep 7; doi: 10.1136/gutjnl-2015-309889. pii: gutjnl-2015-309889. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 161.Camilleri M, Busciglio I, Acosta A, Shin A, Carlson P, Burton D, Ryks M, Rhoten D, Lamsam J, Lueke A, Donato LJ, Zinsmeister AR. Effect of increased bile acid synthesis or fecal excretion in irritable bowel syndrome-diarrhea. Am J Gastroenterol. 2014;109:1621–1630. doi: 10.1038/ajg.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Corazza GR, Menozzi MG, Strocchi A, Rasciti L, Vaira D, Lecchini R, Avanzini P, Chezzi C, Gasbarrini G. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98:302–309. doi: 10.1016/0016-5085(90)90818-l. [DOI] [PubMed] [Google Scholar]
- 163.Stotzer PO, Kilander AF. Comparison of the 1-gram (14)C-D-xylose breath test and the 50-gram hydrogen glucose breath test for diagnosis of small intestinal bacterial overgrowth. Digestion. 2000;61:165–171. doi: 10.1159/000007753. [DOI] [PubMed] [Google Scholar]
- 164.Kerlin P, Wong L. Breath hydrogen testing in bacterial overgrowth of the small intestine. Gastroenterology. 1988;95:982–988. doi: 10.1016/0016-5085(88)90173-4. [DOI] [PubMed] [Google Scholar]
- 165.Rhodes JM, Middleton P, Jewell DP. The lactulose hydrogen breath test as a diagnostic test for small-bowel bacterial overgrowth. Scand J Gastroenterol. 1979;14:333–336. doi: 10.3109/00365527909179892. [DOI] [PubMed] [Google Scholar]
- 166.Riordan SM, McIver CJ, Walker BM, Duncombe VM, Bolin TD, Thomas MC. The lactulose breath hydrogen test and small intestinal bacterial overgrowth. Am J Gastroenterol. 1996;91:1795–1803. [PubMed] [Google Scholar]
- 167.Yamini D, Pimentel M. Irritable bowel syndrome and small intestinal bacterial overgrowth. J Clin Gastroenterol. 2010;44:672–675. doi: 10.1097/MCG.0b013e3181ef3476. [DOI] [PubMed] [Google Scholar]
- 168.Pyleris E, Giamarellos-Bourboulis EJ, Tzivras D, Koussoulas V, Barbatzas C, Pimentel M. The prevalence of overgrowth by aerobic bacteria in the small intestine by small bowel culture: relationship with irritable bowel syndrome. Dig Dis Sci. 2012;57:1321–1329. doi: 10.1007/s10620-012-2033-7. [DOI] [PubMed] [Google Scholar]
- 169.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 170.Walters B, Vanner SJ. Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: comparison with 14C-D-xylose and healthy controls. Am J Gastroenterol. 2005;100:1566–1570. doi: 10.1111/j.1572-0241.2005.40795.x. [DOI] [PubMed] [Google Scholar]
- 171.Parisi G, Leandro G, Bottona E, Carrara M, Cardin F, Faedo A, Goldin D, Pantalena M, Tafner G, Verdianelli G, Zilli M AISGE Group. Small intestinal bacterial overgrowth and irritable bowel syndrome. Am J Gastroenterol. 2003;98:2572. doi: 10.1111/j.1572-0241.2003.08686.x. [DOI] [PubMed] [Google Scholar]
- 172.Nguyen GC, Smalley WE, Vege SS, Carrasco-Labra A AGA Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the medical management of microscopic colitis. Gastroenterology. 2016;150:242–246. doi: 10.1053/j.gastro.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 173.Dierksen J, Gonsoulin M, Walterscheid JP. Poor man's methadone: a case report of loperamide toxicity. Am J Forensic Med Pathol. 2015;36:268–270. doi: 10.1097/PAF.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 174.Kashyap PC, Marcobal A, Ursell LK, Larauche M, Duboc H, Earle KA, Sonnenburg ED, Ferreyra JA, Higginbottom SK, Million M, Tache Y, Pasricha PJ, Knight R, Farrugia G, Sonnenburg JL. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology. 2013;144:967–977. doi: 10.1053/j.gastro.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dior M, Delagrèverie H, Duboc H, Jouet P, Coffin B, Brot L, Humbert L, Trugnan G, Seksik P, Sokol H, Rainteau D, Sabate JM. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol Motil. 2016 Apr 5; doi: 10.1111/nmo.12829. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 176.Bellini M, Rappelli L, Blandizzi C, Costa F, Stasi C, Colucci R, Giannaccini G, Marazziti D, Betti L, Baroni S, Mumolo MG, Marchi S, Del Tacca M. Platelet serotonin transporter in patients with diarrhea-predominant irritable bowel syndrome both before and after treatment with alosetron. Am J Gastroenterol. 2003;98:2705–2711. doi: 10.1111/j.1572-0241.2003.08669.x. [DOI] [PubMed] [Google Scholar]
- 177.Franke L, Schmidtmann M, Riedl A, van der Voort I, Uebelhack R, Mönnikes H. Serotonin transporter activity and serotonin concentration in platelets of patients with irritable bowel syndrome: effect of gender. J Gastroenterol. 2010;45:389–398. doi: 10.1007/s00535-009-0167-y. [DOI] [PubMed] [Google Scholar]
- 178.Foley S, Garsed K, Singh G, Duroudier NP, Swan C, Hall IP, Zaitoun A, Bennett A, Marsden C, Holmes G, Walls A, Spiller RC. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology. 2011;140:1434–1443. e1. doi: 10.1053/j.gastro.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 179.Playford RJ, Domin J, Beacham J, Parmar KB, Tatemoto K, Bloom SR, Calam J. Preliminary report: role of peptide YY in defense against diarrhoea. Lancet. 1990;335:1555–1557. doi: 10.1016/0140-6736(90)91378-n. [DOI] [PubMed] [Google Scholar]
- 180.Bilchik AJ, Hines OJ, Adrian TE, McFadden DW, Berger JJ, Zinner MJ, Ashley SW. Peptide YY is a physiological regulator of water and electrolyte absorption in the canine small bowel in vivo. Gastroenterology. 1993;105:1441–1448. doi: 10.1016/0016-5085(93)90149-7. [DOI] [PubMed] [Google Scholar]
- 181.Bilchik AJ, Hines OJ, Zinner MJ, Adrian TE, Berger JJ, Ashley SW, McFadden DW. Peptide YY augments postprandial small intestinal absorption in the conscious dog. Am J Surg. 1994;167:570–574. doi: 10.1016/0002-9610(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 182.Liu CD, Newton TR, Zinner MJ, Ashley SW, McFadden DW. Intraluminal peptide YY induces colonic absorption in vivo. Dis Colon Rectum. 1997;40:478–482. doi: 10.1007/BF02258396. [DOI] [PubMed] [Google Scholar]
- 183.Zhang H, Yan Y, Shi R, Lin Z, Wang M, Lin L. Correlation of gut hormones with irritable bowel syndrome. Digestion. 2008;78:72–76. doi: 10.1159/000165352. [DOI] [PubMed] [Google Scholar]
- 184.Moriya R, Shirakura T, Hirose H, Kanno T, Suzuki J, Kanatani A. NPY Y2 receptor agonist PYY(3–36) inhibits diarrhea by reducing intestinal fluid secretion and slowing colonic transit in mice. Peptides. 2010;31:671–675. doi: 10.1016/j.peptides.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 185.Han B. Correlation between gastrointestinal hormones and anxiety-depressive states in irritable bowel syndrome. Exp Ther Med. 2013;6:715–720. doi: 10.3892/etm.2013.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceralnociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 187.Schiller LR, Sellin JH. Diarrhea. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 10. Elsevier; New York: 2015. pp. 221–241. [Google Scholar]